-
One hallmark that distinguishes higher plants from animals is the development of germlines within the flowers of adult plants. Thus, unlike in animals, plant germline specification does not occur during embryo development. In the majority of flowering plants, only a single germline precursor cell − the megaspore mother cell (MMC) − is differentiated from the subepidermal somatic cells within the distal ovule tissue giving rise to the female germline[1,2]. By undergoing meiosis, MMC produces four megaspores. Then, merely one megaspore near the chalazal end develops into the functional megaspore (FM), nevertheless, the remaining three cells degenerate[1,2]. Subsequently, as FM progress through three mitotic and nuclear divisions, a mature female gametophyte (FG), the embryo sac, is finally formed[1] (Fig. 1).
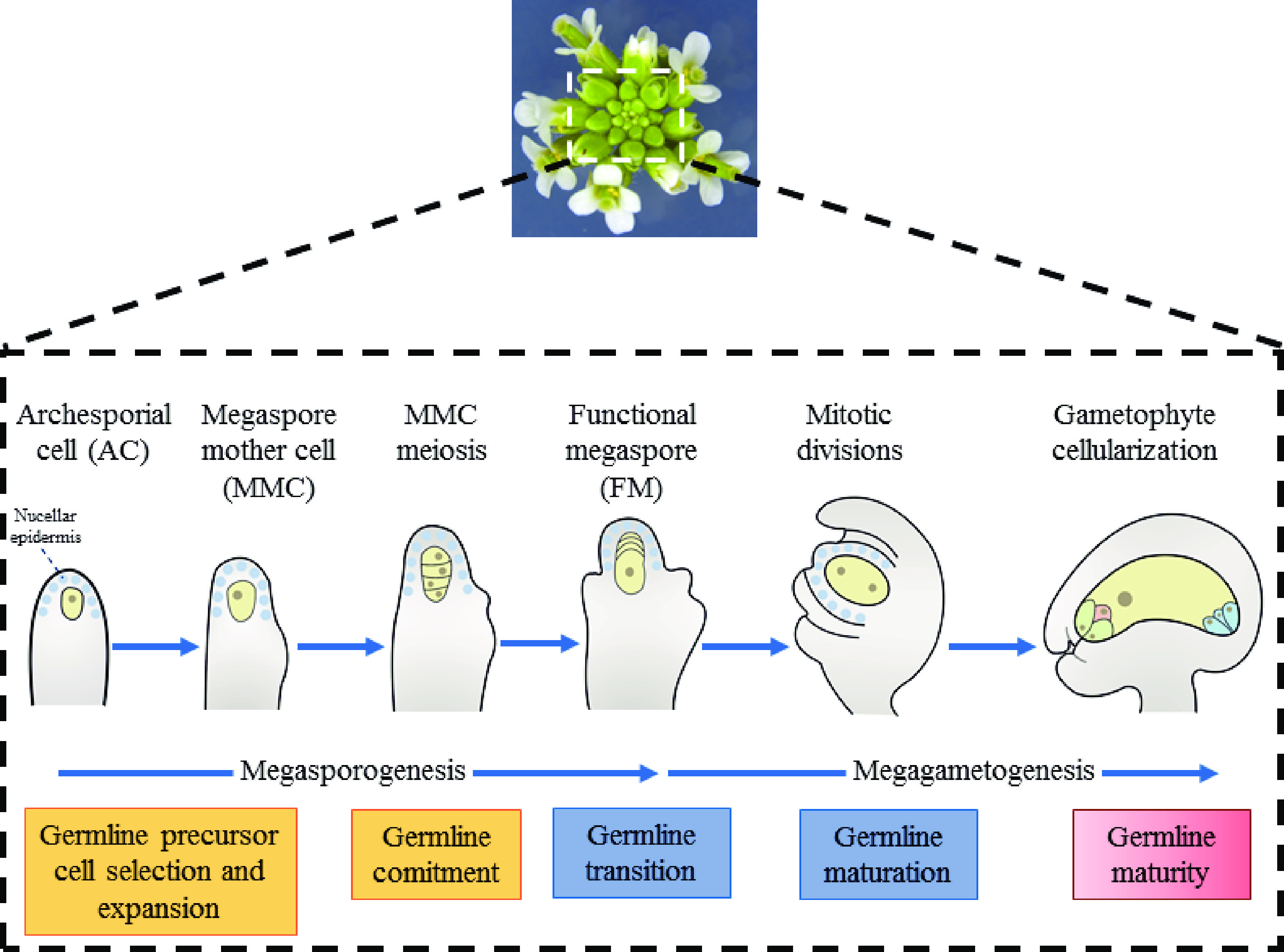
Figure 1.
