-
Trimethylamine N-oxide (TMAO), as an amine, is an indicator of the freshness of aquatic products partly like other amines[1,2]. Meanwhile, biogenic amines also have biological activity[2,3]. TMAO has the function of stabilizing protein structure[4], which is why the TMAO content in deep-sea fish is relatively high[5]. Some studies believe that TMAO is a factor leading to atherosclerosis[6], while other studies suggest that TMAO may be a marker of disease rather than a vector or pathogenic factor[7]. TMAO is also a feed additive which presents a fresh taste[8]. It is an oxidation product of trimethylamine, derived from carnitine, choline, and betaine in food such as liver, fish, and eggs[7,9]. These foods are decomposed into trimethylamine (TMA) in the animal intestine by trimethylamine lyases produced by gut microbiota[6,10,11]. Then, TMA is converted into TMAO by the hepatic flavin monooxygenase 3 (FMO3)[10−12]. After that, TMAO demethylase (TMAOase) decomposes TMAO into dimethylamine and formaldehyde[8].
TMAO, as a nitrogen-containing compound with three methyl groups, has a chemical structure which is similar to methyl donors such as S-adenosylmethionine (SAM)[13]. Research has found that betaine, as one of the precursors of TMAO, can serve as a methyl donor for SAM[14,15]. Spermidine (SPD) is formed by SAM decarboxylation[16], while this process is influenced by mTOR[17]. The mechanistic target of the rapamycin (mTOR) signaling pathway can regulate the growth of organisms and affect many biological metabolic processes[18] wherein mTOR complex 1 (mTORC1) has functions such as inducing protein synthesis[19] or regulating the metabolism of polyamines[17]. A study used TMAO as a food attractant in Litopenaeus vannamei[20]. Another study found that the appetite regulation and feed intake of Chinese mitten crab (Eriocheir sinensis) can be improved by a diet with 0.27% TMAO[21]. Adding low doses of some amines may promote feed digestion and growth in Litopenaeus stylirostris[22].
In China, Chinese mitten crab is favored by Chinese consumers due to its rich nutritional value and unique freshness[23]. In 2023, the aquaculture output of E. sinensis in China reached 888,600 tons (Chinese fishery statistical yearbook, 2023). Many farmers used frozen fresh fish to feed E. sinensis instead of formula feed, which may be due to the fresher flavor of the crabs fed by frozen fresh fish[24,25]. However, frozen fresh fish can cause adverse consequences such as water quality deterioration[26]. TMAO is common in marine fish and has the highest content in muscle[27]. At present, there is no research on the effect of TMAO on the flavor and metabolism of amines in E. sinensis. This research is about the effect of TMAO on umami, the metabolism of amino acids, and the growth of E. sinensis.
-
A study on Hucho taimen found that the optimal amount of TMAO added is 0.025%[38]. Another study on Megalobrama terminalis suggested that adding 0.04% TMAO to the feed can increase the weight gain rate[39]. In addition, we conducted a preliminary experiment with juvenile crabs and found that crabs fed with 0.01% TMAO formula feed had a higher survival rate. Considering the above factors, we set the addition concentration to 0.01 (low-dose group) and 0.04 (high-dose group) to investigate the metabolic regularity of TMAO in Chinese mitten crabs. We designed three diets including TMAO 0% (control), 0.01%, and 0.04%. In order to minimize experimental errors, we used trimethylamine N-oxide to replace peanut meal, which had the highest proportion added to the feed. The fundamental diet formula is shown in Table 1.
Table 1. Ingredient formulation in this feeding trial.
Ingredients (%) 0% TMAO 0.01% TMAO 0.04% TMAO Fish meal 25.00 25.00 25.00 Rapeseed meal 2.50 2.50 2.50 Soybean meal 8.00 8.00 8.00 Cottonseed meal 3.00 3.00 3.00 Peanut meal 27.60 27.59 27.56 α-Starch 20.50 20.50 20.50 Fish oil 1.20 1.20 1.20 Soybean oil 3.90 3.90 3.90 Cholesterol 0.20 0.20 0.20 Carboxymethyl cellulose 2.00 2.00 2.00 Ca(H2PO4)2·H2O 2.20 2.20 2.20 Lecithin 0.20 0.20 0.20 Zeolite 0.40 0.40 0.40 Premixa 1.00 1.00 1.00 Mixtureb 2.30 2.30 2.30 Trimethylamine N-oxidec 0.00 0.01 0.04 Total 100.00 100.00 100.00 Proximate composition Crude protein 36.12 36.09 36.34 a Premix supplied the following vitamins (IU or mg/kg) and minerals (g/kg): vitamin A, 900,000 IU; vitamin B1, 320 mg; vitamin B2, 1,090 mg; vitamin B5, 2,000 mg; vitamin B6, 500 mg; vitamin B12, 1.6 mg; vitamin C, 10,000 mg; vitamin D, 200,000 IU; vitamin E, 4,500 mg; vitamin K3, 220 mg; pantothenate, 1,000 mg; folic acid, 165 mg; choline, 60,000 mg; biotin, 100 mg; and myoinositol, 15,000 mg; ZnSO4·7H2O, 22 g; FeSO4·7H2O, 25 g; CuSO4·5H2O, 2 g; MnSO4·4H2O, 7 g; CoCl2·6H2O, 0.1 g; KI, 0.026 g; Na2SeO3, 0.04 g. b Mixture includes the following ingredients (%): mildew-proof agent 2.35%; antioxidants 1.72%; choline chloride 4.75%; Lvkangyuan 59.61%; salt 22.06%; and biostime 9.51%. c Trimethylamine N-oxide was purchased from Nanjing Lattice Chemical Technology Co., Ltd, Nanjing, Jiangsu, China. All ingredients were screened through a 60 mm sieve, weighed, and mixed. Later, 30% distilled water was continuously added into the mixture and mixed manually to ensure full and uniform mixing. The mixture was then treated with a 2.5 mm mold in a single screw meat grinder extruder, ventilated and air-dried. After that, the feed was ground to proper size and stored at −20 °C.
Crabs and feeding trial
-
One hundred and twenty intact crabs were chosen (37.50 ± 0.5 g) which were purchased from Pukou, Jiangsu province, China (118.426051° E, 32.074386° N). The experiment was conducted from early June to the end of September. The crabs were divided into three groups. Each group had four replicates and each replicate had five male crabs and five female crabs. Crabs were fed in cement pools. Each pool (0.8 m × 1.0 m × 1.0 m, Height × Length × Width) had 14 pipes as shelters and each pipe had a diameter of 15 cm and a length of 20 cm. The crabs were temporarily raised for a week to ensure that they had adapted to the environment of the pool. After that, the crabs were fed with the corresponding experimental diets once a day at 18:00 to ensure they were full. The experiment lasted for 15 weeks. The remaining feed in the pool was cleaned up after crab feeding was completed. During the experiment, 1/3 of the water was replaced daily. The pH in the water was 7.3–8.4, the dissolved oxygen was 5.0–7.0 mg/L, and the temperature was 22–28 °C.
