-
With the rapid development of the modern poultry industry, broiler breeding, as an important branch of animal husbandry, has attracted increasing attention for its production efficiency and animal health. The gut serves as the primary site for nutrient absorption in broilers. A healthy gut enables more efficient digestion and uptake of nutritive components present in the feed, thereby improving the utilization rate and conversion rate of feed is the highest priority for broiler raising. In this process, feed additives play a very important role. Acidifiers, as an additive can significantly improve the intestine environment of broilers and improve feed utilization[1]. Studies have shown that the utilization of acidifiers in broilers can regulate palatability, promote intestinal development, change bacterial environment, improve digestibility, enhance nutritional absorption, and optimize slaughter performance[2]. The equilibrium of the intestinal microbiome wields a pivotal influence in preserving the metabolic homeostasis of broilers. Conversely, if it is disrupted, the health of the host may be affected. Acidifiers can effectively inhibit harmful intestinal flora and maintain intestinal health through various mechanisms such as reducing pH value, bacteriostatic, bactericidal, and improving digestibility[3]. Studies have shown that acidifiers added to the diet of broilers through feed or drinking water, stimulated the proliferation of epithelium cells within the intestinal tract, increased the height of enteric villus, and increased the effective absorption area of the intestine[4]. Acidifier addition also facilitates the proliferation of beneficial microorganisms while impeding the growth of pathogenic bacteria, and enhancing pepsin activity[5]. Nevertheless, a significant shortcoming of traditional acidifiers pertains to their prompt liberation within the intestinal lumen following ingestion by animals. This rapid-release phenomenon has the propensity to exert detrimental effects on the continuous digestive and absorptive processes within the intestine. To circumvent this impediment, the acidifier utilized in our experimental investigation was enteric-soluble benzoic acid, with the intention of effectuating a gradual release within the intestinal milieu, thereby potentially ameliorating the negative consequences associated with rapid release and enhancing the overall functional efficacy of the intestine. Thus, this trial was meticulously designed to assess the influence of two distinct doses of acidifiers on various parameters in broilers, encompassing growth performance, digestive tract pH, intestinal morphology, antioxidant capabilities, and the levels of appetite-related hormones within the intestine.
-
All animal experiments were carried out in compliance with the Guidelines for the Care and Use of Laboratory Animals, prepared by the Institutional Animal Welfare and Ethics Committee of Nanjing Agricultural University, Nanjing, China [Certification No. SYXK (Su) 2022-0031].
A total of 192 broilers, each with an average weight of 45.10 ± 0.05 kg, were purchased from Chuzhou Poultry Hatchery Co., Ltd. (Anhui, China). They were then randomly divided into three groups, with eight cages per group and eight chickens per cage (measuring 1.2 m × 1.0 m × 0.45 m). The experiment spanned from day 1 to day 21 of the birds' age. The normal control group (NC) was administered a basal diet. Meanwhile, the low-dose group had 330 g/t of acidifier supplemented to their basal diet, and the high-dose group had 660 g/t of acidifier incorporated therein. The acidifier, known as Kefeisuan (supplied by Guangzhou Insighter Biotechnology Co., Ltd.), primarily consists of enteric-coated benzoic acid and has a content of 50%. The constituents and nutritional gradations of the basal diet are succinctly illustrated in Table 1.
Table 1. Ingredient composition and calculated nutrient levels of the basal diet
Items Starter stage
(1 to 21 d)Grower stage
(22 to 42 d)Ingredient Corn, % 55.24 59.37 Soybean meal, % 36.92 31.90 Soybean oil, % 3.50 5.00 Limestone, % 1.12 1.23 Dicalcium phosphate, % 2.10 1.50 ʟ-Lysine HCl, % 0.22 0.11 ᴅʟ-Methionine, % 0.28 0.27 Salt, % 0.30 0.30 Vitamin premix1, % 0.03 0.03 Mineral premix2, % 0.20 0.20 70% Choline chloride, % 0.09 0.09 Nutrient level Metabolisable energy (MJ/kg) 12.34 12.97 Crude protein 21.00 19.00 Calcium 1.00 0.90 Total phosphorus 0.67 0.56 Non-phytate phosphorus 0.45 0.35 Lysine 1.20 1.00 Methionine 0.52 0.46 Methionine + cystine 0.85 0.80 1 Vitamin premix provided per kilogram of diet: vitamin A, 12,000 IU; vitamin D3, 2,500 IU; vitamin E, 11 mg; menadione, 1.3 mg; thiamine, 2.21 mg; riboflavin, 7.8 mg; nicotinamide, 40 mg; calcium pantothenate, 16.5 mg; pyidoxine·HCl, 4 mg; biotin, 0.04 mg; folic acid, 1.2 mg; vitamin B12, 15 μg. 2 Mineral premix provided per kilogram of diet: iron, 80 mg; copper, 8 mg; manganese, 110 mg; zinc, 65 mg; iodine, 1.1 mg; selenium, 0.3 mg. Sample collection and preparation
-
On the 21st day, all broilers underwent an 8-h fasting period. Following this, two birds from each cage with body weights closest to the group's average were chosen. These selected birds were subjected to electrical stunning using a 400 Hz current for 5 s at a voltage of 50 v (alternating current). Immediately post-stunning, exsanguination was performed via the left carotid artery. Blood samples were collected in heparinized tubes and centrifuged at 3,000 rpm for 10 min. When the abdominal cavity was opened, the proventriculus and gizzard were carefully cut apart and examined, ensuring all attached materials were removed, and the combined weight of all contents and adhering substances was documented. Subsequently, the intestinum tenue of each broiler was split into three different segments. The duodenum extended from the ventriculus to the pancreo-biliary duct, following it, the jejunum stretched from the pancreo-biliary duct to the yolk stalk, and the ileum went from the yolk stalk to the ileocecal junction. About 1 cm of the middle part of the duodenum, jejunum, and ileum was carefully cut out, flushed with a sterile 0.75% (w/v) saline solution, and in 4% paraformaldehyde to maintain their morphological characteristics for further analysis. Thereafter, the intestinal contents were gently expelled, and the remaining small intestine was sectioned into smaller pieces. Then, using a clean glass slide, the mucosa was painstakingly removed by scraping. It was promptly frozen in liquid nitrogen and stored at −80 °C immediatelyfor subsequent downstream examinations.