Female germline development in Arabidopsis thaliana. The developmental process of female
gametophyte can be divided into two steps, including megasporogenesis and megagametogenesis, in Arabidopsis. Megasporogenesis starts from a subepidermal cell at the top of the ovule primordium and the differentiates into MMC. After one meiosis, the cell formed four haploid megaspores. Among them, the three megaspores near the micropyle end degenerated rapidly, and only the one remaining megaspore survives, which is then named as the functional megaspore (FM). Functional megaspores undergo mitosis to form syncytia and then undergo cellularization to form a mature female gametophyte with multicellular structure. In Arabidopsis, the position of the single germline precursor is strictly restrained. The precise spatiotemporal coordination of cell divisions that multiply the somatic cell or switch to the meiotic cell cycle to become the MMC is vital for the acquisition of female reproductive fate, which begins during the formation of the ovule primordium[3]. Once MMC differentiates, suppression of the core cell cycle machinery is necessary to stabilize its meiotic commitment. However, how the intercellular signaling among somatic cells results in the differentiation of only one hypodermal somatic cell to MMC remains unclear. In this review, we emphasize the role of positional signals in MMC specification.
-
Early in ovule development, the cells in the outermost layer of the nucellus are defined as the epidermal cell layer (also called L1 cells), where numerous essential genes express in the epidermal cell layer and establish the female germline[3]. Particularly in the apical epidermal cell layer SPOROCYTELESS/NOZZLE (SPL/NZZ) and WUSCHEL (WUS) have been identified (Fig. 2a). SPL/NZZ encodes a nuclear localization protein similar to the MADS box transcription factors[4]. As an adaptor-like transcriptional repressor, SPL/NZZ recruits TOPLESS/TOPLESSRELATED (TPL/TPR) corepressors to repress the activities of CINCINNATA (CIN)-like TCP transcription factors to control ovule development in Arabidopsis[5,6]. In the spl/nzz mutant, MMCs cannot form and loss-of-function of TPL1 and overexpression of TCP transcription factors display aborted ovules that resemble the phenotypes of those observed in the spl/nzz mutant[5]. WUS encoding a transcription factor is another crucial regulator involved in MMC formation[7]. Like the spl/nzz mutant, the wus mutant displays no MMCs, suggesting that SPL/NZZ and WUS may belong to the same genetic pathway. Since the expression of WUS is decreased in the spl/nzz mutant, WUS was verified to act downstream of SPL/NZZ during MMC development. In addition, it has been shown that WUS controls MMC formation by indirectly activating the expression of two redundantly acting genes, WINDHOSE 1 (WIH1) and WIH2, which encode small peptides in plants[7]. Although wih1 or wih2 single mutants showed no obvious phenotype in MMC development, wih1 wih2 double mutants showed no MMCs[7]. The possible partners for WIH1 and WIH2 may be TORNADO 1 (TRN1) and TRN2, as the twisted organs of wih1 wih2 have similar phenotypes to trn1 and trn2 single mutants[7]. It has been reported that TRN1 is a cytosolic protein and TRN2 is a multi-transmembrane protein[8], suggesting that TRN1 or TRN2 are not the receptors of WIH1/2. Altogether, the above reports describe an essential genetic pathway for MMC formation, which involves SPL/NZZ, WUS, WIH1/2 and TRN1/2.