Sample collection
-
All crabs were starved for 24 h before sampling. From each replicate, four crabs were selected and hemolymph was collected from them on the leg joints with syringes. The hemolymph was mixed 1:1 using anticoagulant which was composed of 450 mmol/L NaCl, 26 mmol/L citrate, 15 mmol/L EDTA, 100 mmol/L glucose, and 30 mmol/L citric acid with a pH of 7.2. The mixture was then centrifuged at 4,500 rpm, 4 °C for 15 min. The supernatant was taken and stored at −20 °C for detection and analysis. Meanwhile, muscle and hepatopancreas samples were collected and stored at −80 °C for subsequent analysis.
Enzymes in the hepatopancreas related to the metabolism of TMAO and digestion
-
Trypsin (cat. no. A080-2-1, Jiancheng Bioengineering Co., Nanjing, China), trimethylamine N-oxide demethylase (TMAOase) (cat. no. ANG-E329436S, Nanjing Aoqing Biotechnology Co., Ltd., China), and flavin monooxygenase 3 (FMO3) (cat. no. ANG-E54112F, Nanjing Aoqing Biotechnology Co., Ltd., China) in the hepatopancreas were measured through the reagent kit and its instructions.
Electronic tongue
-
The muscle sample (2.0 ± 0.01g) of the crab to be tested was accurately weighed, steamed for 10 min, then 10 mL of deionized water was added, homogenized, and stewed for 10 min. It was then centrifuged at 12,000 r/min for 10 min, the upper oil layer was then removed, filtered, and diluted to 40 mL. The diluent was then poured into a dedicated injection cup for electronic tongue measurements at room temperature. The data collection time was 120 s for each sample, and one data was collected in 1 s. The response value of the 120th s on the umami sensor was selected as the original data of the electronic tongue. After each detection, the electronic tongue sensor was cleaned with deionized water for 10 s.
Determination of biogenic amines
-
Five ml of methanol was added into the muscle or hepatopancreas sample (1.0 ± 0.01 g), homogenized, and centrifuged at 12,000 r/min for 10 min. TMAO and TMA were determined using LC-MS/MS which included Tandem Mass Spectrometry (MS/MS, QTRAP® 6500+) and Ultra Performance Liquid Chromatography (UPLC, ExionLCTM AD). Mobile phase: A, ultrapure water (including 0.3% ammonia and 10 mM ammonium acetate); B, 90% acetonitrile/water (V/V). The flow rate was 0.35 mL/min. The applied parameters were as follows: iionizing voltage, 5,500 V; ion source temperature, 500 °C; collision gas, 50 psi; ion source gas, 50 psi; curtain gas: 35 psi. Scanning mode adopted multiple reaction monitoring mode. Each ion pair was scanned and detected based on the optimized collision energy and declustering potential.
Several biogenic amines (including spermidine, spermine, putrescine, cadaverine, tyramine, and histamine) were determined by LC-MS/MS referring to the method of Zhang et al.[2].
Amino acid analysis
-
Referring to Yuan et al.[28], the amino acid content was determined using acid hydrolysis. The specific steps were as follows: Samples hydrolyzed with 6 N HCl at 110 °C for 24 h, then the HCL was removed with a nitrogen blowing instrument. The samples were redissolved with 0.1 N HCl and passed through a 0.22 μm microporous membrane filter. Finally, an amino acid analyzer (Hitachi L-8900) was used to detect amino acids.
Gene expression
-
Referring to the methods described previously by Dong et al.[29], the total RNA insolation and complementary DNA (cDNA) reverse-transcription were performed. Total RNA was extracted from the hepatopancreas using the SteadyPure RNA Extraction Kit (Accurate Biotechnology, Hunan, China); then, the purity and concentration of RNA was measured using a NanoDrop ND-1000 UV Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) based on OD 260/280 spectrophotometry. The reaction system of RT-qPCR included 10 μL 2 × ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China), 7.2 μL DEPC-water, and 2 μL cDNA template, and the primers of forward and reverse were 0.4 μL (10 μM). Subsequently, the expression levels of specific genes were determined by quantitative Real-time PCR (qRT-PCR) under the Bio-Rad CFX96 system (Hercules, USA). The PCR procedure referred to the methods of Hua et al.[30]. The PCR procedure included 95 °C for 30 s, 95 °C for 10 s, and 60 °C for 28 s for 40 cycles. The melting curve step was from 95 °C to 60 °C for 15 s and 60 °C for 1 min; then, the temperature was raised to 95 °C at a rate of 1.6 °C per second for 15 s. Table 2 shows the specific primer sequences of genes in this experiment. The gene expression level was calaculated by the 2−ΔΔCᴛ[31] method and β-actin used as a reference gene.
Table 2. Primer pair sequences and length of the genes used for real-time PCR (qPCR).
Gene Position Primer sequence Genebank
accessionLength Ref. mTOR Forward AGAAGCTGCATGACTGGGAC c148249_g1 20 [37] Reverse CGGTCACACGACACACTGTA 20 S6 Forward TTCCGAGGGTGAACAAGACG c141087_g1 20 [37] Reverse CTGGCCCATACGCTTCTCAT 20 S6K1 Forward TCAATAGCGTCGTCATCG c74214_g1 18 [37] Reverse CCCTGCGTGTAGTGGTTG 18 4E-BP1 Forward GCAACACGCCAACTAAACTC c114480_g1 20 [37] Reverse GCGACACCACCTAATATCCA 20 eIF4E Forward CAAGGCTGAGCAGGACTTCA c56848_g1 20 [37] Reverse AGCTGATCCAGGTCACAAGC 20 β-actin Forward TCGTGCGAGACATCAAGGAAA KM244725.1 21 [38] Reverse AGGAAGGAAGGCTGGAAGAGTG 22 mTOR: mammalian target of rapamycin; S6: ribosomal protein S6; S6K1: ribosomal S6 protein kinase; 4E-BP1: eukaryotic translation initiation factor 4E-binding protein 1; eIF4E: eukaryotic translation initiation factor 4E. Western blotting
-
The determination of protein expression was carried out with reference to the study by Dai et a.l[32]. A 100 mg muscle sample was washed and RIPA lysis buffer was added, then homogenized. The homogenate was centrifuged and the supernatant retained. The protein concentrations were measured using a BCA protein assay kit (Beyotime Biotechnology, China) and the protein concentrations unified. A thermal cycler was used to denature the proteins. A 10 μl protein sample was then taken and added to the sampling hole. 100 V constant voltage electrophoresis was carried out for 1.5 h. After electrophoresis, the separation gel was taken and the target protein band cut out according to the molecular weight of the protein maker. 120 V constant voltage horizontal electrophoresis was performed for 1.5 h to transfer the protein to the PVDF membrane. The PVDF membrane was then transferred to the protein and placed in a 5% BSA solution. The sample was then sealed at room temperature for 2 h. The 5% BSA solution was then discarded, and the primary anti-mTOR antibody added (No. D221467, Sangong Biotech, China) and incubated at 4 °C for 12 h. The first antibody was then recovered, placed on the table concentrator, and cleaned three times with PBS for 5 min each time. Diluted HRP-conjugated goat anti-mouse IgG (No. D110058, Sangong Biotech, China) was then added and incubated at room temperature for 2 h. It was then placed on the table concentrator and cleaned three times with PBS. ECL protein developer was added to scan protein bands in the hypersensitive chemiluminescence gel imaging system. The intensity of the target bands were quanitfied using Image J software (US National Institutes of Health, USA).
