-
Melon (Cucumis melo L.) is an important fruit vegetable of the family Cucurbitaceae and widely cultivated worldwide. Its production is often subjected to a variety of pathogenic challenges, such as powdery mildew. Melon powdery mildew is mainly caused by Golovinomyces cichoracearum and Podosphaera xanthii, of which P. xanthii races 1 and 2F are the predominant groups in China[1]. At the early stage of powdery mildew, white spots usually appear on the leaves, while these spots can rapidly spread to the petioles, stems or even fruits with the development of pathogenic infection[2]. At the colonization sites, the pathogenic fungi can hijack not only water but essential nutrients such as sugar, nucleotides and proteins from host plants to feed themselves via the haustorium, a specialized intracellular organ that is covered by host-originated membranes[3]. The infected melon organs, particularly leaves, can be yellowed and withered, thus leading to lowered photoassimilates and yield[4].
Powdery mildew is now becoming one of the most deleterious fungal diseases and severely threatens the global production of melon[5]. Creation or screening of high-efficiency and environment-friendly fungicides is considered a critical strategy for powdery mildew management in melon production[6]. However, there are still a multitude of problems in current fungicide-aided control of melon mildew, such as shortage of proper formula in mixed medicament, pathogenic fungus tolerance to the chemicals resulting from long-term and excessive application of single chemical fungicide, which not only decrease the effectiveness of disease management but also cause environmental pollution[7]. Inorganic salts have been demonstrated as having the capacity to inhibit a variety of plant pathogens, thus being thought of as potential and powerful weapons for protecting plants from disease stress[8]. For example, in a study carried out by Soliman et al., the activation of polyphenoloxidase (PPO), peroxidase (POD), chitinase and β-1,3-glucanase, together with the increment of total phenol and protein contents, has been observed in the leaves of okra plants by spraying either chitosan or potassium salt solution, achieving the effective prevention of powdery mildew[9]. Karabulut et al. have reported that the germination of pathogenic spores and the elongation of bud tubes are apparently restricted on the sodium bicarbonate (NaHCO3)-sprayed wheat leaves upon Venturia inaequalis invasion, leading to the significantly reduced occurrence of rust disease[10]. The effective control of powdery mildew, which is caused by Erysiphe corylacearum, has been revealed for hazelnut plants via exogenous application of NaHCO3 solution[11]. For currant plants, Wenneker & Kanne pointed out that the damage from powdery mildew are dramatically alleviated via the weekly spraying of potassium bicarbonate (KHCO3) solution[12]. Moreover, the protective effects of KHCO3 have been observed for sugar beet infected by Cercospora beticola[13], and grape infected by Botrytis cinerea[14], etc. However, it remains largely unknown whether the resistance of melon plants can be improved by KHCO3 when being challenged by P. xanthii, and if so, what are the physiological mechanisms underlying KHCO3-mediated resistance improvement in P. xanthii-infected plants.
In this study, the effects of KHCO3 spraying were physiologically investigated on the adaptability of melon plants to powdery mildew. The apparently lowered disease index was observed for melon seedlings being sprayed with KHCO3 solution at both 7 d after inoculation (DAI) and 2 d before inoculation (DBI). This improvement in mildew resistance might be attributed to the stimulation of both phenylpropanoid metabolic and reactive oxygen species (ROS) scavenging pathways in KHCO3-treated samples. Further investigation for melon seedlings, which were sprayed with H2O, KOH, KCl and KHCO3 solutions at 2 DBI respectively, demonstrated the functions of KHCO3 in controlling powdery mildew was mainly attributed to HCO3−. Altogether, these observations expand our understanding of inorganic salt-mediated protection of melon plants from powdery mildew infection, and could make technical contributions to the environmentally-friendly production of melon in the future.
-
Melon (Cucumis melo L. ssp. agrestis) inbred line 'Yangjiaomi', which displays high powdery mildew susceptibility[15], was used in this study. The germinated seeds were sown in 72-cell seedling trays with nutrient medium (peat : vermiculite : perlite = 2 : 1 : 1), and placed in a growth chamber that was set as 18-h light period with air temperature of 26 °C, 6-h dark period with air temperature of 20 °C, and relative humidity of 60%. At the two-leaf stage, the melon seedlings were transplanted to plastic pots filled with the nutrient soil and incubated under the abovementioned growth conditions. At the three-leaf stage, three exogenous spraying experiments were performed for P. xanthii-inoculated melon seedlings according to the following designs: (1) KHCO3-7DAI spraying experiment, wherein the seedlings were sprayed with H2O and 2.5 g·L−1 KHCO3 (pH 8.54) at 7 d after inoculation (DAI) respectively, and the physiological parameters [P. xanthii proliferation, mildew spot number, disease index, enzymatic activities, as well as the contents of ROS, malonaldehyde (MDA), and secondary phenolic substances] were determined at 0, 1, 3, and 5 d after spraying (DAS); (2) KHCO3-2DBI spraying experiment, wherein the seedlings were sprayed with H2O and KHCO3 at 2 d before inoculation (DBI) respectively, and the abovementioned parameters were determined at 4 and 8 DAI; (3) multiple potassium salt spraying experiment, wherein the seedlings were sprayed with H2O, KOH (pH 8.54), 1.86 g·L−1 KCl and 2.5 g·L−1 KHCO3 (pH 8.54) at 2 DBI respectively, and the determination of physiological parameters (mildew spot number, disease index, enzymatic activities, as well as the contents of total phenols and flavonoids) was carried out at 8 DAI. All experiments were executed in State Key Laboratory of Crop Biology, Shandong Agriculture University, China, from May of 2021 to June of 2022. Three biological repeats were prepared for each parameter.
Phenotypic investigation
-
The representative leaves of melon seedlings in different treatments were photographed, and the disease incidence was recorded on the basis of whether or not disease spots appeared. Thereafter, powdery spots were counted on each leaf of sprayed plants, and the disease index was calculated according to the previously described formula: Disease index = (Sum of numerical disease ratings) / (Number of plants evaluated × maximum of disease rating scale) × 100[16].
The growth of P. xanthii was molecularly evaluated with the previously described qRT-PCR method[17] with minor modifications. In brief, the genomic DNA mixture was first extracted from the pathogen-colonized melon leaves. Using the extracted DNA mixture as a template, two molecular marker genes, P. xanthii TUB2 (PxTUB2) and melon ACT7 (CmACT7), were quantitatively amplified on a 7900HT Fast Real-Time PCR System (ABI, USA). The fungal content was finally determined by calculating the ratio of PxTUB2 to CmACT7 as described by Vela-Corcίa et al.[18]. All primers used for the PCR-based quantitative assay are provided in Table 1.
Table 1. Primers used in qRT-PCR analysis.
Gene Forward primer (5′→3′) Reverse primer (5′→3′) PxTUB2 TTGTAGGAATCACATCCCTTTCTC TTCTTCCGGTTGCATGGGTGGTTC CmACT7 GGCTGGATTTGCCGGTGATGATGC GGAAGGAGGAAATCAGTGTGAACC Assay for enzymatic activities
-
For evaluation of the antioxidant system, 0.3 g of liquid nitrogen-frozen leaves were ground to a fine powder with a mortar and pestle, and then homogenized with 3 mL extraction buffer (50 mM NaHPO4, 0.2 mM EDTA, pH 7.8). After 20-min centrifugation with 12,000 rpm at 4 °C, the resulting supernatants were kept for the determination of superoxide dismutase (SOD), POD and catalase (CAT) activities. Regarding SOD, a reaction mixture was prepared by adding 50 μL of enzyme extract into 3 mL of NBT (nitro-blue tetrazolium) reaction medium (50 mM K2HPO4, 13 mM methionine, 63 mM NBT, and 1.3 mM riboflavin), and subjected to 5-min light treatment at 25 °C with a parallel reaction mixture under darkness as the blank sample. SOD activity was determined using the spectrophotometer method previously described[19]. POD activity was determined according to the method described by Liu et al.[20] with some modifications. In brief, a POD-mediated reduction was initiated by adding 100 μL of enzyme extract into a 2-mL reaction medium [20 mM H2O2, and 1% (w/v) guaiacol]. The enzymatic activity was calculated by monitoring the absorbance increase at 460 nm. For CAT assay, a 1-mL reaction mixture [25 mM sodium phosphate buffer (pH 7.0), 10 mM H2O2, and 0.1 mL enzyme extract] was prepared, and the enzymatic activity was determined by recording the absorbance variations at 240 nm per min.
