-
Humans have used plants and herbs as a source of therapeutic and curative agents since the early ages. Historically, mankind has relied on medicinal and aromatic plant bio-actives to promote overall health and longevity. The growth of herbal plants and their surroundings have been linked to certain factors that qualitatively or quantitatively alter the amount and composition of secondary metabolites, improving the efficacy and bioactive potential of natural products[1,2]. Due to their fragrant character, numerous domestic and foreign exotic species of Lamiaceae have frequently been known in folk medicine. These species have been utilized to treat a variety of skin issues, respiratory infections and digestive disorders. The herbs have noteworthy applications in culinary practices as herbal seasonings[3].
The aromatic Coleus aromaticus Benth., a huge perennial, and succulent herb that belongs to the genus Coleus and family Lamiaceae is native to the Indian subcontinent and is now widely cultivated in other Asian and South American nations. Asian households frequently employ this traditional aromatic plant[4]. These leaves were also used in cooking due to their powerful perfume and flavor. The dried leaves are used as a herbal seasoning for meat products and other food products, and they have an oregano-like texture which makes them perfect as a culinary food supplement[5]. The fresh herb leaves have a wide range of uses, including the treatment of convulsions, epilepsy, asthma, bronchitis, cough, malarial fever, and hepatitis[6]. These medicinal qualities of C. aromaticus namely antioxidant, anti-inflammatory, analgesic, and anti-microbial properties relate to the biological potential of the essential oil[7−10].
Thymol, carvacrol, eugenol, and chavicol and other volatile components of the essential oils of C. aromaticus are known for their anti-microbial properties. The oxygenated monoterpenes, carvacrol and thymol are well known for their numerous practical uses in the food and pharmaceutical industries[11]. Additionally, perfume and cosmetics are made from the fragrant oils. Allelopathic potential, antibacterial properties, insecticidal capabilities, free radical scavenging properties, and radio-protective activities are just a few of the numerous bioactivities of the carvacrol/thymol-rich oil[12−15]. The composition of essential oils have been reported to be impacted by various growth settings, phenological stages, varieties, and other factors which in turn affects the biological efficacy of the oil[16,17].
To the best of our knowledge, Coleus aromaticus has been extensively studied for its biological activities such as antioxidant, anti-inflammatory, and anti-microbial activities but no information regarding its pesticidal capability was found. The primary objective of the present study was to phytochemically characterize the chemical constitution of the aerial parts of C. aromaticus gathered from the agroclimatic region along the foothills of Uttarakhand (India). Further, the essential oil was assessed for its pesticidal activities namely nematicidal activity against Meloidogyne incognita, herbicidal activity against Raphanus raphanistrum, and insecticidal activity against Spodoptera litura. The pesticidal efficacy of the observed major components of the oil was verified using AutoDock software tools on certain proteins/enzymes, i.e., acetylcholinesterase (PDB ID: 6XYS), carboxylesterase (PDB ID: 5IVH), and acetohydroxyacid synthase (PDB ID: 1YHZ).
-
Fresh aerial parts (leaves with stems) of C. aromaticus Benth. were sourced from experimental farms of Medicinal Plants Research and Development Centre, Haldi, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, Uttarakhand, India (29°02′14′′ N, 79°48′74′′ W, 243.8 m elevation) in October 2021. A voucher specimen (GBPUH-1038/13-07-2021) was deposited with the Department of Biological Sciences, College of Basic Sciences and Humanities, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, Uttarakhand, India after the plant was identified by Dr. D.S. Rawat (Plant Taxonomist). The fresh aerial parts of the plant (4,000 g) were chopped and hydrodistilled using a Clevenger-type apparatus for 3−4 h[18] to produce a pale yellow essential oil.
GC/MS analysis
-
The stored oil was analyzed by GC/MS using a Perkin Elmer gas chromatograph model GC Clarus SQ 8C paired with a single quadrupole mass spectrometer model MS SQ8 to determine the bioactive components. The conditions for the columns were as follows: PE-5 capillary column, with dimensions of 30 m × 0.25 mm I.D × 0.25 µm, working in the electron influence method at 60 eV. Air free helium gas was employed as a carrier gas in addition to a fixed stream of 1.32 ml/min at a volume of 1 µl. The split ratio for a injection volume was 0.02 µl was 1:30. Temperature adjustments were made to bring the ion source and injector source to 210 and 250 °C, respectively. The oven temperature was controlled as follows: the oven temperature was first raised from 60 to 310 °C/min at a rate of 20 °C/min before being isotherm finished for 10 min at 310 °C. MS spectra were captured at 60 eV, with a scan range of 30−1,100 m/z. The results obtained were compared with those of the spectral data received from the Wiley Library and NIST.14 databases[19].
Evaluation of nematicidal activity
Isolation, extraction, and identification of nematodes
-
Tomato plant roots infected with root-knot nematodes (Meloidogyne incognita) were gathered from the farmed experimental areas of the Vegetable Research Centre, GBPUA&T, Pantnagar, India. Roots with root-knot nematodes attached to them were cut into short pieces, and they were then placed in a container with a 1.0% NaOCl solution. The suspension was put through a sieve after the bottle was hand-shaken for 5.0 min. The residue was collected from top to bottom sieves 100-mesh and then 400-mesh and put into the 250-ml beaker after being washed with tap water for 1 min. With the use of a counting chamber set up with several eggs or juveniles per mL, the suspension of the fluid was observed[20]. Female perineal patterns were carefully examined in order to identify the species.
