-
A variety of essential nutrients are required by crops, and ensuring the effective supply of elements in agricultural production is an important factor in crop yield and fruit quality[1]. Currently, due to the excessive pursuit of high crop yields, sufficient supplementation of nitrogen (N), phosphorus (P), and potassium (K) has been the main focus in agricultural application[2−4], while investigations on medium/micro mineral nutrients such as calcium (Ca), magnesium (Mg), and zinc (Zn) have lagged behind. Although plants do not need these elements in large quantities compared to N, P, and K[5], their insufficient supply can seriously affect plant growth and development. Examples of issues caused by mineral deficiencies include: Ca deficiency-induced tomato fruit cracking[6] and apple bitter pox disease[7], Mg deficiency-caused yellowing of wheat leaves[8], and Zn deficiency-caused small leaf disease in apple trees[9].
Ca, as an essential mineral element for human health[10], is also a central regulator in plant growth and development and response to environmental stress, where it plays an important role in cell structure and physiological/biochemical metabolic processes[11]. It is now generally accepted that Ca in the soil is mainly transported to the plant root surface in the form of Ca2+ cations via mass flow, diffusion, and root interception, and it is transferred to the root ducts via the apoplastic or symplast pathway[12]. Subsequently, it is transported to above-ground organs, mainly by the process of transpiration pull[13]. However, there are differences in the magnitude of transpiration pull among plant tissues, especially in young leaves and fruits, where transpiration is significantly weaker than that of a large number of mature leaves[14]. As a result, Ca deficiencies often appear in young leaves and fruits, leading to their decreased commercial value. For fruit-bearing crops, such as apples, this phenomenon has a serious financial impact on agricultural producers[15].
High Ca demand is required across the lifespan of apple trees, especially in the later stages of fruit development, and the calcium content in the apple fruit correlates strongly with their quality, resistance to postharvest storage, and shelf life[16]. For example, Ca-deficient apples usually show reduced storage resistance, and the trees can be susceptible to diseases such as bitter pox, water heart disease, and pox spot, which seriously affects the health of the tree and the quality of the fruit[17]. Therefore, sufficient Ca supplementation is crucial for ensuring the quality of apple fruit and thereby the economic value of apples trees[18].
Numerous calcium fertilizers have been used with apples and during their whole growth period[19]. Many studies have shown that regular application of traditional calcium fertilizers not only improves fruit quality and extends the storage period of the fruit, but also prevents and solves various physiological diseases caused by Ca deficiency[20]. Furthermore, calcium fertilizers improve the commercial value of the fruit and increases economic returns. Additionally, traditional Ca fertilizers improve the Ca content of the fruit. However, the poor solubility of Ca and the tendency of Ca to calcify in both soil and plant tissues, results in low fertilizer uptake efficiencies, high loss of fertilizer, and negative environmental consequences[21]. Furthermore, the element-antagonism effects between Ca2+ and macronutrients (K+,
${\text{NH}^+_4} $ Recently, numerous interests have been attracted by the use of fertilizers with nanomaterials. These 'nanofertilizers' offer a potentially new approach to the delivery of elements to plants[23]. The high volume to surface ratio of nanomaterials reduces the dosage and increases efficiency compared to conventional fertilizers[24,25]. Nanofertilizers can enhance the absorption of fertilizer by plants and reduce fertilizer loss and fixation in the soil. Many nutrient nanofertilizers have been developed and applied at the laboratory and field scale[26]. For example, Ca-based nanomaterials have been designed for crop Ca supplementation nano calcium carbonate[27], nano calcium phosphate[28]. However, efficient Ca supplementation for fruit and fruit trees remains to be explored, probably due to the problems of poor Ca solubility, the tendency for calcification, and element-antagonism. In this study, we synthesized Ca-encapsulated carbon dots (Ca-CDs) with excellent solubility and calcification-free characteristics and proved that they are an excellent source of Ca in that the Ca is efficiently utilized by both leaves and fruits. Furthermore, the passive transport feature of Ca-CDs prevents element-antagonism effects. Finally, field application demonstrated that applying Ca-CDs by both leaf-spraying and root irrigation significantly enhanced Ca content in apple fruits.
-
The Ca-CDs were prepared using a hydrothermal synthesis method. Typically, 30 g of calcium gluconate and 30 g of urea were dissolved in 1,000 mL of double distilled water (ddH2O), and the mixed solution was placed in the hydrothermal synthesis autoclave reactor and reacted at 180 °C for 4 h to obtain a crude solution of Ca-CDs. The solid samples of Ca-CDs were obtained with lyophilization for characterization and experimental application.
The UV-Vis absorption and fluorescence spectra of Ca-CDs were measured on the spectrophotometer (Nicolet iS5) and fluorescence spectrofluorometer, respectively. The morphology was observed using a JEOL JEM 2100 Plus transmission electron microscope (TEM), and the size of the Ca-CDs was measured using Nano Measurer 1.2 software. The XRD spectrum was measured using a Rigaku Smartlab SE instrument. The component and surface functional groups of Ca-CDs were analysed using X-ray photoelectron spectrometer (XPS, Thermo Scientific K-Alpha) and Fourier transform infrared spectrophotometer (FT-IR, Nicolet IS5), respectively. The images to measure calcification were taken before and after the addition of 1 g sodium carbonate (Na2CO3) to both the raw material solution (calcium gluconate and urea, 1 mL) and the crude Ca-CD solution (1 mL) in cuvettes.
