-
In 2018, the World Health Organization (WHO) reported that there were 1.8 million new cases of colorectal cancer globally, leading to 881,000 deaths[1]. In 2022, based on the Global Cancer Observatory (GLOBOCAN) data, there were 35,676 cases of Colorectal Cancer (CRC) in Indonesia, accounting for around 8.7% of all cancers, making it the fourth most common cancer in the country[2]. The recurrence and resistance of the cancer following therapy pose significant challenges for patients who have undergone resection or chemotherapy. Metastasis is the primary contributor to morbidity and mortality in patients with cancer[3,4]. The Tumor-Node-Metastasis (TNM) staging system developed by the Union for International Cancer Control (UICC) and the American Joint Committee of Cancer (AJCC) is one of the most widely used prognostic systems in clinical practice[5,6]. However, the prognosis in patients with the same tumor stage can vary significantly due to other factors, such as histological parameters[6].
While morphological parameters continue to serve as crucial predictors of cancer patient outcomes, the emergence of various promising immunohistochemical biomarkers offers hope to assist the assessment of patient prognosis[7]. The proto-oncogene DEK and the involvement of p53 and Bcl-2 in tumorigenesis and the pathogenesis of CRC have been linked to the prognosis of CRC[8]. DEK was found to be overexpressed in CRC compared to normal colorectal mucosa, making it a promising new marker for assessing CRC prognosis based on cancer stage and lymph node metastasis[8,9].
DEK is located on chromosome 6 and is composed of 375 amino acids with an estimated molecular weight of 46 kDa. Since its first discovery as a translocation (t6;9) target in a subset of acute myeloid leukemia (AML), DEK has been related to cell proliferation and tumor development[9,10]. DEK is involved in suppressing the process of cell senescence, apoptosis, differentiation, and epithelial transformation both in vitro and in vivo[8−11]. DEK can influence the expression of several molecular signaling pathways, namely the wingless/β-catenin (Wnt/β-catenin) pathway. Activation of the Wnt pathway sustains a paracrine/autocrine canonical to β-catenin signaling loop, which is involved in tumor cell proliferation, invasion, and metastasis[12].
Furthermore, DEK may also be involved in the inability of cell apoptosis by destabilizing the TP53 gene as a tumor suppressor gene. Under conditions where DEK is suppressed, p53 can act as a tumor suppressor by activating p53 target genes such as the Bcl-2-associated X protein (BAX)[13,14]. BAX facilitates cellular apoptosis while competing with Bcl-2, a proto-oncogene known to impede programmed cell death[15]. Abnormal activation of the Bcl-2 gene may facilitate tumor development. A high percentage of positive Bcl-2 cells in CRC has a significantly lower rate of spontaneous apoptosis rate[16]. When DEK destabilizes the TP53 gene, the interplay of Bcl-2 as pro-apoptotic and BAX as anti-apoptotic is disrupted, causing CRC cases with higher Bcl-2 expression related to tumor progressivity and less favorable prognosis[13,17,18].
To date, no studies in Indonesia have analyzed the expression of DEK, p53, and Bcl-2 in CRC patients. It is hoped that these three markers are associated with the stage of tumor and lymph node metastasis in CRC, thus serving as a prognostic indicator for CRC cases beyond TNM staging.
-
A search was conducted for paraffin blocks and histopathological forms of colon resection specimens from 51 patients diagnosed with CRC in 2022, which were obtained from the archive of the Department of Anatomical Pathology, Universitas Indonesia/Dr. Cipto Mangunkusumo Hospital. Clinical data of the patients were acquired from the electronic medical records of Dr. Cipto Mangunkusumo Hospital. CRC staging followed the TNM classification of the AJCC[6]. The tumor staging is divided into localized tumor (Stage I-IIA) and advanced tumor (stage IIB-IV), where the tumor starts to invade other structures or organs[6,19]. The tumor location was classified into the colon (64.7%), and rectum (35.3%).