Growth performance
-
At the start and end of this trial, the body weights of the broilers were carefully recorded for each replicate cage. Concurrently, the feed consumption across every moment of the experimental period for each of the three groups was documented. Based on these recordings, the average daily gain (ADG), average daily feed intake (ADFI), and the feed-to-gain ratio (F/G) were determined through computation in strict accordance with standard procedures.
On the 21st day, 12 broilers were chosen from each group, with the selection criterion being two birds per pen whose weights were closest to the mean value of that particular pen. These selected broilers were first weighed precisely and then humanely euthanized. The process involved stunning the birds before immediate exsanguination via the left jugular vein. This ensured that the samples obtained were representative and suitable for further analysis.
pH value of the digestive tract
-
The glandular stomach, gizzard, duodenum, jejunum, ileum, and cecum were carefully dissected and isolated. A tiny and accurate incision was then made in each of these organs using a sharp scalpel. Immediately after, a calibrated acidity meter (METTler Toledo S220) was inserted into the incisions to measure the pH value of the contents within each intestinal segment rapidly. This method ensured accurate and rapid data acquisition for further analysis.
Intestinal length and morphological development
-
After the broilers were slaughtered, the lengths of the duodenum, jejunum, and ileum were carefully measured. Approximately 1 cm sections of each of these intestinal segments were then promptly fixed using para-formaldehyde to prepare slice specimens. Subsequently, these specimens were placed under a biological microscope for the observation of intestinal morphological alterations. At three randomly selected locations where the villi were intact and straight, the villus height (VH) and crypt depth (CD) were measured precisely, and the VH/CD ratio was calculated for the quantitative assessment of the intestinal morphological properties.
Intestinal digestive enzyme activities and antioxidant indexes
-
For the assessment of the physiological status of the intestinal mucosa, commercial reagent kits procured from the Nanjing Institute of Bioengineering in China were utilized. These kits enabled the quantification of key digestive enzyme activities and antioxidant parameters. Specifically, the activities of amylase, sucrase, and trypsin, which play crucial roles in the digestive process, were measured. Additionally, several antioxidant markers were examined, namely total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and total antioxidant capacity (T-AOC). It is important to highlight that when conducting inter-sample comparisons, the data obtained from these measurements were not adjusted or correlated with the total protein content within each sample. This decision was made to preclude any potential biases that could arise from differences in protein concentrations and to ensure a more straightforward and reliable comparison among the samples under study.
Assay for appetite-related hormone levels
-
Approximately 0.5 g of intestinal mucosal segments and 4.5 mL of cold physiological saline (0.75%, weight/volume) were homogenized. By centrifuging at 3,500 rpm for 10 min, a supernatant was produced and kept at 4 °C for analysis of intestinal hormones. The manufacturer's instructions were followed for using the commercially available chicken-specific ELISA kit (Angle Gene Biotechnology Co., Ltd., Nanjing, China) for detection of the concentration of ghrelin, cholecystokinin (CCK), and peptide YY (PYY) in the serum and intestine. The reagent kits utilized for this purpose have a 95% reliability. All samples were examined twice, and the determination of the concentration was carried out by using linear regression based on a standard curve.
Total RNA extraction and real-time PCR
-
By using Trizol reagent (from Takara Biotechnology Co. Ltd., Dalian, China), total RNA was separated from duodenal, jejunal, and ileal mucosa samples. A Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) was used to measure the purity and quantity of the RNA. Using a PrimeScript RT Master Mix kit (Takara Biotechnology Co. Ltd.), the reverse transcription of the total RNA was completed, thus generating cDNA. Meanwhile, the RNA of all samples had A260/280 ratios falling within the range of 1.8 to 2.0. For the RT-qPCR, the SYBR Premix Ex Taq kit (Applied Biosystems, Foster City, CA, USA) was used in the QuanStudio6 RT-qPCR detection system (Applied Biosystems, Foster City, CA, USA). The cycling conditions used in the experiment were as follows: 1 cycle at 95 °C for 30 s, and 40 cycles with steps at 95 °C for 5 s, 60 °C for 30 s, 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s. The total reaction volume was 20 µL, which was composed of 0.4 µL of a forward primer (10 µM), 0.4 µL of a reverse primer (10 µM), 1 µL of cDNA, 8.2 µL of sterilized double distilled water, and 10 µL of SYBR Premix Ex Taq II (2 ×). The specific primer sequences are listed in Table 2. The relative levels of mRNA expression were calculated using the 2−ΔΔCᴛ method, taking GAPDH as the reference gene[6].
Table 2. Sequences used for real-time PCR primers.
Genes1 GeneBank
accession no.Primer sequences (5' to 3' direction) Product size (bp) GAPDH NM_204305.1 F: GAGGGTAGTGAAGGCTGCTG 113 R: CATCAAAGGTGGAGGAATGG Ghrelin NM_001001131.1 F: AACTGCTCTGGCTGGCTCT 137 R: CTCCCTCTGTTTCATCTGTAT CCK NM_001001741 F: CAGCAGAGCCTGACAGAACC 121 R: AGAGAACCTCCCAGTGGAACC 1. GADPH = glyceraldehyde-3-phosphate dehydrogenase; CCK = cholecystokinin. Statistics and analysis
-
The statistical analysis of the accumulated data was carried out by employing the one-way analysis of variance (ANOVA) method with the assistance of SPSS statistical software, version 20.0 (SPSS Inc., Chicago, IL, USA). The mean of two birds in each pen was taken as the replicate value (n = 8). The results were shown as mean values along with their standard errors. Duncan's multiple range tests were performed to examine the differences among the three groups, and differences were deemed statistically significant at a p-value of less than 0.05.
-
As can be seen from Table 3, compared with the control group, whether low-dose or high-dose groups had no negative effects on the growth performance of broilers (p > 0.05).
Table 3. Effects of dietary acidifiers on growth performance of broilers at 21 d of age.