Plant material and growth conditions
-
In this experiment, tissue culture plantlets of Malus domestica var. 'Royal Gala', 'Orin' apple calli, and the model plant Arabidopsis thaliana were used as the laboratory research materials. Tissue culture plantlets of 'Royal Gala' were propagated for four weeks on proliferation medium containing Murashige and Skoog (MS) medium supplemented with 0.5 mg/L 6-benzylaminopurine (6-BA), 0.2 mg/L 1-naphthlcetic acid (NAA), 0.1 mg/L gibberellins (GA), 30 g/L sucrose and 0.8% agar. Robust and uniformly grown tissue culture 'Royal Gala' plantlets were transferred to rooting medium containing 1/2 MS, 2 mg/L indole-3-acetic acid (IAA), 20 g/L sucrose and 0.8% agar, and cultured under light for four weeks. The rooted plantlets were domesticated and transferred to cavity trays for cultivation.
The 'Orin' apple calli were grown on MS medium containing 1.5 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), 0.4 mg/L 6-BA, 30 g/L sucrose and 0.8% agar, and incubated at 25 °C in the dark, with successions every 15 d.
All Arabidopsis plants used in this study were Columbia (Col-0) Arabidopsis seeds. They were sterilised with 2% sodium hypochlorite (NaClO) for 10 min and washed three times with sterile ddH2O. After sterilisation, the seeds were vernalized at 4 °C for 3 d and then sown on MS medium and placed in an incubator for 10 d for later treatments.
Uptake, transport, and distribution of Ca-CDs
-
The rooted tissue culture plantlets of 'Royal Gala' were transferred to Hoagland solution containing 500 mg/L Ca-CDs and incubated for 2 d. The control groups were cultured with Hoagland solution alone. The elemental composition of Hoagland is shown in Supplemental Table S1. Then, the LSM880 (Zeiss, Jena, Germany) was used to observe the distribution of Ca-CDs in roots, stems and leaves of seedlings. 'Orin' apple calli were incubated for 4 h at 24 °C and 4 °C in an aqueous solution containing Ca-CDs (500 mg/L), collected by centrifugation, and washed 2–3 times with ddH2O and then transferred to a LSM880 confocal microscope for observing fluorescence change.
To investigate whether Ca-CDs entry into plant cells is dependent on calcium ion channels, and the effects of element-antagonism on Ca-CDs uptake by plants, we used calcium channel inhibitors (lanthanum (III) chloride, LaCl3) and calcium chelator (ethylene glycol tetraacetic acid, EGTA). 'Orin' apple calli were pre-treated with 5 mM LaCl3 and 5 mM EGTA, respectively. After pre-treatment for 2 h, Ca-CDs (500 mg/L) were added to the treatment solution and the 'Orin' apple calli were incubated for 2 h. The fluorescence of Ca-CDs was observed using the LSM880.
To study the fate of Ca-CDs in seedlings, the 'Royal Gala' apple seedlings were transferred to Hoagland solution containing 500 mg/L Ca-CDs for 20 d and then transferred to Hoagland solution without Ca-CDs for 30 d. Subsequently, the fluorescence of Ca-CDs in the seedlings were measured by the LSM880.The excitation and emission wavelengths of the detection signal were 405 nm and 498 nm, respectively.
Ca deficiency and exogenous Ca-CDs treatment in Arabidopsis
-
Arabidopsis seedlings with uniform growth phenotypes were irrigated with Hoagland nutrient solution (with or without Ca element) for two weeks. Then, Arabidopsis cultured with Ca-free Hoagland solution were divided into three groups. Group T1: the seedlings grown continuously in a calcium-deficient environment; group T2: the seedlings treated with 1,000 mg/L Ca-CDs; and group T3: the seedlings treated with 5 mM CaCl2. The Arabidopsis seedlings were harvested after 10 d of the above treatments for following experiments.
Measurement of chlorophyll content, MDA content, and relative conductivity (RCD)
-
Leaf chlorophyll content was determined using the 95% ethanol immersion method[29]. The absorbance were measured at 665 nm and 649 nm. Determination of chlorophyll content was made using the following formulas: chlorophyll a content (Ca) = 13.95 × A655 − 6.88 × A649, chlorophyll b content (Cb) =24.96 × A649 − 7.32 × A665, and chlorophyll content = Ca + Cb.
The Malondialdehyde (MDA) content was determined by the thiobarbituric acid (TBA) method to reflect plant cellular stress damage. The absorbance at 532 and 600 nm were measured, and the MDA content was calculated according to Wang et al.[30] RCD values were obtained using a conductivity meter.
Field experiments
-
Field trials were conducted at the standard orchard of Taian, Shandong Province, China (from June 1, 2022 to October 1, 2022). Five-year-old Fuji apple trees were used for the trials, and three trees with similar growth size and location were selected as replicates in each group. The apple trees were treated at 15-d intervals under the premise that the concentration and dosage used were consistent for each treatment. The treatments were divided in two ways: foliar spraying and root irrigation. Spraying included the following three treatments: spraying with clear water (T1), spraying with the original solution of raw materials (calcium gluconate and urea) before synthesis as a positive control (T2), and spraying with 3,000 mg/L Ca-CDs (T3). Each tree was sprayed with 600 mL of the corresponding solutions containing 0.05% of Tween-80. Before the root irrigation treatments, a hole of 30 cm × 30 cm × 30 cm (20 cm from the root neck) was dug under each tree. In each hole, 2 L of the corresponding solution was poured. Two root irrigation treatments were set up, one with raw material solution (T4 group) and the other with 3,000 mg/L Ca-CDs (T5 group).