After homogenization of 0.5 g leaf samples in 5 mL boric acid buffer (pH 8.8), the resulting mixture was subjected to 15-min centrifugation with 8,000 rpm at 4 °C, and the supernatant was kept for determining phenylalanine ammonia lyase (PAL) activity. A 4-mL reaction system [1 mL sodium borate buffer with 0.02 M L-phenylalanine (pH 8.8), 1 mL enzyme extract, and 2 mL distilled H2O] was incubated at 30 °C for 1 h, and 0.2 mL of 6 M HCl was added to the mixture to stop the reaction. The PAL activity was determined using the absorbance variation at 290 nm according to the previously described method[21].
For PPO assay, 0.5 g of liquid nitrogen-frozen leaf samples were ground to a fine powder and homogenized in 4 mL of 0.05 M phosphate buffer (pH 7.0). The homogenates were centrifuged at 8,000 rpm for 10 min at 4 °C, and the resulting supernatants were used for determining the PPO activity according to the method of Zhang et al.[22]. In brief, a reaction mixture, which included 3.9 mL of 0.1 M sodium phosphate buffer (pH 6.8), 0.1 mL of enzyme extract and 1 mL of 0.1 M catechol solution, was prepared, and the mixture was then kept at 37 °C for 10 min. After stopping the reaction with 2 mL of 20% (w/v) trichloroacetic acid (TCA), the resulting solution was centrifuged at 9,000 rpm for 10 min at 4 °C, and the enzymatic activity was determined by recording the absorbance values of supernatants at 525 nm.
Determination of ROS and MDA content
-
To visualize the ROS accumulation, a total of nine leaf discs were randomly collected from three sprayed leaves, and then used for either DAB staining detection of H2O2 or NBT staining detection of O2.− according to the protocols described previously[23]. The stained samples were transferred to 90% (v/v) ethanol and kept in a 95 °C water bath for 30 min, and then photographed. The contents of H2O2 and O2.− in the sprayed leaves were further determined with the H2O2 content assay kit (BC3590, Solarbio) and the superoxide anion activity assay kit (BC1295, Solarbio), respectively, by following the manufacturer’s instructions.
For MDA assay, 1 mL of the supernatant, which was the same as that for the determination of antioxidant enzymatic activities, was mixed well with 3 mL of TCA buffer [0.5% (w/v) thiobarbituric acid, and 20% (v/v) TCA]. After 30-min incubation at 95 °C, the reaction was stopped using an ice bath, and the absorbance to resulting solution was measured at 530, 450 and 600 nm, respectively. The MDA content was finally calculated according to the formula described by Wang et al.[24].
Determination of resistance-related metabolite content
-
For tannin assay, 0.2 g of frozen-dried leaves were ground to a fine powder, and transferred to 5 mL of 70% (v/v) methanol for 24-h incubation at room temperature. After 10-min centrifugation at 5,000 rpm at 4 °C, the supernatants were well mixed with 3 mL of 4% (w/v) vanillin, 1.5 mL of 37% (w/v) HCl and 0.5 mL of 70% (v/v) methanol, and the condensed tannin content was determined using the previously described spectrometry method[23].
Regarding total phenols and flavonoids, 0.2 g of fresh leaves were well ground on an ice bath and suspended with 10 mL of 1% (v/v) HCl-methanol solution for 20 min extraction at 4 °C under darkness. After being filtered, the resulting solutions were subjected to spectrometric analysis at 280 nm for the determination of total phenol content, and at 320 nm for the determination of flavonoid content as described by Toor & Savage[25].
Lignin content of melon leaves was determined by following the previously described protocol[26] with minor modifications. In brief, after ethanol-mediated homogenization and low-speed centrifugation, the precipitates were dissolved in 0.5 mL of 25% (v/v) Acetyl bromide and incubated at 72 °C for 30 min. The reaction was terminated with 0.9 mL of 2 M NaOH, and then 5 mL of glacial acetic acid and 0.1 mL of 7.5 M hydroxylamine hydrochloride were added. After 5-min centrifugation at 4,500 rpm, 0.1 mL of the resulting supernatant was mixed with 3 mL of glacial acetic acid for the spectrometric analysis at 280 nm and the calculation of lignin content.
Hydroxyproline (HYP) content was measured with the Solarbio HYP assay kit (BC0250, Solarbio), and used as an indicator for the content of endogenous HRGP in the sprayed leaves[27].
Data analysis
-
All data were processed with Microsoft Excel 2013 software, and displayed as mean of three biological repeats ± standard errors (SE). The statistical analysis at a 0.05 significance level was carried out with DPS v9.01 software by following the rules of Duncan's new multiple range test.
-
To unveil the effects of inorganic salts on melon powdery mildew, when powdery spots became apparently observable on melon leaves at 7 DAI, the same volume of H2O and 2.5 g·L−1 KHCO3 solution (pH 8.54, KHCO3-7DAI) were sprayed, respectively, and disease development was then monitored. As shown in the left panel of Fig. 1a, the size and number of powdery spots on the H2O-sprayed leaves quickly increased, while disease spreading was significantly prevented for the KHCO3-7DAI samples. Consistent with the phenotypic results, fungal growth of KHCO3-sprayed seedlings was lowered by 80.9% at 1 DAS, by 75.0% at 3 DAS, and by 78.3% at 5 DAS in comparison to the H2O-treated ones (Fig. 1b). Meanwhile, spot number on the KHCO3-7DAI leaves was decreased by 6.4% at 1 DAS, by 13.9% at 3 DAS, and by 34.9% at 5 DAS relative to the H2O-treated ones (left panel of Fig. 1c). As a result, the significant reduction in disease index was observed for the KHCO3-7DAI seedlings over the investigation course (left panel of Fig. 1d). This evidence demonstrated the positive roles of KHCO3 in protecting melon plants from P. xanthii infection.