Items NC LD HD SEM p-value ADFI (g/d) 61.79 61.63 68.10 1.33 0.06 ADG (g/d) 34.26 34.42 34.88 0.26 0.36 F/G 1.84 1.74 1.88 0.04 0.22 NC group: basal diet; LD group, basal diet with 330 g/t acidifiers; HD group, basal diet with 660 g/t acidifiers; SEM: Standard Error of the Mean; ADFI = average daily feed intake; ADG = average daily gain; F/G = feed/gain. pH value of the digestive tract
-
As shown in Table 4, there were no significant effects on the digestive tract pH values (p > 0.05) when dietary acidifier was supplemented in the low- and high-dose groups in comparison to the NC group.
Table 4. Effects of dietary acidifiers on pH value of digestive tract of broilers at 21 d of age.
Item NC LD HD SEM p-value Duodenum 7.16 6.63 6.64 0.13 0.12 Jejunum 6.18 6.54 6.66 0.07 0.39 Ileum 6.41 6.65 6.71 0.05 0.06 Caecum 7.08 6.80 7.28 0.08 0.14 NC group: basal diet; LD group, basal diet with 330 g/t acidifiers; HD group, basal diet with 660 g/t acidifiers; SEM: Standard Error of the Mean. Intestinal length and morphology
-
As depicted in Fig. 1, broilers in the LD group exhibited a significantly increased duodenal length in comparison to those in the NC group and the HD group on day 21 (p < 0.05).
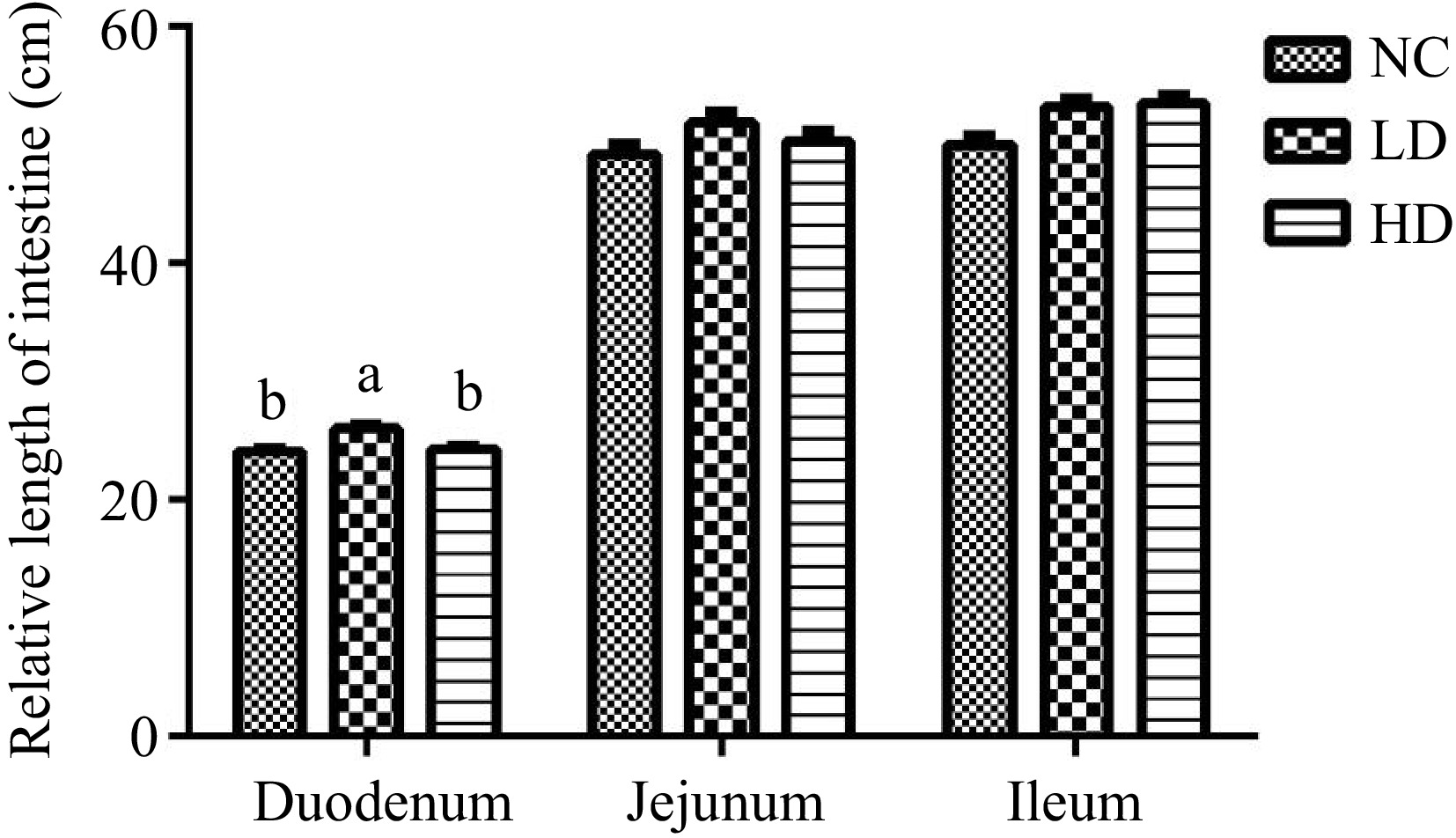
Figure 1.
Effects of dietary acidifiers on intestinal length of broilers at 21 d (cm). NC group: basal diet; LD group, basal diet with 330 g/t acidifiers; HD group, basal diet with 660 g/t acidifiers. Lower case letters a & b above columns indicate that values with different superscripts were significantly different (p < 0.05). Data were expressed as mean ± SE (n = 8).
As can be seen from Table 5, both the villus height and VH/CD ratio of the duodenum were significantly increased (p < 0.01). The crypt depth of the duodenum was significantly decreased (p < 0.05). The VH/CD ratio of the ileum and jejunum were significantly increased (p < 0.05). Both the VH and CD of the ileum and jejunum were not significantly increased (p > 0.05).
Table 5. Effects of dietary acidifiers on intestinal morphology of broilers at 21 d of age.
