-
The genus Lycium contains more than 90 species from temperate to subtropical areas of Australia, Eurasia, Southern Africa, North and South America, and other regions of the world[1]. Among them Lycium barbarum L. (宁夏枸杞), L. chinense Mill. (中华枸杞), and L. ruthenicum Murr. (黑果枸杞), are wildly cultivated in China and commonly known as goji berry[2]. Goji berry can be used in both fresh and dried forms; moreover, dry root bark and fruit has functional importance in the medicinal industry particularly as traditional Chinese medicine (TCM). Goji berry's concentrated extracts and infusions with alcohol, utilized as components to manufacture goji beverages; in addition, it is prepared into a sauce as a kind of therapeutic nourishment[3,4]. Dried natural products are popular medicines used for therapeutic purposes and as a useful diet in Asian countries, including Thailand, Vietnam, Japan, Korea, and China[5]. Goji berries contain an abundance of important chemical compounds, such as carotenoids (lutein and zeaxanthin), betaine, Lycium barbarum polysaccharides (LBPs), and flavonoids, which are mainly involved in antioxidant activity and provide a broad range of potential benefits for anti-aging, well-being, neuroprotection, fatigue, metabolism improvement, glucose control in diabetes, immunomodulation, glaucoma, and cytoprotection[6,7]. The consumption of goji berries is largely acknowledged as a tonic that promotes health benefits and ultimately improves the immune system to fight various diseases[8]. Goji berries are also recognized as boxthorns, matrimony berries, and wolfberries. Japanese speakers refer to it as 'kuko', 'red medlar', or 'the Duke of Argyll's tea tree'. Mandarin-speaking Chinese individuals refer to it as 'gouqi' or '枸杞' while Cantonese-speaking Chinese individuals refer to it as 'keitze'. Local Koreans refer to it as 'gugija', Vietnamese people refer to it as 'củkhởi', and Thai people refer to it as 'găogèe'[3]. In East Asia, especially China, L. barbarum and L. chinense are closely related species[9]. L. chinense is widely distributed and cultivated in East and South Asia[10], whereas L. barbarum is extensively cultivated in Northwest China (Ningxia Hui Autonomous Region) and West China (Xinjiang Uygur Autonomous Region). Ningxia as 'Daodi' region of goji production, widely renowned in China. However, owing to recent market demand, goji cultivation areas have extended to new regions over different climatic zones covering from 82° E and 115° E to 30° N and 45° N. In particular, the cultivation of L. barbarum is largely extended to semi-arid temperate continental (Ningxia, Gansu, Inner Mongolia), upland continental (Qinghai), and continental arid climates (Xinjiang)[11]. However, L. chinense is notably confined to temperate monsoon climates, such as Hebei[12]. Both L. chinense and L. barbarum have a history of more than 2,000 years as TCM, with records from the Tang dynasty (1000−1400 AD)[13]. Geographical knowledge is crucial for differentiating goji fruits in terms of composition and factors such as climate, soil type, and cultivation techniques, which directly impact their biological characteristics and usage[14]. For example, growth, phytochemical content, nutritional profile, root development, and organic matter content significantly influence the overall growth and development of berry size, quality, and yield[15,16]. However, the combined effects of these factors shape the ultimate health benefits and economic value of goji berries. Despite their high nutritive value, goji berries exhibit tolerance and adaptability under salinity stress and are planted as halophyte species in Northwest China with high economic returns[17]. Goji berry production has become acclimatized in Northwest China, especially Ningxia (Zhongning County), possibly due to Zhongning's geographical position, which has the improved quality of berries in China, including fruit firmness with longer shelf life, larger berries of bright red color, and sweeter taste[17]. Historically, Qin (221−206 BC), Han (206 BC−220 AD), and Tang (618−907 AD) dynasties had initiated various irrigation projects and provided a way for water passage from the Yellow River to irrigate the farmland in Yinchuan (Ningxia), which provided favorable conditions for the goji berry production in Zhongning's areas[18]. However, during the Ming (1368−1644 AD) and Qing (1644−1912 AD) dynasties, L. barbarum production greatly increased in Zhongning, reaching approximately 200 ha[18,19]. Furthermore, goji berry domestication has been transformed over 600 years with continuous and conscious efforts by growers, including the harvesting of selective plants with large fruits[20]. Several characteristics, including non-shattering of seeds, germination, growth habit, fruit size and coloration, fruit taste (sweetness and bitterness), and fruit edibility, are involved in the domestication program of wild relatives of goji berries to improve cultivars[21]. To date, conventional approaches such as individual plant selection with distinct characteristics and hybridization have been combined to produce key cultivars of the Ningqi series (Ningqi-1 to Ningqi-10)[18,22]. Furthermore, various biotechnological techniques, such as in vitro culture of meristems, anthers, embryos, and endosperms, have been developed and can be used to induce mutations. Genetic transformation of goji berries mediated by