Histological data were obtained from the reassessment of Hematoxylin and Eosin (H&E)-stained specimens by two pathologists who were blinded to of the clinical data of the patients. Paraffin blocks from each case were cut into 3−5 μm thick sections and subsequently subjected to immunohistochemical staining using a primary antibody panel of DEK, p53, and Bcl-2. Staining of the cell nucleus indicated DEK and p53 expression (Fig. 1a−d). High expression of DEK was considered if > 25% of the cells were stained, and low expression was considered if ≤ 25%. Positive expression of p53 was considered if > 55% of cells were stained and negative if ≤ 55%. Cell cytoplasm and nucleus membrane staining indicated Bcl-2 expression (Fig. 1e & f). Positive expression of Bcl-2 was considered if > 5% of cells were stained and negative if ≤ 5%.
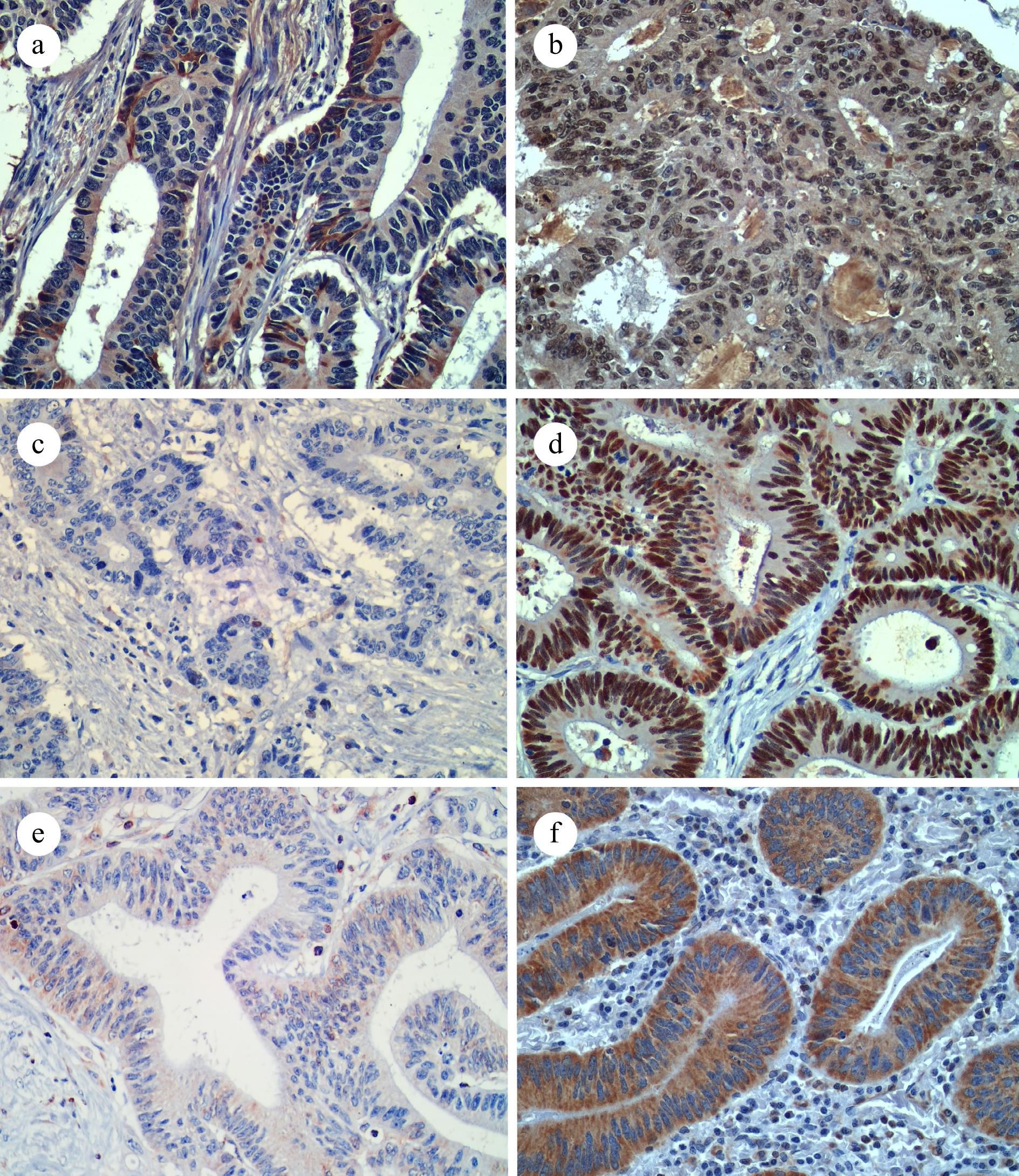
Figure 1.