Determination of fruit weight, hardness, soluble sugars, and titratable acids
-
Fruit sampling was conducted once a month until apple harvest. For each treatment, six fruits per tree were picked to detect the fruit weight. The transverse diameter (mm) and longitudinal diameter (mm) of apple fruits were measured separately using Vernier calipers. The hardness of apples was determined using a Stable Micro Systems TA. XT plus mass spectrometer. An equal amount of pulp was taken from each sample apple, and the juice droplets were squeezed out on an electronic refractometer to determine the soluble solids content.
Total soluble sugars were analyzed spectrophotometrically at 620 nm. About 100 mg of apple tissue was grounded by adding 1 mL ddH2O, and transferred to a 1.5 mL tube at 95 °C for 10 min, followed by centrifugation at 8,000 g for 10 min. The supernatant was measured by the anthrone colorimetric method[31].
A 5 g sample of apple tissue was grounded, and transferred to a 100 mL volumetric flask that was attached to a scale and shaken well. The titration was performed using a calibrated 0.01 M sodium hydroxide solution, and the amount of sodium hydroxide titrant was recorded to calculate the titratable acid content.
Determination of calcium content and cell wall materials
-
Fresh plant tissue samples were oven dried at 80 °C for 48 h. Next, the dried samples were grounded and sieved, and 0.3 g of the samples were accurately weighed and digested overnight by adding 5 mL of concentrated sulfuric acid. Then the Ca2+ content was determined using an atomic absorption spectrophotometer.
A fresh sample of apple fruit (1.0 g) was ground, added to 25 mL of 95% ethanol, heated in a boiling water bath for 30 min, and centrifuged at 8,000 g for 15 min. The sample was then washed three times with 95% ethanol (25 mL), once with methanol-chloroform (1:1, V/V), and once with acetone. Then, the supernatant was discarded. The material obtained after drying was the crude cell wall material (CWM)[32]. Water-soluble pectin (WSP), chelated pectin (CSP), and alkali-soluble pectin (ASP) were obtained by extracting CWM with ddH2O, 0.5 M imidazole solution, and 50 mM sodium carbonate (Na2CO3), respectively[33]. The pectin content was determined by the carbazole colorimetric method, and galacturonic acid was used as a standard[34]. The dried CWM (5 mg) was added 0.5 mL ddH2O and 0.75 mL concentrated sulfuric acid. The solution was left for 30 min before being centrifuged (8,000 g, 4 °C, 10 min). The supernatant was used for the determination of cellulose content, measured at 620 nm. The cellulose content was determined by the anthrone colorimetric method with glucose as the standard[34].
Statistical analysis
-
All experimental data were set up in three or more independent biological and technical replicates and analyzed for significant differences using DPS statistical software. Graphs of the data were achieved using GraphPad Prism 8.0 software.
-
Initially, to obtain Ca-encapsulated CDs (Ca-CDs) with good solubility and calcification-free properties, calcium gluconate and urea were hydrothermally treated at 180 °C for 4 h (Fig. 1a). A brown crude Ca-CDs solution was obtained, and no participates were found after autoclaving, suggesting that the Ca element was either in solution or encapsulated in Ca-CDs. The as-prepared Ca-CDs were directly characterized after the removal of moisture with vacuum freeze-drying. (Fig. 1 & Supplemental Fig. S1). As shown in Fig. 1b, the Ca-CDs exhibited obvious blue fluorescence (FL) with a maximum emission peak at 420 nm upon the excitation at 340 nm. And TEM images of Ca-CDs showed that they had good monodispersity with an average diameter of 1.83 nm (Fig. 1c). In agreement with the results of thermogravimetric analysis (Supplemental Fig. S1), the Ca concentration in Ca-CDs was measured to be 1.5 w% according to the atomic absorption spectrum, suggesting their intrinsic Ca-doped property.
