-
Temperature is perhaps the most fundamental of the environmental factors affecting the growth of tree-fruit species. In production horticulture its primary effect is on the quality and yield of the fruit but a secondary effect is on the geographical distribution of each tree-fruit species. As the meteorological effects of global warming intensify, with more frequent occurrences of extreme weather events, including of exceptionally hot weather, the adverse influences of heat stress (HS) on fruit growth and agricultural production are becoming more serious[1]. Fruit is an important component of a healthy human diet, providing our bodies not only with abundant vitamins and minerals but also with a wide range of secondary metabolites that help them respond to numerous disorders and diseases[2]. In the field, HS leads not only to decreases in fruit yield and in fruit quality, but it also has negative effects on human dietary balance. Therefore, it is worthwhile to further elucidate the responses and adaptation mechanisms of tree-fruit species to HS.
High temperatures can influence fruit trees at multiple levels, including morphological, physiological and molecular. At the phenological level, high temperatures can decrease flowering, premature fruit drop and the incidence of fruit deformities and it can also slow fruit growth. While at the physiological level, HS can affect the rates of photosynthesis and transpiration, and the concentrations of various hormones and soluble osmolytes. HS activates defense mechanisms in fruit trees to mitigate damage though such mechanisms as induction of antioxidant enzymes, heat shock transcription factors (HSFs) and heat shock proteins (HSPs)[3−6]. Plants evolve both resistance and adaptation mechanisms dealing with high temperatures. Heat resistance mechanisms include heat avoidance and heat tolerance. In fruit trees, heat resistance usually involves heat tolerance, which means plants have ways either to reduce or to repair damage caused by HS through changes in metabolism or production of proteins, that help maintain normal physiological activities.
-
Chloroplast structure is susceptible to disruption by high temperatures, with the outer and thylakoid membranes being especially vulnerable. After treating grape seedlings to high temperature (45 °C) for 3 h, the thylakoid lamellae of the leaf chloroplasts became loose and disordered, with many bulky lipid species and vacuolation appearing in the chloroplast interior[7]. High temperatures can also damage the cell membrane, affecting membrane protein structure, increasing the content of unsaturated fatty acids and altering membrane permeability. Heat treatments increase the liposomes in mesophyll cells of pear, immobilizing chromatin in the nucleus and disrupting membrane systems, especially those of the chloroplast thylakoid membrane and the mitochondrial inner membrane[8].
Growth and development
-
A suitable temperature is one of the key determinants of healthy growth and development in a fruit tree. When temperatures exceed the optimal range for a particular tree-fruit species, depending on phenological stage, a whole range of growth effects will appear, including poor pollination or fertilization, slowed fruit growth and early senescence of the leaves. For example, the percentage germination of peach pollen is reduced, or stops altogether, after 5 h of treatment at 35 °C[9]. Sweet cherry pollen mother cells, treated at 35 °C for 4 h during meiosis, increased the proportion of abnormal polyploids to 14-times that of the untreated controls. Also, some pollen disintegrated, anthers desiccated, and the remaining pollen did not germinate in isolated culture[10].
HS can also cause sunburn and wilting of the fruit, both of which impair fruit quality. Fruit sunburn is associated with chlorophyll degradation in the epidermis, disruption of cellular compartmentalization and a loss of balance between the intracellular antioxidant system and the metabolism of superoxide anion radicals, leading to oxidation of polyphenolic compounds and fruit skin browning[11, 12]. Sunburn can be divided into three stages: browning, necrosis and photo-oxidation; the first two being most common. The most common sunburn, browning, is a sub-lethal injury that usually results in yellow/brown spots on the fruit skin[13]. In the early stages of sunburn in apple, the pericarp shows browning, partial disintegration of its vesicle-like membrane structure and indistinct mitochondrial perimeters[14]. Necrotic sunburn is the most obvious. It is caused by high temperatures and strong light, and usually manifests as brown/black necrotic specks on the skin, which in severe cases can cause fruit cracking and drying[15, 16]. When the surface temperature of apple rises above 52 ± 1 °C, even for only for 10 min, the skin cells die and brown/black necrotic spots appear on the sunny side of the fruit. Later, the spots become sunken as the underlying cells die and collapse[17]. When 'Cabernet Sauvignon' grapes are sunburned, pale yellow streaks appear first on the fruit surface, this turns to a darker yellow with dotted crinkling on the sunny side of the fruit. Moreover, with the aggravation of sunburn, black necrotic spots appeared on the fruit peel, the center of the sunburned area sinks and even shrinks[18].
A relationship also exists between high temperature stress and fruit color. High temperatures can inhibit the biosynthesis and transport of anthocyanins in kiwifruit, resulting in poor coloration of red-fleshed kiwifruit[19]. Studies have shown continuous HS leads not only to poor coloration in berry but also can affect fruit quality in kinnow, inhibiting sugar accumulation and lowering anthocyanin content[20−22]. With the gradual progress of research into the effects of high temperatures, there is more fundamental information on the molecular mechanisms through which HS affects fruit coloration. Under high temperatures, heat shock transcription factors MdHSF3b/4a in apple are able to specifically bind the heat shock element (HSE) on MdBBX24 promoter, which in turn activates the expression of MdBBX24, thereby inhibiting the synthesis of anthocyanin in the pericarp and causing poor fruit coloration[23]. At high temperatures, microRNA858 expression is induced in red-fleshed kiwifruit, inhibiting the expression of AaMYBC1, so making the MBW complex unable to form and failing to activate the expression of the late anthocyanin synthesis genes, thus limiting the accumulation of anthocyanin and preventing normal coloration[24].