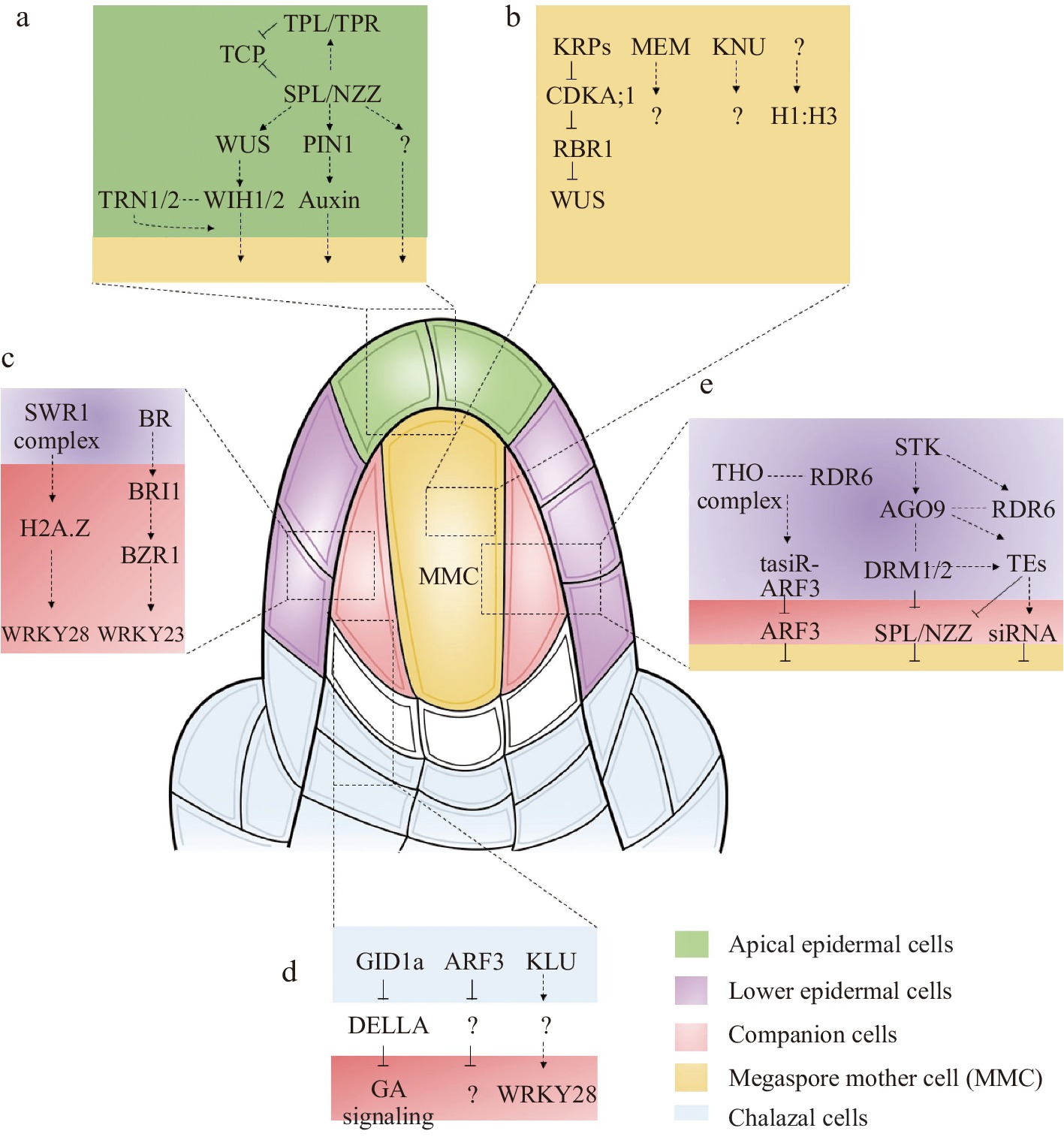
Figure 2.
General mechanisms and key pathways involved in MMC specification. (a) The major signaling pathways of MMC specification in the upper epidermal nucellus. (b) The major signaling pathways of MMC specification in the MMC. (c) The major signaling pathways of MMC specification in the companion cells. (d) The major signaling pathways of MMC specification in the chalaza. (e) The key pathways of ubiquitously expressed genes involved in MMC specification.