Item NC LD HD SEM p-value Duodenum VH (μm) 935.09b 993.07a 944.44b 8.05 <0.01 CD (μm) 29.16a 27.17b 25.03c 0.56 0.02 VH/CD 33.35c 37.81b 40.45a 0.82 <0.01 Ileum VH (μm) 620.95 614.71 650.46 7.87 0.11 CD (μm) 29.21 28.26 26.82 0.47 0.14 VH/CD 21.54b 22.48ab 24.80a 0.47 0.01 Jejunum VH (μm) 863.22 901.45 917.96 10.24 0.08 CD (μm) 28.11 26.95 26.42 0.43 0.26 VH/CD 31.94c 34.40b 36.36a 0.64 0.03 VH: Villus height; CD: Crypt depth; NC group: basal diet; LD group, basal diet with 330 g/t acidifiers; HD group, basal diet with 660 g/t acidifiers. Lower case letters a, b & c indicate that values with different superscripts were significantly different (p < 0.05). SEM: Standard Error of the Mean. Intestinal antioxidant indexes and digestive enzyme activities
-
As shown in Fig. 2, the T-SOD values in the HD group of ileum were significantly higher than that in the NC group (p < 0.05). SOD values of the HD group were higher than NC group both in the jejunum and ileum (p < 0.05), GSH-Px values among the three groups had no significantly difference (p > 0.05).
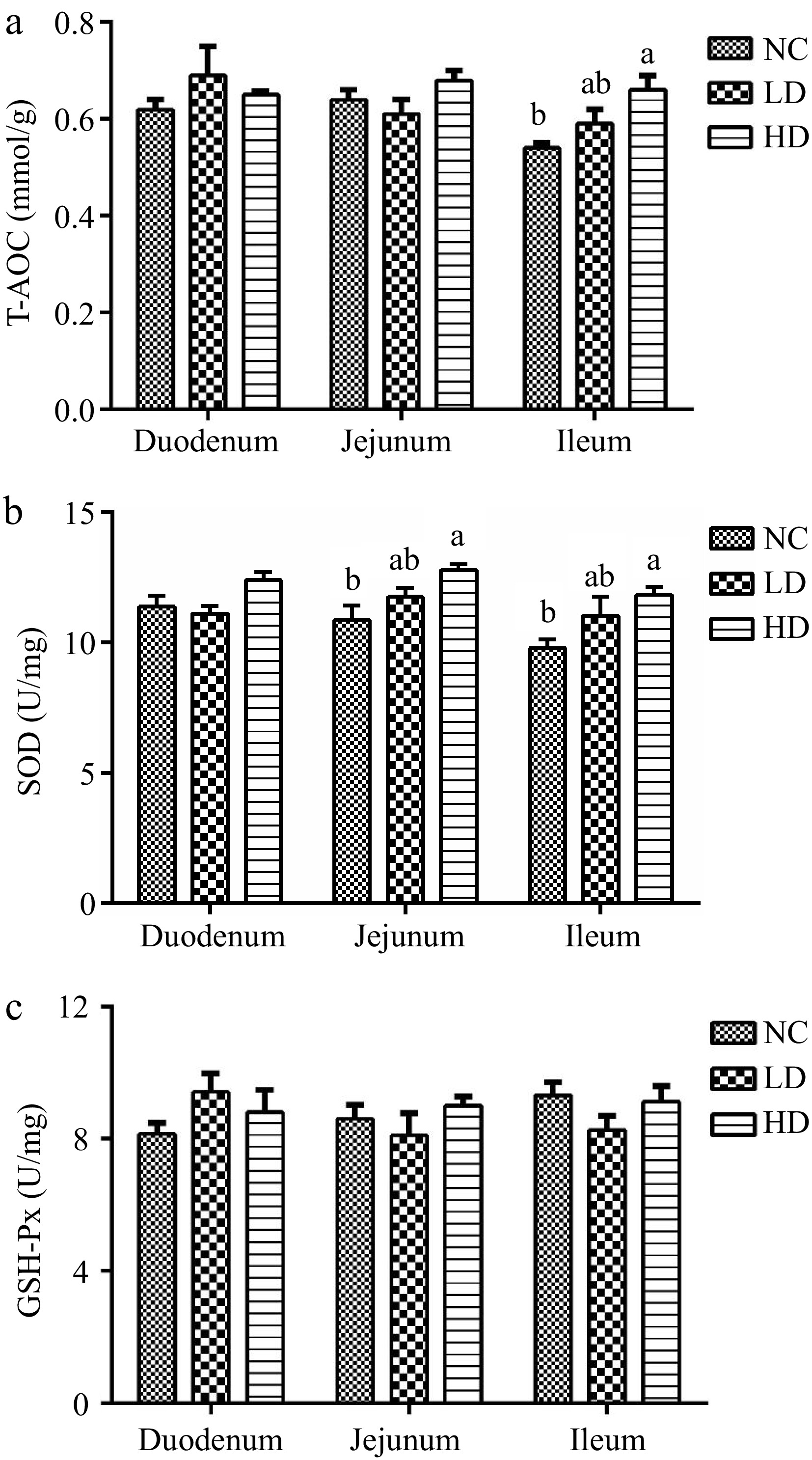
Figure 2.
Effects of dietary acidifiers on (a) T-AOC, (b) SOD, and (c) GSH-Px values of intestinal mucosa for broilers at 21 d. T-AOC: total antioxidant capacity. SOD: superoxide dismutase. GSH-Px: glutathione peroxidase. NC group: basal diet; LD group, basal diet with 330 g/t acidifiers; HD group, basal diet with 660 g/t acidifiers. Lower case letters a, b above the columns indicate that values with different superscripts were significantly different (p < 0.05). Data were expressed as mean ± SE (n = 8).
As can be seen from Fig 3, the amylase and trypsin activities among the three groups in the intestinal had no significantly difference (p > 0.05). Whereas, the duodenal and jejunal sucrase activities in the HD group were significantly higher than those in the NC group and LD group (p < 0.05).