-
Photosynthesis a key life process in plants, and it is also one of the physiological processes most susceptible to temperature change. Photosynthesis involves two photosystems, photosystem I (PSI) and photosystem II (PSII), which are the smallest units in higher plants that absorb solar radiation for primary reactions. It is PSII that is more likely to be inactivated at high temperatures. HS can degrade the oxygen evolving complex (OEC) of PSII, upsetting the balance of electron flow from OEC to the PSII acceptor side along the direction of PSI reaction center[25, 26]. In grapes, a temperature of 35 °C has no effect on photosynthesis, but 40 °C damages the PSII donor side and reaction center, and raising the temperature to 45 °C, the PSII acceptor side is also adversely affected. This indicates the PSII donor side and reaction center in grape leaves are more sensitive to high temperatures[27]. Moreover, PSII may exhibit organizational characteristics in its tolerance to high temperatures. For example, in apple, under the same high-temperature treatment, the OEC in the fruit skin is more degraded than that in the leaves. This indicates that PSII in the fruit skin is more sensitive to high temperatures than that in the leaves[28]. However, more evidence is still needed to confirm the generality of this feature of PSII.
HS affects not only the light response of photosynthesis, but it also damages the structure of photosynthetic organs. HS may cause damage of the D1 and/or to the D2 proteins[29]. For example, the dual stresses of high temperature and high light can lead to the impairment of D1 protein and to a reduction in photosynthetic rate in the PSII reaction center of sand pear leaves. This suggests the reduction of D1 protein content induced by HS and high light is mainly attributable to the suppression of D1 protein synthesis at translation stage[30]. Similarly, during HT and HL, both D1 protein and FTSH proteases were damaged, affecting the photosystem II function of bayberry[31]. Chlorophyll fluorescence (Fv/Fm) is a physiological parameter related to heat tolerance[32]. Xu et al. found an evaluation method for the heat tolerance of grape, based on their chlorophyll fluorescence parameters. Using this, they were able to evaluate a large number of grapevines quickly[33]. This method overcomes the defects of measuring malondialdehy (MDA) content and electrical conductivity linkage (EL) s, which are only able to be used in small samples.
Osmolytes
-
A key adaptation mechanism of plants to abiotic stress is the accumulation of soluble osmolytes[34]. These substances can reduce the osmotic potential in cells, thereby alleviating the harmful impacts of stress. Different tree-fruit species may respond to HS by accumulating a range of soluble osmolytes, including sugar, sugar alcohol (polyol), proline, and soluble proteins[35, 36]. Among these, sugars play significant roles in growth and development as well as in biological defense responses in fruit trees. Under high temperature and other stresses, high levels of fructose and glucose can be accumulated in peach and citrus fruits to resist these stresses, and grape berry can mitigate stress injury by synthetizing raffinose[37−39]. The osmolyte proline is a small molecule and, normally, the content of free proline in fruit trees is relatively low but, when exposed to HS, the free proline content increases significantly, enhancing the heat tolerance of the fruit. Under high-temperature treatments, proline accumulates rapidly in pear and jujube leaves, reducing both the intra- and extracellular osmotic potential and maintaining cellular ion homeostasis, thus alleviating the damage to fruit trees from high-temperature stress[40]. Similarly, even a short heat treatment, induces the accumulation of large amounts of proline in the leaves of plum, to help cope with unfavorable conditions[41].
Soluble proteins are essential nutrients in fruit trees, while also maintaining cellular osmotic balance and helping the trees resist heat damage. Under high temperature conditions, the soluble protein concentrations in both apple and mango leaves increased significantly, improving the water retention capacity of cells and thus enhancing the heat resistance of fruit trees[42, 43]. Similarly, in strawberry leaves, soluble proteins are strongly accumulated under HS, enhancing their heat tolerance[44].
Hormones
-
Hormones are the principal agents in the endogenous signals that regulate the physiological and molecular responses of plants to the environment[45]. HS can induce changes in hormone concentration and activity and so affects the corresponding physiological processes, so as to enhance the adaptability of tree-fruit species to adversity. Abscisic acid (ABA) is one of the most important hormones in plants, and is involved in their response to a range of abiotic stresses. Wang et al. found that the ABA content increased significantly in leaves and helped grapes to resist the serious HS after heat acclimation on grape seedlings for 1 h[46]. Moreover, external application of ABA can mitigate the harmful influences of HS on growth and development of fruit trees. The damage caused by HS to the leaves of 'Moldova' grapes can be significantly reduced when ABA is sprayed on them. The ABA increased the maximum photochemical efficiency of PSII as well as the actual photochemical efficiency and improved the overall photosynthetic performance index[47]. Another study showed that under HS, ABA can also lead to the accumulation of soluble proteins and regulate carbohydrate levels and energy status, thereby improving plant heat tolerance[48].