Calculations
-
The parameters in this experiment were calculated as follows:
$ \mathrm{W}\mathrm{G}\mathrm{R}\; \left(\text{%}\right)=\dfrac{\mathrm{Fi}\mathrm{n}\mathrm{a}\mathrm{l}\; \mathrm{b}\mathrm{o}\mathrm{d}\mathrm{y}\; \mathrm{w}\mathrm{e}\mathrm{i}\mathrm{g}\mathrm{h}\mathrm{t}-\mathrm{I}\mathrm{n}\mathrm{i}\mathrm{t}\mathrm{a}\mathrm{l}\; \mathrm{b}\mathrm{o}\mathrm{d}\mathrm{y}\; \mathrm{w}\mathrm{e}\mathrm{i}\mathrm{g}\mathrm{h}\mathrm{t}}{\mathrm{In\mathrm{i}}\mathrm{t}\mathrm{a}\mathrm{l}\; \mathrm{b}\mathrm{o}\mathrm{d}\mathrm{y}\; \mathrm{w}\mathrm{e}\mathrm{i}\mathrm{g}\mathrm{h}\mathrm{t}}\times100 $ $ \mathrm{F}\mathrm{I}\;\left(\mathrm{g}\right)=\frac{\mathrm{T}\mathrm{o}\mathrm{t}\mathrm{a}\mathrm{l}\;\mathrm{ }\mathrm{f}\mathrm{e}\mathrm{e}\mathrm{d}\;\mathrm{ }\mathrm{i}\mathrm{n}\mathrm{t}\mathrm{a}\mathrm{k}\mathrm{e}\;\mathrm{ }\mathrm{o}\mathrm{f}\;\mathrm{ }\mathrm{e}\mathrm{a}\mathrm{c}\mathrm{h}\;\mathrm{ }\mathrm{t}\mathrm{a}\mathrm{n}\mathrm{k}}{\mathrm{T}\mathrm{o}\mathrm{t}\mathrm{a}\mathrm{l}\;\mathrm{ }\mathrm{n}\mathrm{u}\mathrm{m}\mathrm{b}\mathrm{e}\mathrm{r}\;\mathrm{ }\mathrm{o}\mathrm{f}\;\mathrm{ }\mathrm{c}\mathrm{r}\mathrm{a}\mathrm{b}\;\mathrm{ }\mathrm{i}\mathrm{n}\;\mathrm{ }\mathrm{e}\mathrm{a}\mathrm{c}\mathrm{h}\;\mathrm{ }\mathrm{ }\mathrm{ }\mathrm{ }\mathrm{ }\mathrm{ }\mathrm{ }\mathrm{t}\mathrm{a}\mathrm{n}\mathrm{k}} $ $ \mathrm{S}\mathrm{R}\; \left(\text{%}\right)=\frac{\mathrm{F}\mathrm{i}\mathrm{n}\mathrm{a}\mathrm{l}\; \mathrm{ }\mathrm{n}\mathrm{u}\mathrm{m}\mathrm{b}\mathrm{e}\mathrm{r}\; \mathrm{ }\mathrm{o}\mathrm{f}\; \mathrm{ }\mathrm{c}\mathrm{r}\mathrm{a}\mathrm{b}\mathrm{s}}{\mathrm{I}\mathrm{n}\mathrm{i}\mathrm{t}\mathrm{i}\mathrm{a}\mathrm{l}\; \mathrm{ }\mathrm{n}\mathrm{u}\mathrm{m}\mathrm{b}\mathrm{e}\mathrm{r}\; \mathrm{ }\mathrm{o}\mathrm{f}\; \mathrm{ }\mathrm{c}\mathrm{r}\mathrm{a}\mathrm{b}\mathrm{s}}\times100 $ $ \mathrm{F}\mathrm{C}\mathrm{R}=\frac{\mathrm{F}\mathrm{e}\mathrm{e}\mathrm{d}\; \mathrm{ }\mathrm{i}\mathrm{n}\mathrm{t}\mathrm{a}\mathrm{k}\mathrm{e}}{\mathrm{Fi\mathrm{n}}\mathrm{a}\mathrm{l}\ \mathrm{b\mathrm{o}\mathrm{d}\mathrm{y}\ w\mathrm{e}}\mathrm{i}\mathrm{g}\mathrm{h}\mathrm{t}-\mathrm{I}\mathrm{n}\mathrm{i}\mathrm{t}\mathrm{a}\mathrm{l}\mathrm{\ b\mathrm{o}}\mathrm{d}\mathrm{y}\; \mathrm{w}\mathrm{e}\mathrm{i}\mathrm{g}\mathrm{h}\mathrm{t}\ +\ \mathrm{D}\mathrm{e}\mathrm{a}\mathrm{d}\ \mathrm{c}\mathrm{r}\mathrm{a}\mathrm{b}\ \mathrm{w}\mathrm{e}\mathrm{i}\mathrm{g}\mathrm{h}\mathrm{t}\ \mathrm{g}\mathrm{a}\mathrm{i}\mathrm{n}} $ $ \mathrm{H}\mathrm{S}\mathrm{I}\; \left(\text{%}\right)=\frac{\mathrm{T\mathrm{o}\mathrm{t}\mathrm{a}}\mathrm{l}\ \mathrm{h\mathrm{e}\mathrm{p}\mathrm{a}\mathrm{t}\mathrm{o}\mathrm{p}\mathrm{a}\mathrm{n}\mathrm{c}\mathrm{r}\mathrm{e}\mathrm{a}\mathrm{s}\ w\mathrm{e}}\mathrm{i}\mathrm{g}\mathrm{h}\mathrm{t}}{\mathrm{F\mathrm{i}\mathrm{n}\mathrm{a}\mathrm{l}\ \mathrm{b}\mathrm{o}\mathrm{d}y\ \mathrm{w}\mathrm{e}\mathrm{i}\mathrm{g}\mathrm{h}\mathrm{t}}}\times100 $ where, WGR is weight gain rate; FI is feed intake; SR is survival rate; FCR is feed conversion ratio; HSI is hepatopancreas somatic indices.
Statistical analysis
-
Statistical analysis of the data was performed using the software SPSS 25 (Chicago, IL, USA). Means and pooled SEM (pooled standard error of means) were presented. The normality and homogeneity of variance were tested before analysis by the Kolmogorov-Smirnov and Levene tests. Then, one-way analysis of variance (ANOVA) and Duncan's multiple comparison tests were performed to estimate the significant differences (p < 0.05) among mean values.
-
FCR and FI were affected markedly by the addition of TMAO (p < 0.05, Table 3). Compared with the control group, two experimental groups with different TMAO addition amounts showed a significant reduction in FCR and FI (p < 0.05). Between the 0.01% TMAO group and 0.04% TMAO group, there was no significant difference between FCR and FI. With the addition of TMAO, WGR showed a slight increasing trend. In addition, two experimental groups had a higher SR compared to the control group. There was no marked difference among all groups in other indicators.