Agrobacterium tumefaciens has been established, and the current germplasm of goji berries has been improved for various traits of interest[23−25]. Goji berries have already been subjected to molecular breeding and marker-assisted selection (MAS) techniques, as traditional breeding strategies are hampered by intensive labor, cost, and time inefficiency, which impede goji berry cultivar improvement[26]. With the rapid advancement of next-generation sequencing (NGS), high-resolution genetic maps of goji berries have been constructed by integrating single-nucleotide polymorphism (SNP) markers, which provide affordable tools for QTL mapping and MAS[26,27−30]. Marker-assisted breeding involves the construction of linkage maps, QTL mapping, positioning and cloning of target genes, and comparative analysis of the genome[31]. The current review provides readers with comprehensive information about the genetic diversity, geographical classification, various known cultivars, germplasm resource preservation and development, global germplasm resources, and botanical descriptions of goji berries. Additionally, we provide a thorough understanding of the goji germplasm generated through conventional breeding, as well as molecular and transgenic breeding of goji berries, with prospects for marker-assisted selection and genome editing in goji berries.
Species diversity and geographical distribution
-
The discovery of L. chinense in the southern region of Hebei, China, around 100 BC, is mentioned in the initial records of goji fruits. Whereas L. barbarum was later discovered in semi-arid areas of China, including Ganzhou, Yecheng, Lanzhou, Jiuyuan, and Lingzhou, approximately 600 AC[18]. In 1960, Lycium fruit production spread to other plateau-shaped regions of China under various climatic conditions, including Qinghai, Xinjiang, Ningxia, and Inner Mongolia[11]. In China, Lycium can be found in seven distinct species and three different varieties, including L. cylindricum Kuang and A.M. Lu, L. yunnanense Kuang and A.M. Lu, and L. dasystemum Pojark (L. dasystemum var. rubricaulium), and L. truncatum Y.C. Wang, L. ruthenicum Murray, L. barbarum (L. barbarum var. auranticarpum) and L. chinense Mill. (L. chinense var. potaninii)[1] (Supplementary Table S1). Ningxia goji berries (L. barbarum) from the Northwestern Province and Ningxia Hui Autonomous Region of China have been extensively developed. It has a long flowering and fruiting season that typically lasts from May to October, and its cultivation has since extended to the central and southern regions of the country[5]. L. barbarum var. auranticarpum, a yellow-fruited goji berry that is native to Yinchuan (Ningxia). Chinese goji berries (L. chinense) are grown for medicinal, vegetable, and landscape purposes in northern China, Hebei, Shanxi, Shaanxi, Southern Gansu, and eastern China. Chinese goji berries (L. chinense var. potaninii), somewhat similar to Chinese goji, and is distributed on sunny slopes and valleys in northern Hebei, Shanxi, Shaanxi, Inner Mongolia, Ningxia, western Gansu, eastern Qinghai, and Xinjiang[18]. Yunnan goji berries thrive and spread in the Luquan and Jingdong Counties of Yunnan Province in moist sandlands or in forests at a height of 1,360 to 1,450 m[18]. Black goji berries are distributed throughout Central Asia, northern Shaanxi, Ningxia, Gansu, Qinghai, and Xinjiang. L. dasystemum var. pojarkova is commonly grown on hillsides and beaches throughout Central Asia, including Xinjiang, Gansu, and Qinghai. Another goji berry cultivar (rubricaulium) of L. dasystemum, is widely distributed in Qinghai Province. Cylindricum goji berries are mainly distributed in Xinjiang, and Truncatum goji berries are primarily found along roadsides and hillsides in Shanxi, northern Shaanxi, Inner Mongolia, and Gansu[18]. Moreover, the South African species L. ferocissimum Miers (African boxthorn) has spread to Australia and New Zealand, is involved in the growth of dense woods, and is designated as a weed in Australia's coastal and semi-arid southern regions[1,32]. L. amarum nov. L.Q. Huang, is native to Xizang in southwest China and typically found growing on rocks and alongside roads in a semi-arid, moderate climate. Additionally, it produces bitter fruits that share morphological characteristics with L. chinense, including linear lanceolate leaves, goblets with three lobes, villous rings at the base of the filament, and an adjacent corolla tube[33]. Goji fruits are grown in Europe, Africa, Australia, and North and South America. Interestingly, with an output of 50 tons in 2016, Italy became the European continent's top producer of goji, mostly in the regions of Calabria, Veneto, Puglia, Lazio, and Tuscany. Location and environmental factors, such as temperature, humidity, sunlight, and precipitation, must be known to obtain significant global goji fruit production, as they have a substantial impact on fruit quality, including fruit size and color, taste, and metabolite profile[34]. Several methods and techniques have been developed to distinguish the geographic locations of goji fruits, including near-infrared spectroscopy, principal component analysis, multivariate and linear discriminant analysis, gas chromatography coupled with mass spectroscopy, mass spectroscopy of stable isotopic ratios, stable isotopic ratios coupled with gas chromatography, and high-performance liquid chromatography[35,36].