Salicylic acid (SA) is a phenolic compound that exists widely in higher plants. It participates in many physiological processes, often acting as a signal molecule to indicate stress intensity. Under abiotic stress, SA can improve heat tolerance of plants by activating the antioxidant mechanisms and stimulating the accumulation of osmolytes and HSPs[49]. Studies indicate that SA can improve photosynthesis in fruit trees suffering HS. Exogenous applications of SA can stabilize the active state of Rubisco in grapevine leaves, maintain PSII activity and increase proline and heat-excited protein content, thereby increasing the heat tolerance of grapes[50]. The mechanisms through which SA regulates responses to HS in fruit trees needs further investigation.
Secondary metabolites
-
The synthesis of most secondary metabolites occurs as an intermediate outcome of primary carbon metabolism through pathways such as of phenylpropionic acid, shikimic acid, mevalonate or methyl erythritol phosphate (MEP)[51]. Phenolic compounds, such as flavonols, anthocyanins, and proanthocyanidins, are essential components of plant secondary metabolism with a range of physiological effects, such as enhancing resistance to abiotic stress factors. Anthocyanins, as antioxidants, can reduce reactive oxygen species (ROS) induced by abiotic stress and so help maintain cellular osmotic homeostasis and help plants adjust to a harsh environment - such as to high temperature[52]. For example, green-skinned pear is more susceptible to temperature changes than red-skinned pear, and also more vulnerable to HS[53]. In addition, quercetin, a flavonoid, has a significant effect on enhancing plant resistance to adversity. Under high temperature environments, external applications of quercetin can alleviate photoinhibition in kiwifruit and improve fruit quality[54].
Isoprene is another family of plant secondary metabolites, which is synthesized in the mevalonate pathway. As an antioxidant, isoprene can inhibit the synthesis of ROS while also enhancing the stability of vesicle membranes to maintain the activity of PSII under HS and protect leaves from photo-oxidative damage, thus enhancing the heat tolerance of plants[55−57]. The more isoprene is synthesized and released under stress, the less is the impact on photosynthesis[58]. It has been shown that after high temperature treatment, grapes with high monoterpene emissions had better PSII stability and higher photosynthetic rates than those with low monoterpene emissions[59].
-
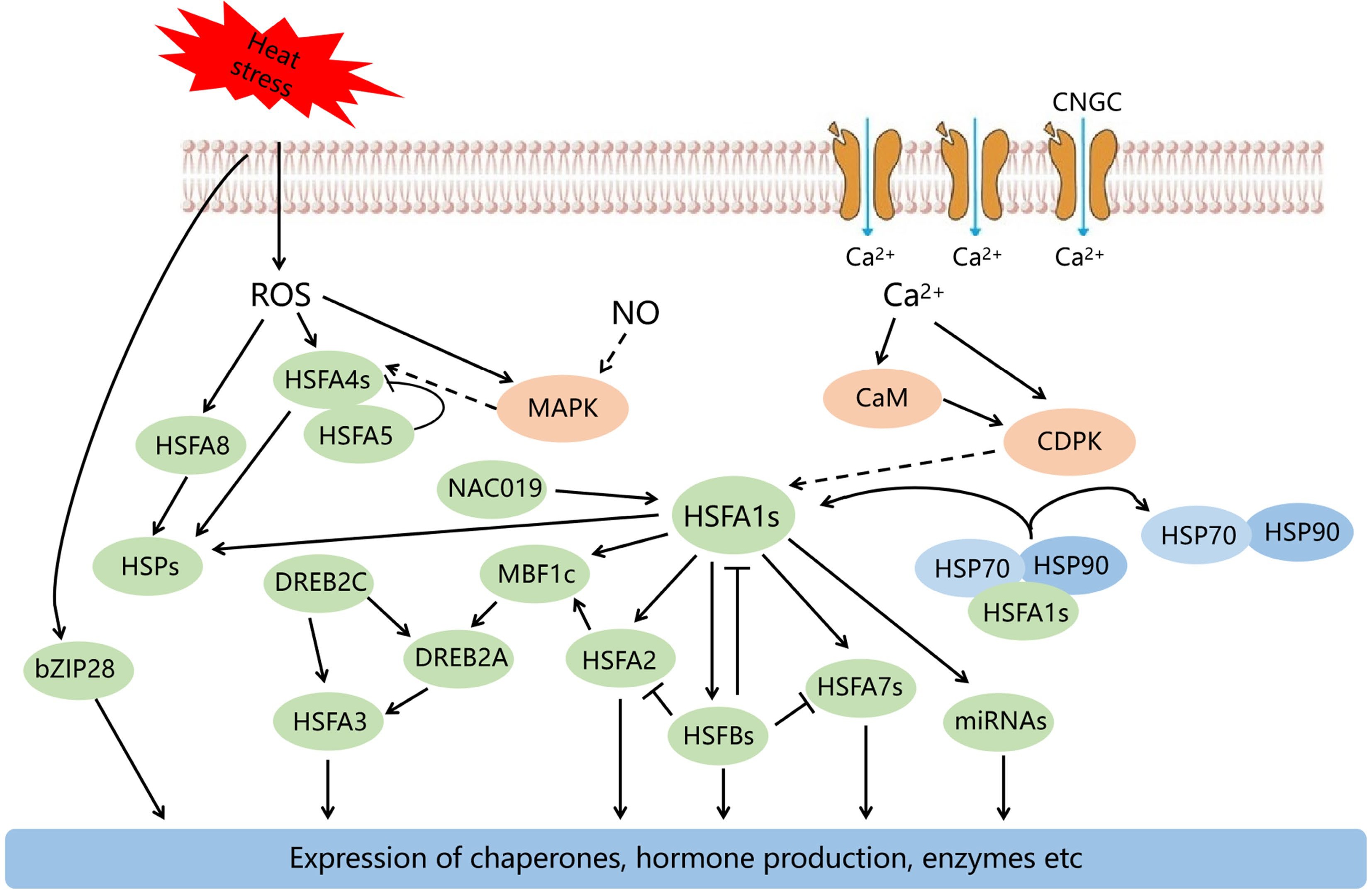
Plants have evolved a range of response mechanisms to improve heat tolerance over long periods of evolution. The responses to HS include a series of processes such as perception of HS signals, signal transduction, and transcriptional regulation (Fig. 1)[1]. In this review, we are focusing as much as possible on studying the responses of fruit trees to HS.
Perception of high temperature signal
-
When plants are subjected to HS, various signaling ions and molecules such as cellular Ca2+, ROS, NO and other molecules recognize the high temperature signals and induce the synthesis of functional proteins as well as the accumulation of metabolites through a series of signal transduction pathways, thus protecting against damage caused by HS.