Table 3. Growth performance of Chinese mitten crab fed experimental diets with different concentrations of trimethylamine N-oxide.
Indexs 0% TMAO 0.01% TMAO 0.04% TMAO p value SR (%) 60 ± 5.77 66.67 ± 6.67 66.67 ± 13.33 0.844 WGR (%) 114.56 ± 5.00 119.20 ± 8.37 124.98 ± 9.26 0.656 FCR 2.72 ± 0.22a 2.09 ± 0.05b 2.02 ± 0.21b 0.049 FI (g) 117.44 ± 4.53a 93.46 ± 4.85b 92.09 ± 2.70b 0.008 HSI (%) 6.12 ± 0.45 6.17 ± 0.02 5.33 ± 0.56 0.342 CW (cm) 57.35 ± 1.05 58.11 ± 1.04 57.01 ± 1.62 0.826 CL (cm) 52.11 ± 0.23 51.29 ± 1.24 51.86 ± 1.68 0.889 BH (cm) 27.89 ± 0.81 26.38 ± 0.65 27.02 ± 0.71 0.399 SR: survival rate; WGR: weight gain rate; FCR: feed conversion ratio; FI: feed intake; HSI: hepatopancreas somatic indices; CW: carapace width; CL: carapace length; BH: body height. The values are the means ± SEM (n = 3). Values in the same row with different superscripts are significantly different at p < 0.05. Enzymes in hepatopancreas
-
In Fig. 1, no marked difference was seen in the enzyme activity of the trypsin between the 0.01% TMAO group and the 0.04% TMAO group. However, compared to the 0% TMAO group, the enzyme activity of the trypsin showed a significant increase in both experimental groups (p < 0.05). No marked difference among all groups was shown in the activity of TMAOase and the content of FMO3.
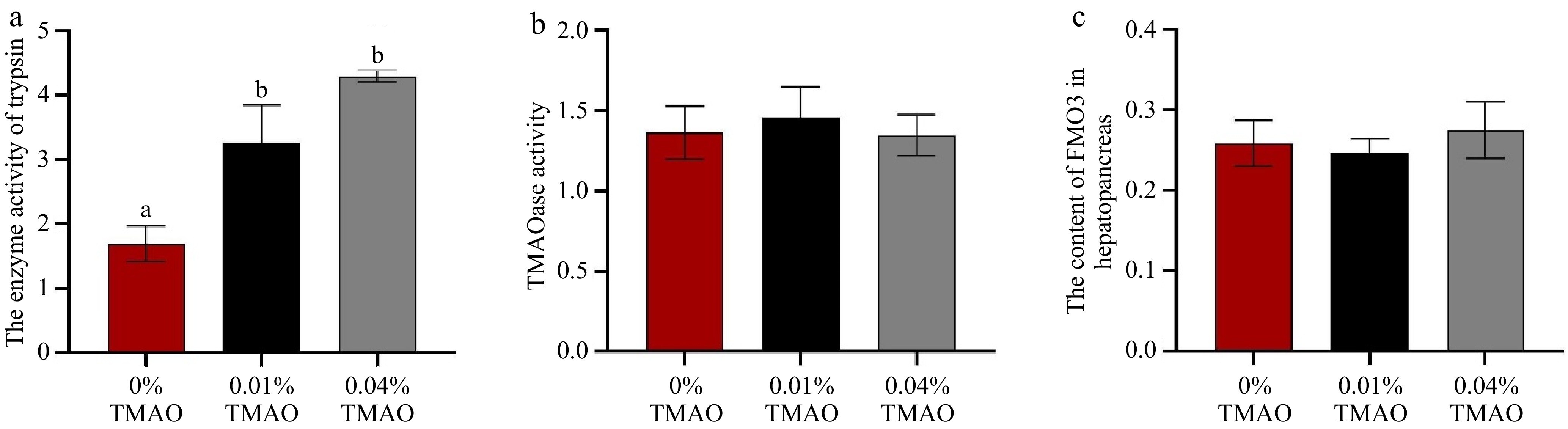
Figure 1.
Effect of dietary TMAO on (a) the enzyme activity of trypsin in the hepatopancreas, (b) the trimethylamine N-oxide demethylase (TMAOase) activity in the hepatopancreas, and (c) the content of flavin monooxygenase 3 (FMO3) in the hepatopancreas. The values are the means ± SEM (n = 3). Different letters indicate significant differences (p < 0.05).
Value of umami
-
Figure 2 shows that the value of umami was significantly higher than that of the 0% TMAO group (p < 0.05). With the further addition of TMAO, the value of umami in the 0.04% TMAO group increased markedly (p < 0.05).
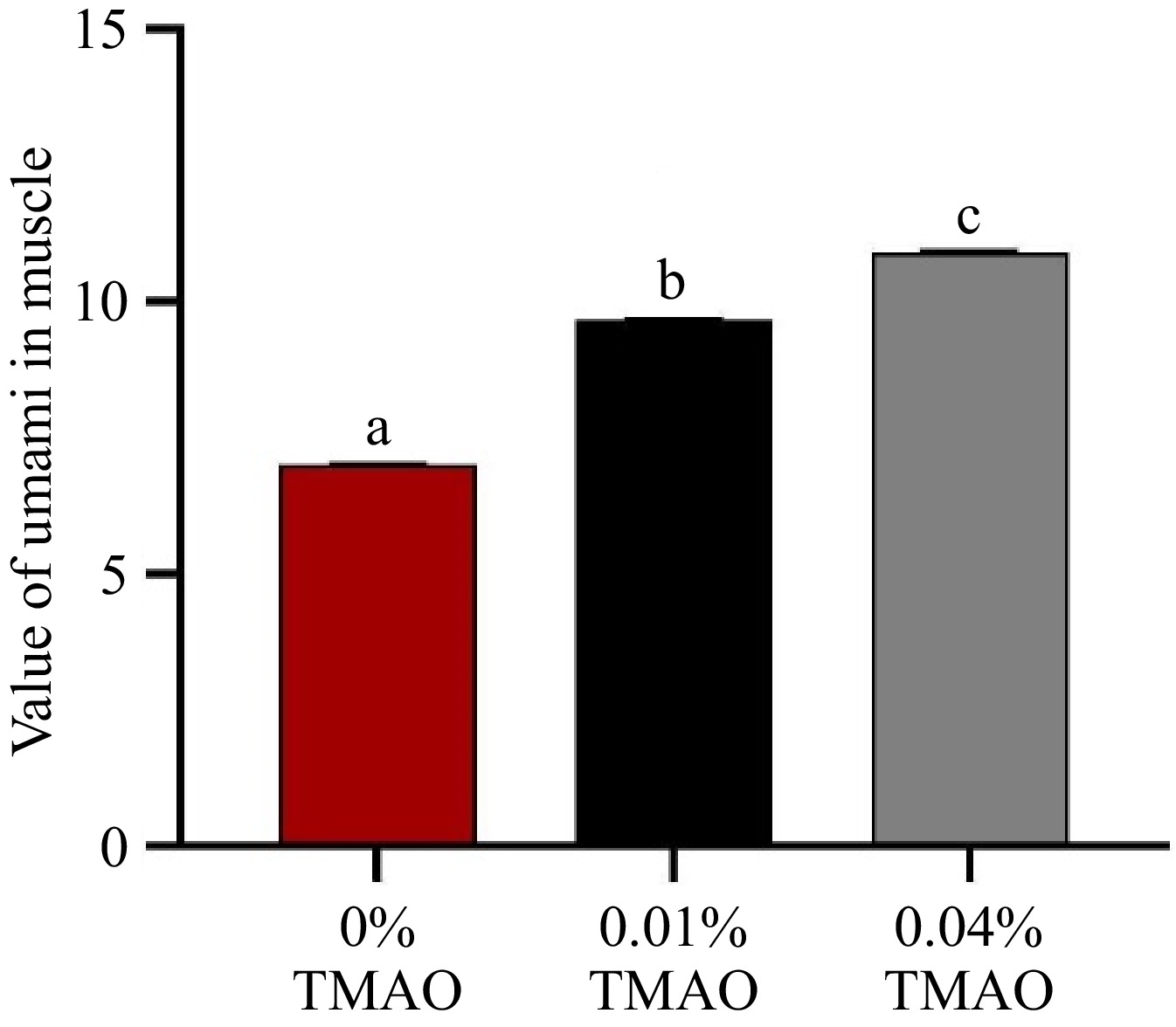
Figure 2.