Representative IHC images for (a) low DEK expression, (b) high DEK expression, (c) p53 negative expression, (d) p53 positive expression, (e) Bcl-2 negative expression, and (f) Bcl-2 positive expression. (400x, IHC).
The chi-square test (alternatively, the Fischer test for a small data size) was used to analyze the relationship between a pair of categorical variables. Multivariate analysis was performed on the significant bivariate analysis (between tumor stage, DEK expression, and p53 expression) using the logistic regression test. Statistical analyses were performed using the Statistical Program for Social Science (SPSS) program. A p-value of < 0.05 indicates statistical significance.
-
The mean age of the patients was 56.84 years, ranging from 26 to 85 years old, with a male-to-female ratio of 27:24. Resection with tumor cells present at the edge was considered a positive margin status (25.5%), while no tumor cells were considered a negative margin status (74.5%). The histological grade of the tumor was recategorized into two categories, with the majority of cases being a low-grade (80.4%) and a smaller proportion of high-grade (19.65%). The depth of tumor invasion was assessed based on the UICC classification and divided according to invasion into the submucosa (0%), muscularis propria (25.5%), subserosa (58.8%), and other organs (15.7%). Lymphovascular invasion was absent in most cases, accounting for 70.6%, while it was present in 29.4% of cases. Additionally, perineural invasion was absent in a substantial proportion of cases, comprising 86.3%, whereas it was identified in 13.7% of cases. Furthermore, lymph node metastasis was observed in 45.1% of the cases, while it was absent in 54.9% of the cases. Regarding tumor stages, 49% of cases were localized, whereas 51% were classified as advanced stage (see Table 1).
Table 1. Clinicopathological characteristics of the research sample (n = 51).
Characteristic No. of cases (%) Age < 50 years 12 (23.5%) > 50 years 39 (76.5%) Mean (standard deviation) 56.84 (± 13.00%) Sex Male 27 (52.9%) Female 24 (47.1%) Tumor location Colon 33 (64.7%) Rectum 18 (35.3%) Largest tumor dimension < 5 cm 17 (33.3%) > 5 cm 34 (66.7%) Margin status Negative 38 (74.5%) Positive 13 (25.5%) Histology grade Low grade 41 (80.4%) High grade 10 (19.6%) Depth of invasion (pT) Submucosa (pT1) 0 (0) Muscularis propria (pT2) 13 (25.5%) Subserosa (pT3) 30 (58.8%) Invades other organs/perforates peritoneal visceral (pT4) 8 (15.7%) Lymphovascular invasion Absent 36 (70.6%) Present 15 (29.4%) Perineural invasion Absent 44 (86.3%) Present 7 (13.7%) Lymph node metastasis Absent 28 (54.9%) Present 23 (45.1%) Tumor stages Localized (I-IIA) 25 (49%) Advanced (IIB-IV) 26 (51%) The relation of numerous markers' expression with clinicopathological characteristics
-
The present study showed a significant relationship between Bcl-2 expression and tumor histological grade (p = 0.006). Bcl-2 expression was positive in all cases of low-grade tumors, which was significantly higher than that in high-grade tumors. All cases with lymphovascular and perineural invasion were positive for Bcl-2 expression. However, no significant association was observed with these findings. No further associations were found between DEK, p53, or Bcl-2 expression with other clinicopathological characteristics (see Table 2).
Table 2. Clinicopathological characteristics and its association with DEK, p53, and Bcl-2 expression in colorectal cancers (n = 51).