Calcium (Ca) is an essential nutrient for plant growth and development. It has a number of extremely important physiological functions in maintaining cell morphology, regulating ion homeostasis and osmotic pressure. Moreover, the Ca2+ ion, as a second messenger, can respond to various biological and abiotic stresses. Some studies have demonstrated that HS alters cell membrane fluidity and affects the structure and function of proteins localized on membranes, such as Ca2+ channels, thus triggering cyclic nucleotide-gated ion channel (CNGC) to mediate Ca2+ inward flow. Inflow of Ca2+ is a key process that stimulates genetic expression to cope with HS and promote the expressions of HSPs and the acquisition of heat tolerance in plants[1, 60−62]. After heat acclimation of grapevine leaves, the Ca2+ content in the leaf cytosol increased but the amount of Ca2+ in the vacuoles and intercellular spaces was less than in the controls. Compared with the controls, the content of Ca2+ in the cytosol of mesophyll cells after heat treatment was higher, and the chloroplast was not damaged. This indicates the Ca2+ homeostasis in mesophyll cells of grapevines leaves is extremely significant in response to temperature stress[63]. The Ca2+ ion also plays a prominent role in signal transduction pathways, and Ca2+ signaling can be transduced through Ca2+-binding proteins such as calmodulin (CaM), calcium-dependent protein kinase (CDPK), and CBL[64−66]. CDPK is an important Ca2+ sensor in the stress responses of fruit trees. For instance, the expression of MaCDPK7 gene was strongly increased after heat treatment in banana[67].
The ROS in plants mainly include: hydrogen peroxide (H2O2), superoxide anion (O2−), singlet oxygen (1O2), and hydroxyl radical (OH). As a major signal molecule, ROS can participate in early responses to HS and plays a dual role to respond to stress in plants[68]. Excessive content of ROS causes peroxidation of membrane lipids and toxic influences on cells. These are the main factors of cell damage by HS. However, the accumulation of small amounts of ROS can also activate plant antioxidant defense systems and accumulate numerous antioxidant enzymes, among these, catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD) can scavenge excessive ROS and mediate ABA signaling and expression of HSFs, helping plants to mitigate HS damage[36]. In addition, the accumulation of H2O2 in plants is also induced by stress. For instance, in citrus, O2− and H2O2 accumulate rapidly under HS, causing deactivation of the PSII reaction center.[69]. H2O2 not only plays multiple roles as a signaling molecule of stress resistance in plants but also can enhance the yield and quality of fruit. For example, applying H2O2 to wax apple can significantly increase photosynthesis and fruit set in tree-fruit species, as well as increasing fruit anthocyanin content[70].
Nitric oxide (NO) plays a significant physiological function not only in heat signal transduction but also in plant responses to HS. Recent studies indicate NO not only interacts with lipid peroxides, which promote lipid peroxidation, increasing membrane stability and reducing electrolyte leakage of cells, and maintaining PSII activity and mitigating photo-oxidative damage under HS[71−73]. When plants suffer HS, the amount of NO increases significantly to help the plant adapt to the stress. For example, during HS in citrus leaves, NO levels increase significantly, which triggers a series of responses such as increases in antioxidant enzymes to reduce stress damage[74]. Although there is increasing evidence for the functional importance of NO in high-temperature signaling, the mechanisms by which NO is regulated in response to HS of fruit trees are not clear.