Effect of dietary TMAO on the value of umami in muscle. The values are the means ± SEM (n = 3). Different letters indicate significant differences (p < 0.05).
Deposition of TMAO and TMA
-
Results of TMAO and TMA content in the muscle and hepatopancreas are shown in Fig. 3. The TMAO content in muscle of the 0.04% TMAO group increased significantly compared to the other two groups (p < 0.05). Regarding the content of TMAO in the hepatopancreas, no significant difference could be shown among all groups. Whether in muscle or hepatopancreas, no marked difference was observed in TMA content among all groups.
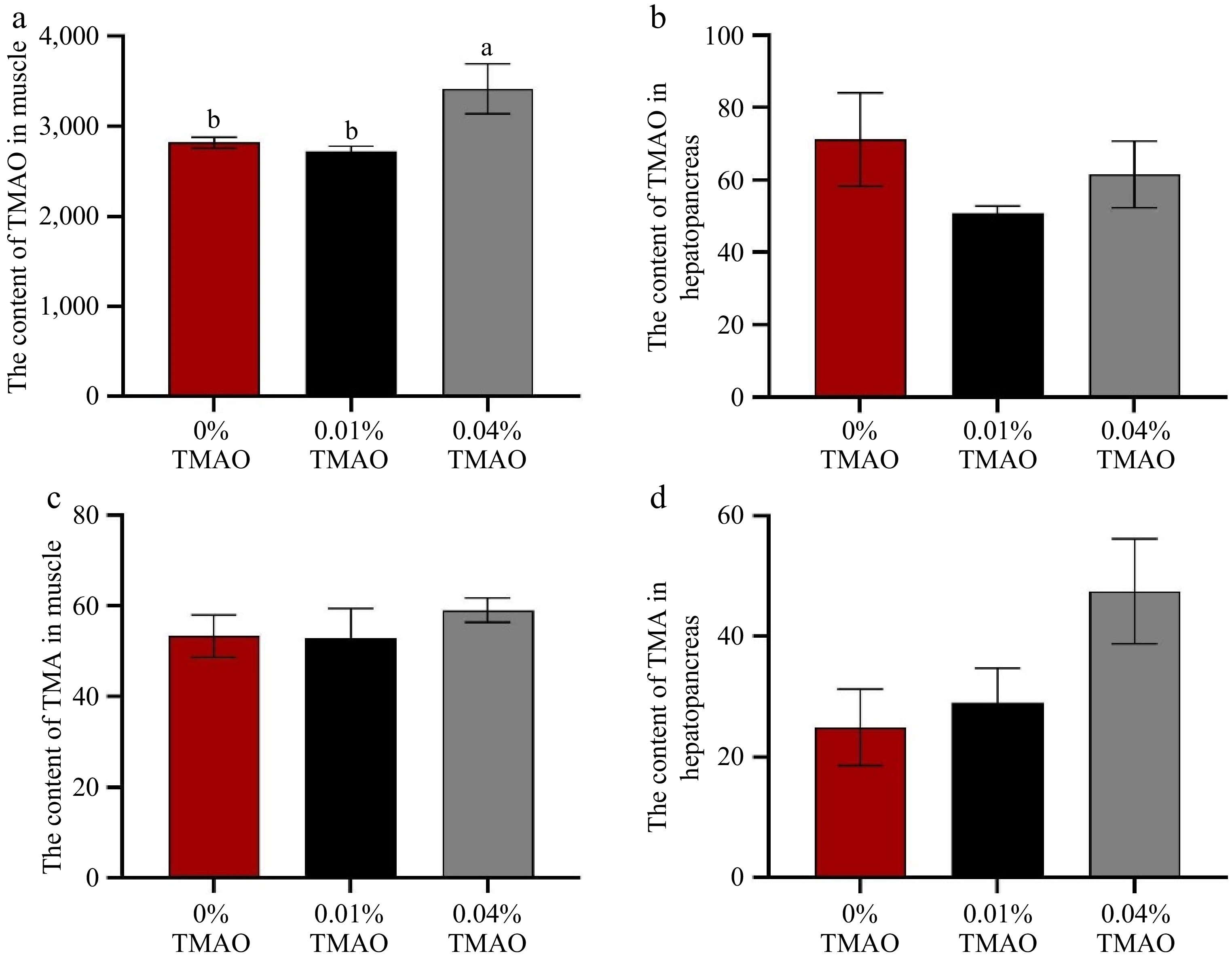
Figure 3.
Effect of dietary TMAO on (a) the content of TMAO in muscle, (b) the content of TMAO in the hepatopancreas, (c) the content of TMA in muscle, and (d) the content of TMA in the hepatopancreas. The values are the means ± SEM (n = 3). Different letters indicate significant differences (p < 0.05).
Amino acid
-
The results of amino acids in the muscle are shown in Table 4. The methionine content in the muscle of the 0.04% TMAO group increased significantly compared to the other two groups (p < 0.05). The content of the remaining 16 amino acids did not show a marked difference between the groups but showed an upward trend with the addition of TMAO. Total amino acid content also showed an upward trend.
Table 4. Effect on the composition of amino acids in the muscle of Chinese mitten crab fed experimental diets with different concentrations of trimethylamine N-oxide.
