-
Pancreatic cancer (PC) is an extremely fatal disease of the digestive system, with a five-year survival rate of merely 10%[1−3]. PC-related deaths accounted for 88.12% of new cases, second only to liver cancer, and became one of the six leading causes of death in China in 2015[4]. Several risk factors are associated with PC, including family history, obesity[5], type 2 diabetes[5], and smoking[6]. Surgical operation remains the only potential cure, though fewer than 20% of patients qualify for surgical treatment[7]. At diagnosis, the majority of PC patients have either locally advanced stage (30%−35%) or distant metastasis (50%−55%)[8]. Systemic chemotherapy is the preferred treatment for advanced PC, focusing on enhancing quality of life and extending survival time. Currently, first-line chemotherapy with the gemcitabine plus albumin-coated paclitaxel (AG) regimen and fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) regimen have shown clinical benefits in advanced PC[9,10].
The synergistic effect of anti-angiogenic drugs in conjunction with chemotherapy has been demonstrated in several cancer types[11,12]. Anti-angiogenic drugs can inhibit abnormal tumor angiogenesis, improve the distribution and penetration of drugs in tumor tissues, and enhance the efficacy and tolerance of chemotherapy[13]. A variety of anti-angiogenic drugs, such as bevacizumab combined with chemotherapy, have been used in many clinical studies in pancreatic cancer. Although improvement of PFS has been observed in some clinical trials[14], the results do not show significant survival advantages. Anlotinib is a novel small molecule multi-target receptor tyrosine kinase (RTK) inhibitor that effectively inhibits vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and stem cell factor receptor (c-Kit) and other kinases, exhibiting significant anti-angiogenesis and tumor proliferation inhibition[15]. Clinical research has demonstrated the effectiveness of anlotinib in treating multiple types of tumors, including pancreatic cancer[16−18], non-small-cell lung cancer[19], soft tissue sarcoma[20], advanced medullary thyroid carcinoma[21], metastatic renal cell carcinoma[22,23], esophageal carcinoma[24], and hepatocellular carcinoma[21].
Several clinical studies have revealed encouraging outcomes from the combination of anlotinib and chemotherapy. In non-small-cell lung cancer, this combination demonstrated strong tumor-suppressing activity with manageable side effects[25]. For metastatic breast cancer, the combination of anlotinib and chemotherapy achieved a disease control rate of 79.3%[26]. Given the hypoxia and nutrient-deprived microenvironment of pancreatic cancer, which is linked to elevated levels of vascular endothelial growth factor (VEGF) and the occurrence of distant metastasis[11,27], exploring the value of anlotinib combined with chemotherapy is clinically significant. At present, the clinical impact of anlotinib in treating pancreatic cancer remains uncertain. Some retrospective studies have reported that anlotinib alone or in combination with gemcitabine plus S-1 could prolong the survival of unresectable or metastatic PC patients[14,28]. A recent phase II clinical study showed that anlotinib in combination with immunotherapy and chemotherapy achieved OS data of 13.7 months in the first-line treatment of advanced PC[29]. More clinical data are still needed to prove the value of anlotinib in PC.
The aim of this study was to evaluate the potential efficacy and safety of anlotinib combined with the AG regimen as a first-line treatment for advanced pancreatic cancer, expanding anlotinib's indications, providing clinical treatment references, and exploring more effective strategies for pancreatic cancer treatment.
-
We retrospectively collected data from patients with advanced pancreatic cancer receiving first-line treatment at the Department of Oncology, Nanjing First Hospital (Nanjing, China) from May 2018 to September 2023. Patients were assigned to two groups based on the treatment protocol: 39 patients in the chemotherapy group (AG regimen) and 32 patients in the combination therapy group (anlotinib plus AG regimen). The criteria for inclusion were as follows: (1) patients diagnosed with pancreatic cancer through histopathology or cytology with at least one measurable lesion as defined by the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1); (2) defined as stage IV pancreatic cancer according to the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) 8th edition staging criteria; (3) pre-treatment ECOG score of 0−2; (4) no prior anti-tumor therapy, or only one neoadjuvant or adjuvant chemotherapy with recurrence more than six months post-chemotherapy; (5) expected survival of at least three months; (6) aged 18−80 years; (7) had received at least four cycles of treatment. The criteria for exclusion were as follows: patients with severe dysfunction of the heart, brain, liver, or kidneys, as well as those with significant coagulation abnormalities, those who were diagnosed with two or more systemic malignant primary tumor types, those who were unable to conduct follow-up and those who were in special populations such as pregnant women, postpartum women, and individuals with mental illness. This study protocol was reviewed and approved by the Nanjing First Hospital, approval number KY20240520-KS-05.