Characteristic DEK p53 Bcl-2 Low High p Negative Positive p Negative Positive p Tumor location Colon 13 20 0.669a 17 16 0.212a 1 32 0.282a Rectum 6 12 6 12 2 16 Largest tumor dimension < 5 cm 6 11 0.838a 7 10 0.69a 2 15 0.255a > 5 cm 13 21 16 18 1 33 Margin status Negative 11 27 0.05a 17 21 0.929a 1 37 0.156a Positive 8 5 6 7 2 11 Histology grade Low grade 13 28 0.146a 16 25 0.154b 0 41 0.006a High grade 6 4 7 3 3 7 Depth of invasion (pT) T1−T2 3 10 0.323a 5 8 0.577a 1 12 1.00a T3−T4 16 22 20 18 2 36 Lymphovascular invasion Absent 11 25 0.125a 18 18 0.276a 3 33 0.546a Present 8 7 5 10 0 15 Perineural invasion Absent 17 27 0.669a 21 23 0.436b 3 41 1.00a Present 2 5 2 5 0 7 The values are presented as No. of cases. a Chi-square test. b Fisher test. Various biomarkers associated with tumor stage and lymph node metastasis
-
This study revealed a significant association between DEK and p53 expression and tumor stage. High DEK expression was significantly more prevalent (p = 0.012) in the localized stage of the tumor (20/32, 62.5%) than in the advanced stage. In contrast, the expression of p53 was significantly higher (p = 0.036) in the advanced stage (18/28, 64.3%) than in the localized stage. Additionally, high DEK expression was significantly associated (p = 0.010) with the absence of lymph node metastasis (22/32, 68.8%). Nonetheless, the expression of the Bcl-2 tumor marker was not significantly associated with tumor stage (p = 1.00) or lymph node metastasis (p = 0.583) (see Table 3).
Table 3. Analysis of DEK, p53, and Bcl-2 association with tumor stage and lymph node metastasis (n = 51).
Parameter Tumor stage p LN metastasis p Localized
(I-IIA)Advanced
(IIB-IV)Absent Present DEK Low 5 14 0.012a 6 13 0.010a High 20 12 22 10 p53 Negative 15 8 0.036a 16 7 0.056a Positive 10 18 12 16 Bcl-2 Negative 1 2 1.00a 1 2 0.583a Positive 24 24 27 21 The values are presented as No. of cases. LN, lymph node. a Chi-square test. The interplay between biomarkers
-
In the present study, the association between the expression of these biomarkers was investigated. The analysis revealed a statistically significant association (p = 0.038) between DEK and p53 levels. Specifically, high DEK expression was more frequently observed in cases with negative p53 expression (18/32, 56.2%), whereas low DEK expression was more common in cases with positive p53 expression (14/19, 73.7%). These findings suggest a potential interplay between DEK and p53 (see Table 4).
Table 4. Analysis of DEK association with p53 and Bcl-2 (n = 51).
p53 p Bcl-2 p Negative Positive Negative Positive DEK Low 5 14 0.038a 17 2 0.547a High 18 14 31 1 The values are presented as No. of cases. a Chi-square test. Multivariate analysis between two significant biomarkers with advanced-stage tumors
-
Logistic regression analysis was used to assess the relationship between various biomarkers that showed significance in bivariate analysis. The present results indicated that DEK expression was a significant predictor of tumor grade (p = 0.044), with high DEK expression as the reference category. This study found that low DEK expression was more likely in advanced-stage tumors (OR = 3.756). Conversely, p53 expression did not show a significant association (p = 0.133) with predicting tumor stages, suggesting that DEK serves as an independent predictor of tumor staging (see Table 5).
Table 5. Multivariate analysis (using binomial logistic regression) of DEK and p53 association with advanced-stage tumor (n = 51).