Muscle 0% TMAO 0.01% TMAO 0.04% TMAO p value Essential amino acid Threonine 0.54 ± 0.05 0.57 ± 0.01 0.60 ± 0.05 0.610 Valine 0.52 ± 0.04 0.56 ± 0.01 0.60 ± 0.05 0.441 Methionine 0.30 ± 0.03a 0.31 ± 0.02a 0.41 ± 0.01b 0.014 Isoleucine 0.45 ± 0.05 0.53 ± 0.01 0.55 ± 0.04 0.171 Leucine 0.81 ± 0.07 0.92 ± 0.01 0.95 ± 0.07 0.287 Phenylalanine 0.50 ± 0.04 0.53 ± 0.01 0.57 ± 0.06 0.456 Lysine 0.93 ± 0.07 1.01 ± 0.02 1.03 ± 0.08 0.492 Histidine 0.28 ± 0.03 0.29 ± 0.00 0.32 ± 0.03 0.539 Arginine 1.18 ± 0.08 1.24 ± 0.03 1.36 ± 0.09 0.268 Non-essential amino acid Aspartic acid 1.10 ± 0.08 1.21 ± 0.02 1.26 ± 0.10 0.373 Serine 0.51 ± 0.04 0.54 ± 0.01 0.59 ± 0.05 0.413 Glutamic acid 1.78 ± 0.13 1.95 ± 0.03 2.01 ± 0.15 0.378 Glycine 0.87 ± 0.05 0.90 ± 0.05 0.88 ± 0.02 0.876 Alanine 0.83 ± 0.06 0.91 ± 0.03 0.98 ± 0.12 0.490 Cystine 0.14 ± 0.02 0.14 ± 0.02 0.17 ± 0.02 0.548 Tyrosine 0.40 ± 0.04 0.44 ± 0.02 0.50 ± 0.12 0.278 Proline 0.47 ± 0.06 0.46 ± 0.03 0.51 ± 0.03 0.659 Total 11.61 ± 0.85 12.52 ± 0.29 13.27 ± 1.01 The values are the means ± SEM (n = 4). Values in the same row of each tissue with different superscripts are significantly different at p < 0.05. Biogenic amines
-
In Table 5, with the addition of TMAO, the content of spermidine and spermine in the 0.01% TMAO group decreased, and then decreased significantly in the 0.04% TMAO group (p < 0.05). No marked difference was shown in the content of the other four biogenic amines (cadaverine, histamine, putrescine, and tyramine) wherein cadaverine, histamine, and putrescine showed a downward trend.
Table 5. Deposition of biogenic amines in muscle of Chinese mitten crab fed experimental diets with different concentrations of trimethylamine N-oxide.
Amines 0% TMAO 0.01% TMAO 0.04% TMAO p value Spermidine 450.23 ± 51.83a 372.72 ± 12.41ab 334.46 ± 19.94b 0.112 Spermine 88.87 ± 11.95a 39.39 ± 3.09b 28.01 ± 1.35b 0.002 Cadaverine 62.83 ± 7.75 43.58 ± 11.26 34.17 ± 5.31 0.126 Histamine 10.78 ± 0.62 10.48 ± 0.15 10.07 ± 0.07 0.445 Putrescine 345.77 ± 37.71 312.99 ± 70.59 328.98 ± 66.13 0.423 Tyramine 5.89 ± 1.04 6.68 ± 0.78 7.73 ± 0.92 0.421 The values are the means ± SEM (n = 3). Values in the same row of with different superscripts are significantly different at p < 0.05. Gene expression
-
The result of gene expression on the mTOR pathway is shown in Fig. 4. The relative gene expression level of S6K1, S6, mTOR, and eIF4E showed an upward trend in the 0.01% TMAO group. Meanwhile, the relative gene expression level of S6K1 and eIF4E increased significantly (p < 0.05) while 4E-BP1 decreased markedly (p < 0.05). On the contrary, in the 0.04% TMAO group, the above-mentioned upregulated genes had all been downregulated and the relative gene expression level of 4E-BP1 had a slight upregulation compared to the 0.01% TMAO group.
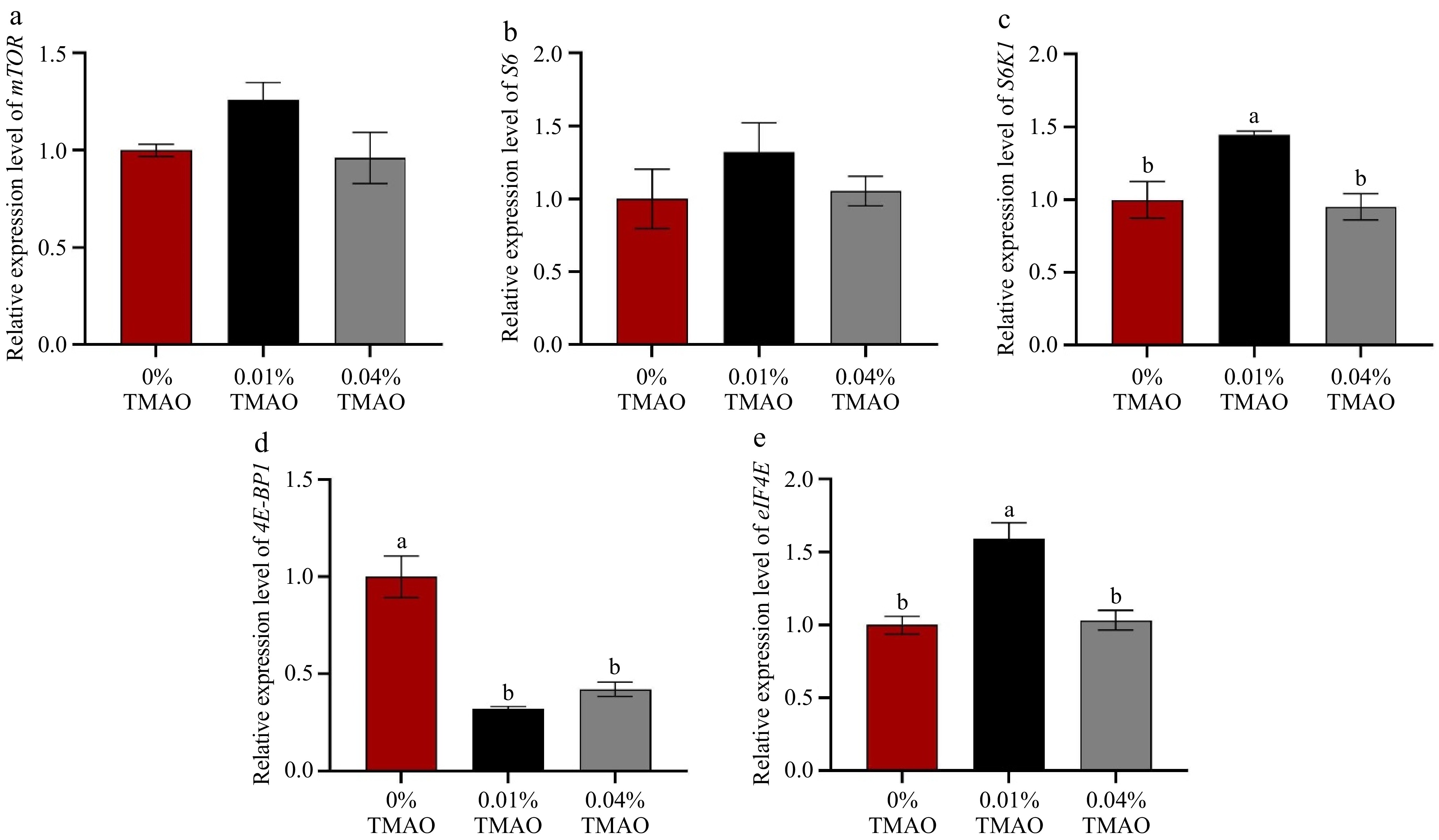
Figure 4.