Hatching and mortality test
-
Fresh tomato plant roots infected with root-knot nematodes (M. incognita) were used to prepare a 100 ml suspension of eggs containing 50 eggs per ml in distilled water. Five mL of egg suspension (50 eggs/ml) and 1.0 ml of each concentration of CAEO at 0.25, 0.5, and 1.0 µl/ml were transferred separately in triplicate into blocks of cavity glass (2.5 cm in diameter). Data was observed over the course of 24-, 48-, 72- and 96-h, respectively. In the control groups, 2.0 ml of egg suspension and 1.0 ml water were kept in blocks of hollow glass[21]. Under a stereo optical microscope (Olympus CX3) microscope (40×), the number of eggs that hatched after the 96-h exposure was counted. The percentage (mean%) of the egg hatchability inhibition was found as a function of CAEO activity and the impact of concentrations and time interval.
M. incognita eggs were placed in distilled water and actively continued for 24 h at room temperature (26 ± 2 °C) to measure the mortality rate. A solution of freshly hatched juveniles (J2) (approx. 50 J2/ml) was made in deionized water. In the block of glass cavity with a diameter of 2.5 cm, 2.0 ml of the suspension of freshly hatched juveniles and 1.0 ml of each concentration of CAEO (0.25, 0.5, and 1.0 µl/ml) were added and kept at room temperature. Three replicates of the experiment were conducted. The block of glass cavity treated as a control contained 1.0 ml of nematode mixture and 1.0 ml of deionized water. Under a light stereo-binocular microscope (Olympus CX3) (6×), the number of deceased juveniles was counted after 72 h of exposure. The percentage (mean%) of dead nematodes used to calculate the immobilization of J2 nematode larvae against CAEO. It was believed that their continued immobility following their submersion in water proved nematode mortality[22].
Phytotoxic activity
-
To examine the phytotoxic effect demonstrated by CAEO, fresh fungal-treated seeds of Raphanus raphanistrum var sativus (radish) were purchased and obtained from Vegetable Research Centre, Pantnagar, Uttarakhand, India. For a period of four weeks, seeds were kept at room temperature in paper bags. Prior to the experiments, the seeds' viability and capacity for germination were tested. Seed surfaces were sterilized in two-steps (a 30 s 70% ethyl alcohol rinse followed by a 20 min treatment with 10% sodium hypochlorite solution), washed three times with sterile distilled water, and air-dried aseptically in a laminar hood. Ten seeds were put in Petri plates with two layers of filter paper on the surface (Whatman No. 2). First a stock of oil in dimethyl sulfoxide (DMSO)/water (1.0%, v/v) was created in order to make precise concentrations of CAEO in water (250, 500, 750, and 1,000 µl/ml). Ten ml of each concentration were finally added to the Petri dishes. 1.0% DMSO in water was used as the control. All of the studies were repeated twice, and there were five replicates of each treatment. Plastic paraffin film tape was used to seal the Petri dishes containing the seeds. After that, Petri dishes were housed in a germinator with a 16-h photoperiod set at 25 °C. In this experiment, root and shoot lengths as well as germination percentage were measured[23].
Evaluation of insecticidal activity
Insects
-
Spodoptera litura eggs lying on castor leaves were obtained from the Crop Research Centre, Pantnagar, Uttarakhand, India, and were confirmed by Dr. R.M. Srivastava (College of Agriculture, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, Uttarakhand, India). For two to three generations, the eggs were artificially subcultured and cleansed in a dark incubator at 28−30 °C with relative humidity maintained at 70% to 80%. A freshly prepared artificial diet constituting of 120 g soybean powder, 96 g wheat germ, 40 g yeast powder, 32 g agar, 16 g casein, 9.6 g ascorbic acid, 6.0 g potassium sorbate, 2.0 g methylparaben, 1.2 g choline chloride, 0.4 g cholesterol, 0.24 g inositol, 0.08 g vitamin B complex, and 1.280 L H2O was fed to the recently hatched larvae kept in the sterile glass chambers (20 cm × 15 cm × 6 cm). After 5 d, each larva was moved into a separate sterile glass tube (10 cm high and 2 cm in diameter), fed a fresh artificial meal, and kept at room temperature (28−30 °C) until they pupated. Male and female adults were coupled and reared with honey water (15%, w/v) in clean containers (40 cm × 30 cm × 10 cm) following their transformation from the pupal stage. On oiled papers that had been positioned in the containers, the eggs of mated adults were gathered. To create the next generation of larvae, the eggs underwent another treatment. With a photoperiod of 14 L:10 D h, a temperature of 27 ± 0.5 °C, and a relative humidity (RH) of 75% ± 5%, the rearing conditions were maintained. For this investigation, third-instar larvae were employed[24].
Insecticidal activity via contact activity
-
The drip approach was applied to the contact activity procedure. Unaffected by gender, 5.0 healthy adults with good activity and steady growth were chosen from the reared adults. They were put into a glass activity test container 5.5 cm high and 2.5 cm in diameter. In order to create a serial testing solution, CAEO was dissolved in 1.0% tween 20 water solution. Four concentrations of CAEO (10 to 50 µl/ml) were found in formal experiments in accordance with the findings of preliminary experiments. Five replications of each treatment and control at various concentrations were performed. The test insects' death/survival was examined and noted 24 h later, and irregular activity was taken to mean that the insects had perished[24].
Molecular docking studies
-
Molecular docking techniques were used to validate all of the pesticide actions. The X-ray crystal structures of the enzymes acetylcholinesterase (PDB ID: 6XYS), carboxylesterase; CaE (PDB: 5IVH), and acetohydroxyacid synthase, AHAS (PDB: 1YHZ) was retrieved from the RCSB protein data bank. The molecular docking studies of thymol on these proteins were carried out using AutoDock4.2 with Discovery Studio and Cygwin64 Terminal tool to determine the binding energy, visualize docking poses, and understand the various ligand-target receptor interactions responsible for the pesticidal activity of CAEO[25].