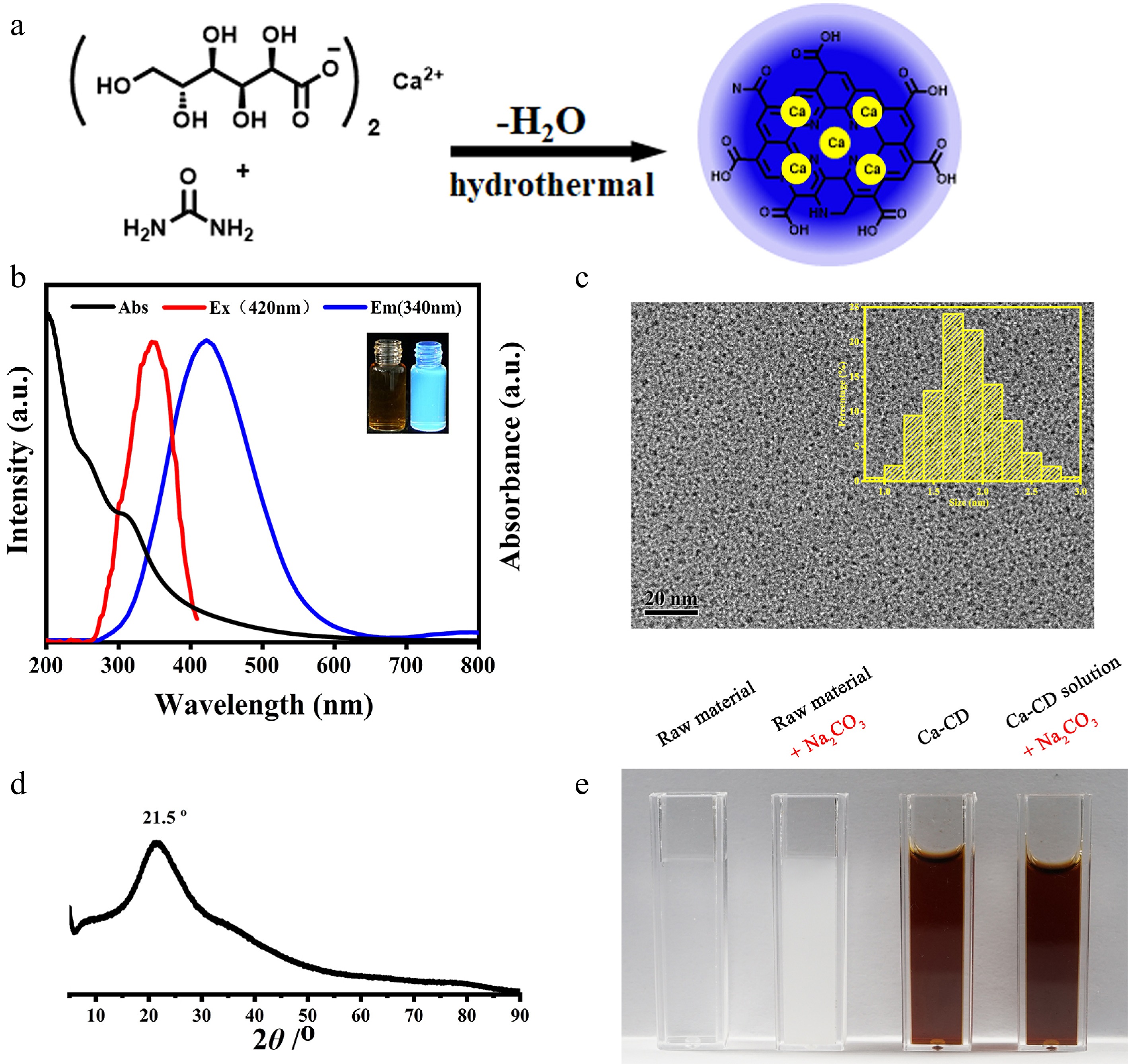
Figure 1.
Characterization of Ca-encapsulated CDs. (a) Illustration of the synthesis of Ca-CDs. (b) UV-Vis absorption, FL excitation and emission spectra of Ca-CDs (insert: Images of Ca-CDs under daylight and 365 nm exciting light illustration). (c) TEM images of Ca-CDs (insert: Size distribution of as-prepared Ca-CDs). (d) XRD spectrum of Ca-CDs. (e) Images after the addition of Na2CO3 into the raw material solution and the crude Ca-CDs solution.
Subsequently, to investigate the available forms of Ca elements in Ca-CDs, XRD and XPS assays were performed. The XRD profile of Ca-CDs (Fig. 1d) proved the amorphous feature of Ca-CDs with a single broad peak at 2θ = 21.5° indexing [002] plane of carbon, and no corresponding peaks of Ca(OH)2, CaCO3, and other inorganic Ca species were found, suggesting no Ca-based inorganic nanomaterials, such as nano-CaCO3, were generated. Furthermore, to exclude the possibility of free Ca2+ cations, Na2CO3 was added into the solution of raw materials and the crude Ca-CDs solution without any purification, respectively. Interestingly, no significant changes were observed for the Ca-CDs solution, while an amount CaCO3 white precipitate was generated in the control group (Fig. 1e), suggesting the calcification-free property of Ca-CDs. In addition, the XPS spectrum (Supplemental Fig. S1c–f) indicated that the Ca-CDs surface contained a high amount of C, O, and N elements, but few Ca elements, since only a poor peak signal for Ca2p (284.8 eV) was found. These results suggest that Ca elements are probably encapsulated inside the Ca-CDs, not on their surface.
The translocation and passive transport of Ca-CDs into plant cells
-
Calcium supplements require translocation and transport into cells. In this respect, there are some similarities but also many differences between traditional Ca nutrients and Ca-CDs. Ca2+ in the soil enters the plant root cells, crosses the endothelium and thin-walled xylem cells and enters the xylem tissue for longitudinal distance transportation[35]. In this process, this plastic extracellular pathway is believed to be the main route for entering xylem cells, and the symplastic route via ion channels-involved active transport is also reported to contribute to this process[36]. In the current study, the blue fluorescence feature of Ca-CDs (Fig. 2) was employed to visibly track Ca-CDs translocation and transport inside seedlings. As is shown in Fig. 2a, after 2d hydroponic cultivation by Ca-CDs, the light-excited blue Ca-CDs could be observed successively in the roots, stems, and leaves of seedlings. The fluorescent Ca-CDs could be observed in both cells and apoplasts of root hairs and leaves, suggesting that besides the plastic extracellular pathway, the symplastic route also played a crucial role in Ca-CDs uptake. It is worth noting that, distinguished from Ca2+ cations, Ca-CDs can hardly be recognized and translocated to cells by ion channel proteins in the symplastic route, suggesting that Ca-CDs are hardly transferred into cells via the active transport pathway.