Germplasm resource conservation and development
-
Most of China's northwestern regions considered 'home to goji'. Seven distinct species and three different varieties of Lycium have been discovered in China[1]. Red goji berries (L. barbarum) are the most popular and widely consumed fruits and medicines in China[9]. In contrast, abundant germplasms of red goji berries have been generated through natural selection and hybridization and naturalized in the northwest to central parts of China[37,38]. The yellow-colored goji berry is a distinct type of L. barbarum germplasm resource among goji germplasm. The fresh fruit of yellow goji '黄色宁夏枸杞' tastes sweet and delicious and rich in trace elements including iron, manganese, and zinc content being relatively high[18]. Recent reports have revealed intra-and interspecific crosses of goji berries, which generate and preserve the highest number of individuals with distinguishing agronomic characteristics. These abundant germplasm resources can be utilized to construct high-density genetic maps of goji berries and mine genomic regions corresponding to traits of interest[26,28,29]. The 'National Goji berry Germplasm Resources Garden' was established in 1986 with an area of 13.33 hectometer (hm2) (38°3800 N, 106°90 E), Yinchuan, Ningxia Hui Autonomous region, China. After more than 30 years of dedication, the 'Ningxia Academy of Agricultural and Forestry Sciences (NXAAS)' now boasts the largest collection of goji germplasm resources along with the greatest number of genes kept in vivo, and the most significant strategic resource reserve base[18,39]. The selection criteria for germplasm collection include genetic diversity, phenotypically distinct characteristics, agronomic characteristics, and yield potential at different geographical locations[18]. The L. barbarum germplasm resource nursery of NXAAS has collected more than 2,000 inbred lines of 10 species, three variants, and unique goji germplasm resources at home and abroad, as well as more than 20,000 plants that have been kept ex situ[39]. Furthermore, these resources include 11 goji berry species in China and five species abroad, 16 new varieties, 20 farm varieties, and 350 wild goji berries (primarily from Gansu, Qinghai, Xinjiang, Ningxia, Sichuan, Hubei, Jiangsu, Shandong, and Hebei, among others), 80 new and excellent lines, and 1,700 intermediate breeding materials for the development program[18].