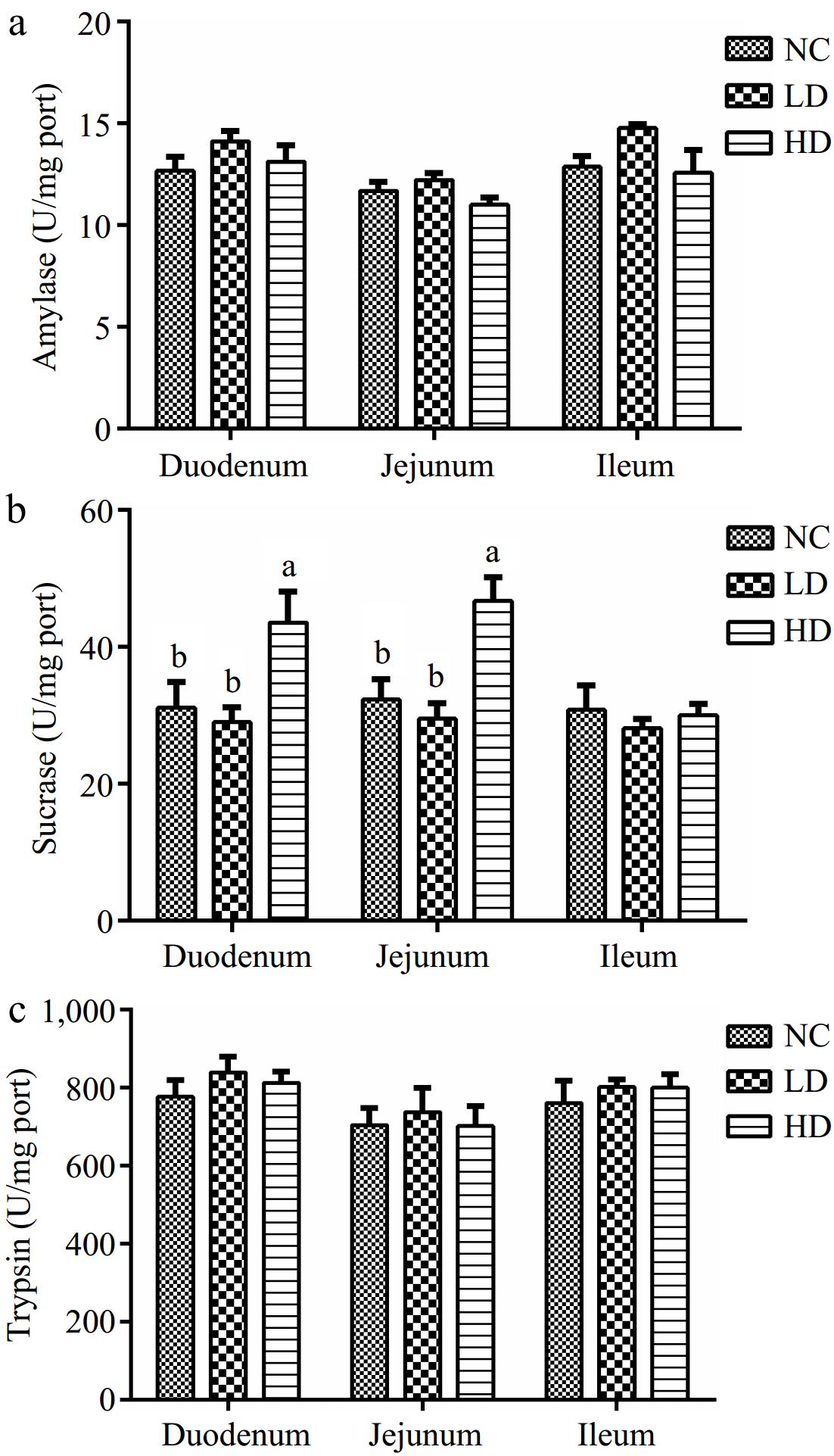
Figure 3.
Effects of dietary acidifiers on (a) amylase, (b) sucrase, and (c) trypsin activities of digestive enzymes in intestinal mucosa for broilers at 21 d. NC group: basal diet; LD group, basal diet with 330 g/t acidifiers; HD group, basal diet with 660 g/t acidifiers. a, b indicate that values with different superscripts were significantly different (p < 0.05). Data were expressed as mean ± SE (n = 8).
Appetite-related hormone levels and gene expression
-
As can be seen from Fig. 4a, the CCK level was significantly increased in comparison with the NC group (p < 0.05). In Fig. 4b, the duodenal and jejunal ghrelin levels of the HD group, along with the jejunal CCK level of the HD group were significantly elevated compared to the LD group (p < 0.05). Meanwhile, a lower acifidier supplement decreased the ghrelin in the ileum significantly compared with the NC group. From Fig. 4c, compared with the NC group, the PYY level of the LD group had significantly improved in the duodenum. In addition, the PYY level of the LD group was significantly higher than that in the HD group (p < 0.05).