Most vertebrates, insects, and nematodes have acetylcholinesterase (AChE), (PDB ID: 6XYS), which is the target for the action of organophosphates and carbamate pesticides. AChE hydrolyzes the neurotransmitter acetylcholine (ACh) to acetic acid and choline at the synapses and neuromuscular junction. As a result, inhibiting AChE causes the nervous system to dysfunction and the nematodes perishes[26].
Certain plant-derived substances may have an impact on the enzymatic profile of insect pests. Proteinaceous inhibitors, for instance, may impede proteolytic activity and cause abnormal growth and development. By using the protein ligand's three dimensional structure and its affinity for the detoxifying enzyme carboxylesterase (CaE) (PDB ID: 5IVH), which is located in the head capsule of Spodoptera litura larvae, it may be possible to anticipate the hazardous effects of chemical components of botanicals on S. litura[27].
Numerous commercial herbicides (applied to rice, corn, wheat, and cotton crops) target acetohydroxyacid synthase (AHAS), also known as acetolactate synthase (ALS), with PDB ID: 1YHZ. Low application rates, excellent crop selectivity, and low animals toxicity are the three features that distinguish pesticides as AHAS inhibitors. The AHAS enzyme failed to complete the conversion into isoleucine, leucine, and valine, also known as BCAAs, which is why AHAS inhibitor has an indirect impact on protein synthesis in plants by reducing the production of these branched-chain amino acids[28].
In silico PASS studies
-
A web-based online software program evaluated the pesticidal activities of the main constituents identified in CAEO. The experiment predicted probable activity (Pa) and probable inactivity (Pi). Using PASS online software, the structures of key constituents were translated into their SMILES forms and utilized to forecast the biological spectrum. Only the activities that have Pa > Pi are thought to be likely for a specific drug prediction.
Statistical analysis
-
The means ± standard deviation of three parallel measurements represented the experimental results. The statistical calculations used to determine the mean values and standard deviation. Three replicates for three to five concentrations in each sample were used in the experiment to test the nematicidal, insecticidal, and herbicidal activity. The 2-factor and 3-factor CRD (ANOVA) were used to analyze the raw data, and statistical analysis was used to determine the mean values and standard deviation (SD). Percentage data were subjected to angular transformation[29].
-
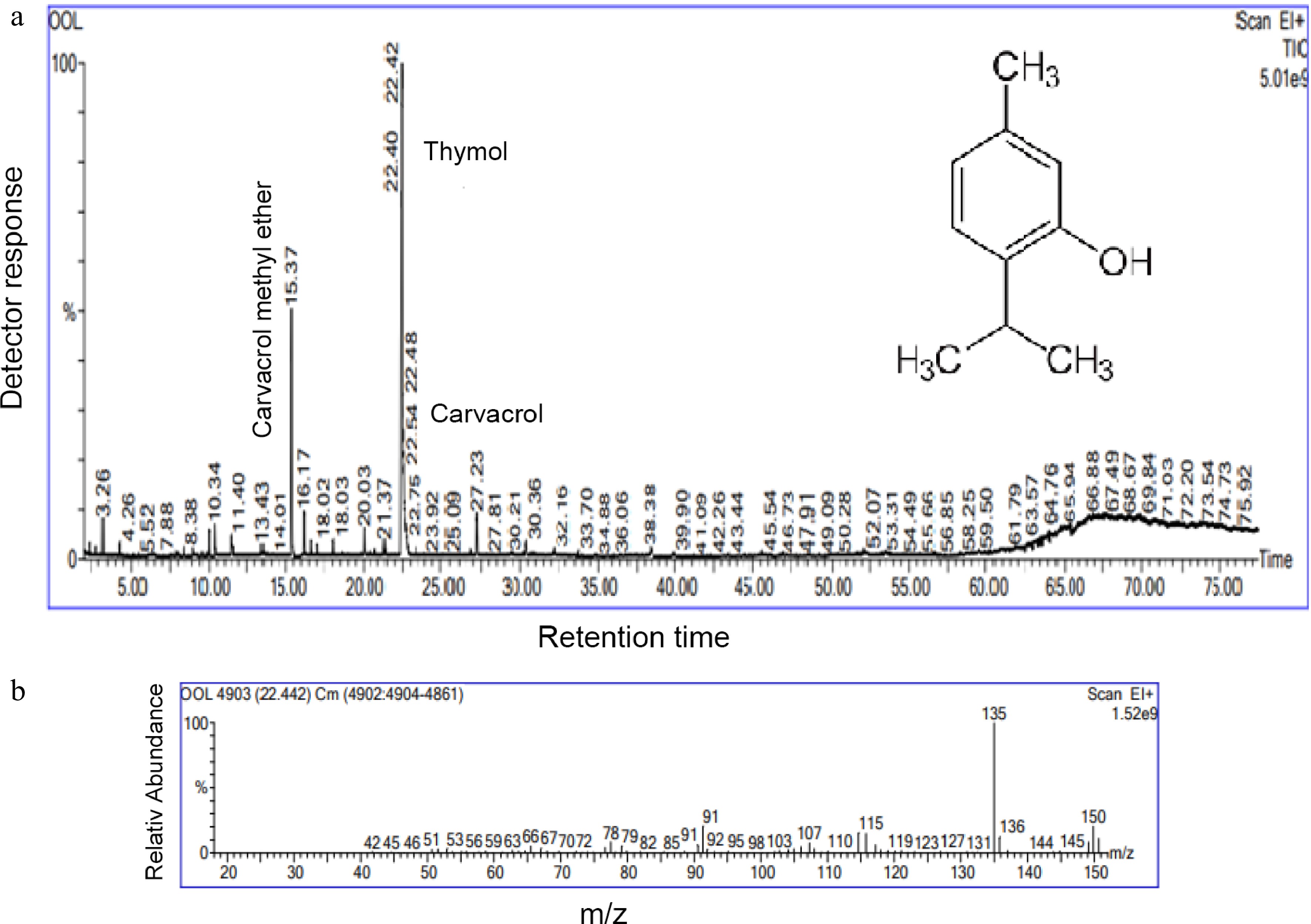
A viscous, pale-yellow liquid, with an intense bitter aroma was the product of CAEO at 0.2% (v/w). The GC-MS analysis showed that 12 terpenoid compounds were present, with a total identification rate of 97.67%. Thymol (69.60%) was the predominant component, followed by p-cymene (3.95%), (E)-caryophyllene (3.69%), carvacrol (3.27%), α-thujene (3.25%), γ-terpinene (2.95%) and carvacrol methyl ether (2.26%), which were all in intermediate concentration (Table 1). Figure 1a & b shows the gas chromatogram and mass spectrum of thymol. Oxygenated monoterpene (72.87%), hydrocarbon monoterpene (10.15%), hydrocarbon sesquiterpene (6.17%), and oxygenated sesquiterpene (1.09%) are the different types of these molecules. 