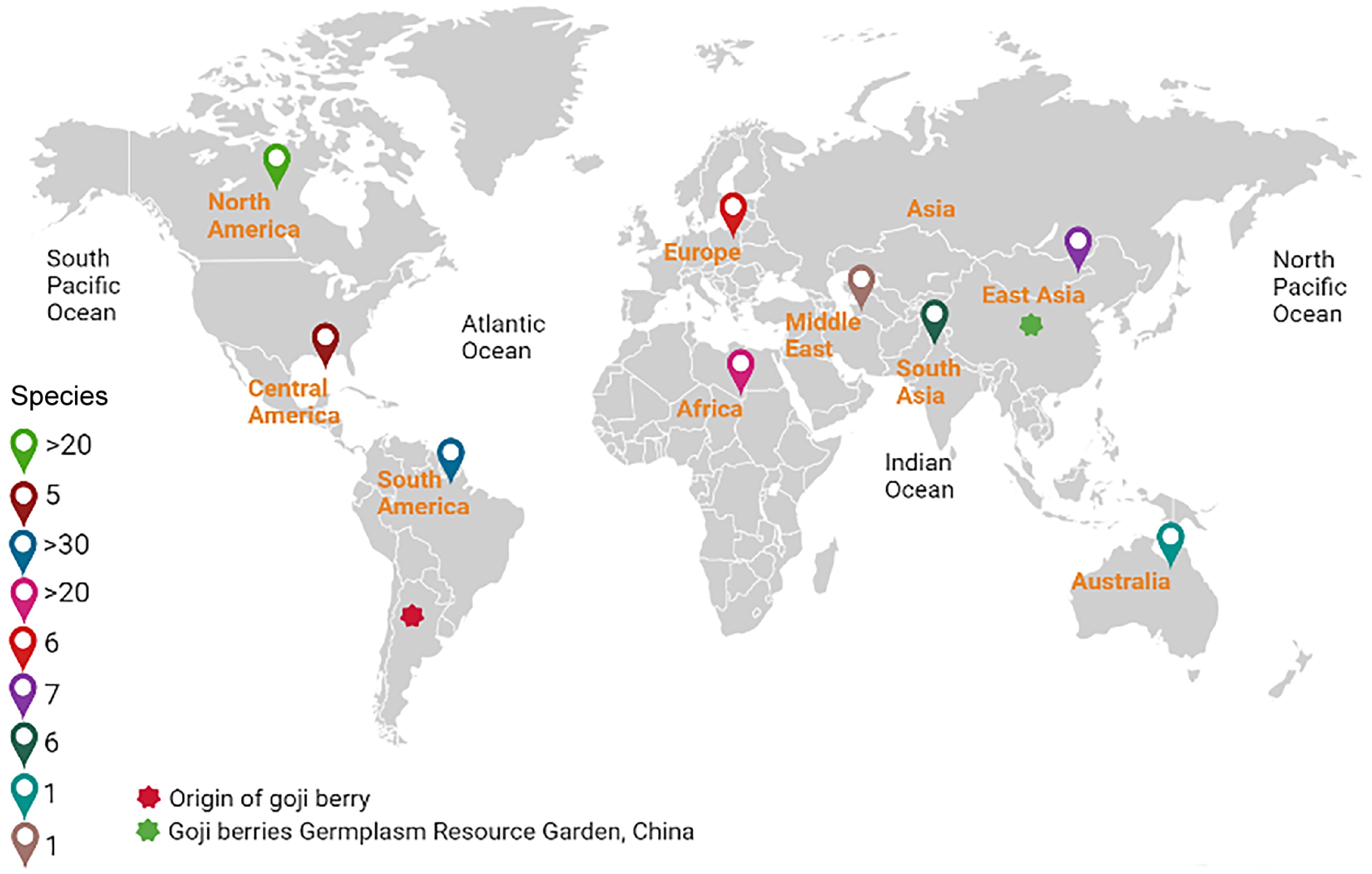
Figure 1.
The conservation and distribution of global genetic resources of goji berries. Different colored icons show number of species across all the continents. Red and green stars indicate origin of goji berries in South America and germplasm resource garden of goji berry in (Northwest Ningxia Province) China also known as the 'home of goji', respectively. North America, more than 20, Central America, five; South America, more than 30; African continents, more than 20; Europe, six; Oceania (Australia), one; Asia, 14.
The Jinghe County Goji Berry Germplasm Resources Center collected 36 goji strains, including 26 original varieties (lines). Qinghai University and Hebei Science and Technology Teachers College gathered goji germplasm resources[38,40]. A particular project of the State Forestry Administration, 'Introduction of the US wild goji berry germplasm resources, cultivation and utilization technology', hosted by the Gansu Academy of Forestry Sciences, introduced seven varieties of American wild goji from Arizona and other US locations, five of which were successfully propagated and grown[41]. However, the difficulties and success rates associated with growing American wild goji berries are related to problems with seed dormancy, variations in seed viability, and environmental stressors such as drought and vulnerability to pests and illnesses. Furthermore, the genetic makeup of wild goji may result in differences in berry fruiting, growth, and development, complicating conventional cultivation methods[41,42]. Wild L. americana Jacq. can be used as a source of goji germplasm for distant hybridization[42,43]. Notably, the primary areas of focus for goji germplasms in other countries, including the US, are biogeography, reproductive evolution, systematics, and bioorganic chemistry[41,42]. The American wild goji species (L. exsertum A. Gray, L. cooperii A. Gray, and L. brevipes Benth.), including 11 other domestically developed goji species and two wild species, can be split into two groups for further development of unique germplasm resources with distinct characteristics[44,45]. However, L. exsertum was introduced, and 19 unique plants were produced by the National Wolfberry Engineering Research Centre (NWERC) of NXAAS[41,46]. The description specification and data standard of goji germplasm resources and 'new plant varieties' specificity, consistency, and stability testing guidelines for goji berry were developed by the 'National Goji berry Genetic Resource Garden', and provided standard texts for further standardizing the description and records of goji germplasm resources, as well as guidelines for testing new plant varieties. Furthermore, a foundation for the directional breeding of new goji varieties was laid by initially developing a platform for collecting information on germplasm resources that covered more than 200 evaluation indicators and systematically completed a comprehensive evaluation of agronomic, quality, resistance, and other traits of more than 500 goji berry germplasm resources[18,39].