Effect of dietary TMAO on (a) mammalian target of rapamycin (mTOR), (b) ribosomal protein S6 (S6), (c) ribosomal S6 protein kinase (S6K1), (d) eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), and (e) eukaryotic translation initiation factor 4E (eIF4E). The values are the means ± SEM (n = 3). Different letters indicate significant differences (p < 0.05).
Protein expression related to the mTOR pathway
-
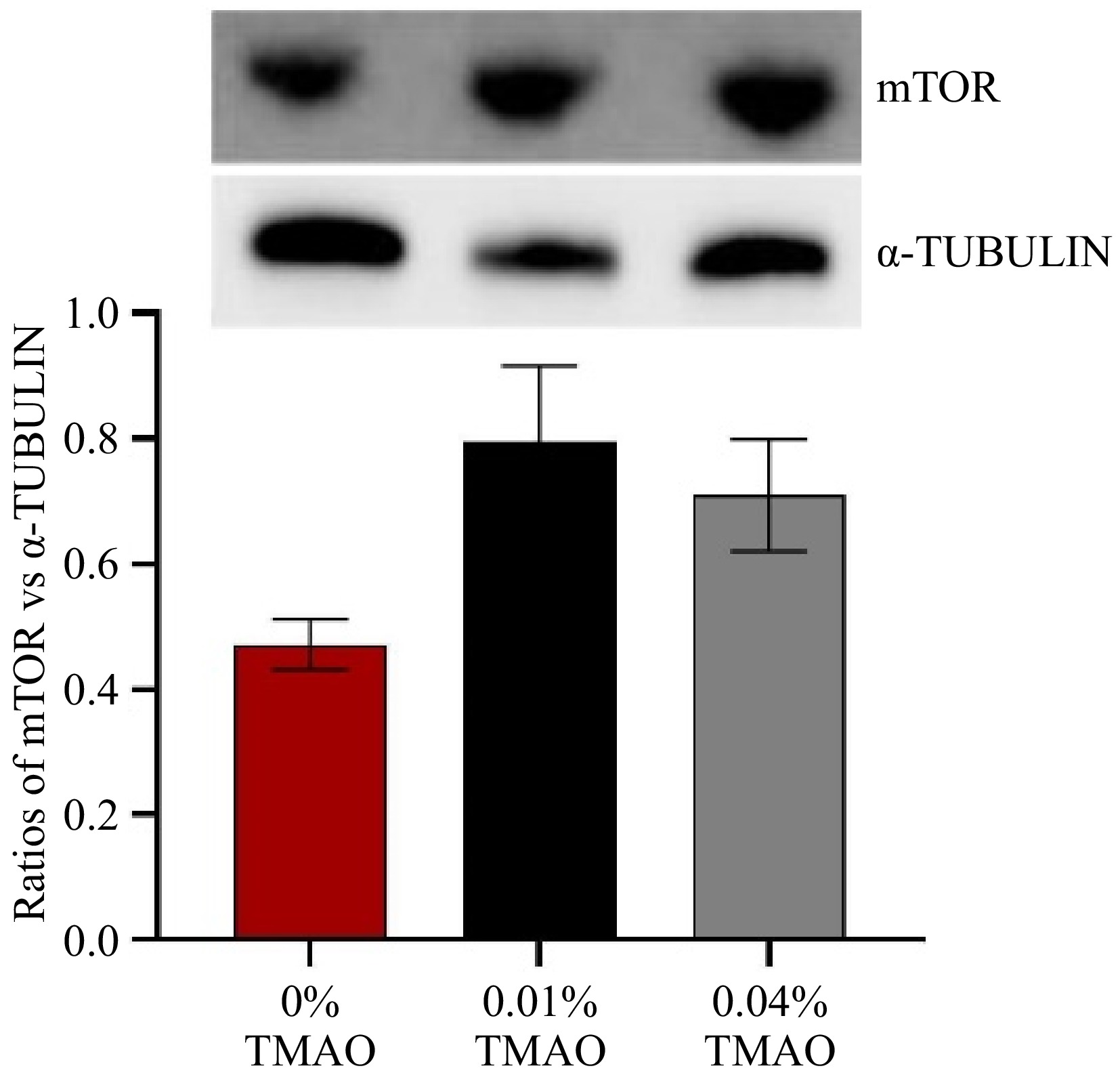
From Fig. 5, the protein expression of mTOR in muscle had no significant difference among all groups. There was a slight increase of mTOR in the 0.01% TMAO group compared with the 0% TMAO group and then showed a downward trend in the 0.04% TMAO group.
-
Nowadays there are still many farmers feeding frozen fresh fish in the E. sinensis breeding industry of China, one reason for this is the low utilization rate of formula feed. In addition, there is a problem of higher FCR when feeding E. sinensis formula feed compared to other aquaculture animals[21]. However, frozen fresh fish have problems with uncontrollable quality and unstable sources and it can lead to deterioration of water quality[26,33]. Frozen fresh fish comes from the ocean and some previous reports found that to better regulate osmotic pressure and protect proteins, the muscles of marine fish contains a large amount of TMAO[5,7]. We speculated that this may be why E. sinensis that eat frozen fresh fish have a fresh or even fishy smell.
The results of this study suggest that crab formula feed with a moderate amount of TMAO had a slight improvement effect on SR and WGR and it could decrease the FCR significantly. However, the FI of two experimental groups with TMAO decreased at the same time and this may be the main reason for the decrease in FCR. Decrease of the FI was observed. This might be due to the low amount of attractant added, which did not achieve the attractant effect. There might also be a decrease in palatability leading to a reduction in feed intake. A similar situation was also observed in another study on rainbow trout (Oncorhynchus mykiss)[40]. Meanwhile, due to the long breeding cycle, this result was also a problem that food attractants may encounter after long-term use. In addition, WGR showed an upward trend with the addition of TMAO, and HSI increased in the 0.01% TMAO group. This indicated that moderate TMAO had a promoting effect on the growth of E. sinensis. From the results of the enzyme activity, there is a marked upward trend in trypsin activity with the addition of TMAO. It indicated that adding a moderate amount of TMAO into the diet could promote digestion and this contributed partly to the decrease of FCR.