Heat shock transcription factors
-
The expression of heat shock transcription factors (HSFs) is induced by HS, and can activate the transcription and expression of downstream genes and other target genes by recognizing and binding to HSEs, which have significant influences on heat tolerance of plants[75, 76]. The number of HSF members varies among species. For instance, strawberry and peach have 17 members, citrus has 18, grape has 19, Chinese white pear has 29, Pyrus communis has 33 and apple has 25. Moreover, the basic structure of HSFs is relatively conservative across species.
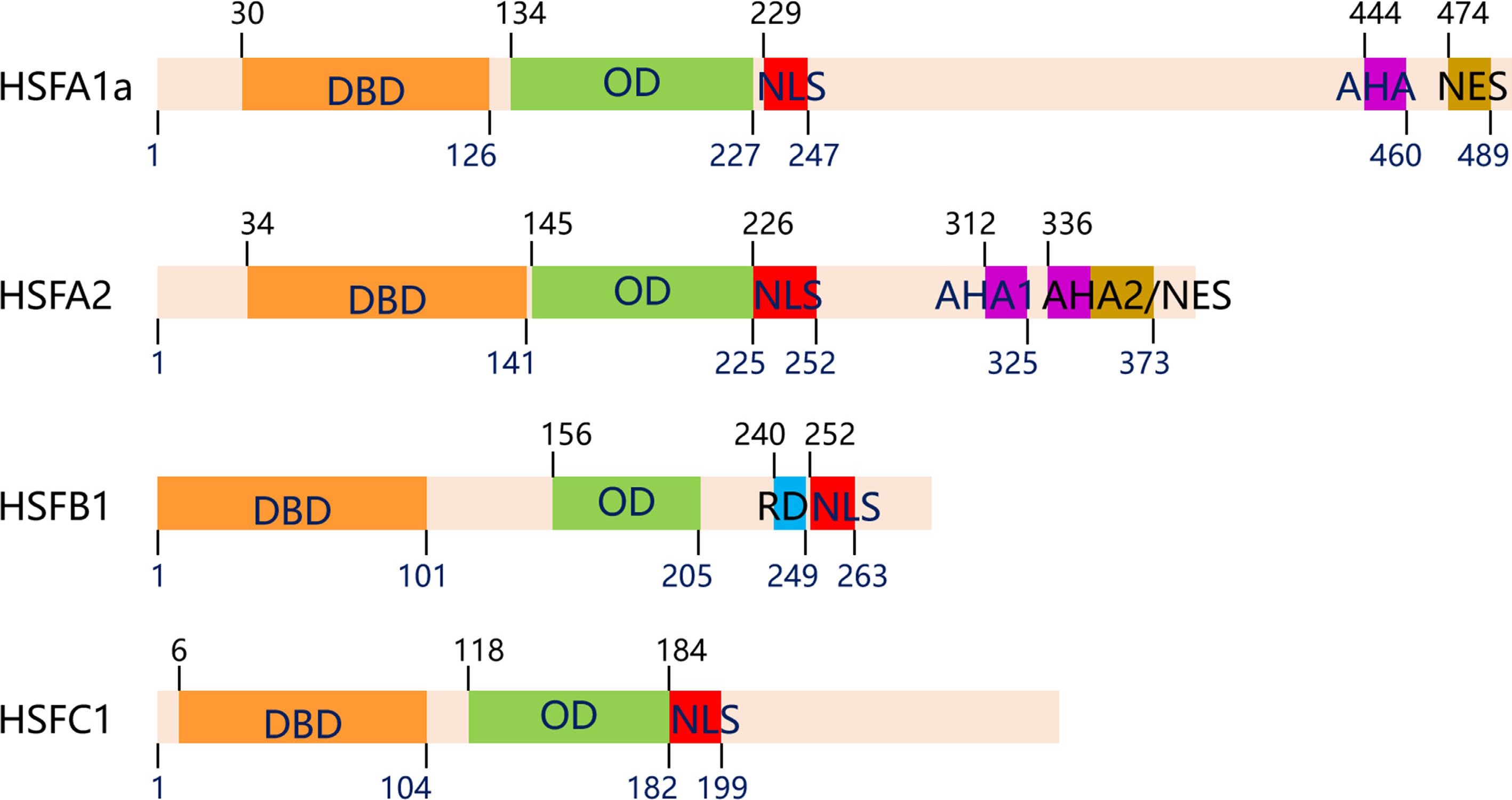
The core structural domains of HSF include the DNA-binding domain (DBD), oligomerization domain (OD or HR-A/B), and nuclear localization signal (NLS). The DBD is a highly conserved segment, located at the N-terminal of the HSF, which is composed of three helical structures (H1, H2 and H3) and four antiparallel β-folds (β1, β2, β3 and β4). The highly conserved structure of helix-turn-helix (H2-T-H3) in the hydrophobic center of the DNA binding domain can accurately recognize the HSE sequence (5'-GAAnnTTC-3'). The oligomerization domain (OD), located at the C-terminal of HSF, is made up of two hydrophobic heptapeptide repeats A and B (HR-A/B), and connected to the DBD region by fragments of 15 to 80 alkaline amino acids[75]. The NLS is necessary for protein import into the nucleus and consists of a cluster of alkaline amino acid residues, most of which neighbor the HR-A/B at the C-terminal. Other functional modules of HSF include the nuclear export signal (NES) and the C-terminal domain (CTD). The activation domain, also known as the AHA motif, is particularly high in hydrophobic and acidic amino acid residues, with great variability and transcriptional activation[75, 76]. The genes of plant HSFs have wide structural complexity and functional diversity. According to the quantity of amino acid residues between HR-A/B, HSFs can be classified into three major categories, A, B, and C (Fig. 2)[77−79]. It is worth noting that HSFB members, other than HSFB5, contain a special tetrapeptide –LFGV- in the C-terminal domain, which acts as a repressor domain[76].