Global germplasm repositories of goji
-
Temperate and subtropical climates worldwide encourage the growth of goji berries. Recent statistics on goji berry germplasm conservation reported that there are more than 90 Lycium species, along with six distinct varieties recognized throughout all continents around the globe[1] (Fig. 1, Supplementary Table S1). South America, specifically Argentina and Chile, and North America had the highest concentration of species, totaling 32 and 24, respectively (Fig. 1, Supplementary Table S1). About 24 species have been found in South Africa, 12 in Europe and Asia, two in Africa and Eurasia, one in Australia (L. australe F. Muell), and one in the Pacific Islands (L. sandwicense A. Gray)[1,47]. However, L. carolinianum Walter (Moc. and Sessé ex Dunal) C.L. Hitchc is indigenous to North America and the Pacific Islands (Fig. 1, Supplementary Table S1). Based on the examination of chloroplast DNA sequences and nuclear markers and numerous phylogenetic and biogeographic studies, it was suggested that Lycium originated in Southern and Northern America[48]. Furthermore, these investigations uncovered unique clades that were geographically matched to species found in both North and South America, suggesting that the genus may have originated in these regions before spreading worldwide[48]. A common ancestor of American origin exists for all species in southern Africa, Australia, and Eurasia. L. sandwicense distinguished itself from species in South and North America, and Australian and Eurasian species once descended from a Southern African progenitor. L. barbarum, L. chinense, and L. ruthenicum, were Eurasian species, and therefore associated with L. europaeum L., according to phylogenetic analysis[1]. The Australian species L. australe and the Southern African species L. afrum L., L. cinereum Thunb., L. ferocissimum Miers, L. pilifolium C.H. Wright, L. prunus-spinosa Dunal, L. schizocalyx C.H. Wright, and L. villosum Schinz were closely related to the Eurasian species such as L. intricatum Boiss, L. berlandieri Dunal and L. pallidum Miers[1,49,50]. Species from the Pacific Islands and North or South America were grouped together[48]. About six Lycium species, including L. ruthenicum, L. makranicum Schonebeck-Temesy, L. shawii Roem. and Schult., L. dasystemum, L. edgeworthii Miers, and L. depressum stocks have been identified in the Middle East and South Asia (mainly Pakistan). These plant species are cultivated on a small scale for food and medicinal purposes and are recognized for their distinguishing characteristics[1,51] (Fig. 1, Supplementary Table S1).