After entering the intestine, substances such as L-carnitine or choline are decomposed into TMA by trimethylamine lyase which is produced by gut microbiota[10]. TMA is then oxidized by FMO3 to TMAO in the liver[9]. Unnecessary TMAO will decompose into dimethylamine under the action of TMAOase, ultimately generating methanal and it being excreted[8]. In this research, no marked difference was shown in FMO3 among all three groups. It indicated that TMAO could be absorbed into the hepatopancreas directly. From the results, TMAO could be largely deposited into the muscle of E. sinensis while it could hardly be deposited in the hepatopancreas. In addition, TMA, as a downstream product of TMAO, could also hardly deposit in muscle and hepatopancreas. In this situation, TMAOase, which is an enzyme necessary for the decomposition of TMAO into TMA, had no difference among all groups. It indicated that the TMAO added into the diet did not degrade but instead deposited into the muscle.
TMAO presents a fresh taste[21]. Due to the existence of large amounts of biogenic amines in aquatic products after death, which are toxic to humans, biogenic amines such as spermidine, spermine, etc., are used as indicators to determine the freshness of aquatic products[2]. From the results, the content of several biogenic amines including spermidine, spermine, cadaverine, histamine, and putrescine in the muscle showed a downward trend with the addition of TMAO. Among them, spermidine and spermine decreased significantly in the 0.04% TMAO group. Meanwhile, the value of umami in muscle showed an opposite trend, corresponding to the trend of biogenic amine deposition one by one. It indicated that the increase in the value of umami in muscle might be caused by the decrease of the biogenic amines, which represented freshness levels. In addition, due to the umami of TMAO and its ability to deposit in the muscle of E. sinensis as observed from the results, the increase in umami might also be partly due to the deposition of TMAO in muscle. TMAO has three methyl groups[34], so it is highly likely to act as a methyl donor. From the results of the amino acid analysis, the content of most amino acids showed an upward trend and methionine increased significantly in the 0.04% TMAO group. Due to the function of methionine as a methyl donor[14], the addition of TMAO in the diet might be used by the organism as a methyl donor instead of methionine, thereby saving methionine and causing an increase in methionine content in the muscle of E. sinensis.
Protein synthesis is a crucial part of animal growth and can be regulated by the mTOR pathway[18]. mTOR includes mTORC1 and mTORC2. Wherein mTORC1 phosphorylates its downstream S6K and 4E-BP1 target protein. Then S6K1 phosphorylates S6 to promote protein synthesis and 4E-BP1 separates from eIF4E after phosphorylation, thereby relieving the limitation of protein translation[35]. From the results, the protein expression of mTOR in muscle increased in the 0.01% TMAO group and the gene expression of mTOR, S6K, S6, and eIF4E in the hepatopancreas increased while 4E-BP1 decreased in the 0.01% TMAO group. It indicates that 0.01% TMAO in the diet could promote protein synthesis via the mTOR pathway. A previous report found that methionine activated the mTOR pathway through S-adenosylmethionine[36]. In another study on E. sinensis, it was also found that methionine promoted protein synthesis by activating the mTOR pathway[37]. The result of mTOR pathway might be due to the methionine which was saved by being replaced by TMAO to perform the function of methyl donors, this result might also be attributed to a general increase in the levels of most amino acids in muscle. A previous study on E. sinensis found that excessive methionine could lead to inhibition of the mTOR signaling pathway[37]. From the research, when adding 0.04% TMAO into the diet, the protein and gene expression, beneficial for protein synthesis, decreased. This might be due to the excessive amount of methionine saved after adding more TMAO.
The results indicated that a moderate amount of TMAO could positively affect the growth performance and the taste of muscle. This experiment aimed to find a food attractant that could not only improve the growth performance but also improve the flavor in E. sinensis. However, how to accurately regulate flavor and the upper limit of this regulation are not yet clear. Therefore, research can further increase the concentration gradient of TMAO addition and explore its efficiency in improving flavor.
-
This experiment shows that adding 0.01% TMAO could improve growth performance appropriately. In addition, adding 0.01% and 0.04% TMAO into the diet could increase the umami of muscle by affecting the metabolism of biogenic amines. 0.01% TMAO in the diet may activate the mTOR pathway by affecting amino acid metabolism.
This research was supported by Agriculture Technical System of Jiangsu, China (JATS [2023]433). The authors are also grateful to all the laboratory members for their continuous technical advice and helpful discussions.
-
Ethics Review Approval: All procedures were reviewed and preapproved by the Animal Care and Use Committee of Nanjing Agricultural University (Nanjing, China) (Permit nos 31572403 and 31402075, 2 June 2022).
-
Adherence to the 3Rs Principle: The research followed the 'Replacement, Reduction, and Refinement' principles to minimize harm to animals.
-
Animal Welfare: This article provides details on the housing conditions, care, and pain management for the animals, ensuring that the impact on the animals was minimized during the experiment.
-
Informed Consent in Clinical Studies: This research does not involve pets or other domestic animals.
-
The authors confirm contribution to the paper as follows: study conception and design: Liu W, Jiang G, Hua H; data collection: Hua H, Guo H, Liu Z, He C, Huang Y, Xiong W, Wang X, Wen Y, Yin S, Wang P; analysis and interpretation of results: Jiang G, Hua H, Cao X, Guo H, Wang A; draft manuscript preparation: Hua H. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Hua H, Guo H, Liu W, Liu Z, He C, et al. 2025. Trimethylamine N-oxide promotes the growth of Chinese mitten crab (Eriocheir sinensis), deposition of amines, and activates the mTOR pathway by affecting metabolism of amino acids. Animal Advances 2: e008 doi: 10.48130/animadv-0025-0005
Trimethylamine N-oxide promotes the growth of Chinese mitten crab (Eriocheir sinensis), deposition of amines, and activates the mTOR pathway by affecting metabolism of amino acids
- Received: 14 September 2024
- Revised: 11 January 2025
- Accepted: 21 January 2025
- Published online: 17 March 2025
Abstract: This study was conducted to evaluate the changes in amino acid metabolism and amine deposition in Chinese mitten crab (E. sinensis) fed with a diet containing Trimethylamine N-oxide (TMAO) to promote its growth and flavor. Three diets containing 0% TMAO, 0.01% TMAO, and 0.04% TMAO were designed respectively for E. sinensis (37.50 ± 0.5 g) for 15 weeks. According to the results, two experimental groups with TMAO had significant decrease in feed conversion ratio and feed intake (p < 0.05) while it showed the opposite trend in the enzyme activity of trypsin. With the addition of TMAO, the value of umami in muscle increased significantly (p < 0.05). Compared with the other two groups, the 0.04% TMAO group showed a significant increase in TMAO deposition and the content of methionine in the muscle (p < 0.05). Meanwhile, the deposition of spermidine and spermine significantly decreased in this group (p < 0.05). When feeding crabs with the diet containing 0.01% TMAO, the relative gene expression of S6K1 and eIF4E increased significantly while 4E-BP1 decreased markedly (p < 0.05). The protein expression of mTOR in muscle showed an upward trend in the 0.01% TMAO group. In summary, 0.01% TMAO in the diet could improve the FCR by increasing the enzyme activity of trypsin and improving growth performance by activating the mTOR pathway. 0.04% TMAO could increase the value of umami and the deposition of TMAO in muscle.
-
Key words:
- Trimethylamine N-oxide /
- Chinese mitten crab /
- Nutrition /
- Flavor /
- Biogenic amines