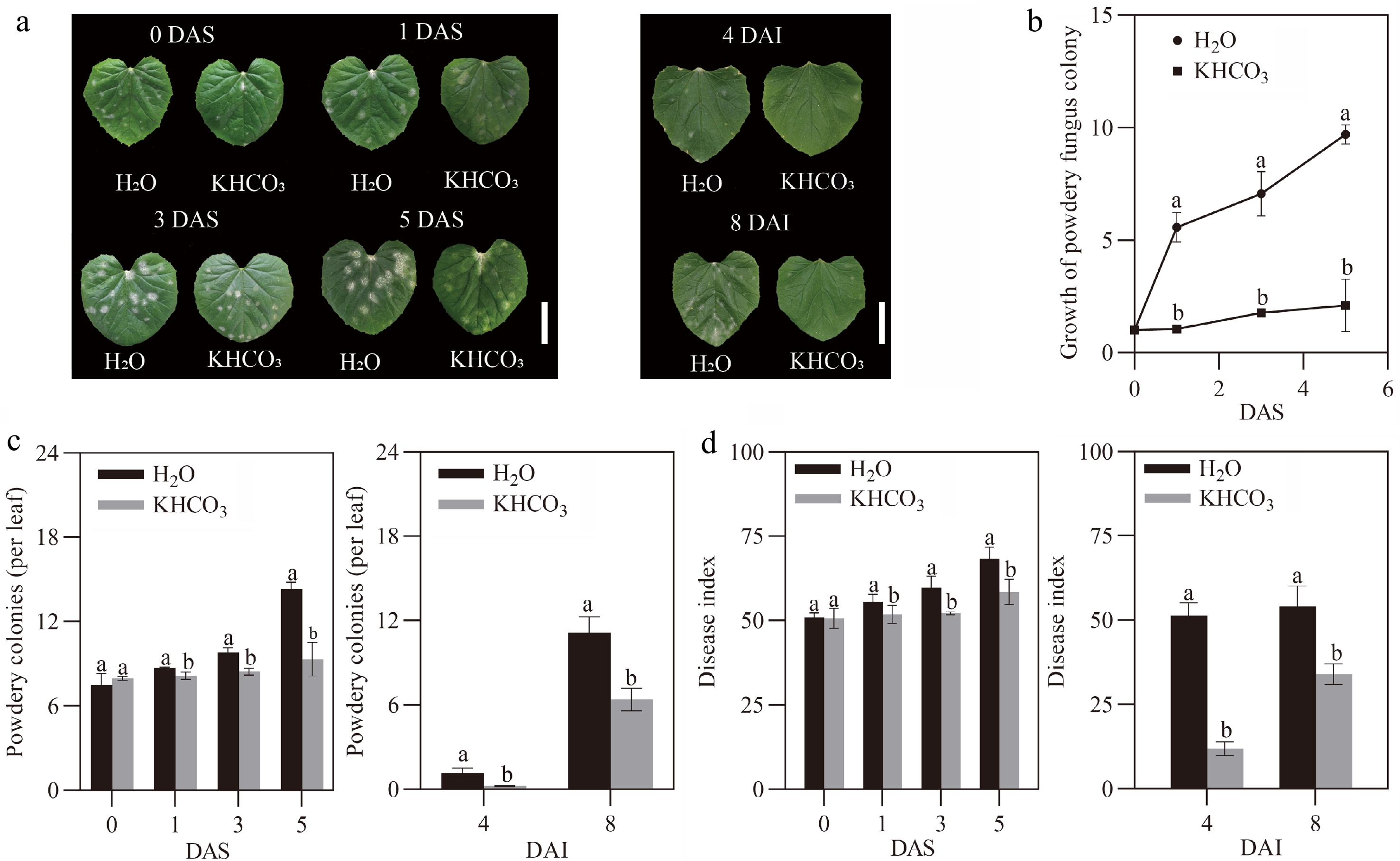
Figure 1.
Effects of KHCO3 spraying on melon seedlings under powdery mildew. (a) Phenotypic comparison between H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and between H2O-sprayed (control) and KHCO3-2DBI melon seedlings at 4 and 8 DAI (right panel). Scale bar = 5 cm. (b) Proliferation evaluation of P. xanthii on the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS. (c) Number of powdery mildew spots on the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-7DAI seedlings at 8 DAI (right panel). (d) Disease index of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-7DAI seedlings at 4 and 8 DAI (right panel). DAS: days after spraying; DAI: days after inoculation. In (b)−(d), the data of each parameter are displayed as mean of three biological repeats ± standard errors (SE), and the different letters indicate significant differences in the comparisons between H2O-sprayed and KHCO3-7DAI/-2DBI samples at a 0.05 statistical level.
We wondered whether KHCO3-mediated protection derived from the direct inhibition of P. xanthii or the improvement of endogenous immunity system in melon plants. To this end, another experiment, wherein the melon seedlings were sprayed with H2O and 2.5 g·L−1 KHCO3 solution (pH 8.54) at 2 DBI (KHCO3-2DBI), respectively. The results showed that powdery spots appeared on the leaves of H2O-sprayed seedlings while remained invisible on the KHCO3-2DBI leaves at 4 DAI; at 8 DAI, powdery spots began to be observable on the KHCO3-2DBI leaves, whereas the much more severe disease symptoms, including the significantly enhanced incidence rate and spot number, were detected in the H2O-sprayed seedlings (right panels of Fig. 1a & c). The disease index of KHCO3-2DBI melon plants was 76.8% lower at 4 DAI, and 37.2% lower at 8 DAI than that of the H2O-treated ones (right panel of Fig. 1d). These results implied that the alleviatory effects of KHCO3 on powdery mildew may be attributed to the induction of innate immunity system in melon seedlings. However, it should be noted that the possibility that KHCO3 imposes inhibitory effects on P. xanthii could not be excluded, which deserves further investigation in the future.
KHCO3 spraying activates phenylpropanoid metabolic pathway in P. xanthii-infected melon plants
-
To survive under biotic stress, plants have developed a series of specific metabolic pathways to cope with pathogenic challenges over the long-term evolution period[28]. In the present study, we explored the phenylpropanoid metabolic pathway, which is closely associated with disease resistance[28], in different melon samples. Compared to the control samples, PAL and PPO activities in the leaves of KHCO3-7DAI melon seedlings were increased by 19.15% and 24.83% at 3 DAS, and by 15.91% and 48.53% at 5 DAS, respectively (left panels of Fig. 2a & b). Meanwhile, it was observed that the content of condensed tannin was increased by 42.62% at 1 DAS, 106.38% at 3 DAS and 58.7% at 5 DAS in the KHCO3-treated leaves (left panel of Fig. 3a). Furthermore, the contents of lignin, HRGP, total phenols and flavonoids were significantly increased by 17.47%, 17.65%, 30.22% and 38.55% at 3 DAS, as well as by 16.40%, 22.94%, 16% and 26.54% at 5 DAS by KHCO3 treatment, respectively (left panels of Fig. 3b−e).
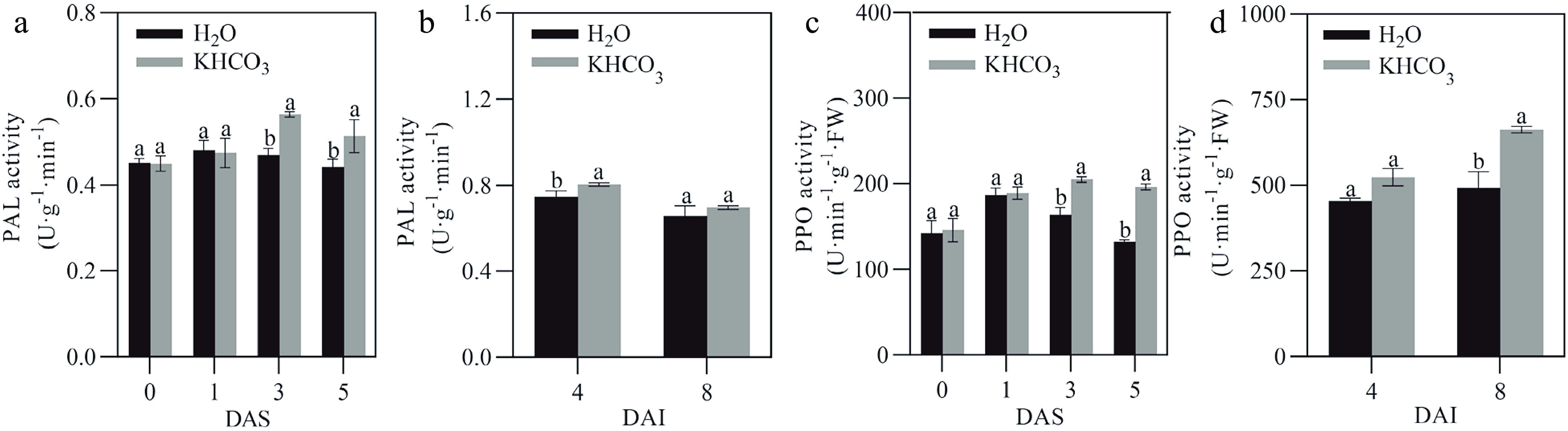
Figure 2.