7.39% of additional chemicals were found in the oil. The outcomes were consistent with those of an analysis of the chemical variability of aerial parts of C. aromaticus gathered from the experimental farms of Purara, Bagheswar, and Diary farm, Pantnagar conducted by Verma et al.[30]. The thymol content of both the oils ranged from 85.9% to 98.9%. Our findings were consistent with those of Tewari et al.[31], who identified thymol as the main component. The current findings differ from earlier studies published worldwide[11, 12, 32−34], where carvacrol was the main constituent of the aerial section of C. aromaticus. These chemical compositional discrepancies could be caused by geographical distribution, genetic, environmental, developmental, and other factors.
Table 1. Chemical composition of CAEO.
S.N. Compound R.I. Lit R.I. Exp % Mol. formula M.F.P. Monoterpene hydrocarbon 1. α-thujene 931 929 3.2 C10H16 M+ = 136; m/z: 121, 119, 105, 93 (100%), 91, 77, 65, 53, 51, 43, 41, 27 2. p-cymene 1022 1023 3.9 C10H14 M+ = 134; m/z: 132, 120, 119 (100%), 103, 91, 77, 65, 55, 41, 39 3. γ-terpinene 1054 1054 2.9 C10H16 M+ = 136; m/z: 121, 119, 107, 105, 93 (100%), 91, 79, 77, 65, 43, 41, 39, 27 Total (%) 10.0 Monoterpene oxygenated 4. Thymol 1288 1283 69.6 C10H14O M+ = 150; m/z: 136, 135 (100%), 115, 91, 79, 77, 65, 51, 39 5. carvacrol 1296 1297 3.2 C10H14O M+ = 150; m/z: 136, 135 (100%), 117, 107, 91, 77, 65, 51, 39, 27 Total (%) 72.8 Sesquiterpene hydrocarbon 6. Bicyclogermacrene 1502 1501 2.5 C15H24 M+ = 204;
m/z: 189, 176, 161, 147, 136, 133, 121, 107, 93 (100%), 79, 67, 53, 41, 39, 297. (E)-caryophyllene 1421 1423 3.7 C15H24 M+ = 204; m/z: 175, 147, 133, 120, 107, 93 (100%), 91, 79, 69, 55, 41, 39, 27 Total (%) 6.2 Sesquiterpene oxygenated 8. β-eudesmol 1648 1645 1.1 C15H26O M+ = 222; m/z: 189, 175, 141, 131 (100%), 79, 75, 73, 55 Total (%) 1.1 Others 9. 1-(3-ethyloxiranyl)-ethanone − − 2.6 C6H10O2 M+ = 114; m/z: 85, 71, 57, 44, 43 (100%), 38, 31 10. Carvacrol methyl ether 1247 1251 2.3 C11H16O M+ = 164; m/z: 161, 149 (100%), 91, 79, 71, 53 11. Thymyl acetate 1355 1355 1.3 C12H16O2 M+ = 192; m/z: 150, 136, 135 (100%), 91,43 12. Carvacrol ethyl ether 1456 1457 1.2 C12H24O M+ = 184; m/z: 138, 124, 109, 95, 82, 67, 57 (100%), 55, 43, 41, 39, 29 Total (%) 7.4 Total Composition (%) 97.5 CAEO: Coleus aromaticus essential oil; R.T.: Retention time; R.I. Lit.: Retention index (DB-5 column) acquired from literature; R.I. Exp.: Retention index acquired from experimental data; M.F.P.: Mass Fragmentation Pattern. Thymol, the main component in the current study, is an isomeric form of carvacrol and is a phenolic monoterpenoid with a pleasant aroma. It is also found to be a derivative of p-cymene[35]. Thymol is considered to be the marker compound of the Lamiaceae family that is typically found in the Thymus, Oreganum, Coleus, Satureja, and Thymbra. Thymol and carvacrol are popularly utilized as additives in cosmetics, the food industry, perfumery, and aromatherapy due to their pleasant odour and flavour. They are prized for their antioxidant, anti-inflammatory, antibacterial, antispasmolytic, and antitumor activity in the pharmaceutical industries since they are known to be the precursors of thymohydroquinone and thymoquinone. The production of γ-terpinene from geranyl diphosphate (GDP) with the help of P450 monooxygenases and dehydrogenase initiates the whole biosynthetic route of thymol and carvacrol[36].