As the key transcription factor or transcription factor-like protein, WUS and SPL/NZZ must be associated with key genes of other pathways and/or involved in their transcriptional regulation. A recent report showed that loss of function mutant of nucleolar histone deacetylases 1 (HDT1) and HDT2 affects the expression of WUS and generates multiple MMC-like cells[9]. The regulation between plant hormones and WUS or SPL/NZZ proteins might also be necessary to generate the microenvironment to establish the female germline. Plant hormones play an important role in the physiological processes of plant development, especially by offering location information to support local differentiation events[10]. Auxin may play an important role in female germline formation since auxin creates a phytohormone gradient in apical epidermal cells (based on the synthetic auxin sensor, DR5)[11−13]. In addition, PIN-FORMED1 (PIN1), which localizes in the epidermal cell layer of the nucellus adjacent to the region where the MMC is formed, can facilitate polar auxin transport in Arabidopsis[14,15]. Although auxin accumulates in the tip of the apical epidermal cell layer, auxin carriers mutants show defects at later stages rather than the initiation of female gametophyte development, suggesting that auxin is essential for female gametophyte development. Still, its role in germline specification remains unclear[14].
-
The cells below the epidermis cell layer of the nucellus can be defined as the sub-epidermal cell layer (also called L2 cells)[3]. During the ovule initiation stage, the sub-epidermal cell layer functions as a sporogenous tissue full of the nucellar dome. Only one cell in the sub-epidermal cell layer becomes the generative cell through differentiation, first developing into an archesporial cell (AC) and then into the MMC[3]. After MMC formation, the sub-epidermal cells can be divided into MMC and companion cells[3]. Since the MMC is specialized from sub-epidermal cells, the precise regulation of sub-epidermal cells is very important. With the deepening of research, more and more genes expressed in MMC or companion cells have been reported to participate in MMC specialization (Fig. 2b & c).
Once the MMC begins to expand, its epigenetic and transcriptional status changes quickly from that of the circumambient sporophytic tissues, and it involves changes in chromatin content and chromatin reprogramming, including the condensed chromatin or heterochromatin dynamics, the key histone modifications and changes in the core histone variants[16−18]. Recent studies using non-denaturing whole-mount DNA staining and confocal imaging revealed that the MMC differentiation exhibits chromatin decondensation and a decrease in heterochromatin[19]. Furthermore, linker histone (H1) regulates chromatin compaction by binding linker DNA and core nucleosomes to stabilize the high-order chromatin structure[20]. There are three H1 genes in the Arabidopsis genome, including H1.1, H1.2, and H1.3[21]. Compared to the surrounding somatic cells, the levels of H1.1 and H1.2 quickly decrease in the early MMC stage and are undetected at the consecutive stage, indicating that the reduction of H1 might be the result of somatic cell specialization into MMC[18,19,22]. Similar to H1.1 and H1.2, histone 3 variant H3.1 is evicted in the MMC during MMC differentiation[23]. Moreover, the gene expression levels also change by distinct levels of histone H3 modifications and active RNA polymerase II[19]. Besides, histone H3 lysine 4 trimethylation (H3K4me3) and H3K27me3 are two prominent histone methylation marks, which act as active and repressive transcription marks, respectively[24]. Immunofluorescence assays suggest that H3K4me3 increases while H3K27me3 reduces in the MMC in contrast to those in the circumambient cells, suggesting that many genes are required during the MMC specialization process[19].