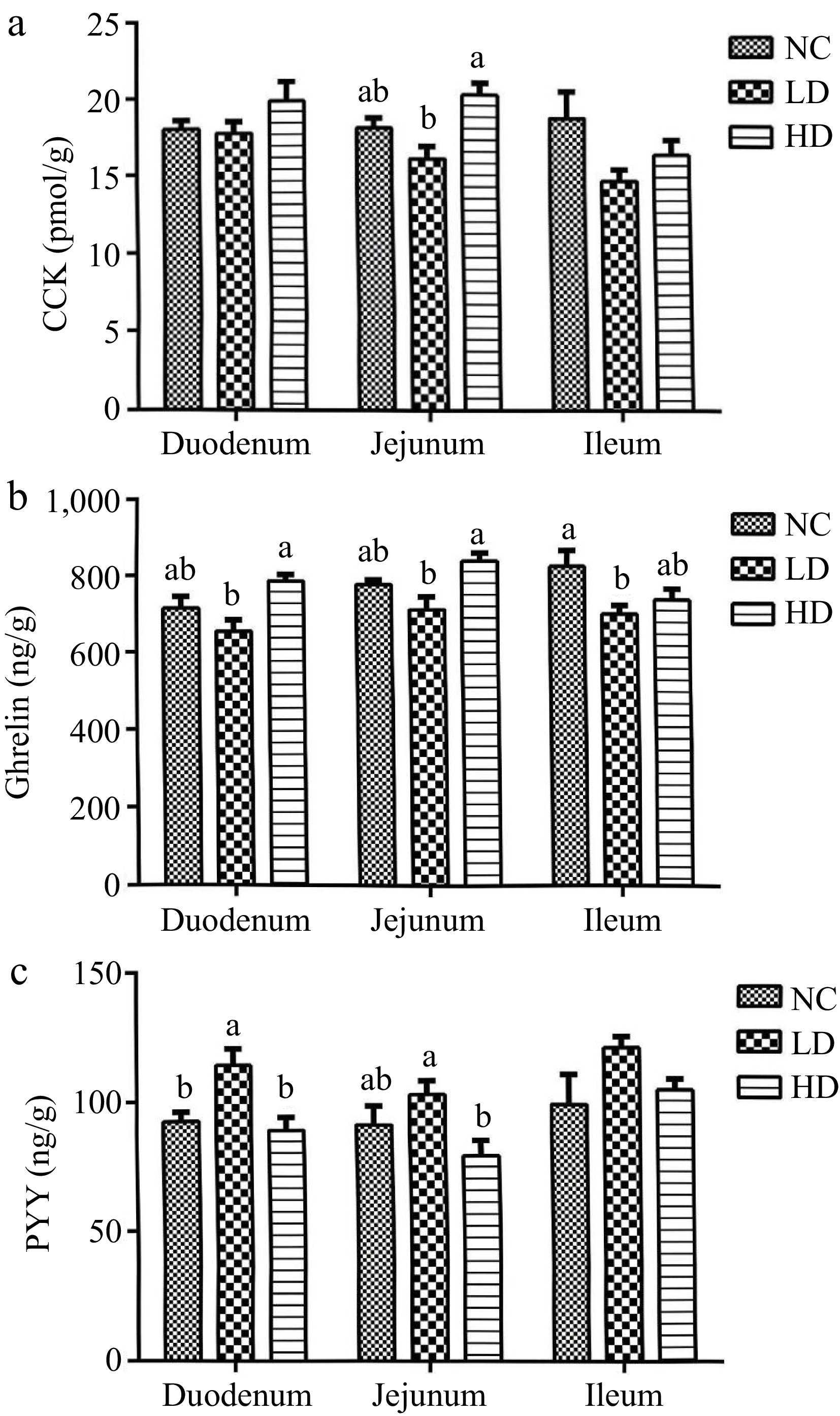
Figure 4.
Effects of dietary acidifiers on (a) CCK, (b) ghrelin, and (c) PYY levels of intestinal mucosa for broilers at 21 d. CCK: cholecystokinin. PYY: Peptide YY. NC group: basal diet; LD group, basal diet with 330 g/t acidifiers; HD group, basal diet with 660 g/t acidifiers. a, b indicates that values with different superscripts were significantly different (p < 0.05). Data were expressed as mean ± SE (n = 8).
As shown in Fig. 5a, a significant elevation was observed in the relative mRNA expression levels of the CCK gene in the jejunum and ileum when compared with the NC group (p < 0.05). As we can see in Fig. 5b, it was evident that the relative mRNA expression of the ghrelin gene in the HD group, particularly in the duodenum and jejunum, was markedly up-regulated compared to the LD group (p < 0.05). However, the relative mRNA expression of the CCK and ghrelin genes had no significant differences throughout the entire intestine when compared to the NC group (p > 0.05).
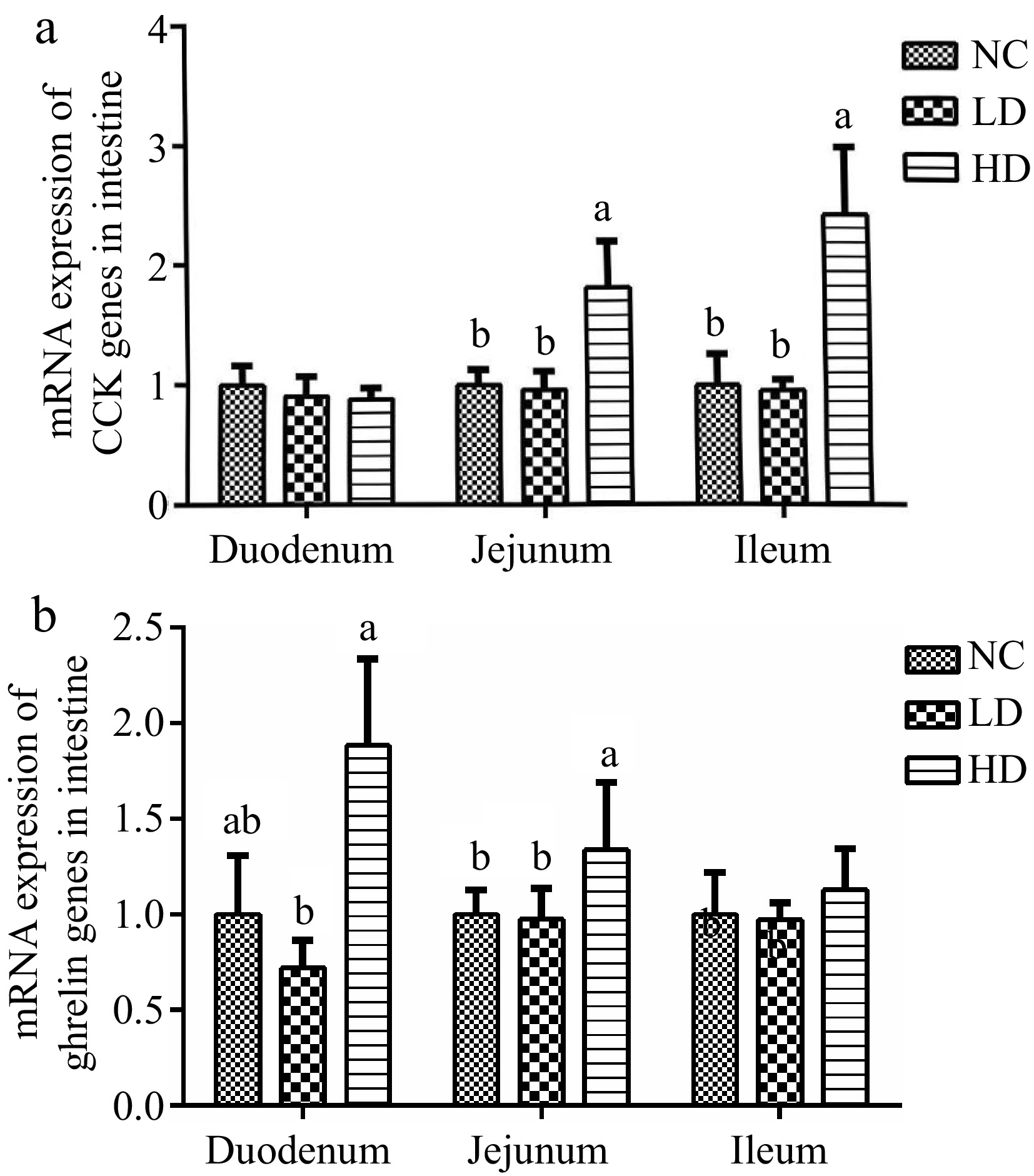
Figure 5.