Treatment
-
(1) Chemotherapy group: Gemcitabine 1,000 mg/m2 via intravenous infusion on days 1 and 8, followed by albumin-bound paclitaxel 125 mg/m2 via intravenous infusion on days 1 and 8, 21 days per cycle.
(2) Combined treatment group: Anlotinib hydrochloride capsules 12 mg orally in the morning for 14 consecutive days, then discontinued for one week, gemcitabine 1,000 mg/m2 via intravenous infusion on days 1 and 8, and albumin-bound paclitaxel 125 mg/m2 via intravenous infusion on days 1 and 8, 21 days per cycle.
Outcome assessment
-
Patients were followed up monthly during treatment and every three months post-treatment. The deadline for follow-up was March 2024. Medical history, CA199 level (detected by electrochemiluminescence immunoassay), and imaging examinations were collected during follow-up. Primary endpoints were OS and PFS, with OS defined as the duration from the initiation of treatment to either death or the most recent last follow-up, and PFS from treatment start to tumor progression or death. The secondary endpoints were as follows: ORR, DCR, and time to treatment failure.
Tumor treatment response was assessed using RESECT 1.1, with outcomes categorized as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). ORR was determined by dividing the total of CR and PR by the total number of cases. DCR was calculated by dividing the sum of CR, PR, and SD by the total number of cases.
Adverse events were assessed and recorded according to CACTE version 4.0, focusing on grade III/IV events and the patient's tolerance.
Statistical analysis
-
Data were analyzed using SPSS 25.0 and GraphPad Prism 9.0. Continuous variables conforming to normal distribution were expressed as mean ± standard deviation and qualitative variables as rates (%). Independent samples t-test, non-parametric test, and χ2 or Fisher's exact tests were used to compare baseline characteristics, survival, efficacy, and safety. Kaplan-Meier curves were generated to estimate OS and PFS. Independent risk factors affecting survival were identified through univariate and multivariate Cox regression. In this study, p < 0.05 was considered statistically significant.
-
This study examined the clinical data of 71 patients diagnosed with pancreatic cancer between May 2018 and September 2023, including 32 patients in the A + AG regimen group and 39 patients receiving AG regimen chemotherapy. Patients' gender, age, past history, body mass index (BMI) (calculated as weight in kilograms divided by the square of height in meters), Eastern Cooperative Oncology Group (ECOG) score[30], pathological type, metastasis site, carbohydrate antigen 19–9 (CA19-9) level, and thioredoxin reductase (TR) are presented in Table 1. A comparative analysis revealed no statistically significant differences in the baseline characteristics between the two groups (p > 0.05).
Table 1. Patient characteristics.