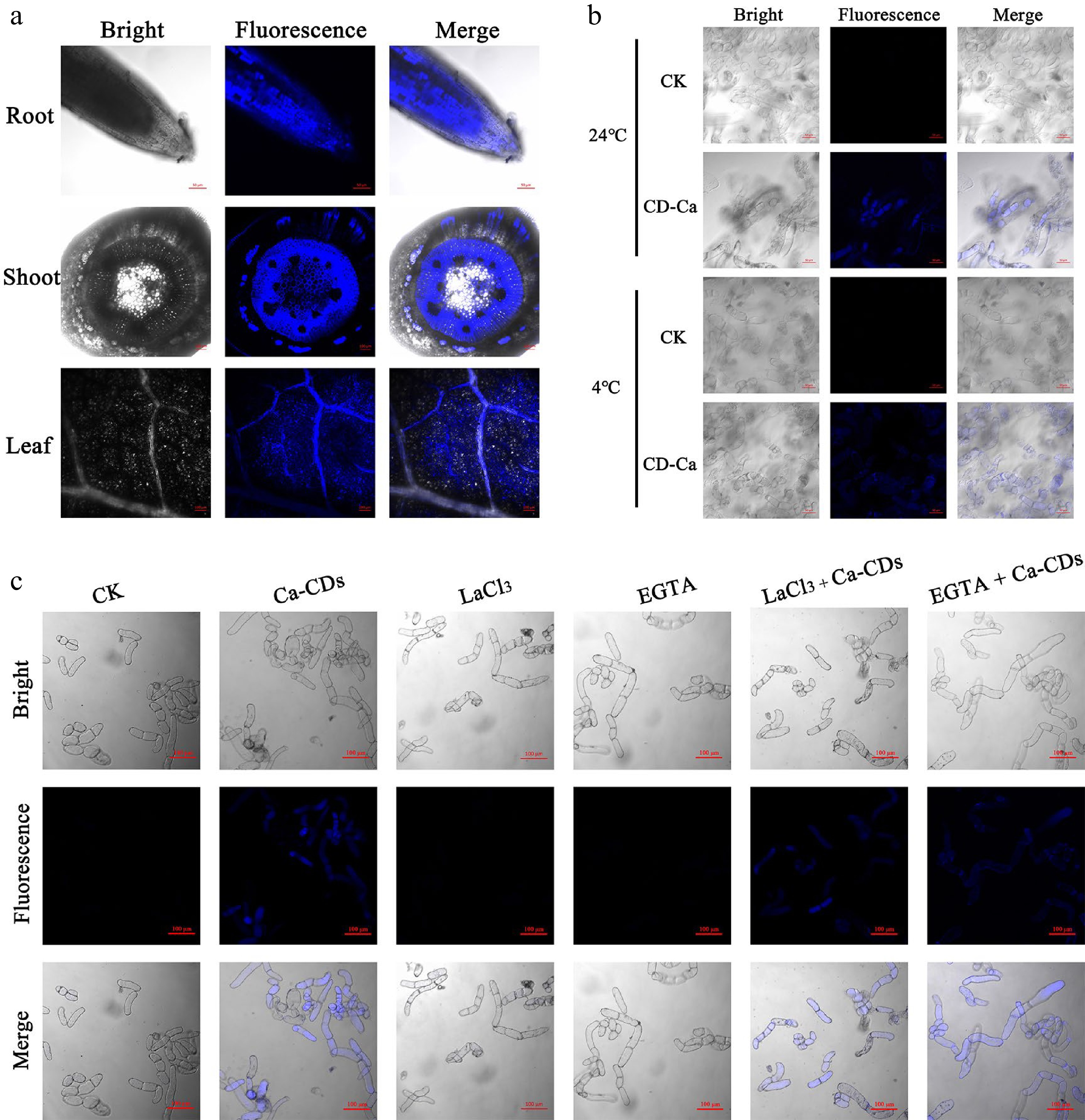
Figure 2.
Uptake, transport and distribution of Ca-CDs. (a) Confocal microscopy images of Ca-CDs localization in the roots, stems and leaves of apple seedlings. (b) Uptake of Ca-CDs in 'Orin' apple calli cells at 24 °C and 4 °C. (c) Images of calli cells after different treatments with Ca-CDs, EGTA and LaCl3. The confocal images were obtained with the LSM880 confocal microscope (405 nm excitation, 410−585 nm collection).
It is known that active transport of Ca2+ in plants requires energy consumption, and this transport is inhibited at low temperatures[37]. Accordingly, to further explore the Ca-CDs capacity of transporting into/out of the cells, 'Orin' apple calli was investigated under different temperature treatments. Inspiringly, Ca-CDs were successfully observed inside calli cells at both room temperature and low temperature (4 °C) (Fig. 2b). These results indicated that Ca-CDs entered into cells via a passive transport pathway, which was an ion channel-independent, energy-free process. What's more, this passive transport pathway was further established using the calcium-ion-chelating agent EGTA and the calcium ion channel blocker LaCl3 (Fig. 2c). Notably, the passive transport proporty of Ca-CDs can hardly inhibited by element-antagonism effects. Furthermore, in order to study the effects of Ca-CDs on seedling growth, apple seedlings were incubated in a Ca-CDs solution (500 mg/L) for 20 d. It was found that the seedlings were still able to grow normally without wilting, and new leaves grew on the top of the plant stem (Supplemental Fig. S2a). Meanwhile, the blue fluorescence Ca-CDs in seedlings disappeared after 30 d during Ca-CDs-free hydroponic cultivation (Supplemental Fig. S2b−c), suggesting that Ca-CDs could be biologically degraded in the plant body, and available Ca could sustain slow release and provide long-term Ca-nutritional support.
Ca-CDs alleviate calcium deficiency stress
-
It has been shown that calcium deficiency in plants exhibits unfavourable phenotypes such as dwarf growth, root growth inhibition, and faded greenish-yellow leaves[38]. To demonstrate whether Ca-CDs can provide supplemental Ca for plant growth, Arabidopsis seedlings were treated with Ca-CDs. As shown in Fig. 3, compared to seedlings grown under normal conditions (CK), Ca-deficient seedlings (T1) showed significant growth inhibition, including yellowing leaves, even scorched leaf margins, and significant increases in stress-related leaf MDA content (Fig. 3d) as well as RCD (Fig. 3e). Furthermore, after the addition of Ca-CDs solution (1,000 mg/L, T2 group) to these Ca-deficient seedlings, significant recovery phenotypes were obtained, including increased shoot and root fresh weight (Fig. 3b), chlorophyll content enhancement (Fig. 3c), and significantly reduced MDA content (Fig. 3d) and RCD content (Fig. 3e). Also, these results matched the results obtained for the calcium chloride-treated group (CaCl2, 5 mM, T3 group).