Apart from chromatin reprogramming, the changes in expression dynamics of key genes of the sub-epidermal cells (MMC or companion cells) play key roles in female germline initiation. The first type of gene is expressed explicitly in MMC. Among them, the KNUCKLES (KNU) gene, encoding a C2H2 zinc-finger protein, is involved in the initiation of female germline development[25]. Although KNU has been shown to accumulate in the MMC and is commonly used as a marker of MMC identity, its functions in MMC specialization are still unknown. Another MMC identity marker is ARGONAUTE 9 (AGO9), which localizes in the MMC nucleus[26]. The localization of the AGO9 protein is also very important because of its vital function in limiting female germline identity. And initial immunolabeling studies showed that AGO9 protein was limited to the epidermal cell of the nucellus[27]. However, further studies suggested AGO9 protein localization in the nucleus of MMC and the cytoplasm of adjacent L2 ovule cells as well as in the epidermal cell[27]. Unlike KNU, the mutant of ago9 displayed approximately 50% multiple MMC-like cells[26]. Interestingly, mutations in the other AGO proteins (AGO4, AGO6, and AGO8) in the same clade of AGO9 also show multiple MMC-like cells[28]. In addition, mutations of AGO9 related epigenetic pathway genes, RNA-DEPENDENT POLYMERASE 6 (RDR6) and SUPPRESSOR OF GENE SILENCING 3 (SGS3), also promote the L2 cells surrounding the MMC into MMC[29−31]. The third gene specifically expressed in MMC is MNEME (MEM), which encodes a possibly ATP-dependent RNA helicase and belongs to epigenetic regulators[32]. Mutations in MEM lead to the formation of multiple MMC-like cells in the nucellus[32]. However, it is still unclear how the activation of MEM in the germline regulates somatic cell identity. Furthermore, a ligand-receptor signaling pathway in germline formation has been established in rice. The peptide ligand TAPETUM DETERMINANT-LIKE 1A (OsTDL1A) is specifically expressed in MMC, and its receptor MULTIPLE SPOROCYTE 1 (MSP1), expressed in the somatic cells surrounding the MMC, are required for MMC formation[33,34]. A similar regulation mechanism has also been reported in maize, where MULTIPLE ARCHESPORIAL CELLS 1 (MAC1), an ortholog of OsTDL1A, is specifically expressed in the MMC and represses multiple MMC formation in maize[35,36].
Relative to L2 cells, the other type is the gene specifically expressed in companion cells. The first reported gene specifically expressed in companion cells is WRKY28, a zinc-finger WRKY TF[37]. In the wrky28 mutant, multiple MMC-like cells were present in ovules[37]. Further analysis showed that WRKY28 is activated by cytochrome P450 gene KLU through H2A.Z incorporation mediated by the chromatin remodeling complex SWR1, suggesting that transcription of WRKY28 is induced by diffusion of the mobile signal generated by KLU into sube-pidermal nucellar cells[37]. It is believed that other local signals in companion cells may also regulate WRKY28 transcription. The WRKY23 is another gene specifically expressed in companion cells[38]. Recently, it was demonstrated that BR signaling restricts multiple sub-epidermal cells in ovule primordia at distal nucellus from acquiring MMC-like cell identity through transient activation of the WRKY23[38]. Since it is derived from the same ancestor cell, it is believed that the characteristics of L2 cells are similar, and the signal exchange may be more frequent. If the gene expressed in companion cells is ectopically expressed in MMC, will it induce multiple MMC phenotypes or restrict MMC formation? To answer this question, WRKY23 and WRKY28 were ectopically expressed in MMC using the KNU promoter. The results showed that ectopically expressed WRKY23 by KNU promoter causes multiple MMC-like cells in ovules[38]. However, no obvious abnormal ovules were observed in ectopically expressed WRKY28 transgenic plants, suggesting that WRKY23 and WRKY28 act in different signaling networks.
-
It has been reported that the ovule primordium elongates and develops three parts: the nucellus, chalaza and funiculus. The chalaza (the area where the integument and nucellus fuse) is in contact with the placenta in the ovary wall, and the tissue is not clear. The integument originates from the base of the ovule primordium. According to the structural observation, chalaza comprises four layers of cells. Most genes expressed in the chalaza may play key roles in integument development (Fig. 2d), such as INNER NO OUTER (INO)[39], AINTEGUMENTA (ANT)[40] and ABERRANT TESTA SHAPE/KANADI4 (ATS/KAN4)[41]. Since the position of the chalaza is relatively far from the L2 layer cells, how the signaling in the chalaza regulates female germline formation is largely unknown.