Clinical characteristics A + AG group
(n = 32)AG group
(n = 39)p-value Gender 0.551 Male 20 (62.5%) 27 (69.2%) Female 12 (37.5%) 12 (30.8%) Age (years, $ \overline{x}\pm s $ ) 64.0 ± 10.0 66.9 ± 8.7 0.199 Smoking history 10 (31.3%) 10 (25.6%) 0.601 Drinking history 11 (34.4%) 15 (38.5%) 0.722 Past medical history Hypertension 14 (43.8%) 15 (38.5%) 0.652 Diabetes 10 (31.3%) 13 (33.3%) 0.852 BMI (kg/m2), $\overline{x}\pm s $ ) 22.6 ± 2.5 21.4 ± 2.8 0.089 ECOG score 0.801 0−1 19 (59.4%) 22 (56.4%) 2 13 (40.6%) 17 (43.6%) Pathological type 0.782 Adenocarcinoma 28 (87.5%) 36 (92.3%) Other types 4 (12.5%) 3 (7.7%) Metastatic site Liver 25 (78.1%) 23 (59.0%) 0.086 Lung 10 (31.3%) 15 (38.5%) 0.527 Peritoneum 4 (12.5%) 9 (23.1%) 0.252 Lymph node 17 (53.1%) 21 (53.8%) 0.952 CA19-9 (U/ml) 0.307 ≤ 27 9 (28.1%) 7 (21.9%) > 27 23 (71.9%) 32 (78.1%) TR (u/ml) 0.185 ≤ 4 12 (37.5%) 9 (23.1%) > 4 20 (62.5%) 30 (76.9%) The figures in parentheses indicate the proportion of patients as percentages. Tumor reaction
-
All patients had measurable lesions, and the clinical efficacy was evaluated following RECIST version 1.1 in both groups. No significant differences in ORR and DCR were observed between the A + AG group and the AG group (ORR: 12.5% vs 5.1 %, p = 0.399; DCR: 87.5% vs 74.4%, p = 0.233) (See Table 2).
Table 2. Comparison of tumor response status between the two groups.
Tumor response A + AG group (n = 32) AG group (n = 39) p-value CR 0 0 PR 4 2 SD 24 27 PD 4 10 ORR 4 (12.5%) 2 (5.1%) 0.399 DCR 28 (87.5%) 29 (74.4%) 0.233 Survival analysis
-
With follow-up up to March 2024, PFS was observed in 71 patients and OS in 65 patients, while the remaining six patients were lost to further analysis. The overall mPFS was 5.8 months (95 % confidence interval (CI) = 4.883−6.717) (Fig. 1a) and mOS was 11.4 months (95% CI = 9.355−13.505) (Fig. 1b). In the