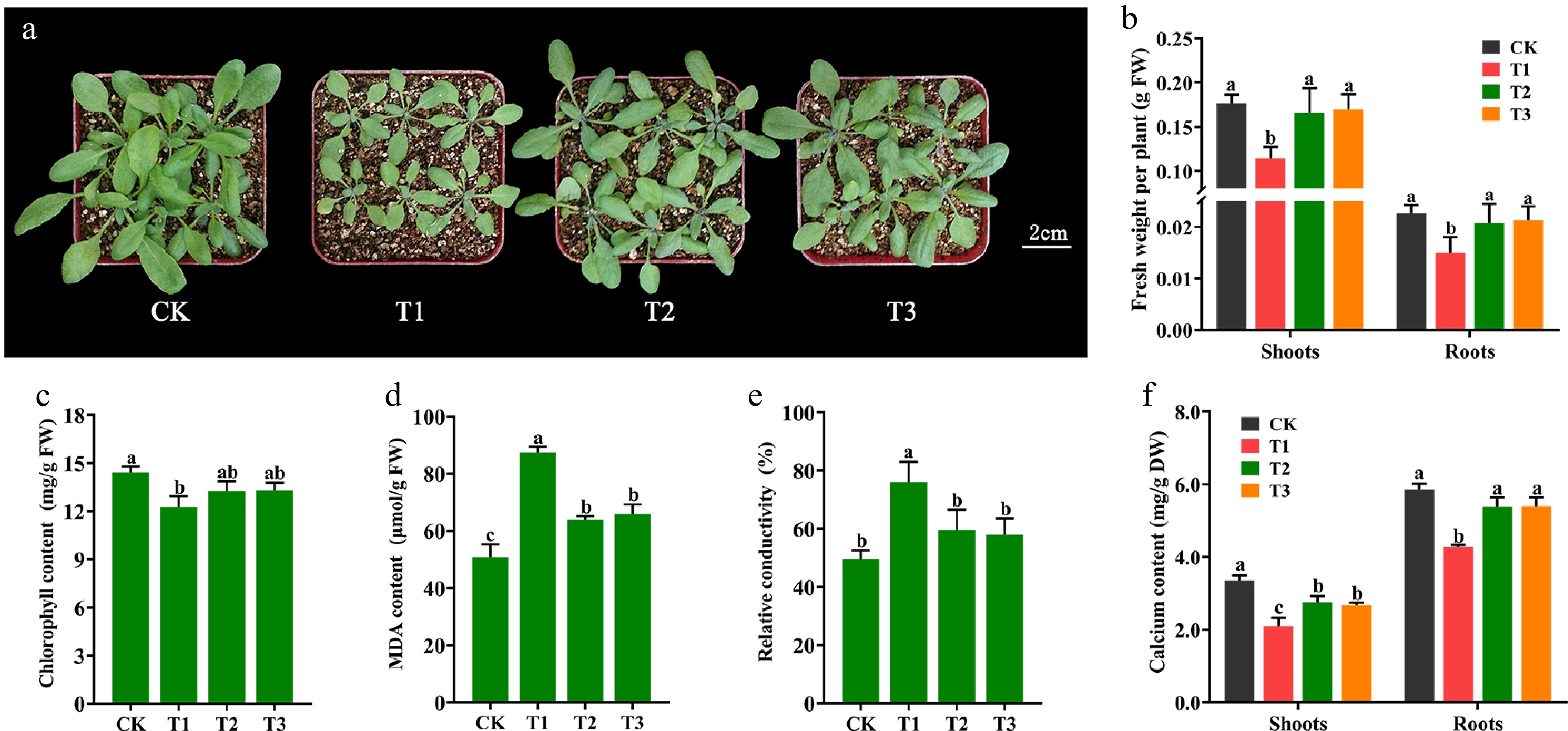
Figure 3.
Effect of Ca-CDs treatment on Arabidopsis seedlings. (a) Arabidopsis seedlings grown under Ca-sufficient (CK) and -deficient (T1) conditions for 14 d. T1 seedlings were then treated with 1,000 mg/L Ca-CDs (T2) and 5 mM CaCl2 (T3) solution for 10 d, respectively. (b) Fresh weight, (c) chlorophyll content, (d) MDA content, (e) RCD and (f) calcium content of Arabidopsis seedlings.
To evaluate more visually the calcium replenishment capacity of Ca-CDs, the Ca content in Arabidopsis seedlings was measured. Consistent with the envisioned results, the T2-treated Arabidopsis had higher Ca content in both shoots (30.95%) and roots (26.22%) than the T1 treatment, almost recovering to normal levels (Fig. 3f). These results suggest that Ca-CDs treatment can alleviate the stress caused by Ca deficiency.
Exogenous application of Ca-CDs enhanced Ca content in apple fruit
-
Calcium concentration is a crucial nutrition factor in apple fruits, and calcium is also closely related to fruit quality, resistance to storage, and resistance to physiological diseases. Ca deficiency may cause bitter pox disease in apples as well as a diminished level of fruit appearance and quality[39]. To verify whether Ca-CDs could promote Ca uptake in apple fruits, 5-year-old Fuji apple trees were supplied with Ca-CDs with the following five treatments: T1, foliar spraying with water; T2, foliar spraying with raw material solution; T3, foliar spraying with Ca-CDs solution; T4, root irrigation with raw material solution; and T5, root irrigation with Ca-CDs solution.