According to several studies, C. aromaticus essential oil possesses pharmacological qualities including anti-oxidant activity, anti-diabetic activity, antimicrobial activities, and insecticidal activity[12, 37−39]. In addition, fungicidal, insecticidal, mosquito larvicidal, and antifeedant effects of thymol derived from several plants of the Lamiaceae family have been described[40−43]. The present study evaluated the various pesticide activities of C. aromaticus essential oil.
In-vitro nematicidal activity of CAEO via mortality and egg hatchability assay
-
In this investigation, the bio-nematicidal potential of the oil was assessed. The oils demonstrated very high levels of inhibition in the case of egg hatchability, with 95.39% at 0.25 µl/ml and 96.87% at 1.0 µl/ml dosing levels (Table 2). A similar dose level was used to test the % mortality of M. incognita 2nd stage larvae. Surprisingly, CAEO was observed to report a moderate mortality rate of 52.32% at a dose of 1.00 µl/ml (Table 3). As the oil was concentrated, the rate at which larvae hatched increased steadily, reflecting the fact that the concentration was a factor in the juvenile hatching of root-knot nematode, M. incognita. In the control setting, a considerable proportion of juveniles hatched, and there was very little mortality. After 72 h and 96 h durations, respectively, the highest concentration of 1.00 µl/ml resulted in the greatest amount of larval mortality and egg hatchability inhibition. As a result, it was discovered that the actions were concentration and time -dependent.
Table 2. % Egg hatchability inhibition of CAEO against M. incognita in laboratory conditions.
Dose (µL/mL) Number of eggs hatched in time Mean % Egg hatchability 24 h 48 h 72 h 96 h 0.25 4.66 5.66 7.33 11.33 7.25 95.39 0.50 4.66 5.66 6.66 8.66 6.42 95.92 1.00 3.33 5.00 5.66 5.66 4.92 96.87 Control 106.00 143.00 173.66 207.66 157.58 ± 43.35 S.E.M 0.34 0.29 0.59 C.D. 1% 1.35 1.17 2.34 C.D. 5% 0.99 0.86 1.73 C.V. 56.90 CAEO: Coleus aromaticus essential oil; C.D.: Critical Difference; C.V.: Coefficient of Variance, ** p < 0.05. Table 3. % Mortality of 2nd stage larvae of M. incognita in different concentrations of CAEO.
Dose (µL/mL) Number of larvae dead in time Mean
larvae dead% Mortality 24 h 48 h 72 h 0.25 9.33 27.33 28.33 21.66 ± 10.69 13.47 0.50 25.00 38.33 39.33 34.22 ± 8.00 27.78 1.00 55.66 66.00 66.33 62.66 ± 6.06 52.32 Control 2.00 8.66 11.66 7.44 ± 4.94 S.E.M. 2.05 2.05 3.55 C.D. 1% 8.34 8.34 14.45 C.D. 5% 6.09 6.09 10.55 C.V. 15.56 CAEO: Coleus aromaticus essential oil; C.D.: Critical Difference; C.V.: Coefficient of Variance, ** p < 0.05. Acetylcholinesterase enzyme (PDB ID: 6XYS) molecular docking investigations were also carried out to confirm the nematicidal activity testing results. Using a binding energy of -6.20 kcal/mol, root mean square deviation of 96.68 Å and estimated inhibition constant of 28.68 µM, thymol formed strong bonds with the amino acid residues Tyr334, Ser81, and Gly80 through van der Waals forces, Tyr442 and Ile439 through pi-alkyl interactions, and Trp432 through pi-sigma interactions. With a binding energy value of -6.45 kcal/mol, carbofuran was shown to interact with many amino acids when compared to the other ligands that were examined (Fig. 2). After thorough clinical trials, additional research is required to assess the safety of the botanicals for the use in humans.
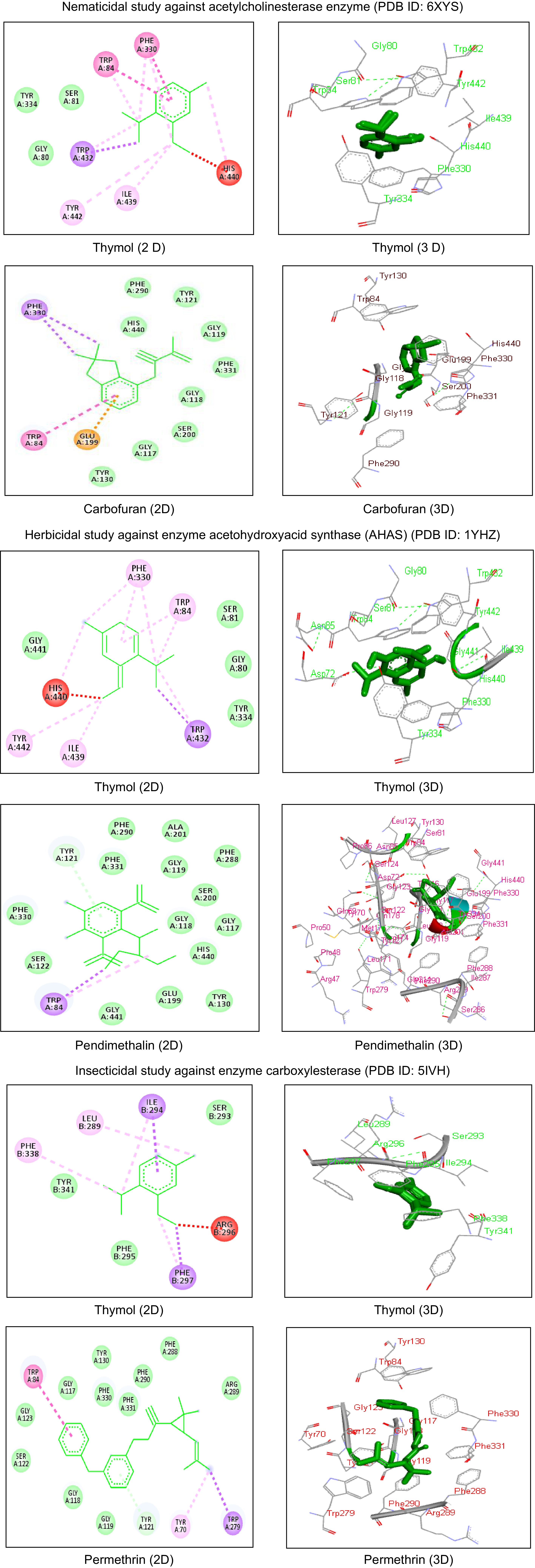
Figure 2.