AG regimen group, mPFS was 5.1 months (95% CI = 3.632−6.568) and mOS was 9.6 months (95% CI = 8.703−10.557). In the A + AG group, mPFS was 7.4 months (95% CI = 4.212−10.588), and mOS was 15.0 months (95% CI = 9.496−12.564). Survival outcomes in the A + AG group significantly exceeded those of the AG group (PFS: p = 0.003; OS: p = 0.001), indicating meaningful clinical benefits (Fig. 1c, d). However, no significant differences in OS were observed within clinical subgroups, such as ECOG score or metastasis location (p > 0.05) (Fig. 1e & f).
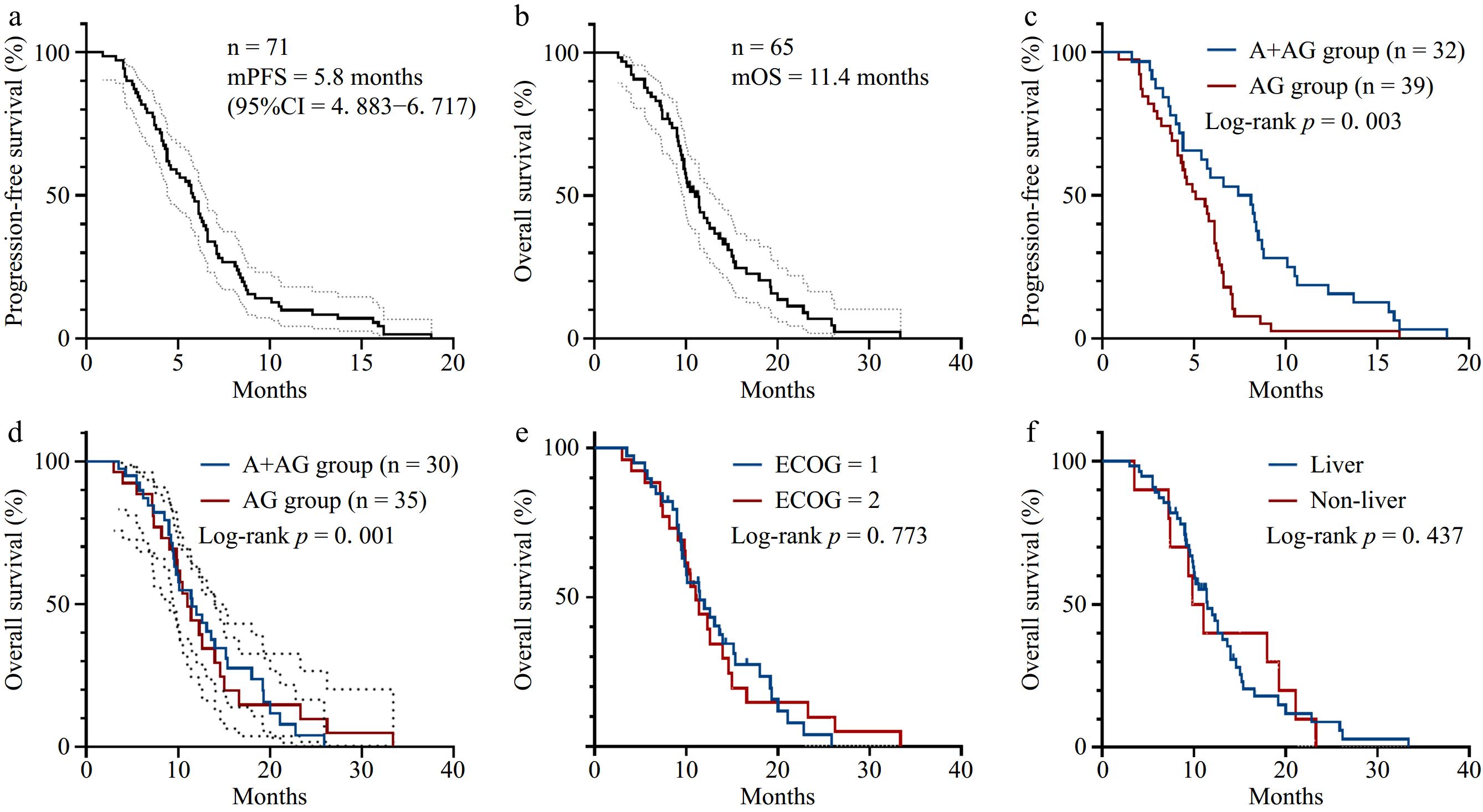
Figure 1.
Survival curves of patients in this study. (a) PFS survival curve of the overall population with mPFS of 5.8 months (95% CI = 4.883−6.717). (b) OS survival curve of the overall population with mOS of 11 months (95% CI = 9.355−13.505). (c) PFS survival curves of the A + AG regimen group and the AG regimen group (p = 0.003). (d) OS survival curves of the A + AG regimen group and AG regimen group (p = 0.001). (e) OS survival curves according to the ECOG score. (f) OS survival curves according to the metastasis site.
Univariate and multivariate analyses of PFS and OS
-
A COX analysis based on baseline characteristics was performed on the PFS of patients in the AG and A + AG groups. Univariate analysis identified pathology type as a relevant clinical factor affecting PFS (Table 3). while pathology type and CA199 level were the relevant clinical factors affecting OS (Table 4). In the A + AG group, univariate analysis identified TR as the relevant clinical factor affecting PFS (Table 5), and TR was the relevant clinical factor affecting OS (Table 6). Multivariate analysis showed no clinical factors correlated with PFS and OS in both groups.
Table 3. Univariate and multivariate analysis of factors influencing PFS in patients in the AG regimen group.