B SE Wald OR CI (95%) p Lower Upper DEK 1.323 0.658 4.040 3.756 1.034 13.652 0.044 p53 −0.936 0.623 2.260 0.392 0.116 1.329 0.133 B, Coefficient. SE, standard error. Wald, Wald statistic. OR, odd ratio. CI, confidence interval. -
CRC has a notable metastatic potential and is associated with mortality; therefore, it is imperative to investigate specific biomarkers for assessing CRC prognosis[1,2,20]. In this study, the aim was to evaluate the disease prognosis by identifying tumor staging and metastasis to adjacent lymph nodes. Previous studies have highlighted DEK's involvement in carcinogenesis and poor prognosis across various cancer types, such as colorectal, lung, breast, liver, and melanoma[9,21−24]. For instance, Zhou et al. demonstrated DEK overexpression in lung cancer tissues, promoting tumorigenesis, and cell survival[21]. Similarly, Kappes et al. observed stronger DEK expression in deeper, more malignant melanocytic lesions[25]. In a study on CRC by Lin et al., higher DEK expression was linked to poor prognosis, late-stage tumors, lymph node metastasis, and reduced survival time[9].
The well-established theory on how DEK induces cell proliferation is through the Wnt and β-catenin signaling pathways, especially Wnt10, which has already been observed to have a strong association with some cancers, such as breast cancer[12,22]. Increased levels of DEK expression lead to heightened transcription and release of Wnt ligands, which in turn can stimulate cell proliferation via a paracrine mechanism mediated by β-catenin signaling[10]. Previous investigations utilizing cell culture, murine models, and xenograft tumor models have demonstrated that overexpression of DEK induces cell proliferation[25−27]. The β-catenin signaling pathway is known for its role in promoting cell invasion; however, the precise molecular mechanisms underlying its influence on cell invasion remain elusive. Mouse embryonic fibroblasts (MEFs) with a DEK knocked out exhibit reduced cell migration due to reduced chemotaxis-driven migration through collagen[25]. Our previous review observed the interplay between DEK and other cancer biomarkers, including p53 and Bcl-2. DEK influences p53 by destabilizing the TP53 gene and disrupting its function as a tumor, thereby contributing to tumorigenesis. The BAX protein, a product of p53 target genes, acts as a pro-apoptotic factor that counterbalances the anti-apoptotic properties of Bcl-2. However, destabilized p53 impedes BAX production, thereby disrupting the balance between Bcl-2 and BAX to regulate cell death[14].
The findings revealed a significant increase in p53 expression during the advanced stages of tumor (IIB-IV). Consistent with our results, several studies have reported similar associations between p53 expression and tumor stage[28−30]. For example, Kim et al. observed that a missense mutation in TP53 leads to the production of mutant p53 protein, which resists degradation by MDM2 via ubiquitylation. Consequently, the accumulation of mutant p53 in the cell nucleus results in p53 overexpression, which is notably higher in advanced-stage tumors[28].
Increased expression of p53 is often associated with higher tumor staging because p53 plays a crucial role in the response of cells to DNA damage and cell cycle regulation. In advanced cancers, mutations or dysfunctions in p53 are frequently observed, leading to a failure in cell cycle control and allowing cancer cells to proliferate uncontrollably. Normally, p53 induces DNA repair, cell cycle arrest, or apoptosis when genetic damage occurs. However, in high-stage cancers, p53 often becomes mutated or loses its normal function, causing its expression to increase in response to increased DNA damage resulting from uncontrolled cell proliferation. This contributes to a more aggressive malignancy, increasing the tumor's potential for metastasis and invasion of the surrounding tissues. Moreover, elevated p53 expression in high-stage tumors can reflect disruptions in apoptosis regulation and cellular stress responses. In the advanced stages, tumors typically experience oxidative stress and an accumulation of genetic mutations, triggering increased p53 expression as part of an adaptive mechanism to ensure tumor cell survival. Although p53 generally suppresses tumor growth in high-stage cancers, this regulatory mechanism is often disrupted, and high p53 expression contributes to faster and more invasive cancer cell proliferation. Therefore, increased p53 expression in high-stage tumors often indicates the breakdown of normal cellular control mechanisms, enabling the tumor to grow more rapidly and spread more extensively[31,32].