Effects of KHCO3 spraying on the activities of phenylpropanoid metabolic enzymes in melon seedlings under powdery mildew. (a) PAL activity in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). (b) PPO activity in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). DAI: days after inoculation; DAS: days after spraying. The data of each parameter are displayed as mean of three biological repeats ± standard errors (SE), and the different letters indicate significant difference in the comparison between H2O-sprayed and KHCO3-7DAI/-2DBI samples at a 0.05 statistical level.
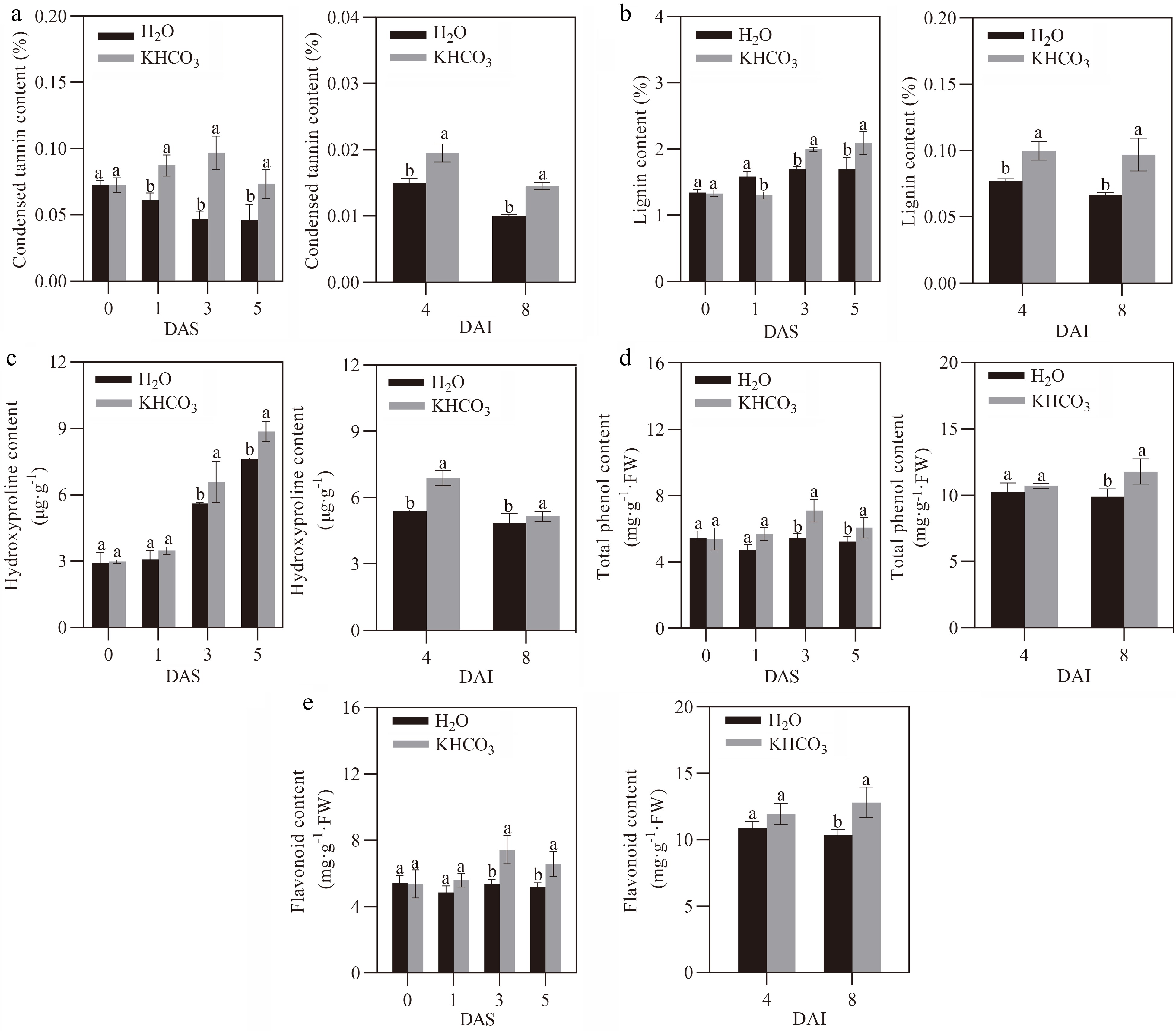
Figure 3.
Effects of KHCO3 spraying on phenylpropanoid metabolite content in melon seedlings under powdery mildew. (a) Condensed tannin content in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). (b) Lignin content in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). (c) Hydroxyproline-rich glycoprotein (HRGP) content in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). (d) Total phenol content in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). (e) Flavonoid content in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). DAS: days after spraying; DAI: days after inoculation. The data of each parameter are displayed as mean of three biological repeats ± standard errors (SE), and the different letters indicate significant difference in the comparison between H2O-sprayed and KHCO3-7DAI/-2DBI samples at a 0.05 statistical level.
Similarly, the higher activity was observed for PAL and PPO at 4 DAI, and for PPO at 8 DAI in the leaves of KHCO3-2DBI seedlings than the H2O-treated samples (right panels of Fig. 2a & b). We also found that the contents of condensed tannin and lignin were enhanced by 33.33% and 29.87% at 4 DAI, and by 44.78% and 40% in the KHCO3-2DBI leaves at 8 DAI, respectively (right panels of Fig. 3a & b). Furthermore, the higher content was detected for HRGP at 4 DAI, and for total phenols and flavonoids at 8 DAI in the KHCO3-treated samples than those of the H2O-treated samples (right panels of Fig. 3c, d & e).
Antioxidant system is stimulated in melon plants upon P. xanthii infection by KHCO3 spraying
-
We explored the responses of the antioxidant system, a highly conserved pathway that is closely associated with the environmental adaptive capacity of plants[29], in KHCO3-sprayed melon seedlings under powdery mildew. For KHCO3-7DAI seedlings, we observed that the activities of SOD, POD and CAT, three well-known enzymes responsible for ROS scavenging[30], were significantly increased by 21.98%, 23.85% and 25.42% at 1 DAS, by 22.89%, 39.98% and 31.75% at 3 DAS, and by 59.68%, 43.28% and 34.15% at 5 DAS in comparison to the H2O-treated samples, respectively (left panels of Fig. 4a, b & c). Similarly, the activities of SOD, POD and CAT were increased by 16.20%, 66.85% and 32.63% at 4 DAI, and by 3.8%, 37.10% and 8.56% at 8 DAI in the leaves of KHCO3-2DBI seedlings relative to the H2O-treated samples, respectively (right panels of Fig. 4a, b & c).