Clinical features Univariate analysis Multivariate analysis HR 95% CI p HR 95% CI p Gender Male 1.00 Female 0.70 0.34−1.44 0.331 Age 1.03 0.99−1.07 0.141 BMI (kg/m2 ) 1.09 0.96−1.24 0.205 Hypertension True 1.000 False 0.909 0.467−1.769 0.778 ECOG score 1.24 0.64−2.38 0.526 Pathological type Adenocarcinoma 1.00 1.00 Other types 10.20 2.24−46.53 0.003 1.07 0.18−6.52 0.943 Lymph node metastasis True 1.00 False 0.72 0.37−1.42 0.342 Liver metastasis True 1.00 False 1.28 0.67−2.46 0.461 Pulmonary metastasis True 1.00 False 0.79 0.40−1.55 0.492 Peritoneal metastasis True 1.00 False 0.70 0.32−1.53 0.372 CA199 level Negative 1.00 Positive 1.97 0.85−4.58 0.113 TR 0.96 0.85−1.07 0.457 Table 4. Univariate and multivariate analysis of factors influencing OS in patients in the AG regimen group.
Clinical features Univariate analysis Multivariate analysis HR 95% CI p HR 95% CI p Gender Male 1.00 Female 1.30 0.62−2.73 0.487 Age 1.00 0.96−1.05 0.981 BMI (kg/m2 ) 1.01 0.90−1.14 0.858 Hypertension True 1.00 False 0.59 0.28−1.22 0.156 ECOG score 1.13 0.56−2.26 0.737 Pathological type Adenocarcinoma 1.00 1.00 Other types 11.28 2.45−51.98 0.002 3.34 0.51−21.82 0.208 Lymph node metastasis True 1.00 False 0.88 0.44−1.77 0.719 Liver metastasis True 1.00 False 0.64 0.30−1.37 0.253 Pulmonary metastasis True 1.00 False 0.88 0.44−1.78 0.725 Peritoneal metastasis True 1.00 False 1.35 0.58−3.14 0.482 CA199 level Negative 1.00 1.00 Positive 2.95 1.18−7.35 0.020 3.34 1.12−10.22 0.031 TR 1.05 0.93−1.18 0.461 Table 5. Univariate and multivariate analysis of factors influencing PFS in patients in the A + AG regimen group.
Clinical features Univariate analysis Multivariate analysis HR 95% CI p HR 95% CI p Gender Male 1.00 Female 0.72 0.35−1.50 0.383 Age 1.00 0.96−1.04 0.941 BMI (kg/m2 ) 0.98 0.85−1.14 0.789 Hypertension True 1.00 False 0.79 0.38−1.64 0.521 ECOG score 0.97 0.47−2.00 0.934 Pathological type Adenocarcinoma 1.00 Other types 1.27 0.43−3.70 0.664 Lymph node metastasis True 1.00 False 0.98 0.48−1.99 0.950 Liver metastasis True 1.00 False 1.92 0.78−4.72 0.155 Pulmonary metastasis True 1.00 False 1.40 0.62−3.18 0.424 Peritoneal metastasis True 1.00 False 0.78 0.27−2.26 0.649 CA199 level Negative 1.00 1.00 Positive 2.31 0.93−5.77 0.073 2.39 0.94−6.06 0.067 TR 1.17 1.03−1.33 0.014 1.11 0.97−1.28 0.128 Table 6. Univariate and multivariate analysis of factors influencing OS in patients in the A + AG regimen group.
Clinical features Univariate analysis Multivariate analysis HR 95% CI p HR 95% CI p Gender Male 1.00 Female 1.34 0.55−3.25 0.521 Age 1.06 0.96−1.05 0.056 1.06 0.98−1.15 0.170 BMI (kg/m2 ) 1.07 0.88−1.31 0.475 Hypertension True 1.00 1.00 False 0.37 0.14−1.00 0.050 0.84 0.24−2.92 0.789 ECOG score 0.78 0.31−1.95 0.588 Pathological type Adenocarcinoma 1.00 Other types 1.38 0.39−4.89 0.614 Lymph node metastasis True 1.00 False 1.18 0.49−2.87 0.709 Liver metastasis True 1.00 False 0.56 0.18−1.75 0.321 Pulmonary metastasis True 1.00 False 0.86 0.35−2.10 0.735 Peritoneal metastasis True 1.00 False 1.48 0.34−6.47 0.605 CA199 level Negative 1.00 Positive 1.83 0.67−5.05 0.242 TR 1.18 1.01−1.38 0.035 1.07 0.88−1.31 0.496 Adverse reactions
-
Adverse reactions were evaluated concerning CACTE version 4.0. The typical side effects related to anlotinib treatment included hypertension, fatigue, loss of appetite, hyperlipidemia, hand-foot syndrome, proteinuria, and hepatic dysfunction[31]. Notably, the A + AG group showed a markedly higher incidence of hypertension compared to the AG group (p = 0.020). However, the incidence of grade III/IV hypertension did not differ statistically between the two groups. The incidence of other adverse reactions did not differ significantly between the two groups, as shown in Table 7. During the treatment period, no patients died due to grade V adverse reactions, and all observed adverse reactions remained manageable.