Botanical description of goji
-
Plants from the Lycium genus are often thorny shrubs that grow up to 3 m tall, located in plateau areas with arid and semi-arid climates, between 700 and 2,700 m above sea level, and are referred to as 'Boxthorn or Matrimony Vine'. The term 'Boxthorn' most likely originates from the thorny nature of these shrubs; whereas, 'Matrimony Vine' refers to the cultural symbolism associated with the plants with origin in traditional Chinese culture, where goji berries are considered as a symbol of fertility, enduring relationships, and making it symbolically linked to marriage[18,19]. The root system of goji berries consists of primary, lateral, and fibrous roots. It begins with the seed radicle and develops into primary roots as it grows and matures[18]. A lateral root is considered fibrous if it consists of thin branches, whereas it is considered lateral if it has thick branches. The leaves were fleshy and narrowly linear, and the shrubs had overlapping thorny and leafy branches. Tiny flowers have a purple or blue-violet corolla and can grow alone or in clusters of up to 2 cm in length. The first body of the branch, leaf, flower, and other organs is the bud, which starts the shoot, leaves, and flowers during plant growth and development[20]. According to their developmental shape and position, goji buds are classified as normal, adventitious, leaf, or mixed[18]. Bisexual goji berries have flowers with a calyx, petals, stamens, and pistils. The goji flower consists of five stamens, yellowish oblong anthers, uneven filament length, somewhat higher or slightly lower than the stigma, and a campanulate calyx with two lobes. In addition, corolla purple-red with funnel-shaped, apex five-lobed, rarely four or six to seven lobed with elliptical shape, base with ears, superior two-chambered pistil ovary, and green filiform style[18]. Goji berries exhibit a bittersweet taste with a reddish, orange to black color. The exocarp (skin), mesocarp (pulp), and lignified endocarp around the seeds comprise the pericarp walls of the fruit. The fruit is delicate and extremely susceptible to mechanical injury because of its thin exocarp[52]. L. barbarum, L. chinense, and L. ruthenicum are the most frequently mentioned species in the literature with regard to nutritional and phytochemical composition, in vitro and in vivo biological studies, and food, cosmetic, and pharmaceutical applications[1,11,35]. Among the aforementioned species, L. ruthenicum has fruits with brownish seeds and a black-purple color. Black goji berries are perennial shrubs that are photosynthetically active and resistant to drought, cold, salinity, vigorous root tillering, low soil content, and high nutrient content. It is also useful for managing saline-alkaline land, protecting slopes, and preventing soil erosion, with a wide range of economic prospects. Black goji, also known as 'soft gold', has far greater therapeutic and physiological benefits than regular red goji berries[53,54] (Fig. 2). Red goji (L. barbarum) is 1–3 m tall with a vining growth habit. The leaf shape is narrow, oval to ovate, and goji fruit size is 2.5 cm with two or three chambers, fleshy orange to red, juicy sweet to bitter having few or up to 15 seeds per fruit[29]. Yellow-fruited goji (L. barbarum var. auranticarpum), an upright shrub with moderate vigor, thin dark-green lanceolate leaves, and a flat or inverted surface with orange to yellow color[18]. Chinese goji (L. chinense) is a densely branched shrub with single, alternating, or clustered leaves that are oblong and lanceolate. The bright red berries are 1.2 cm in size and oval in shape[29] (Fig. 2). Another variety, Chinese goji (L. chinense var. potaninii) exhibited lance-shaped leaves, sparsely ciliated corolla lobes, and vaguely auriculated basal lobes. Yunnan goji (L. yunnanense Kuang et A.M. Lu) is an erect shrub with a robust and thick trunk, narrowly ovate to lanceolate leaf blades, and berries of spherical shape with a yellowish-red color[18]. Goji dasystemum (L. dasystemum var. pojarkova), a shrub with many greyish yellow branches, lanceolate leaves, and red ovoid or oblong berries. Another cultivar of L. dasystemum var. rubricaulium goji berries differ mainly in their old brownish-red branches, and their corolla lobes are not ciliated. Goji cylindricum (L. cylindricum Kuang et al. Lu), a white or yellowish inflexible branching shrub with sessile leaf blades and a yellowish red ovoid berry. Goji truncatum (L. truncatum Y.C. Wang), a 1.5 m tall shrub with a greyish white or yellow cylindrical branch, single leaves on long shoots, and red oblong berries[18]. L. amarum has linear, lanceolate leaves, goblets with three lobes, a villous ring at the base of the filament, and an adjacent corolla tube[33]. Moreover, goji berries have an indeterminate growth habit, flowering, and continuous fruiting during the growing season and grow well in various soils[55]. Throughout the blossoming period, flowers are produced daily, and flowering can be affected or delayed by low temperatures, high humidity, or cloudy conditions. More flowering can be seen in the afternoon when the day temperature is below 18 °C, and even more in the morning when the temperature increases above 18 °C[18]. In summer, fruit berries bloom from late May to mid-July and last for the entire month of August. However, in autumn, goji fruiting begins in late September to mid-October, which is considered a less vigorous and unproductive season for goji berries[55].