There was a significant correlation between Bcl-2 expression and the histological grade. Notably, Bcl-2 positivity is predominantly associated with low-grade tumors. According to theory, negative or low Bcl-2 levels are associated with poorer cancer outcomes. For instance, Cai et al. found significantly higher Bcl-2 negativity in patients with advanced tumor stages[14,33]. Conversely, Liukkonen et al. reported a predominance of Bcl-2 negativity in early tumor stages. This observation aligns with a previous study indicating that abnormal activation of the Bcl-2 gene tends to occur early in apoptosis inhibition[34].
Unexpectedly, the present study revealed that DEK expression was significantly higher in earlier tumor stages and the case with the absence of lymph node metastasis. These findings seem counterintuitive to those of numerous previous studies where high DEK expression was associated with advanced tumor stage and lymph node metastasis[9,35,36]. Yang et al., in their study on breast cancer, reported significantly higher DEK expression (p = 0.030) in both stages of the tumor, localized (I-II) and advanced (III-IV)[22]. On the contrary, Lin et al. observed lower DEK expression in all tumor stages of prostate cancer[37]. These findings demonstrated the inconsistency of DEK expression by tumor stage, and the mechanism or pathophysiology of these findings was not observed in either of the studies.
An increase in DEK (a protein) density in the context of cancer is associated with lower tumor staging because DEK plays a critical role in cell proliferation and invasion. In early-stage cancer, cancer cells tend to grow more rapidly and aggressively, which can lead to the increased expression of proteins such as DEK. This protein regulates cell division and supports the survival of cancer cells; therefore, an increase in DEK may reflect higher biological activity in the early stages of a tumor, which typically indicates a lower malignancy. Low tumor staging (such as stage I or II) usually suggests that the cancer is still confined to a localized area and has not spread widely, allowing for a more controlled and organized molecular process, including DEK's role in cell cycle regulation. Moreover, the increase in DEK in low-stage cancers may also be related to its ability to regulate epithelial-mesenchymal transition (EMT), a mechanism that enables cancer cells to migrate and invade surrounding tissues. In the early stages, although cancer cells show limited invasion potential, high DEK expression can indicate that the molecular processes underlying cancer progression are ongoing, even though metastasis has not yet significantly occurred. Studies have shown that elevated DEK is often found in cancer cells that have not yet undergone significant invasion or metastasis, supporting the hypothesis that DEK may serve as a biomarker for cancers in their early stages, which have not yet been widely spread[22,38,39]. The discrepancy between the present findings and those of previous studies could be attributed to differences in patient cohorts, tumor microenvironments, or genetic heterogeneity. Further research is necessary to elucidate the role of DEK in colorectal cancer progression and to determine whether it can serve as a reliable biomarker for early-stage disease.
In multivariate analysis, DEK was identified as an independent predictor of tumor stage. Moreover, positive p53 expression was significantly more prevalent in cases with low DEK expression. This suggests that DEK and p53 may be active at different times or even at distinct stages of cancer progression. While many studies have indicated that p53 expression is more common in advanced cancer stages, the present results appear to contradict earlier findings from our review, where DEK was thought to align with p53 by destabilizing its gene, thus playing a role in cancer pathophysiology[14,28,30].
The best explanation for the results is that DEK plays a notable role in the early initiation of tumorigenesis in CRC. A review article by Privette Vinnedge et al. hypothesized that DEK expression stimulates cell proliferation and potentially initiates the initial stages of differentiation by regulating nucleic acid structure. Through its involvement in modulating nucleic acid topology, DEK may facilitate chromatin organization, transcription, DNA repair, DNA replication, and alternative mRNA splicing, consequently influencing various signal transduction pathways that are crucial for differentiation[40]. Shibata et al. discovered that DEK regulates various transcriptional regulators, including the transcriptional activator-repressor complex (MED12L, SETBP1, HDAC9, and PRDM2), histone acetyltransferase (MYST4), trithorax complex (MLLT2 and MLLT3), methyl-DNA binding proteins (DNMT3L, DNMT3B, and MBD2), and numerous transcription factors[41]. These oncoproteins are believed to play a crucial role in the epigenetic regulation of cancer stem cells (CSCs), and their investigation revealed that the downregulation of these molecules affects colony formation activity and the expression of CSC markers. Therefore, DEK may contribute to maintaining the tumor-initiating activity, partly through the modulation of these transcriptional regulators[12]. In their examination of breast cancer, Privette Vinnedge et al. determined that while DEK is not essential for tumor metastasis, it enhances cellular invasion and proliferation of metastatic tumors. Interestingly, the loss of DEK did not prevent metastasis but did lead to a significant reduction in the burden of metastatic tumors[12].