The 'master regulator' HSFA1, is crucial in the regulatory network of heat stress response (HSR)[80]. HSFA1s can directly regulate several heat stress-related transcription factors, including HSFA2, HSFA7a, HSFBs and MBF1c[81]. The function of HSFA1s was first verified in Arabidopsis, where four HSFA1 homologous genes are present. In recent years, the functions of HSFA1 members in horticultural crops has also been verified. HSFA1a improves pollen viability and fertility in transgenic tomatoes by inhibiting ROS accumulation, regulating protein repair and degradation to maintain cellular homeostasis and pollen heat tolerance[82]. In addition, the expressions of FvHSFA1d, FvHSFA2a and FvHSFA3a genes in strawberry (Fragaria vesca) showed an increasing trend at high temperature (42 °C )[83].
HSFA2 is similar to HSFA1 in structure. Its expression is induced by stress and is thought to be a heat-stress induced enhancer. HSFA2 is a key regulator in responses to stresses in plants and can form heterologous oligomeric complexes with HSFA1. HSFA2 not only induces the expression of HSP-like molecular chaperones but also structural genes, such as galactinol synthase1 (GOLS1) and APX2 to help plants withstand HS[39, 84]. In apples, the expression of most MdHSFs is induced when exposed to natural high temperature for long periods, especially MdHSFA2a, MdHSFA2b, MdHSFA3b and MdHSFA3c whose expression levels are significantly increased after the stress incident[85]. One study showed that after heat treatment, the expression of HSFA2 in the heat-tolerant Vitis davidii ('Tangwei') was significantly higher than in the heat-sensitive Vitis vinifera ('Jingxiu'), suggesting that HSFA2 may be the key factor determining the heat tolerance of grapes[86]. Meanwhile, the coding regions of HSFA2 from 'Jingxiu' and 'Tangwei' are different. Moreover, the variation of a single amino acid in the AHA motif resulted in higher transcriptional activation activity of HSFA2 in 'Tangwei' than that in 'Jingxiu'. This was confirmed by the analysis of 41 Vitis germplasms. It was also shown that HSFA2 was a positive regulator of heat tolerance in grape by gene overexpression[87].
HSFA3 also has significant effects on establishing heat tolerance in plants. For instance, in lilies, HSFA3, like HSFA1 and HSFA2, has transcriptional activating activity. HSFA3 of lilies has two homologous genes: LlHSFA3A and LlHSFA3B. Overexpression of these in Arabidopsis enhanced its thermotolerance by enhancing proline metabolism[88]. HSFA4 not only participates in the response to HS but is also associated with salt tolerance in plants. Heterologous expression of lily HSFA4 in Arabidopsis enhances basal heat tolerance by regulating ROS metabolism[89]. Expression of CmHSFA4 in chrysanthemum can be induced by salt stress. Overexpression of CmHSFA4 in chrysanthemum under salt stress can suppress the accumulation of Na+ while maintaining the concentration of K+ and reducing the content of H2O2, thus enhancing the salt tolerance[90]. Unlike other HSFs, HSFA5 could specifically bind to HSFA4 and is an inhibitor of HSFA4. Moreover, the HSFA4/5 dimer is not involved in the reaction to HS; its main role may be related to the regulation of pathogen infection and/or to ROS induced cell death[91].
HSFA6 has significant effects on the response to ABA and acquired heat tolerance, and the appropriate ABA signal is of great significance for the expression of HSFA6. Moreover, HSFA6b can combine with DREB2A promoter to promote the expression of DREB2A. As a downstream regulatory gene of the stress response mediated by ABA, HSFA6b is necessary for heat tolerance in plants[92]. Consistent with other class A HSFs, expression of HSFA7 is also activated by HS. For example, the expressions of HSFA2a, HSFA7a and HSFB2 genes showed marked increases in grapevine after heat treatment at 45 °C[93]. In addition, HSFA7 takes part in the mechanism of citric acid degradation under heat treatment by regulating the expression of genes associated with citrate metabolism[94].