First, to demonstrate the effective supplementation of Ca that Ca-CDs can induce, Ca content in apple fruit was examined at different time periods. The apple fruit samples were picked once a month to evaluate the effect of Ca-CDs application in the field. As shown in Fig. 4b, the Ca contents decreased sharply at the young fruit stage, and then slowly as the fruit matured, which was in agreement with previous reports[40]. As expected, the Ca content of apples in the T3 and T5 groups presented much higher Ca contents than those in the T1, T2, and T4 groups (Fig. 4d). Inspiringly, the foliar spraying (T3) and root irrigation (T5) of Ca-CDs yielded approximately 2.5- and 3.0-fold enhancements in Ca content of apple fruits relative to the T2 and T4 groups, respectively. Interestingly, the application of raw material (calcium gluconate and urea) solution (T2 and T4 groups) presented no obvious changes in apple Ca content compared to the control group (T1), suggesting the significant Ca supplementation in T3 and T5 groups was caused by the application of Ca-CDs.
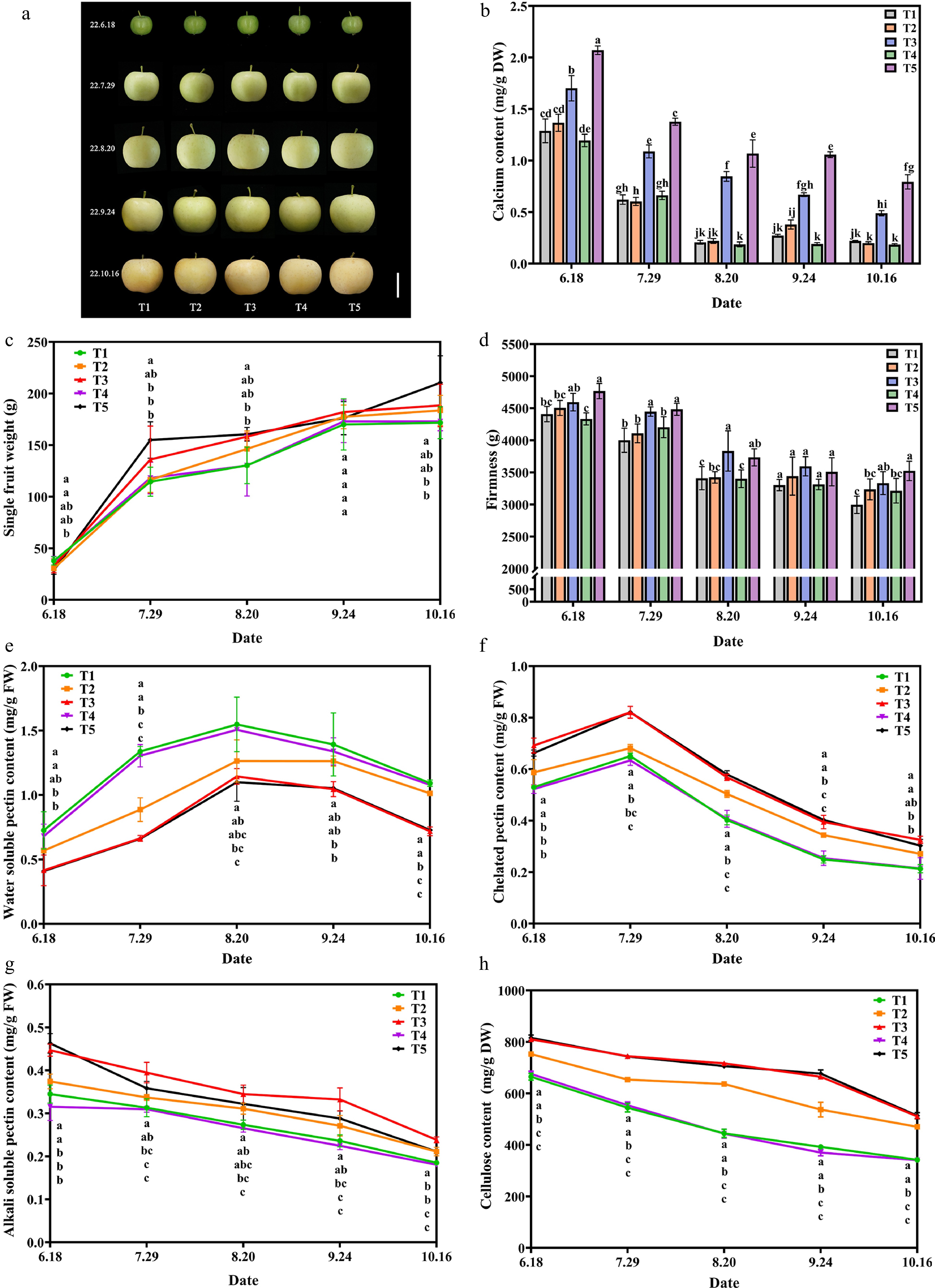
Figure 4.
Effect of exogenous Ca-CDs on apple fruit. (a) Phenotypic images, (b) calcium content, (c) weight per fruit, (d) fruit hardness, (e) water-soluble pectin, (f) chelated pectin, (g) alkali-soluble pectin and (h) cellulose content of apple fruits under different treatments.
By tracking the data of apples during treatment, it was found that, over the fruit expansion period, the fruit weight increased continuously in all groups (Fig. 4c). Encouragingly, in August, apple fruit weights in T3 and T5 groups with Ca-CDs application were higher than that in T1 and T4 groups without Ca-CDs application, with an increase of 21.34% and 23.29%, respectively.