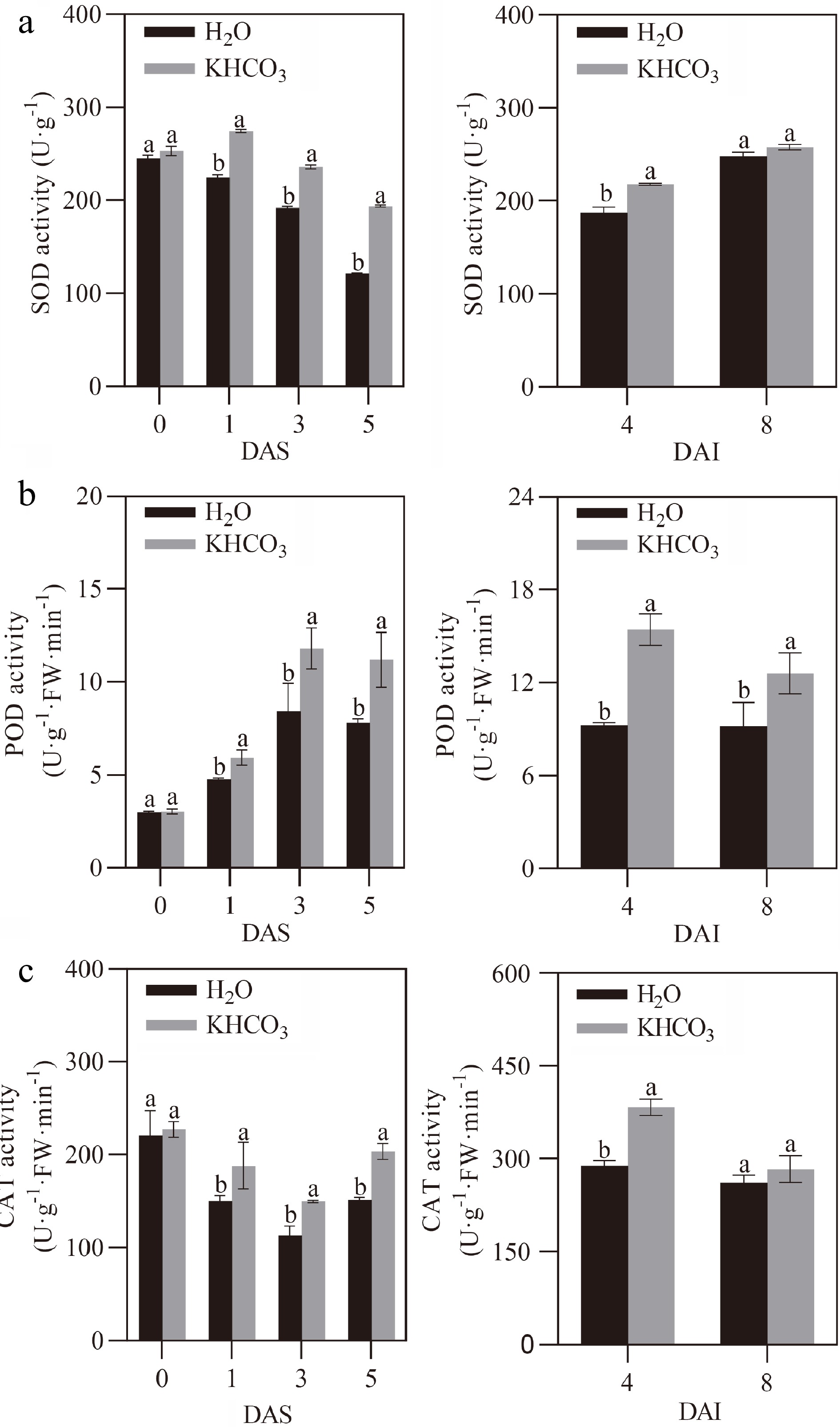
Figure 4.
Effects of KHCO3 spraying on the activities of antioxidant enzymes in melon seedlings under powdery mildew. (a) SOD activity in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). (b) POD activity in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). (c) CAT activity in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). The data of each parameter are displayed as mean of three biological repeats ± standard errors (SE), and the different letters indicate significant difference in the comparison between H2O-sprayed and KHCO3-7DAI/-2DBI samples at a 0.05 statistical level.
The effects of KHCO3 spraying on ROS production were further evaluated in melon seedlings under powdery mildew. DAB and NBT staining results showed less accumulation of both H2O2 and O2.− at 1 DAS, 3 DAS and 5 DAS in the KHCO3-7DAI leaves, and at 4 and 8 DAI in the KHCO3-2DBI leaves, when compared to the H2O-treated samples (Fig. 5a & b). To determine whether the observed differences in ROS accumulation reached a statistical significanct level, spectrometric assay was carried out to quantify H2O2 and O2.− content in melon leaves at different investigation time points. For KHCO3-7DAI seedlings, we observed that the contents of H2O2 and O2.− were 19.75% and 19.64% lower at 1 DAS, 23.47% and 26.76% lower at 3 DAS, and 27.52% and 22.37% lower at 5 DAS than those of the H2O-treated samples, respectively (left panels of Fig. 5c & d). Similarly, for KHCO3-2DBI seedlings, the lower content was also detected for both H2O2 and O2.− relative to those of the H2O-treated samples (right panels of Fig. 5c & d). Consistent with the stimulated antioxidant enzymes and the reduced ROS accumulation, we observed that MDA content was dramatically reduced in the leaves of both KHCO3-7DAI and -2DBI seedlings relative to the H2O-treated samples (Fig. 5e).
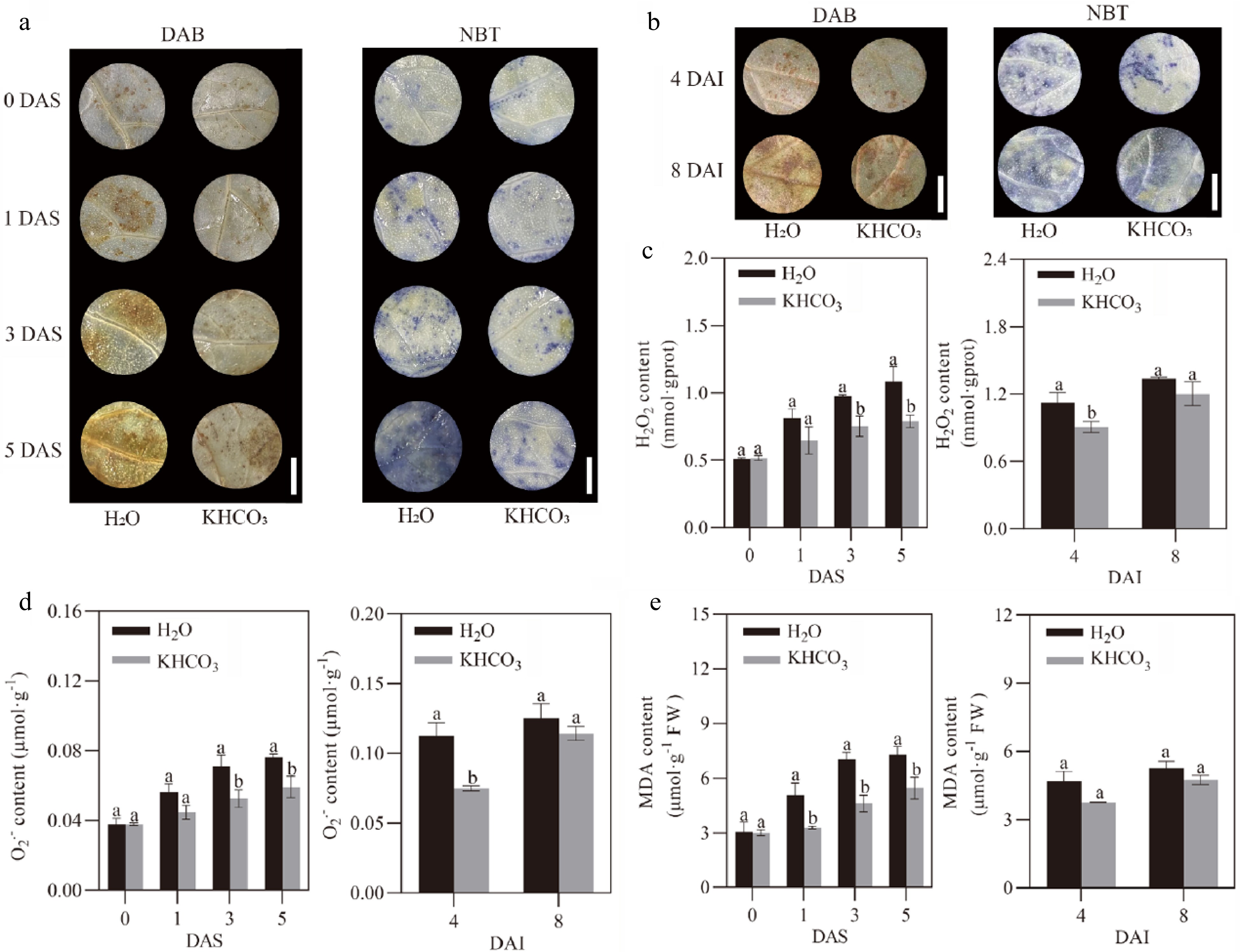
Figure 5.