Regarding HSFA8, studies have shown that HSFA8 is related to the drought stress response. MdHSFA8a has been reported to be beneficial to the synthesis of flavonoids and the scavenging of ROS in apple. MdHSFA8a can interact with MdRAP2.12 and activate the expression of downstream genes under drought stress. Meanwhile, MdHSFA8a can also promote the expressions of ABA signaling-related genes, thereby participating in the induction of stomatal closure by ABA[95]. In addition, HSFA8 is extremely sensitive to oxidative stress and is regarded as a molecular sensor of ROS in plants[96]. Unlike other HSF members, HSFA9 is generally expressed only in seeds and participates in regulating their physiological processes. For instance, in Arabidopsis, overexpression of VvHSFA9 increased seed germination rate but delayed flowering time[97]. In addition, HSFA9 has been associated with the late embryonic and early photomorphogenesis stages[98].
In plants, most class B HSF members have negative regulatory effects compared to class A HSFs. In tobacco, overexpression of HSF1 from Vitis pseudoreticulata, a B2 family member of HSF, reduced basal tolerance but increased the acquisition of heat tolerance[99]. However, there are also some members of class B HSFs that have transcriptional activations similar to class A ones. In Arabidopsis, heterologous expression of HSFB5 from peach inhibited root length and number but enhanced the thermotolerance of overexpressed lines under HS[100]. The class C HSF family is the least member of the three classes of HSFs, it participates in response to HS as well as to salt and cold stresses in plants. For example, the expression of VaHSFC1 in Vitis amurensis is activated by abiotic stresses, such as high temperature, cold, and salt. Heterologous expression of VaHSFC1 increases plant resistance to heat and cold stresses by increasing membrane stability and reducing electrolyte leakage in transgenic strains of Arabidopsis[101].
-
In plants, multiple bridge factor 1 (MBF1), a transcriptional coactivator, has significant influences on developmental processes and stress responses by building a bridge between TFs and TATA box binding protein (TBP). MBF1 contains an N-terminal domain and a C-terminal helix-turned-helical structural domain. The N-terminal is responsible for binding to TFs and the C-terminal is the binding domain of TBP, which is highly conservative among species. MBF1 has important effects on responses of stresses such as to heat, drought, and pathogens[102]. In grape, the expression of VvMBF1a is markedly increased by both drought stress or by ABA treatment. The overexpression of VvMBF1a improved plant drought tolerance by upregulating two genes in the ABA-dependent drought stress response pathway. These results show that VvMBF1a may participate in drought response in grape and enhances drought resistance through an ABA-dependent signal transduction way[103]. In addition, the overexpression and knockout of MBF1c in suspension cells, demonstrated that MBF1c positively regulates heat tolerance in grape[87]. In summary, although the structure of MBF1 is relatively conserved, the function of MBF1 varies greatly among different plant species.
AP2/EREBP TFs
-
APETALA2/ethylene-responsive element binding proteins (AP2/EREBP) is a class of transcription factors unique to plants, including four subfamilies AP2, associated with ABA insensitive3/viviparous 1 (RAV), dehydration-responsive element binding protein (DREB) and ethylene-responsive factor (ERF). Little research has been done on the AP2 and RAV subfamily members in heat tolerance of plants, so we focus here on the DREB and ERF subfamilies. The expression of VpERF1 and VpERF2 were both activated by HS, peaking after 2 h and 6 h, respectively, of heat treatment at 44 °C, suggesting they might participate in HSR of grape[104].
bZIP TFs
-
bZIP is a class of transcription factor also induced by HS, which participates in the response of HS through pathways other than HSFA1. HS leads to protein mispairing of endoplasmic reticulum in plant cells and induces unfolded protein response (UPR), which causes damage. However, bZIP can participate in the UPR process to help plants withstand the damage of HS[105]. Fifty-five bZIP members have been identified in grapes, of which the expressions of 21 bZIP members are affected by heat treatment. This suggests bZIP members take part in the HSR of grapevine[106]. Zha et al. cloned VvbZIP60 and VvbZIP60s (alternative splicing of VvbZIP60) of grapevine and verified their functions by heterologous expressions in Arabidopsis. It was shown that VvbZIP60s is a key gene in the HSR of grapevines, and that it can bind to VvHSP83[105].
MYB TFs
-
MYB is one of the largest families of TFs and it can participate in the regulation of a many physiological processes as well as the responses to various abiotic stresses. In apple, McMYB4 participates in the response to abiotic stresses including temperature, by regulating biosynthesis of flavonols and the hormone signaling pathway[107]. Some MYB TFs also play negative regulatory roles. For example, blueberry VcMYB4a, which has a C2/EAR repressor motif, was induced to be expressed by HS, while overexpression of VcMYB4a reduced the resistance of blueberry callus to various stresses, such as to low and high temperatures[108].
NAC TFs
-
It has been shown NAC TFs are also related to the response to HS in plants. Some studies indicate that NAC regulates heat tolerance in fruit trees. For example, heterologous expression of VvNAC17 from grapes in Arabidopsis improved the heat resistance of the transgenic lines by promoting proline synthesis and the expressions of genes relevant to stress[109]. PpNAC56 enhanced the heat resistance of transgenic tomato by activating the expression of PpHSP17.4 and promoting the accumulation of osmolytes[110].