Table 7. Comparison of adverse reactions between the two groups.
Grade III adverse reactions Grade III/IV adverse reactions Group A + AG Group AG p value Group A + AG Group AG p value Leucopenia 17 (53.2%) 23 (58.9%) 0.800 13 (40.6%) 12 (30.8%) 0.387 Decreased hemoglobin 17 (53.1%) 16 (41.1%) 0.437 4 (12.5%) 7 (17.9%) 0.763 Thrombocytopenia 15 (46.9%) 21 (53.9%) 0.729 11 (34.4%) 8 (20.5%) 0.189 Hypoalbuminemia 13 (40.6%) 20 (51.3%) 0.511 8 (25.0%) 7 (17.9%) 0.469 Diarrhea 19 (59.3%) 18 (46.1%) 0.384 2 (6.3%) 1 (2.6%) 0.585 Pleural and abdominal fluid 8 (25.0%) 3 (7.7%) 0.094 3 (9.4%) 5 (12.8%) 0.936 Nausea and vomiting 18 (56.2%) 26 (66.7%) 0.513 7 (21.9%) 6 (15.4%) 0.482 Fatigue and poor appetite 24 (75.0%) 29 (74.4%) 1.000 5 (15.6%) 3 (7.7%) 0.500 Hepatic dysfunction 11 (34.4%) 13 (33.3%) 1.000 9 (28.1%) 4 (10.3%) 0.053 Hemorrhage 3 (9.4%) 1 (2.6%) 0.471 1 (3.1%) 0 0.921 Hypertension 8 (25.0%) 5 (12.8%) 0.312 14 (43.8%) 11 (28.2%) 0.172 Hand-foot syndrome 6 (18.7%) 5 (12.8%) 0.153 2 (6.3%) 0 0.200 Hyperlipidemia 10 (31.2%) 10 (25.6%) 0.797 6 (18.8%) 3 (7.7%) 0.301 Proteinuria 16 (50.0%) 17 (43.6%) 0.764 4 (12.5%) 2 (5.1%) 0.399 -
Currently, chemotherapy is the sole effective option for treating unresectable pancreatic adenocarcinoma. Since 1997, gemcitabine has been the primary chemotherapy medication for individuals with locally advanced or metastatic pancreatic cancer, while the survival data is merely 5.7 months[32]. The current National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO) guidelines recommend regimens including the AG regimen, gemcitabine plus capecitabine (GX regimen), and FOLFIRINOX regimen for patients with unresectable locally advanced or metastatic PC[33,34]. Compared to the FOLFIRINOX regimen, the AG regimen is more widely used in clinical practice due to relatively mild adverse effects. However, regardless of the clinical choice of the FOLFIRINOX or the AG regimen, the overall survival (OS) of advanced PC patients still does not exceed 1 year[9].
It is well recognized today that anticancer drugs often are most effective when used in combination. Targeted drug combination chemotherapy is an important research direction for pancreatic cancer. A multicenter, randomized phase IIb trial assessing the efficacy of gemcitabine combined with nimotuzumab in KRAS wild-type pancreatic cancer revealed improved OS in the combination group in comparison with the placebo group. This suggests that optimizing systemic chemotherapy and exploring broad-spectrum targeted therapies remain important directions for future research in pancreatic cancer.