Comparative 2D and 3D interactions of thymol and standard drugs with different target proteins used in the study. 6XYS: PBD ID for the crystal structure of enzyme acetylcholinesterase from the gut of Meloidogyne incognita larvae, 5IVH: PDB ID for the crystal structure of enzyme carboxylesterase from the head capsule of Spodoptera litura larvae, 1YHZ: PDB ID for the crystal structure of enzyme acetohydroxyacid synthase (AHAS) from the weed Raphanus raphanistrum sub sativus, amino acid residues in green rings are showing van der Waals interactions, amino acid residues in pink rings are showing pi-alkyl interactions, amino acid residues in purple rings are showing pi-sigma interactions, amino acids in red rings are showing unfavorable bumps.
The current literature search turned up no accounts on the nematicidal activity of C. aromaticus. Coleus forskohlii belonging to the same genus exhibited nematicidal activity against M. javanica[44]. Even so, several species of Lamiaceae plants, including Mentha pulegium, Origanum vulgare, Origanum dictamnus L., Melissa officinalis, Ruta graveolens, Satureja montana and Thymbra capitata, have been studied for their nematicidal potential[45−47]. Carvacrol was examined for its potent activity against M. incognita as well as its synergistic potency with other terpenes[48]. According to Choi et al.[49] and Abdel-Rahman et al.[50], the main compound in this study, thymol, also showed impressive nematicidal action against Bursaphelenchus xylophilus and Caenorhabditis elegans. Thus, supporting the findings of earlier investigations, the substantial nematicidal activity in the present study can be attributed to the high concentration of thymol.
Effect of CAEO against Raphanus raphanistrum seed germination
-
To evaluate the bioherbicidal effect of C. aromaticus EOs at various doses, a germination bioassay was conducted. CAEO at 250 µl/ml demonstrated a broad herbicidal spectrum of 63.70% against R. raphanistrum seed germination. With a rise in EOs concentration, the germination inhibition significantly increased. In comparison to the control setup, CAEO showed the maximum germination inhibition rate in R. raphanistrum seeds at the highest concentration of 1000 µl/ml, which was 97.75%. These findings show that CAEO, even at lower doses, had a negative impact on seed germination. Additionally, as compared to the untreated control, all four concentrations dramatically reduced the lengths of the seedlings' roots and shoots (Table 4).
Table 4. % Phytotoxic activity of CAEO against R. raphanistrum seeds in laboratory conditions.
Dose (µL/mL) Number of seeds germinated in different time intervals Mean seed germinated % Growth inhibition % Root growth inhibition % Shoot growth inhibition 24 h 48 h 72 h 96 h 108 h 250 1.66 2.66 3.33 3.66 4.66 3.20 ± 1.12 63.70 74.79 91.93 500 0.66 1.00 1.66 2.33 3.00 1.73 ± 0.95 80.34 85.91 94.99 750 0.00 0.66 1.00 1.33 2.00 1.00 ± 0.74 88.66 97.36 98.76 1000 0.00 0.00 0.33 0.33 0.33 0.20 ± 0.18 97.75 98.11 100 Control 7.00 7.00 10.00 10.00 10.00 8.80 ± 1.64 0.0 0.0 0.0 Pendimethalin 0.0 0.0 0.0 0.0 0.0 0.0 100.0 100.0 100.0 C.D. 1% 0.53 C.D. 5% 0.39 C.V. 18.13 CAEO: Coleus aromaticus essential oil; C.D.: Critical Difference; C.V.: Coefficient of Variance, ** p < 0.05. Acetohydroxyacid synthase (AHAS) (PDB ID: 1YHZ) was used in molecular docking studies to corroborate the experimental findings of the herbicidal activity. Using binding energy of −6.02 kcal/mol, root mean square deviation of 97.88 Å and estimated inhibition constant of 38.39 µM, thymol strongly bonded with Tyr334, Ser81, Gly441, and Gly80 amino acid residues with van der Waals forces, Phe330, Trp84, Tyr442, and Ile439 with pi-alkyl interactions, and Trp432 with pi-sigma interactions. With a binding energy of −7.50 kcal/mol, pendimethalin was shown to interact with several amino acids when compared to the examined ligands (Fig. 2). After thorough clinical trials, additional research is required to assess the safety parameters of the botanicals for human use.