-
Goji berries have been domesticated for almost 2,000 years, with its origins mostly in traditional Chinese medicine. The oldest known mentions of goji berries may be found in ancient Chinese records such as the 'Shen Nong Ben Cao Jing' (Divine Farmer's Materia Medica), which was written around 200 CE and emphasizes the virtues of the berries for longevity and energy[7,18,20]. Goji berries were widely grown throughout China during the Tang Dynasty (618–907 CE), especially in Ningxia. During the Ming and Qing dynasties, goji berries were an essential part of traditional Chinese medicine and cuisine, which led to the development of varieties suitable for various climates and purposes[7,18]. It is not known how L. barbarum was naturalized in northwest China and concentrated in Zhongning County, Ningxia. It is possible that Zhongning's geographic location is one reason why the berries of L. barbarum grown in this county had the best quality in terms of fruit size, color, texture, and sweetness. The Yinchuan plains in Ningxia were irrigated with water from the 'Yellow River' as part of one of many irrigation projects in antiquity, which further improved the circumstances for goji berry cultivation in Zhongning as well as across China[18]. Moreover, growers may deliberately remove fully ripe fruits from specific trees and then isolate or store the seeds before replanting them the following year[20]. Plants that have undergone such cautious practices of selective breeding or domestication for thousands of years may have accumulated superior morphological and physiological traits from their wild ancestors[20]. Domestication may be related to several agronomically important traits such as fruit size, berry color, fruit edibility and sweetness, seed retention and germination, and growth habits. Landraces were established as a result of domestication, whereas the existence of landraces in Zhongning was not acknowledged until the 1960s and later discovered ten other landraces along with Damaye, Xiaomaye, Heyemaye, and Baitiaogouqi[56]. 'Damaye' was found in the garden of renowned goji grower 'Zhuohan Zhang' and regarded as the most reliable landrace since it consistently yields huge, uniform fruits with little variation over time[20]. The development and processing industry of goji in China has increased with the progress of hybrid breeding, selection of individuals, chromosome manipulation, biotechnological advances, and the recent use of marker-assisted selection (MAS) studies. Each step of the goji breeding support system made a substantial contribution to expanding production standards and scale.
Fruit and vegetable-oriented cultivars of goji
-
High quality and yield, resilience to both biotic and abiotic stresses, and wide adaptability are desirable qualities of goji cultivars. Large fruits, dried fruits with a polysaccharide content of more than 3%, and carotene concentrations of more than 0.85 mg/kg are other indicators of superior quality. In 1973, NXAAS selected and crossed potential 'Damaye' progeny with fruit-oriented Ningxia cultivars, including Ningqi-1 to Ningqi-5. The clonal variety Ningqi-6 originated from a spontaneous hybrid seedling found in the goji berry germplasm bank of the Ningxia Forestry Research Center in 2003 and was produced by asexual expansion. A solitary plant with promising potential was found in the Ningqi-1 plantation in 2002, and NWERC used it to produce the clone variety Ningqi-7[18]. Ningqi-8 was first introduced by the Ningxia Forestry Research Institute (NFRI) in Xinjiang, Zhongning, and Inner Mongolia. NWERC chose and multiplied Ningqi-9 using unique individuals from L. barbarum crops in Inner Mongolia. The Zhongning Qixin Goji Berry Cooperative Station (ZQGCS) bred Ningqi-10 with Ningqi-5 (females) and Ningqi-4 (males). Damaye line, a clone that was selected and multiplied by potential individuals at the Zhongning goji berry management station[20]. Moreover, Ningnongqi-1 to Ningnongqi-5, Ningnongqi-8, Ningnongqi-10, Keqi-6081, and -6082 were bred at NWERC; whereas, Qixin-3 bearing distinct features of early fruiting, fast growth, stress resistance, self-compatibility, large fruit of even size, and good taste, was bred by ZQGCS. NWERC created a leafy vegetable-oriented variety (Ningqicai-1) that neither blooms nor bears fruit through interspecific hybridization crossing Ningqi-1 and local wild goji. This leafy cultivar is rich in nutrition and health care and accumulates 18 amino acids, crude protein, vitamins, a variety of essential minerals, and trace elements. Another triploid green vegetable variety, Ningqi-9, was developed by NFRI via hybridization and ploidy breeding. The autotetraploid cultivar exhibits a great capacity for branching, immature shoots that grow quickly, sensitive leaves, long blades, and good flavor, and is induced from Ningqi-1 and hybridized with Hebei goji during inbreeding[18,20]. Northeast, northwest, southwest, central, and southern China are heavily populated with wild goji berries. Numerous other variants have been grown and developed using selection and hybridization in several parts of China, including leafy vegetable varieties of goji developed by Hebei Normal University of Science and Technology, Baoqi-1, 2, Changxuan-1, Tiangjing-1, -3, and -8, and fruit-oriented varieties grown in Hebei Province. Three Jingqi-4, -5, and -7 goji cultivars were developed in Jinghe County between 2005 and 2013 by the Jinghe Goji Development Center and Xinjiang Academy of Forestry Sciences. Furthermore, the Qinghai Academy of Agricultural and Forestry Sciences developed Qingqi-1, -2, and Qingheiqi-1, clones of L. ruthenicum, between 2013 and 2015. Mengqi-1 (L. barbarum), a goji cultivar, was created in Inner Mongolia by the Horticultural Institute of the Inner Mongolia Academy of Agriculture and Animal Husbandry in 2005[18].