Effects of dietary acidifiers on the mRNA expression of (a) CCK, and (b) ghrelin in the intestinal mucosa of broilers at 21 d. CCK: cholecystokinin. NC group: basal diet; LD group, basal diet with 330 g/t acidifiers; HD group, basal diet with 660 g/t acidifiers. a, b indicates that values with different superscripts were significantly different (p < 0.05). Data were expressed as mean ± SE (n = 8).
-
The growth performance of broilers is a key economic characteristic. Acidifier supplementation creates an acidic environment in the gastrointestinal tract of broilers, which facilitates the proliferation of beneficial bacteria, inhibits the growth of harmful bacteria, and maintains a balanced intestinal micro-ecosystem. It is widely recognized that acidifiers could enhance the digestion and absorption of energy and protein, boost feed efficiency, improve nutrient digestibility, bolster intestinal immunity, and elevate growth performance and product quality in broilers. Inorganic acids can chemically interact with specific feed nutrients, such as hydrochloric acid potentially degrading vitamins and minerals. Enteric-soluble benzoic acid signifies an advancement in benzoic acid technology, enabling targeted release in particular intestinal regions (e.g., the small intestine) to perform its acidifying functions more precisely, including antibacterial effects and regulation of intestinal pH. Thus, enteric-soluble benzoic acid is an upgrade and innovation over traditional benzoic acid acidifiers, offering a novel approach. Additionally, the traditional benzoic acid's odor, similar to formaldehyde, can deter animals from eating, negatively impacting feed intake and hindering growth and production performance. In our current study, the addition of acidifiers, regardless of whether at low or high concentrations, did not change the growth performance and intestinal pH of broilers, which might contradict findings from other studies[7]. A previous study indicated that combining 2-hydroxy-4-methylthiobutyric acid with other acidifiers facilitates intestinal development, optimizes the microbiome structure, and boosts growth performance in broilers[8]. This discrepancy could be attributed to the use of powdered feed in our experiment, as opposed to pelletized feed. Traditional acidifiers may rapidly reach the intestinal epithelial mucosa, where they are detected by taste receptors, thereby stimulating intestinal functions. Furthermore, variations between our study's outcomes and those of other research could be due to differences in dosage and the method of acidifier administration.
Acidifiers can also provide a suitable environment for digestive enzymes such as pepsin, thus affecting their activity[8]. Digestive enzymes mainly play a role in the small intestine. Different digestive enzymes break down feed components that are not good, and large molecules are broken down into small molecules, so that these nutrients can be more easily absorbed and utilized by the intestine[9]. The digestive tract of juvenile animals is not fully developed, and the secretion of gastric acid in the intestinal tract is insufficient. In our study, the high-dose addition of acidifier significantly enhanced the activity of sucrase in the duodenum and jejunum, which was in accordance with the findings of other researchers who used potassium diformate and encapsulated organic acids to improve intestinal function and digestive enzyme activities[10,11]. Sucrase is an enzyme that hydrolyzes sucrose into its two component monosaccharides, glucose, and fructose. It indicates that enteric-soluble benzoic acid supplements could improve carbohydrate digestion.
The intestine, as a site for nutrient digestion and absorption, is crucial for the development of intestinal morphology. Intestinal villi extend from the tip to the base of the recess, and their height is directly correlated with the rate of nutrient absorption in the small intestine. As the height of the villi increases, the absorption area of the small intestine becomes larger, thereby enhancing the digestion and absorption of nutrients[12]. The ability of broilers to absorb nutrients is contingent upon the integrity of their morphology of the intestinal tract. The intestinal crypt represents the vertical distance extending from the opening of the crypt to the base. A shallower CD is associated with a higher proliferation rate of intestinal cells[13]. The VH/CD ratio is a comprehensive index that reflects the nutrient absorption and digestion capacity of the small intestinal mucosa in broilers. A lower ratio of VH to CD indicates a decrease in digestive capacity and intestinal mucosal damage in broilers, serving as a crucial indicator of the decline in intestinal health[14]. When the intestinal integrity is compromised, the villi height may be reduced, intestinal cells and immune cells may be destroyed, resulting in abnormal intestinal development, abnormal intestinal epithelial cell transport function, and affecting intestinal immune function and nutrient digestion and absorption[15,16]. The results of the present experiment indicated that the duodenal length and villus height were increased significantly, while crypt depth decreased significantly. Additionally, their ratio increased significantly, suggesting that the addition of an acidifier enhanced the intestinal surface area of the duodenum and augmented the nutrient absorption capacity of the duodenum, ileum, and jejunum. These findings are consistent with those of peer research[17]. Simultaneously, our findings indicate that the intestinal mucosal epithelium exhibited a significant increase in sucrase, potentially due to the beneficial stimulation of the intestinal mucosa by acidifiers. This is consistent with previous studies[18].