Recent reports showed that trans-acting small interfering RNAs known as tasiR-ARFs work non-cell-autonomously to inhibit the expression of AUXIN RESPONSE FACTOR3 (ARF3) in Arabidopsis and prevent the formation of multiple MMCs[42]. The expression of ARF3 was detected in the central chalazal zone, while the expression of ARF3m, which was resistant to tasiR-ARF, diffused from the chalazal region to the distal nucellus except for the MMC, including the cells adjacent to the MMC, resulting in multiple MMC formation[42]. Furthermore, the expression of ARF3-GFP in tasiR-ARFs biogenesis mutant tex1 extended in the lateral direction to the epidermal cells[42], which resemble the pattern of ARF3m-GFP, indicating that TEX1- and TAS3-mediated restriction of ARF3 expression limits supernumerary MMC formation non-cell-autonomously. Moreover, when WRKY28 promoter was used to express ARF3m, it showed ARF3m expression in hypodermal cells surrounding the MMC but not in epidermal cells, leading to more than one MMC formation[43]. These findings suggest that tasiR-ARFs inhibit ectopic MMC fate by repressing ARF3 in these hypodermal cells. In addition, arp6 klu double mutant and wrky28 mutant ovules present multiple MMC[37]. KLU, expressed in the inner integument primordia, promotes the expression of WRKY28 non–cell-autonomously through SWR1-mediated H2A.Z deposition at WRKY28, which suggests that KLU genetically interacts with SWR1 to suppress germline identity in the sub-epidermal nucellar cells through generating a mobile signal that diffuses from the base of the inner integument to adjacent tissues[37].
Another gene expressed in the chalaza region is GIBBERELLIN-INSENSITIVE DWARF1a (GID1a), which encodes a gibberellin receptor[44]. There are three GID1 genes, GID1a, GID1b and GID1c, in the Arabidopsis genome[44]. Functional analysis of gid1a showed no obvious phenotype in ovule development. However, overexpression of GID1a using 35S promoter and ovule-specific SEEDSTICK promoter (STK) displayed additional MMC-like cells[44], suggesting that GID1a is involved in MMC differentiation. Further analysis showed that in pSTK:GID1a ectopic overexpressing ovules, there are multiple subepidermal nucellus cells that enlarge, but only one of them obtains the germline identity, while the remaining enlarged cells do not develop further.
After the ovule primordium begins to develop, the three PD (Proximal-Distal) pattern elements are successively established during primordium outgrowth, starting from the distal nucellus, then the central chalaza and finally the proximal funiculus. This implies that chalaza may play an important role in the communication between nucellus and funiculus. It is anticipated that more important factors involved in MMC specialization in the chalaza region will be identified and cloned in the future.
-
In addition to the specifically expressed genes, some ubiquitously expressed genes in the early ovules also regulate MMC differentiation (Fig. 2e). Germline specification and subsequent acquisition of meiotic competence require cell cycle regulators. During the cell cycle in Arabidopsis, the inhibitory effect of the Rb homolog RETINOBLASTOMA-RELATED 1 (RBR1) is repressed by cyclin-dependent kinase A;1 (CDKA;1) when it is phosphorylated[45]. Several functionally redundant CDK inhibitors of the KIP-RELATED PROTEINs (KRPs, also called ICKs) repress CDKA;1[46]. Recent studies showed a gene regulatory network (GRN), KRPs-CDKA;1-RBR1, restricting MMC formation to a single cell[45]. All of the genes in this GRN are ubiquitously expressed in the early ovules. rbr1 mutant and krp4 krp6 krp7 triple mutant show ectopic MMC-like cells that appear to be caused by mitosis of the MMC[45]. Interestingly, the expression of KRP7 under the control of the KNU promoter largely rescued the krp triple-mutant phenotype, indicating that the KRPs expressed in MMC, rather than those ubiquitously expressed in the whole ovule, may be the dominant part of MMC specialization[45]. At the same time, Cao et al. showed a similar phenotype in the septuple ick1 ick2 ick3 ick4 ick5 ick6 ick7 (named as ick1/2/3/4/5/6/7) mutant[47]. Surprisingly, WUS mutation significantly restored the phenotype of multiple MMCs in the rbr1 mutant[45]. Nevertheless, ectopic expression of WUS failed to induce the MMC to enter mitosis[45]. In contrast, ectopic expression of SPL/NZZ induces multiple MMC. A recent report showed a GRN, STK-AGO9-DRM1/2-SPL/NZZ is required to restrict the expression of SPL/NZZ to ensure a single MMC formation[48]. The DRM methyltransferase double mutants drm1drm2 present multiple MMCs, similar to the stk, ago9 and rdr6 mutants[48]. Ectopic expression of SPL/NZZ was observed in these mutants, indicating that the excessive MMC-like cell development might be due to the ectopic activation of SPL/NZZ[48]. In addition, a series of heterochromatin regulators, including MET1 (a DNA methyltransferase responsible for CG methylation)[49], ARID1 (ARID domain-containing 1)[49], and TRAMGaP (TRAF Mediated Gametogenesis Progression)[50], are ubiquitously expressed during MMC formation. Mutations in these genes exhibit additional MMC-like cells in the ovules.