In this study, DEK was found to be an independent predictor of tumor stage. These results differ from those of previous studies. DEK overexpression has been linked to a poor prognosis and resistance to therapy. Therefore, it is less specific or well-established as a predictive biomarker than KRAS and BRAF. KRAS and BRAF are well-established oncogenes that are frequently mutated in CRC, serving as critical drivers of tumorigenesis and as predictive biomarkers for targeted therapies. Mutations in KRAS lead to constitutive activation of signaling pathways that promote uncontrolled cell proliferation. These mutations are associated with resistance to anti-EGFR monoclonal antibody therapies and generally indicate poor prognosis in patients with CRC[42]. Similarly, BRAF mutations, most notably the V600E mutation, result in continuous activation of the MAPK pathway, contributing to aggressive tumor behavior and poor clinical outcomes. BRAF-mutant CRCs are typically unresponsive to anti-EGFR therapies, underscoring the importance of BRAF status in guiding treatment decisions[43]. In contrast, DEK is an emerging oncoprotein involved in chromatin remodeling and transcription regulation. Overexpression of DEK has been observed in various cancers, including CRC, where it promotes tumorigenesis and chemoresistance. Unlike KRAS and BRAF, DEK does not commonly harbor activating mutations. Instead, its overexpression contributes to cancer progression by enhancing tumor cell proliferation and survival[44]. While research on DEK is ongoing, it holds potential as a novel therapeutic target, though it has not yet reached the same level of clinical application as KRAS and BRAF. DEK, while less understood, represents a promising area of research with potential implications for future CRC therapies.
In conclusion, the present study emphasizes the high expression of DEK in localized-stage tumors and the absence of lymph node metastasis. Furthermore, DEK emerged as an independent predictor of tumor stages, with increased expression associated more frequently with tumors in the localized stage. Conversely, p53 positivity was predominantly observed in advanced stages, whereas Bcl-2 showed no significant association with tumor stage or lymph node metastasis. Given the cross-sectional design of this study, it was difficult to observe the correlation between biomarkers and clinicopathological characteristics in patients. A cohort study would be more appropriate for evaluating long-term prognosis, such as 5-year survival rates and overall outcomes, using various biomarkers. The present findings suggest that DEK may play a pivotal role in the early stages of carcinogenesis, providing a foundation for future research exploring DEK expression throughout tumorigenesis.
-
What is already known about this subject:
1. High DEK expression was notably prominent in the CRC compared to healthy colon tissues.
2. Increased DEK expression was associated with unfavorable prognosis, advanced tumor stages, lymph node metastasis, and shorter survival times of CRC patients.
3. DEK influences p53 by destabilizing the TP53 gene, which contributes to CRC carcinogenesis.
What are the new findings:
1. High DEK expression is significantly higher in localized-stage tumors and in the absence of lymph node metastasis, suggesting its pivotal role in the early initiation of CRC carcinogenesis.
2. DEK was an independent predictor of tumor stage, where low DEK expression was more likely to be found in the advanced-stage tumors.
3. Positive p53 expression was significantly more prevalent in cases with low DEK expression, suggesting that DEK and p53 may be active at different periods or at distinct stages of cancer progression.