The current intensive farming mode leads to the sub-health state of broilers, and broilers often face stress challenges. Oxidative stress is a common kind of stress, and many exogenous environmental stimuli may cause oxidative stress. Broilers are often in a state of antioxidant imbalance under oxidative stress[19]. Endogenous antioxidant enzymes include T-SOD, GPX, and CAT[20]. T-SOD converts superoxide into non-toxic or slightly toxic H2O2, and then through the action of GPX and CAT, eventually leads to the disproportionation of H2O2, generating water and oxygen[21]. GPX is one of the important enzymes of glutathione (GSH) oxidation, which can not only protect the cell membrane from free radicals but also maintain the normal function of cells, to specifically catalyze the reaction of reducing glutathione and free radicals to produce glutathione disulfide compound (GSSG)[22]. In our study, we found that high doses of acidifier significantly increased the total AOC and SOD of ileum and jejunum SOD values. This is consistent with other studies, which demonstrated that enteric-soluble benzoic acid could affect the antioxidant ability of intestinal mucosa in broilers.
The intestinal tract is not only the main site of digestion and absorption of nutrients but also the largest endocrine organ, which produces a variety of bioactive peptides and hormones[23]. Gastrointestinal hormones have a certain impact on appetite, and these hormones interact with the nervous system to jointly regulate appetite[24]. CCK is a peptide hormone that stimulates the contraction of the gallbladder's smooth muscle and promotes bile discharge into the duodenum, aiding in fat digestion. Additionally, CCK plays a crucial role in appetite regulation, gallbladder contraction, and pancreatic enzyme secretion[25]. Ghrelin is a polypeptide consisting of 28 amino acids that influence the release of appetite-related neurotransmitters, and promote the release of growth hormone by the pituitary gland, and regulate feeding behavior and appetite[26]. PYY is a potent anorexic gastrointestinal hormone that reduces feed intake by affecting the brain's feeding centers, such as the arcuate nucleus, to suppress appetite[27]. In China, there is a phrase, 'waiting for the plums to quench one's thirst' this idiom suggests that the thought of sour fruit can stimulate saliva production, and it is also believed that acidic foods often stimulate the appetite[28]. Our experiment demonstrated that a higher dose of acidifier could increase the levels of ghrelin and CCK, whereas a low dose of acidifier supplementation elevated the PPY level in the intestine. From a certain perspective, the dietary addition of a specific dose of acidifiers may play a role in regulating short-term appetite in broilers.
-
Supplementation with acidifiers had no negative influence on the growth performance, but it can influence the intestine morphology, antioxidant abilities, as well as the hormone levels of broilers. In addition, dietary acidifiers with higher doses could ameliorate the antioxidant abilities of the intestine and improve the digestive enzyme activities of broilers. At the same time, the higher dose of acidifiers may over-stimulate the broilers' intestinal secretion function.
This research was supported by the Natural Science Foundation of Jiangsu Province (BK20240222), Jiangsu Province Industry-University-Research Project (BY2022605), Jinling University of Science and Technology High-level Talent Start-up Project (JIT-B-202036), Jinling University of Science and Technology 2020 university-level Scientific Research Incubation Fund (JIT-FYXM-2020) and University Student Innovation and Entrepreneurship Project (202413573026Z).
-
All procedures were reviewed and preapproved by the Nanjing Agricultural University Institutional Animal Care and Use Committee, with the identification number NJAULLSC2023090, and an approval date of August 14, 2023. The research adhered to the 'Replacement, Reduction, and Refinement' principles to minimize harm to animals. This article details the housing conditions, care, and pain management for the animals, ensuring that their impact during the experiment is minimized.
-
The authors confirm contribution to the paper as follows: resources, conception: He X; writing-original draft: Wang Z, Ma B; investigation: Wang Z; experimental protocol optimization: Han Q; data visualization: Shi X; data analysis & interpretation: Dong X; writing - revision: Ma B. All authors reviewed the results and approved the final version of the manuscript.
-
The data will be made available on reasonable request from the corresponding author.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
He X, Wang Z, Han Q, Shi X, Dong X, et al. 2025. Dietary enteric-coated benzoic acid supplementation improved the intestinal antioxidant and digestive function in broilers. Animal Advances 2: e009 doi: 10.48130/animadv-0025-0007
Dietary enteric-coated benzoic acid supplementation improved the intestinal antioxidant and digestive function in broilers
- Received: 19 December 2024
- Revised: 16 January 2025
- Accepted: 26 January 2025
- Published online: 02 April 2025
Abstract: As an additive to regulate the intestinal pH environment of broilers, enteric-coated benzoic acid has many benefits. This study aimed to explore wether enteric-soluble benzoic acid could induce the appetite based on gut appetite hormone secretion. A total of 192 broilers were randomly partitioned into three groups. Each group consisted of eight cages, and each cage housed eight chickens. Birds were fed the base diet (NC) supplemented without or with 330 g/t (low-dose group, LD) or 660 g/t enteric-soluble benzoic acid (high-dose group, HD). The experiment lasted 21 d. Supplementing acidifiers did not exhibit any negative impacts on the growth performance of broilers. Compared with the NC group, the HD group significantly increased the villus height (VH) and the VH/CD ratio in both the duodenum and jejunum. Conversely, in the LD group, the crypt depth (CD) of the duodenum was significantly reduced. Compared to the LD group, VH, and CD in the duodenum of HD group were decreased significantly, the VH/CD ratio of jejunum and ileum were increased. The digestive enzymes of sucrase in the duodenum and jejunum were elevated in the HD group compared to those in the NC and LD group. The T-AOC and SOD in the ileum of the HD group were significantly higher than those of the NC group. Cholecystokinin (CCK) and Ghrelin both in the duodenum and jejunum were significantly increased in the HD group compared with the LD group (p < 0.05). Ghrelin in the ileum of the LD group, as well as Peptide YY (PYY) in the duodenum of the HD group had significantly increased compared to those of NC group (p < 0.05). Compared with the LD group, the mRNA expression of CCK and ghrelin of jejunal mucosa in the HD group, as well as mRNA expression of ghrelin in duodenal mucosa were significantly up-regulated (p < 0.05). In summary, the supplementation of 330 or 660 g/t of enteric-soluble benzoic acid significantly improves the intestinal morphological features and impacts the secretory function of the intestinal mucosa in broilers. These results suggest a potential role of enteric-soluble benzoic acid in the regulation of appetite-related physiological mechanisms.