-
Although many factors involved in megasporogenesis have been identified by genetic screening, the molecular mechanism controlling germline specification and development remains unclear. A transcriptome is dynamic and a distinct representative of the cellular state. In addition, the convenience of genome-wide analysis using sequencing technologies made transcriptome profiling an integral part of almost all genomic studies of biological processes. Transcriptome profiling has identified multiple pathways acting in the MMC specification in Arabidopsis[32]. Schmidt et al. used a combination of laser-assisted microdissection (LAM) and Affymetrix ATH1 GeneCHIP profiling to analyze the transcriptome of the MMC as well as the surrounding ovule sporophytic tissue and revealed more than 9,000 MMC expressed genes[32]. Further analysis revealed that MEM was exclusively expressed in MMC and more than one MMC was observed in ovules of heterozygous mem/MEM plants[32]. Using the same methods, Tucker and his colleague identified genes expressed in and/or around developing megaspores during the transition to megagametogenesis[51]. They found that AGO5 was expressed around reproductive cells during megasporogenesis, and mutants in AGO5 displayed multiple MMC[51]. Besides, spl mutant is a recessive sporophytic mutation that completely abolishes both male and female sporogenesis. Consistently, a differential gene expression profiling (DEG) using high-throughput Tag-seq analysis was carried out to identify genes with higher expression levels in WT ovules undergoing megasporogenesis compared with those from homozygous spl mutants[52]. This study identified 862 candidate genes and revealed the involvement of KLU in female meiosis[52].
The emerging high-throughput technology of single-cell RNA sequencing (scRNA-seq), which enables the capture of the genetic information of the individual cell, generates extensive genetic profiling of thousands of single cells in a population, reflecting the heterogeneous nature of different cell types and the biological complexity of individual tissues. Hou et al. identified 16,872 single cells of Arabidopsis ovule primordia at three developmental time points during female germline differentiation and observed dynamic waves of gene expression along the developmental trajectory[53]. Furthermore, the study indicated that ERECTA (ER) receptor-like kinase family is important in restricting MMC specification to a single cell and promoting MMC differentiation[53].
-
After the archesporial cell (AC) expands and specializes in MMC, it undergoes meiosis to produce four megaspores. Callose (beta-1,3-glucan) precedes the initiation of meiosis, and callose deposition/dynamics may play important roles in the plant meiosis process through positional signals and cell-to-cell communication[54]. However, it is unclear how the callose contribute to the process of meiosis. In most higher plants, such as Arabidopsis, only one megaspore at the chalaza can develop into the FM, whereas the other three megaspores far from the chalaza degenerate, indicating a positional signal from the chalazal megaspore that may promote degeneration of the other spores, resulting in a single embryo sac per ovule. The genetic basis and molecular mechanisms that control the formation, death, or survival of megaspores remain largely unknown. Only a few genes that participate in the specification of the FM have been identified. ANTIKEVORKIAN (AKV) is the first reported gene involved in the specification of the FM[55]. In the akv mutant, the number and position of surviving megaspores are variable[55]. Similar to the akv mutant, in the ick1/2/3/4/5/6/7 septuple mutant supernumerary FMs were formed[47]. Interestingly, the multiple surviving megaspores are capable of developing into mature embryo sacs in both akv and ick1/2/3/4/5/6/7 septuple mutants[47,55]. Overexpression of ARABINOGALACTAN PROTEIN 18 (AGP18) causes multiple surviving megaspores with an FM identity[56,57]. However, it is insufficient to promote FM differentiation, suggesting that AGP18 may act as a positive regulator for the selection of megaspores[56,57]. By contrast, the FM specification is lost in triple mutants of cytokinin receptor genes (ahk2 ahk3 cre1)[58]. These findings indicate that the positional signals are essential for determining the survival of the megaspore.