Numerous studies demonstrated that monoterpene enriched essential oils significantly reduced the germination of weed. In the current investigation, practically all CAEO-treated concentrations had a negative impact on seed germination as well as seedling shoot and root length growth. The results presented here also indicated that oxygenated monoterpenes were the predominant class, which is consistent with those of Pinheiro et al.,[51], who discovered that essential oils from Plectranthus amboinicus rich in carvacrol and thymol effectively inhibited the germination of Lactuca sativa and Sorghum bicolor seeds. Kanyal et al.[3] also reported the substantial herbicidal potential of the oxygenated monoterpene-rich Coleus barbatus aerial part essential oil and the monoterpene hydrocarbon-rich C. barbatus root part essential oil. A number of herbal plants in the Lamiaceae family have also demonstrated allelopathic effects in recent studies, including Thymus vulgaris against Xanthium trumarium and Avena sterilis[52], Thymus capitatus against Convolvulus arvensis and Setaria viridis[53], Thymus vulgaris and Satureja hortensis against Chenopodium album, Ambrosia artemisiifolia and Sorghum halepense[54] and Monarda fistulosa, Satureja pilosa, Origanum vulgare, Micromeria dalmatica, Thymus longedentatus, and Artemisa campestris against Lolium perenne and Trifolium pratense[55]. Thymol, the primary component of CAEO and carvacrol, has also been shown to inhibit seed germination in several other plants, including Sinapi sarvensis, Sonchus oleraceus, Amaranthus retroflexus, Centaurea salsotitialis, Lolium rigidum, Raphanus raphanistrum, and Rumex nepalensis[40,56,57] which adequately supports our findings that CAEO has high bioherbicidal activities which affect the seed germination and root and shoot growth of R. raphanistrum.
In-vitro insecticidal activity of CAEO against S. litura
-
The maximum insect mortality against S. litura was recorded in CAEO at a dose level of 50 µl/ml, which was up to 71.13%. Table 5 presents the comprehensive findings. In the review of the literature, there are no reports on the insecticidal effects of CAEO. The findings are consistent with the studies of earlier researchers. These results imply that CAEO has the potential for the development of novel insecticidal components/chemicals for the management of stored pests and insects.
Table 5. % Mortality of S. litura against CAEO in laboratory conditions.
Dose
(µL/mL)Insects observed alive at different time intervals Mean insect survival % mortality 12 h 24 h 36 h 10 5.00 5.00 5.00 5.00 ± 0.0 0 20 5.00 4.33 4.00 4.44 ± 0.51 11.13 30 4.66 3.66 3.33 3.88 ± 0.69 22.33 40 4.00 4.00 3.33 3.77 ± 0.38 24.46 50 2.00 1.33 1.00 1.44 ± 0.51 71.13 Control 5.00 5.00 5.00 5.00 ± 0.0 0 Permethrin 0.0 0.0 0.0 0.0 100.0 C.D. 1% 0.5 0.7 1.3 C.D. 5% 0.4 0.5 0.9 C.V. 14.7 CAEO: Coleus aromaticus essential oil; C.D.: Critical Difference; C.V.: Coefficient of Variance, ** p < 0.05. Molecular docking studies were also performed using carboxylesterase enzyme (PDB ID: 5IVH) to corroborate the experimental results of the insecticidal activity. Thymol strongly bonded with Tyr341, Ser293, and Phe295 amino acid residues with van der Waals forces, Leu289 and Phe338 with pi-alkyl whereas Ile294 and Phe297 with pi-sigma interactions using binding energy of −4.61 kcal/mol, root mean square deviation of 107.88 Å and estimated inhibition constant of 416.13 µM. Permethrin was observed to show binding interactions with many amino acids as compared to the tested ligands with a binding energy of −8.78 kcal/mol (Fig. 2). Further clinical trials and research is needed to evaluate the safety of these natural botanicals for human use.
C. aromaticus has been recommended for its effective efficiency against the stored grain pest, Tribolium castaneum[58]. Essential oil of C. aromaticus along with its major component thymol has also been evaluated for its larvicidal activity against Culex tritaeniorhynchus, Aedes albopictus, and Anopheles subpictus[42]. In another study by Govindaraju et al.,[59], Coleus aromaticus essential oil and its major compound carvacrol against Aedes aegypti, Culex quinquefasciatus, and Anopheles stephensi. In addition, Coleus amboinicus leaf essential oil collected from Andhra Pradesh, India was observed to show insecticidal activity against white termites, Odontotermes obesus Rhamb. and confused flour beetle, Tribolium castaneum[60]. According to reports, thymol and carvacrol found in CAEO exhibit insecticidal activities against a variety of agricultural pests and stored grain insects[61,62]. It can be inferred that the major and minor components of the essential oil may work in synchronous to increase the potency for pesticidal activities.
In silico PASS studies
-
All the components identified in CAEO were induced to the PASS program which details the pesticidal activities of the components with respect to the probable activity (Pa) and probable inactivity (Pi). A greater Pa value in comparison to Pi (Pa > Pi) validates better activity to be used as a drug. Thymol, the main constituent of the oil showed better results with high anti-helminthic and insecticidal activity which is in accordance with the present results. The Pa and Pi values of the major components are presented in Table 6 showing the insecticidal, antibacterial, antifungal, and anthelmintic activities.
Table 6. In silico PASS prediction bioactivities of major compounds in CAEO.