-
The small sample size limited the generalizability of the findings to a broader population. Additionally, the cross-sectional design of the study precludes the establishment of causality between DEK, p53, and Bcl-2 expression and their associated clinicopathological characteristics, lymph node metastasis, and tumor staging. The absence of longitudinal data may underestimate the temporal relationships between DEK, p53, and Bcl-2 expression and tumor progression. Although multivariate analyses were conducted, unmeasured confounders such as genetic predispositions or environmental factors may have influenced the results. Future studies involving larger and more diverse populations are necessary to validate these findings. Longitudinal studies would be particularly beneficial for exploring the causal relationships between DEK, p53, and Bcl-2 with clinicopathological features, lymph node metastasis, and tumor staging.
This study was funded by the Publikasi Terindeks Internasional (PUTI) Q2 scheme year 2023 from Universitas Indonesia (Contract No. NKB-672/UN2.RST/HKP.05.00/2023).
-
This study (protocol number: 23-05-0619) was approved by the Ethics Committee of the Faculty of Medicine, University of Indonesia, Cipto Mangunkusumo Hospital under the reference number KET-643/UN2.F1/ETIK/PPM.00.02/2023. Due to the nature of the research and following an informed consent waiver granted by the ethics committee (reference number: ND-389/UN2.F1/ETIK/PPM.00.02/2023), written informed consent from the participants was not required for their inclusion in the study.
-
Nur Rahadiani was the principal investigator, taking charge of project administration, funding, data analysis, manuscript drafting, and the decision to publish. Marini Stephanie and Amelia Fossetta Manatar designed the methodology and managed data collection and analysis. Kathryn Effendi handled data curation and analysis. Ening Krisnuhoni supervised the study and contributed to data curation and analysis. Marini Stephanie, Kathryn Effendi, and Amelia Fossetta Manatar reviewed and edited the manuscript. All authors reviewed the results and approved the final version of the manuscript
-
The data generated in this study remains in use for other ongoing research project, thus are not publicly available. However, they can be made available upon reasonable request from the corresponding author.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Rahadiani N, Effendi K, Stephanie M, Manatar AF, Krisnuhoni E. 2024. From biomarkers to outcomes: investigating DEK, p53, and Bcl-2 expression and their role in colorectal cancer stage and lymph node metastasis. Gastrointestinal Tumors 11: e005 doi: 10.48130/git-0024-0005
From biomarkers to outcomes: investigating DEK, p53, and Bcl-2 expression and their role in colorectal cancer stage and lymph node metastasis
- Received: 30 October 2024
- Revised: 20 December 2024
- Accepted: 23 December 2024
- Published online: 31 December 2024
Abstract: Colorectal cancer (CRC) presents a significant global health burden, with mortality rates being affected by recurrence and resistance to therapy. Emerging immunohistochemical biomarkers, such as DEK, p53, and Bcl-2 offer the potential for enhanced prognosis. DEK's role in tumor progression, particularly its interaction with p53 and Bcl-2, remains underexplored in Indonesian patients with CRC. Investigating these markers may aid in refining CRC management beyond traditional staging methods. Paraffin blocks from 51 patients in 2022 were collected from our archive. Immunohistochemistry assessed the expression levels of DEK, p53, and Bcl-2. The association between biomarkers and clinicopathological characteristics was evaluated using Chi-Square and Fisher Test. Multivariate analysis was performed using logistic regression analysis. High DEK expression was significantly prominent in the localized stage (20/32, 62.5%) and in the absence of lymph node metastasis cases (22/32, 68.8%). Meanwhile, positive p53 expression was significantly more dominant in the advanced stage of the tumor (18/28, 64.3%). Bcl-2 expression was not significantly associated with tumor stage or lymph node metastasis; however, positive Bcl-2 expression was observed in all histologically low-grade tumors (41/41, 100%) Additionally, DEK was also observed as an independent predictor for tumor stage (OR = 3.756). In conclusion, the present study highlights DEK's increased expression in localized-stage tumors and its predictive value for tumor staging. p53 positivity is prevalent in advanced stages, whereas Bcl-2 shows no significant association with tumor stage or lymph node metastasis. The findings suggest DEK's potential role in early carcinogenesis, warranting further investigation.
-
Key words:
- DEK /
- p53 /
- Bcl-2 /
- Colorectal cancer