Fruit firmness is an important indicator of fruit quality. Pre-harvest calcium treatment on apples could significantly increase fruit hardness and the content of fruit soluble solids and improve fruit quality[41]. As shown in Fig. 4d, the hardness of apples decreases as the fruit ripens. Importantly, the fruit hardness of Ca-CDs-treated fruits was higher than that of control groups throughout the whole process. By the time of harvesting (in October), the fruit hardness in T3 and T5 groups (Ca-CDs treatment) was significantly higher than that of the control fruit, and 18.2% and 15.4% enhancements were found compared to those in the T2 and T4 groups, respectively. Taken together, both foliar spraying and root irrigation of Ca-CDs increased fruit hardness. Moreover, besides fruit weight and hardness, significant enhancements of the content of soluble solids, soluble sugars, and titratable acids were found in Ca-CDs-treated groups, improving fruit quality (Supplemental Table S2).
Studies have shown that Ca2+ in fruits facilitates the formation of bridges between pectin residues and adhesions between cells, maintaining the stability of the cell wall and increasing stress resistance[42]. As shown in Fig. 4e−g, the treatments of Ca-CDs (T3 and T5 groups) led to the decrease in water-soluble pectin contents and the accumulation of chelated pectin and alkali-soluble pectin in apple fruits. These results showed that Ca-CDs-supplemented Ca combined with pectin to form Ca-pectin cross-linked polymers to improve the mechanical strength of the cell wall, thus improving fruit hardness and preventing physiological diseases caused by calcium deficiency. Furthermore, both foliar spraying and root irrigation of Ca-CDs (T3 and T5 groups) also increased the cellulose content, one of the main components of the fruit cell wall of fruits, and inhibited cellulose degradation during fruit growth and development (Fig. 4h).
Collectively, these results proved the excellent fruit-targeted Ca-supplementation capacity of Ca-CDs. Previous studies have indicated that nano-calcium can promote plant calcium uptake and increase biomass by affecting root conformation[43]. Moreover, nano-calcium can increase the calcium content in nectarine fruits, alleviate cracking and improve fruit quality[44]. Similar to the above research, we designed and developed Ca-CDs with good calcium supplementation effect, especially for apple fruits, Ca-CDs treatment not only increased fruit calcium content but also improved fruit quality in various aspects such as hardness, water-soluble pectin and chelated pectin. Meanwhile, unlike other calcium nanoparticles, the non-calcifying and highly soluble properties of Ca-CDs make them potentially useful for a wide range of applications in agriculture.
-
In this study, we developed Ca-encapsulated carbon dots (Ca-CDs) with promising characteristics of being calcification-free and having good solubility and mobility. The Ca-CDs showed an excellent capacity for Ca supplementation and alleviating Ca-deficiency stress. Furthermore, orchard application either by foliar spraying or root irrigation proved Ca-CDs could significantly enhance Ca content in apple fruits with obvious enhancements in apple quality, including fruit weight, firmness, chelated pectin, and so on. Our results illustrate the huge potential of Ca-encapsulated nanomaterials for efficient Ca supplementation of fruit crops.
This work was financially supported by Major basic research projects of Shandong Province (ZR2020ZD43), National Natural Science Foundation of China (22207067), Natural Science Foundation of Shandong Province (ZR2021MC073), China Agriculture Research System of MOF and MARA (CARS-27), Taishan Industrial Leading Talents (LJNY202026) and Shandong Province Key R&D Program (2022TZXD008-02).
-
The authors declare that they have no conflict of interest.
- Supplemental Fig. S1 (a) The FT-IR spectrum of Ca-CDs. (b) Thermogravimetric analysis of Ca-CDs under O2 condition. (c) XPS full scan spectrum of Ca-CDs. (d-f) High-resolution XPS spectra of C 1s (d), N 1s (e) and O 1s (f) of Ca-CDs.
- Supplemental Fig. S2 (a) Seedlings treated by Ca-CDs for 20 days. Confocal microscopy images of seedlings treated by Ca-CDs for 20 days (b) and then followed by Ca-CD-free treatment for 30 days(c).
- Supplemental Table S1 Formula table of Hoagland nutrient solution.
- Supplemental Table S2 Effects of different treatments on fruit quality.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang G, Liu X, Liu Y, Zhang Z, You C, et al. 2023. Efficient enhancement of calcium content in apple by calcium-encapsulated carbon dots. Fruit Research 3:10 doi: 10.48130/FruRes-2023-0010
Efficient enhancement of calcium content in apple by calcium-encapsulated carbon dots
- Received: 11 February 2023
- Accepted: 13 March 2023
- Published online: 18 April 2023
Abstract: Efficient application of mineral nutrient supplements, such as calcium (Ca), is essential for ensuring crop yield and fruit quality. Calcium, however, usually has poor solubility, the tendency for calcification and element-antagonism, hindering its efficient absorption, which usually results in Ca deficiency in young leaves and fruits. Here, we developed Ca-encapsulated carbon dots (Ca-CDs) as nanofertilizers. These Ca-CDs have excellent solubility, great mobility, resistance to calcification, and no element-antagonism. Furthermore, this technique has an excellent capacity for being an efficient mode of Ca supplementation. Our results showed that the Ca-CDs promoted efficient Ca nutrition uptake and enhanced fruit Ca concentration. Furthermore, orchard applications proved that both foliar spraying and root irrigation of Ca-CDs could significantly enhance Ca content in apple fruit. This study informs the great agricultural application potential of nanomaterials in fruit calcium supplement.
-
Key words:
- Calcium /
- Apple /
- Nanomaterials /
- Fertilizer