-
Since MMC is the first female germline cell lineage in plants, understanding the molecular mechanism of MMC specialization and differentiation is particularly important for plant reproduction. In recent years, some of the key developmental genes that promote the regulation and differentiation of MMC have been identified using genetic screens, such as nuclear localization proteins (SPL/NZZ), Cyclin-related proteins (KRPs), and transcription factors (WUS). More advanced tools, including RNA-seq, single-cell transcriptomic, and CRISPR have been used to investigate MMC specification as research technology has progressed considerably. Currently, the reviews of MMC mainly elaborate on epigenetics, microRNA, developmental processes, etc. This review is the first to summarize MMC formation from the perspective of the positional signal. This provides space for future research on MMC development, which is even more refined than currently described.
The position and the number of the single female germline precursor cell at the distal part of ovule primordia appear to be very precisely controlled. In the early stage of ovule development, two cell types can be distinguished: epidermis layer cells and subepidermal layer cells. The most distal L2 cell will become the precursor of the female germline, first via differentiation into an archesporial cell (AC) and then into the MMC[59,60]. Although diverse regulatory pathways tightly regulate the initiation of MMC specification at particular positions within the sporophyte in Arabidopsis[59,60], little is known about the interaction of pathways and the integration of polarity cues in the regulation of these processes. In view of the differential patterns of cell layers, a positional signal was established in the developmental process of female germline cell specification and differentiation. Positional signal regulation of growth and development has been reported in different tissues, including the root epidermis[61] and embryo sac[62,63]. Development and cell differentiation in the early Arabidopsis ovule are another attractive and appropriate model system for deciphering the mechanism in the regulation of cells and especially germ cell specification by position signaling. Future studies may focus on the positional signal establishment of key genes in plant reproduction via regulating MMC differentiation. Advanced technology may help to find new germline specification factors.
We apologize for not being able to refer to some related literature due to the page limit. We acknowledge the funding from the National Natural Science Foundation of China (32170352, 31970333, 32270366), the Excellent Youth Foundation of Fujian Province to H.C. (2022J06014), the Excellent Youth Foundation of Fujian Agriculture and Forestry University to H.C. (xjq202108), the Guangxi Distinguished Experts Fellowship to Y.Q., the Science and Technology Major Project of Guangxi (Gui Ke AA22068096) and the Science and technology innovation project of Pingtan Science and Technology Research Institute (PT2021007, PT2021003).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Hainan Yazhou Bay Seed Laboratory. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Cai H, Ma S, Su H, Liu K, Aslam M, et al. 2022. Positional signals establishment in the regulation of female germline specification. Seed Biology 1:6 doi: 10.48130/SeedBio-2022-0006
Positional signals establishment in the regulation of female germline specification
- Received: 07 August 2022
- Accepted: 15 September 2022
- Published online: 02 November 2022
Abstract: The female germline specification process of a single megaspore mother cell (MMC) of ovule primordium (nucellus) is intriguingly complex because it involves the interaction of different pathways tightly linked with positional information. Various Arabidopsis genes, including the stem cell promoting factor WUSCHEL, have already been shown to be involved in this precise regulation process. Recently, there have been some reviews on MMC specialization, mainly from the aspects of epigenetics, microRNAs and gene regulatory networks. However, those reviews have not taken into consideration the function of positional signals in female germline specification. Here, we review major progress in the cell fate control of female germline specification, highlighting the functions of positional cues.
-
Key words:
- Positional signals /
- Female germline specification /
- Megaspore mother cell /
- Ovule