Angiogenesis plays a crucial role in the progression and spread of tumors. It has been revealed that several proangiogenic factors, including VEGF, transforming growth factor (TGF)-β, and platelet-derived growth factor (PDGF)-A overexpressed in PC and involved in disease progression[35−37]. However, the therapeutic effectiveness of traditional drugs targeting angiogenesis, such as bevacizumab, in treating PC patients is far from expected. Sorafenib, a drug that inhibits VEGFR-2 and PDGFR-β, has demonstrated effectiveness against pancreatic cancer in preclinical studies. Unfortunately, the combination of sorafenib and gemcitabine did not improve advanced PC patients' prognosis in a multicenter phase II clinical study[38]. Other studies demonstrated that apatinib, a novel VEGFR-2 tyrosine kinase inhibitor can promote radiosensitivity in the treatment of advanced PC with tolerable side effects[39]. Nonetheless, anti-angiogenic therapies remain clinically promising by normalizing tumor vasculature, improving drug delivery, enhancing the local immune response, and reducing distant metastasis. Targeting angiogenesis remains a viable strategy for treating advanced pancreatic cancer.
Anlotinib, a multi-targeted anti-tumor therapy, specifically targets critical components of the angiogenic pathway, including VEGFR-2, VEGFR-3, PDGFR-α, PDGFR-β, and FGFR1-4, exhibiting a lower inhibitory concentration 50 (IC50). Due to its target concentration, anlotinib has strong efficacy in anti-angiogenic therapy with a low risk of adverse reactions[40]. Furthermore, anlotinib effectively inhibits c-Kit[41,42], the rearranged during transfection proto-oncogene (Ret)[43], FGFR[44,45], and hepatocyte growth factor receptor (c-Met)[46], which collectively suppress the growth and movement of tumor cells. A study indicates that anlotinib activates the endoplasmic reticulum stress pathway( PERK/peIF2α/ATF4 ), inducing reactive oxygen species production, which blocks cell proliferation and causes cell cycle arrest at the G2/M phase thereby inducing apoptosis of PC cells[47]. Multi-omics analysis has shown that anlotinib significantly inhibits ribosomal activity, further curtailing the proliferation of PC cells by regulating cell cycle dynamics, RNA metabolism, and lysosomal function[48]. Anlotinib also can promote tumor vascular normalization, transforming the immunosuppressive tumor microenvironment into one that stimulates immune responses. This action significantly reduces tumor growth and helps prevent systemic immune suppression. Furthermore, when combined with PD-1 checkpoint inhibitors, anlotinib can reverse the immunosuppression caused by PD-L1 upregulation after monotherapy, prolong the period of vascular normalization, and ultimately lead to tumor regression[49]. A recent clinical study reported the benefit of combining S-1, sintilimab, and anlotinib as second-line therapy for pancreatic cancer (PC) patients with liver metastasis, extending overall survival (OS)[50]. These findings suggest that anlotinib-related combination therapy could offer a novel treatment approach for PC patients. Additionally, recent studies have shown that combining penpulimab, anlotinib, nab-paclitaxel, and gemcitabine yields promising clinical outcomes, particularly in enhancing ORR and DCR in metastatic PC[29]. Our study focuses on exploring the combination of anlotinib with gemcitabine and nab-paclitaxel, aiming to provide clinicians with more treatment options.
In this study, we evaluated the effectiveness and safety of combining anlotinib with albumin-bound paclitaxel and gemcitabine as a first-line treatment for PC. We observed that ORR reached 12.5% and DCR was 87.5% in this regimen. Both mPFS and mOS of the A + AG group were superior to the AG group. Moreover, the inclusion of anlotinib did not raise the risk of severe adverse reactions when compared to chemotherapy alone. While patients receiving anlotinib showed a notably higher risk of developing hypertension, there was no significant difference in the occurrence of grade III/IV hypertension, which could be tolerated by most patients, and this adverse reaction can be managed with dose regulation and symptomatic treatment. Intriguingly, some adverse reaction is also correlated with the efficacy of anlotinib, such as hand-foot syndrome is positively correlated with prolonged survival in NSCLC treated with anlotinib[51].