Effects of KHCO3 spraying on ROS production and lipid peroxidation in melon seedlings under powdery mildew. (a) DAB staining of H2O2 (left panel) and NBT staining of O2.− (right panel) in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS. (b) DAB staining of H2O2 (left panel) and NBT staining of O2.− (right panel) in the leaves of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI. (c) H2O2 content in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). (d) O2.− content in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). (e) MDA content in the leaves of H2O-sprayed (control) and KHCO3-7DAI melon seedlings at 0, 1, 3 and 5 DAS (left panel), and of H2O-sprayed (control) and KHCO3-2DBI seedlings at 4 and 8 DAI (right panel). DAI: days after inoculation; DAS: days after spraying. In (a) and (b), scale bar = 1 cm. In (c)-(e), the data of each parameter are displayed as mean of three biological repeats ± standard errors (SE), and the different letters indicate significant difference in the comparison between H2O-sprayed and KHCO3-7DAI/-2DBI samples at a 0.05 statistical level.
HCO3− serves as the core player in KHCO3-mediated protection of melon plants upon P. xanthii infection
-
We wondered which factors in the KHCO3 solution could serve as the core players in the accumulation of resistance-related secondary metabolites and the stimulation of antioxidant enzymes, of which both positively contributed to the enhanced resistance of melon seedlings upon P. xanthii invasion. To this end, the exogenous spraying was carried for melon seedlings at 2 DBI with H2O, KOH (pH 8.54), KCl (1.86 g·L−1) and KHCO3 (2.5 g·L−1, pH 8.54), respectively, and powdery mildew development was then investigated on the sprayed plants at 8 DAI. The apparent restriction of mildew spots was only observed on the leaves of melon seedlings treated with KHCO3 in comparison to the H2O-, KOH- and KCl-sprayed samples (Fig. 6a). Consistent with the phenotypic results, we found that the disease index of KHCO3-treated melon seedlings was significantly lower than that of the other three sprayings at the end of the investigation course (Fig. 6b).
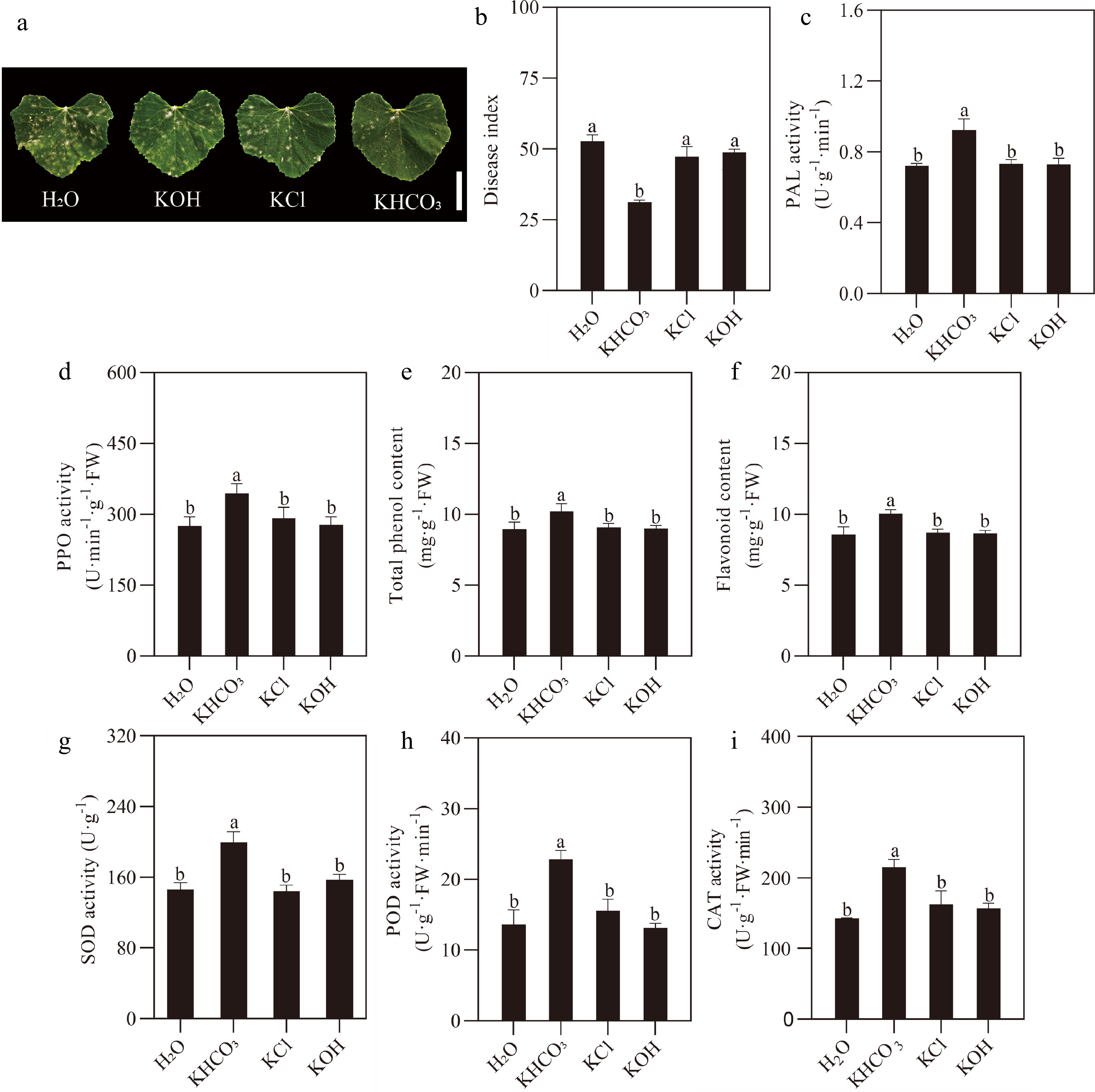
Figure 6.