WRKY TFs
-
WRKY has important effects on the responses to various stresses in plants. The expression of most FvWRKY increases under drought and salt stress in strawberry, while expressions decrease under high temperature stress, inferring that FvWRKY may have a negative regulatory function under HS[111]. The functions and regulation mechanisms of the WRKY family members in fruit trees requires further investigation.
Other regulation factors
-
An increasing number of studies have confirmed that miRNAs have not only significant influences on morphological construction and development of plants but can also participate in stress responses by targeting stress-related genes. For example, the expressions of many miRNAs in banana are inhibited under HS. At the same time, the SQUAMOSA-PROMOTER BINDING-LIKE (SPL) family was found to be a common target gene of miR535 and miR156. This indicates that the miR156-SPL module may take part in the HSR in banana[112]. Similarly, miRNA (Vvi-miR167) also has significant effects on the HSR of grape[113].
Heat shock proteins and other heat induced proteins
-
Heat shock proteins (HSPs) play multiple roles in folding, assembly, translocation and degradation of proteins during the plant life cycle. As molecular chaperones, HSPs can inhibit protein denaturation and aggregation, and help protein refolding under abiotic stress. In this way they have a vital involvement in protecting plants from heat damage[33]. Moreover, HSPs have important impacts on maintaining cell membrane integrity, in scavenging ROS and in the accumulation of antioxidants and osmolytes[114]. HSPs can be divided into five subfamilies based on molecular mass; these include HSP100, HSP90, HSP70, HSP60 and HSP20[115]. Some studies have confirmed that HSPs play positive roles in the HSR of fruit trees. For instances, HSP83, which belongs to the Hsp90 subfamily, participates in the HSR of grapes by promoting the reversal and disintegration of denatured proteins under heat treatment[105]. Expressions of HSP70 and sHSP17.6 were increased after heat and cold acclimation in grapes. sHSP70 and sHSP17.6 may have a key parts in cold- and heat-treatment induced cross-adaptation of grapes to temperature stress[116]. HSP20, also known as a small HSP, takes part in various abiotic stress response processes. Heterologous expression of peach PpHSP20-32 in Arabidopsis not only enhances the heat resistance of the transgenic lines but also increases plant height[117].
In addition to HSPs, there are many other proteins, including SOD, POD, CAT and dehydrin, can also participate in HSR. For example, the expression of antioxidant enzymes (CAT and POD) genes increased markedly in blueberry[118]. Likewise, during high temperature treatment (45 °C), the activity of POD in kiwifruit also increases significantly[119].
-
As global warming continues to intensify, the adverse influences of HS on yield and quality of fruit are becoming increasingly severe, so it is important to elucidate how fruit trees respond to HS. In general, the responses of fruit trees to high temperatures involve a series of processes such as perception of high-temperature stress, signal transduction, expression and regulation of genes, and synthesis of various metabolites. Based on the biological characteristics of fruit and the current status of plant heat-tolerance research, the focus of future research should be on the following: (1) Exploring the specific HSR of various organs and development stages in fruit trees, especially the reproductive stage; (2) Perception of high temperature and signal transduction; (3) Multilevel heat response mechanisms of fruit at the protein and metabolic levels, especially the post-translational regulatory of key TFs,epigenetic regulation and small RNAs. Also determining how the chromatin landscape is affected by HS; (4) Studying the mechanism of thermotolerance diversity among various germplasms of the same tree-fruit species, and then exploring any specially heat-resistant germplasm and genetic resources, and developing molecular markers for these. Eventually, based on the above research, a variety of methodological techniques can be combined to screen and so create heat-resistant fruit germplasm for use in production.
This work was funded by the National Natural Science Foundation of China (Grant no. U21A20227), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant no. XDA23080602). This research was conducted as part of the LIAINNOGRAPE International Associated Laboratory.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li S, Chen H, Yu H, Li Y, Wang L. 2023. Responses and adaptations of fruit trees to high temperatures. Fruit Research 3:23 doi: 10.48130/FruRes-2023-0023
Responses and adaptations of fruit trees to high temperatures
- Received: 06 June 2023
- Accepted: 31 July 2023
- Published online: 11 September 2023
Abstract: The tree-fruit industry is extremely vulnerable to climate change. We are already seeing that global warming is increasing the incidence and severity of extreme high temperature weather. This means fruit trees are more often experiencing damage due to high temperatures which upsets their growth and development. Heat stress disturbs not only cellular homeostasis but also a fruit’s normal physiological and metabolic processes. Together these effects are proving to be a major adverse factor that compromise both fruit yield and fruit quality. Hence, a better understanding of the response mechanisms of fruit trees to repeated incidences of high temperature is hugely significant for the sustainability of tree-fruit production, justifying this research as one of the main focuses of fruit biology today. In this review, we summarize the physiological and molecular mechanisms through which high temperatures affect tree-fruit species and discuss possible new directions for future study.