It is necessary to state that this retrospective study has many limitations, including retrospective data, which has selection bias and information bias that is difficult to avoid. It is necessary to conduct prospective or randomized controlled trials in the future. Secondly, the sample size of this study is limited and it is a single-center study, which may lead to data bias and be limited by treatment conditions, making it difficult to comprehensively reflect the effectiveness and safety of the treatment. Future research should involve multi-center studies with larger sample sizes. In addition, the management strategy for adverse reactions is imperfect, which may affect the accurate evaluation of drug safety.
-
In conclusion, our findings suggest that combining anlotinib with gemcitabine and albumin-bound paclitaxel may be an effective first-line treatment option for advanced pancreatic cancer with significant survival advantages and tolerable adverse reactions, which is of great clinical significance. To further confirm these findings and improve the therapeutic regimen of anlotinib in PC, large-scale, prospective, multi-center randomized controlled trials, along with long-term follow-up data, are required.
We would like to extend our sincere thanks to all the colleagues and patients who contributed to this study. This study was supported by the Natural Science Foundation of Jiangsu Province (Grant No. BK20231127) to Xiaowei Wei.
-
This study protocol was reviewed and approved by the Nanjing First Hospital, approval number KY20240520-KS-05.
-
The authors confirm contribution to the paper as follows: analysis design, clinical data collection, statistical analysis performed: Zheng Y; draft manuscript preparation: Zhou Y; treated patients, manuscript revision: Zhou J, Wei X, Xia H, Liang W. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets produced and/or analyzed in this study are available upon reasonable request from the corresponding author.
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Yawen Zheng, Yuhan Zhou
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zheng Y, Zhou Y, Xia H, Liang W, Wei X, et al. 2025. A clinical retrospective study of anlotinib in combination with gemcitabine and albumin-bound paclitaxel for the first-line treatment of advanced pancreatic cancer. Gastrointestinal Tumors 12: e001 doi: 10.48130/git-0025-0001
A clinical retrospective study of anlotinib in combination with gemcitabine and albumin-bound paclitaxel for the first-line treatment of advanced pancreatic cancer
- Received: 14 October 2024
- Revised: 10 December 2024
- Accepted: 23 December 2024
- Published online: 21 January 2025
Abstract: Anlotinib has demonstrated efficacy and tolerability in treating various tumors. The purpose of this retrospective study is to assess the efficacy and safety of anlotinib combined with gemcitabine and albumin-bound paclitaxel as a first-line treatment for advanced pancreatic cancer. Patients with advanced pancreatic cancer who are not candidates for surgery were divided into two groups: anlotinib plus gemcitabine/nab-paclitaxel (A + AG) (n = 32) and gemcitabine/nab-paclitaxel alone (AG) (n = 39). Data was analyzed for clinical characteristics, survival benefits, and side effects. The median progression-free survival (mPFS) (7.4 m vs 5.1 m, p = 0.003) and the median overall survival (mOS) (15 m vs 9.6 m, p = 0.001) were both prolonged in the A + AG group compared to AG group. The objective response rate (ORR) (12.5% vs 5.1%, p = 0.399) and the disease control rate (DCR) (87.5% vs 74.4%, p = 0.233) only disclosed increasing trends in the A + AG group compared to the AG group. Univariate analysis identified that pathology type (p = 0.002) affects PFS, while pathology type (p = 0.003), and CA199 level (p = 0.020) affect OS in the AG groups. In the A + AG group, the Thymidine kinase (TR) level affected PFS (p = 0.014) and OS (p = 0.035). Multivariate analysis showed no clinical factors correlated with PFS and OS in both the A + AG and AG groups. The most common adverse effects were leukopenia (91.5%), fatigue/poor appetite (85.9%), nausea/vomiting (80.3%), and thrombocytopenia (77.5%). The incidence of grade III/IV adverse reactions was similar in both groups. No grade V adverse reactions occurred. This study demonstrates that anlotinib combined with the AG chemotherapy regimen provides a new first-line therapeutic choice for patients with advanced pancreatic cancer.
-
Key words:
- Anlotinib /
- Pancreatic cancer /
- Gemcitabine and albumin-bound paclitaxel /
- Survival /
- Safety