Responses of melon seedlings to different inorganic salt sprayings under powdery mildew. (a) Phenotypic comparison between H2O- (negative control), KOH-, KCl- sprayed and KHCO3-2DBI melon seedlings (positive control) at the end of 8-day P. xanthii infection course. Scale bar = 5 cm. (b) Number of powdery mildew spots on the leaves of H2O- (negative control), KOH-, KCl-sprayed and KHCO3-2DBI melon seedlings (positive control) at the end of 8-day infection course. (c) PAL and (d) PPO activities in the leaves of H2O- (negative control), KOH-, KCl- sprayed and KHCO3-2DBI melon seedlings (positive control) at the end of 8-day infection course. (e) Total phenol and (f) flavonoid content in the leaves of H2O- (negative control), KOH-, KCl-sprayed and KHCO3-2DBI melon seedlings (positive control) at the end of 8-day infection course. (g) SOD, (h) POD and (i) CAT activities in the leaves of H2O- (negative control), KOH-, KCl- sprayed and KHCO3-2DBI melon seedlings (positive control) at the end of 8-day infection course. The data of each parameter are displayed as mean of three biological repeats ± standard errors (SE), and the different letters indicate significant differences in the comparison between H2O-sprayed and KOH-/KCl-/KHCO3-2DBI samples at a 0.05 statistical level.
Further investigation was carried out for key enzymes/metabolites in phenylpropanoid metabolic and antioxidant pathways. The greatest enhancement in the activities of PAL and PPO and the contents of total phenols and flavonoids was revealed for the KHCO3-treated samples than those of the H2O-, KOH- and KCl-treated ones (Fig. 6c−f). For antioxidant system, we found that, in comparison to the H2O-, KOH- and KCl-treated plants, 36.77%, 27.21% and 38.91% increase in SOD activity was detected for the KHCO3-treated ones, together with 67.91%, 73.92% and 47.07% increase in POD activity, and 27.35%, 37.31% and 32.58% increase in the CAT activity (Fig. 6g−i).
-
In the past years, accumulating evidence has uncovered the positive effects of inorganic salts on plant resistance to disease stress, therefore, the exploration of proper chemical formula for plant protection has been attracting extensive attention from researchers in both basic and applied horticulture. For example, in a study carried out by Yildirim et al. the authors have reported that the resistance of rose plants to powdery mildew, a devastating disease caused by Podosphaera pannosa, is significantly increased by weekly spraying of either NaHCO3 or K2CO3 solution[31]. Cerkauskas et al. have found that powdery mildew caused by Leveillula taurica is apparently decreased in pepper plants by exogenous spraying of potassium dihydrogen phosphate (K2HPO4) solution[32]. More recently, the inhibitory effects of KHCO3 spraying on powdery mildew of apple plants were revealed[33]. Here, the obviously lowered disease incidence rate, spot number and disease index were observed for KHCO3-sparyed melon seedlings upon P. xanthii infection, regardless of whether the treatment was carried out at either 7 DAI or 2 DBI (Fig. 1), revealing its general role in protecting plants from powdery mildew.
A multitude of secondary metabolites, together with the enzymes responsible for their biosynthesis and degradation, are directly involved in plant resistance to biotic challenges[34]. Among these disease-related secondary processes, the phenylpropanoid metabolic pathway is considered a major source of anti-pathogen phenolic substances and other natural compounds, of which the biosynthesis is commonly taken charge of by a series of metabolic enzymes such as PAL and PPO[35]. In the present study, we explored the effects of KHCO3 spraying on the phenylpropanoid metabolic pathway in diseased melon seedlings, and the significantly increased metabolic capacity was observed for PAL and PPO in the KHCO3-7DAI and -2DBI samples relative to the H2O-treated ones (Fig. 2). Consistent with the promoted phenylpropanoid metabolism, the dramatically enhanced contents of phenolic substances including condensed tannin, lignin, total phenols and flavonoids, as well as the increased HRGP content, were detected in the leaves of KHCO3-7DAI and -2DAI melon seedlings (Fig. 3).
Similar results have been reported in previous studies. For example, by executing a series of enzymatic assays for 35 tomato varieties, Alizadeh-Moghaddam et al. have uncovered the positive association of PAL activity with tomato defense against Alternaria alternate, a causal pathogen for early blight[36]. Li et al. have also reported that exogenous application of acetylsalicylic acid (ASA) can greatly enhance the activities of phenylpropanoid metabolic enzymes such as PAL and PPO in melon fruits, leading to the efficient restriction of Fusarium rot and neosolaniol contamination[37]. As a well-known anti-pathogen phenolic compound generated from phenylpropanoid metabolism, lignin has been demonstrated to be rapidly accumulated at infected sites with the aid of HRGP, a class to cell wall-associated proteins being profoundly involved in plant response to various biotic stress[38], and function as physical barriers to block the absorption of water and nutrients from diseased plants, thus completely or mostly restricting the invasion and spread of pathogens[39−41]. Condensed tannins and flavonoids, another two resistance-related phenolic substances, also play essential roles in plant defense against disease pathogens[42]. Based on these observations, we concluded that the KHCO3-mediated melon protection under powdery mildew might, to a large extent, be attributed to the stimulated phenylpropanoid metabolic pathway.
Oxidative burst, a highly conservative defense reaction wherein ROS is rapidly and explosively generated in diseased plants[43], is also considered a crucial component of plant immunity systems. The accumulation of ROS such as H2O2 and O2.−, on one hand, could benefit plant survival via inducing a series of subsequent defense responses and dissolving fungal hyphae at the early stage of pathogen invasion[23], while on the other hand, result in severe disturbance of normal physiological and biochemical processes in plants with the extension of accumulation time[44]. To overcome the negative effects from excessive ROS, plants have evolved an enzymatic detoxification mechanism, which is composed of a series of antioxidant enzymes such as SOD, POD and CAT, to balance ROS production in diseased plants[45−47]. For example, a comparative analysis of antioxidant capacity has been carried out for 25 tomato inbred lines with different resistance to early blight caused by Alternaria solani, and the significantly enhanced POD and CAT activities have been revealed in the resistant plants relative to those in the susceptible ones upon A. solani infection, demonstrating the positive involvement of antioxidant enzymes in tomato defense against early blight[48]. In another report about tomato early blight, the authors have observed that the development of early blight is controlled by combining application of Bacillus subtilis, a well-known biocontrol agent, with two necessary nutrients of potassium and zinc, which results in the significant activation of antioxidant enzymes such as SOD and POD in diseased plants[49]. Our study demonstrated, upon P. xanthii infection, the antioxidant capacity of SOD, POD and CAT in KHCO3-7DAI and -2DBI melon seedlings was significantly increased in comparison to the H2O-treated ones over the investigation course (Fig. 4), evidencing that the stimulation of the antioxidant system might also be involved in the KHCO3-mediated improvement of melon resistance to P. xanthii infection.