Major compounds Predicted biological activities Anti-helminthic (nematodes) Insecticidal Anti-fungal Anti-bacterial α-thujene 0.388 > 0.047 − 0.337 > 0.067 0.130 > 0.098 p-cymene 0.633 > 0.005 0.391 > 0.006 0.368 > 0.058 − γ-terpinene 0.642 > 0.005 − 0.443 > 0.041 0.325 > 0.051 thymol 0.569 > 0.008 0.323 > 0.013 0.464 > 0.037 0.336 > 0.047 carvacrol 0.722 > 0.004 0.351 > 0.010 0.449 > 0.039 0.319 > 0.053 bicyclogermacrene 0.520 > 0.014 0.350 > 0.010 0.439 > 0.042 − (E)-caryophyllene 0.333 > 0.080 0.368 > 0.008 0.582 > 0.020 0.437 > 0.023 β-eudesmol − − 0.401 > 0.049 0.302 > 0.059 carvacrol methyl ether 0.622 > 0.005 0.388 > 0.007 0.362 > 0.059 − thymyl acetate 0.775 > 0.003 0.327 > 0013 0.456 > 0.038 0.324 > 0.052 dodecanal 0.458 > 0.025 0.368 > 0.008 0.314 > 0.075 0.280 > 0.068 Pa > Pi, Pa = Probable activity and Pi = Probable inactivity. -
The purpose of the current study was to disclose the chemical makeup and for the first time, the possible pesticidal bioactivity of the essential oil found in the aerial portions of C. aromaticus. The unique aspect of this study was that the herbal spice material was collected during its vegetative stage from the Tarai region of Pantnagar in order to bio-evaluate its nematicidal, herbicidal, and insecticidal effectiveness. When compared to other studies of Uttarakhand, the geographical conditions, edaphic and climate characteristics, and experimental setup may have had an influence on the difference in composition observed in the GC-MS analysis. The main component of thymol (69.60%) contributed to oxygenated monoterpenes (72.87%) in the essential oil. Other important compounds identified included p-cymene (3.95%), (E)-caryophyllene (3.69%), carvacrol (3.27%), α-thujene (3.25%), γ-terpinene (2.95%) and carvacrol methyl ether (2.26%). Our results prove that CAEO can also be a viable choice for the management of M. incognita nematodes and Spodoptera litura. The bioactivities were also validated using molecular docking techniques. Further clinical experiments have revealed that the oil can potentially be used as a bio-pesticide.
-
Avneesh Rawat: Planning original draft, collated the literature and prepared the manuscript. The study was part of his Ph.D. thesis work. Om Prakash: Advisor of Avneesh Rawat, planned the study of the present work, provided research guidance. Kirti Nagarkoti: Helped in preparing the manuscript, Formal analysis. Ravendra Kumar: Co-advisor of the student, helped in executing the experiments. Mahendra Singh Negi: Helped in providing the plant samples for executing the experiments. Satya Kumar: Member of research advisory committee, guided to conduct the nematicidal studies. Ravi Mohan Srivastava: Member of research advisory committee, guided to conduct the entomological studies.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors acknowledge the G. B. Pant University of Agriculture and Technology, Pantnagar, India, for providing academic support and Central Instrumentation Center, University of Petroleum and Energy Studies (UPES), Bidholi campus, Dehradun, for providing facility for GC-MS analysis.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Rawat A, Prakash O, Nagarkoti K, Kumar R, Negi MS, et al. 2024. Chemical profiling and bioactivity evaluation of thymol rich Coleus aromaticus Benth. essential oil. Medicinal Plant Biology 3: e007 doi: 10.48130/mpb-0024-0007
Chemical profiling and bioactivity evaluation of thymol rich Coleus aromaticus Benth. essential oil
- Received: 27 October 2023
- Revised: 25 January 2024
- Accepted: 22 February 2024
- Published online: 10 April 2024
Abstract: Coleus aromaticus Benth. (Family: Lamiaceae) is a huge perennial, aromatic and succulent herb native to the Indian subcontinent. The dried leaves have an oregano-like texture making them a perfect culinary food supplement to be used as herbal seasoning for meat and other food products. The present study aimed to identify the bioactive components in the essential oil collected from the fresh aerial parts of Coleus aromaticus Benth. Using GC/MS analysis, 12 terpenoid components were identified, accounting for 97.5% of the overall oil content. Thymol (69.6%), p-cymene (3.9%), (E)-caryophyllene (3.7%), carvacrol (3.2%), α-thujene (3.2%), γ-terpinene (2.9%), and carvacrol methyl ether (2.3%) were identified to be the primary constituents in the oil, which was determined to be dominated by oxygenated monoterpenes (72.8%). Additionally, at the highest dose, CAEO showed significant pesticidal activity, inhibiting the egg hatchability of Meloidogyne incognita by 96.9%, immobilizing it by 52.3%, insecticidal activity on Spodoptera litura by 71.13%, and phytotoxic activity on Raphanus raphanistrum seeds by 97.75%. For speculating the potential method of action of CAEO components, the proteins/enzymes namely acetylcholinesterase (PDB ID: 6XYS), carboxylesterase (PDB ID: 5IVH), and acetohydroxyacid synthase (PBD ID: 1YHZ) were employed. The novel aspect of this study was that the herbal spice material was collected during its vegetative stage from the Tarai region of Pantnagar (India) in order to bio-evaluate its nematicidal, herbicidal, and insecticidal effectiveness. It was found that CAEO is an effective alternative source of natural pesticides and opens the way for additional research on its mechanistic techniques and field tests to determine its pesticidal studies.
-
Key words:
- Coleus aromaticus /
- Thymol /
- Nematicidal /
- Herbicidal /
- Insecticidal /
- Docking