Several scenarios have been proposed to explain the positive effects of inorganic salts on plant disease resistance. The first is the cation-dependent hypothesis, wherein the improved resistance was attributed to the cations’ effects in the chemical formula of inorganic salts. This scenario can be supported by the observations by Steinberg et al. that the application of C18-
$ \text{SMe}_2^+ $ $ \text{HCO}_3^- $ $ \text{HCO}_3^- $ $ \text{CO}_3^{2-} $ $ \text{CO}_3^{2-} $ $ \text{HCO}_3^- $ To figure out the determinant factor in KHCO3-mediated protective effects, four parallel treatments were carried out for melon seedlings with H2O, which was used as a negative control, KHCO3 solution, which was used as a positive control, KOH solution, of which the pH was equal to that of the positive solution, and KCl solution, wherein the molar concentration of K+ was equal to the positive solution, before P. xanthii inoculation. The apparent restriction of mildew spots, as well as the activation of phenylpropanoid metabolic and antioxidant pathways, was only observed in melon seedlings treated with KHCO3 relative to the H2O-, KOH- and KCl-treated samples (Fig. 6). We thus deduced that it might be
$ \text{HCO}_3^- $ Based on the results in this study, we propose a working model to explain the physiological mechanism underlying KHCO3-mediated melon protection from powdery mildew (Fig. 7): for melon plants challenged or to be challenged by P. xanthii,
$\text{HCO}_3^- $ 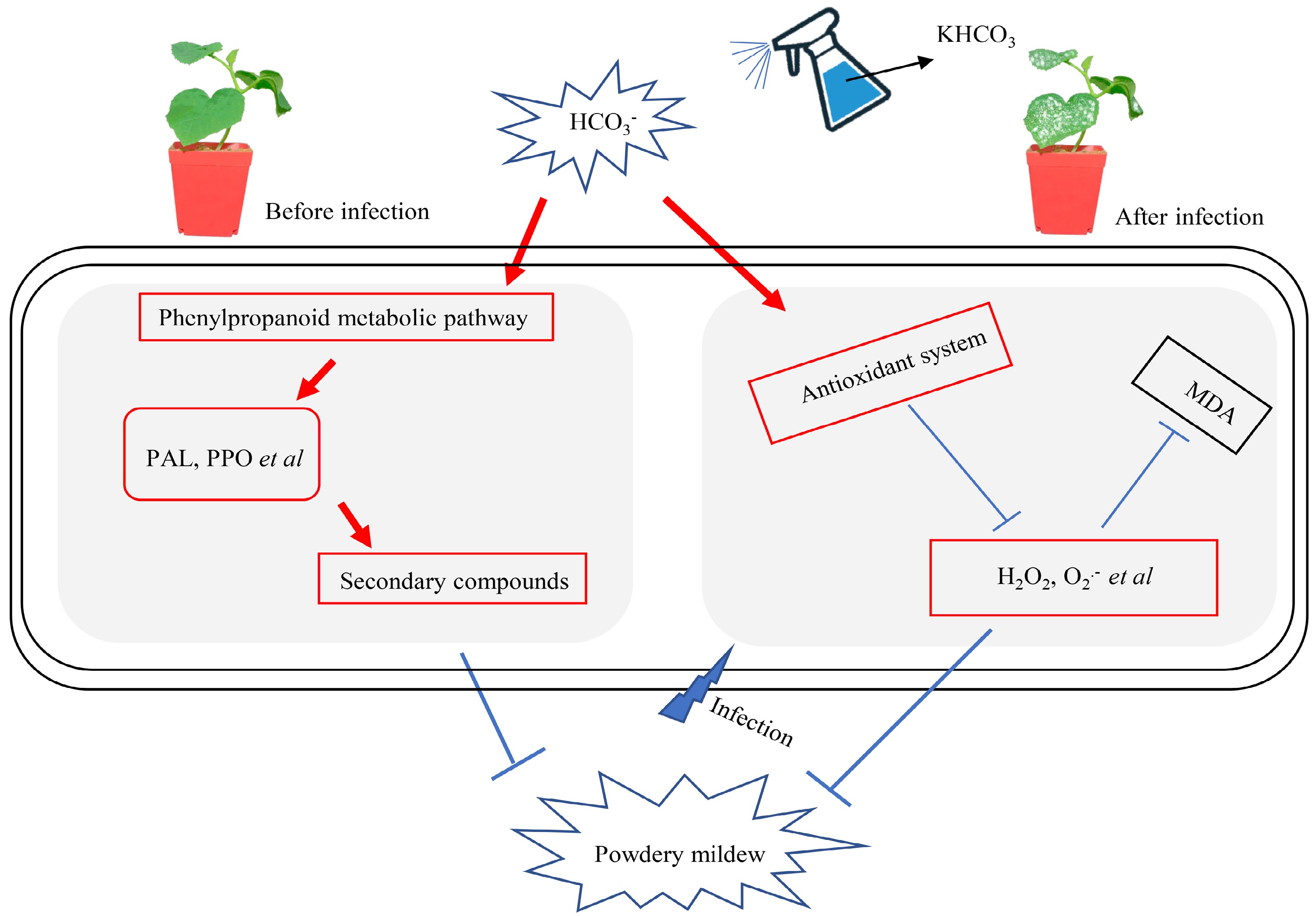
Figure 7.
A proposed model for KHCO3-mediated melon protection from powdery mildew. For melon plants challenged or to be challenged by P. xanthii, HCO3− in the KHCO3 spraying activated phenylpropanoid metabolic pathway to generate more resistance-benefiting metabolites such as HRGP and lignin, conferring inhibitory effects on pathogen development; simultaneously, the activities of key antioxidant enzymes were enhanced to scavenge the overaccumulated ROS, protecting host plants from oxidative damage; and therefore, improved growth performance could be obtained for melon plants under powdery mildew.
-
In this study, we demonstrated that the exogenous spraying of 2.5 g·L−1 KHCO3 apparently alleviated the damage of powdery mildew to melon plants, most likely due to the stimulation of both phenylpropanoid metabolic pathway and the antioxidant system. Further investigation unveiled that
$ \text{HCO}_3^- $ This work was supported by Shandong Vegetable Research System (SDAIT-05), the Key Research and Development Program of Shandong and Chongqing Cooperation (2020LYXZ001), Natural Science Foundation of Shandong Province (ZR2022MC029) and Seed-Industrialized Development Program of Liaocheng City (2021LZ05).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Jianquan Wang, Xiaoye Yu
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang J, Yu X, Hu J, Wang Q, Zheng J, et al. 2023. Positive involvement of HCO3– in modulation of melon resistance to powdery mildew. Vegetable Research 3:3 doi: 10.48130/VR-2023-0003
Positive involvement of HCO3– in modulation of melon resistance to powdery mildew
- Received: 17 October 2022
- Accepted: 06 December 2022
- Published online: 17 January 2023
Abstract: Inorganic salts such as KHCO3 are considered as potential powerful weapons for protecting plants from disease challenges, while their effects remain largely unknown on melon (Cucumis melo L.) resistance to powdery mildew caused by Podosphaera xanthii. In this study, the alleviatory effects of KHCO3 were physiologically explored on P. xanthii-infected seedlings of 'Yangjiaomi', an agrestis melon cultivar being susceptible to powdery mildew, by exogenous spraying at 7 d after inoculation (DAI) and 2 d before inoculation (DBI), respectively. The significantly improved resistance to P. xanthii was observed in melon seedlings sprayed with 2.5 g·L−1 KHCO3 (pH 8.54). Further investigation showed that the activities of PAL and PPO, as well as the accumulation of resistance-benefiting secondary metabolites, were stimulated by KHCO3 treatments. Meanwhile, the activities of SOD, POD and CAT were significantly increased, and the contents of O2.−, H2O2 and MDA were dramatically lowered in the KHCO3-sprayed seedlings than those in the H2O-sprayed seedlings. Another four treatments [H2O, KOH (pH 8.54), 1.86g·L−1 KCl and 2.5g·L−1 KHCO3 (pH 8.54)] were carried out for melon seedlings at 2 DBI. The significantly decreased disease index and the stimulated ROS and phenylpropanoid metabolic pathways were only observed for the KHCO3-sprayed group, suggesting the crucial roles of HCO3− involved in the protection of melon seedlings from powdery mildew. Collectively, our results provide new physiological insights into inorganic salt-mediated plant protection and could benefit the green production of melon in the future.
-
Key words:
- Cucumis melo L. /
- Podosphaera xanthii /
- HCO3− /
- Phenylpropanoid metabolism /
- Antioxidant capacity