Advances in transgenic and marker assisted breeding for goji
-
Several biotechnological methods, including meristems, anthers, embryos, endosperms, and in vitro culture materials used to induce mutations, have been established to protect goji berries against biotic and abiotic stresses. For instance, a line resistant to Fusarium graminearum was created using radiation-treated embryonic calluses cultivated in vitro[57]. Similarly, salt-tolerant plants were generated by selecting ethyl methanesulfonate (EMS)-treated embryonic calluses in the presence of NaCl[58]. Moreover, genetic transformation mediated by Agrobacterium tumefaciens has been established in goji berries[23−25,59]. A successful regeneration and genetic transformation system for black goji (L. ruthenicum) was recently established, and the fruit weight loci, fw2.2, were characterized using CRISPR/Cas9 genome editing. Transgenic goji plants had nine biallelic mutations and four homozygous mutations in the fw2.2 target gene[24]. Similarly, another study successfully established a goji regeneration system and knocked out LrPDS using CRISPR/Cas9[25]. Five carotenogenic genes from red goji berries were functionally examined in transgenic tobacco (Nicotiana tabacum L.) plants as part of a study on the genetic engineering of specific genes. The results showed that all transgenic tobacco plants constitutively expressed these genes, and the amount of beta-carotene in their leaves and flowers increased[60]. These findings suggest that these genes may be employed to boost β-carotene production in red goji berries. Goji has already been subjected to molecular breeding and marker-assisted selection (MAS). Traditional breeding strategies are hampered by intensive labor, cost, and time inefficiency, which impede goji berry cultivar improvement[26]. With the rapid advancement of next-generation sequencing (NGS), high-resolution genetic mapping and single nucleotide polymorphism (SNP) markers have provided affordable tools for QTL mapping and MAS[27]. A genetic map based on the F1 population provides a reliable source for identifying the linkage between economic traits and DNA markers in perennial fruit crops[61,62]. Recently, several significantly important genetic loci, including stable QTLs corresponding to fruit quality and size/weight-related traits, have been identified in goji berries using ultra-dense linkage mapping[29,63].
Highly heterozygous mapping populations were successfully developed by interspecific crosses, and F1 individuals were determined using a pseudo-test cross approach, which is widely applicable to perennial trees and forest herbs[64]. Therefore, the first high-density genetic map of L. barbarum was based on an intraspecific F1 population using ddRAD-seq[28] and a goji berry SNP-based genetic map using SLAF-seq based on interspecific F1 populations[28,29]. Next-generation sequencing (NGS)-based resequencing genetic analysis was performed to construct an ultra-dense high-resolution genetic map of goji berries using 200 potential individuals derived from the F1 population (L. barbarum var. Ningqi-1 × L. yunnanense var. Yunnan goji). A high-density linkage map was established with a total genetic length of 2,122.24 cM along with average inter-marker distance of 0.25 cM and 8,507 SNPs. Furthermore, QTL mapping analysis detected 25 stable QTLs in different linkage groups corresponding to agronomic traits, with maximum LOD values of up to 19.37 and PVE percentages of 51.9%. Eighty-two differentially expressed genes (DEGs) underlying these stable QTLs were identified using RNA sequencing analysis[30]. A recent report on goji berry genome establishment provides strong evidence for the evolution, diversification, and distribution patterns of goji berries worldwide. This study analyzed the genomes of L. barbarum and other 12 perennial indigenous species and discovered new gene families with their expansion and contraction within Solanaceae. Moreover, assimilation with other genomes provides strong evidence in favor of a whole-genome triplication-WGT event that occurred soon after the split between Solanaceae and Convolvulaceae and is shared by all previously sequenced solanaceous plants[65]. Together, these collective findings could provide a solid basis for the genetic breeding of goji berries to further improve domestication and crop improvement, corresponding to genetic architecture and yield enhancement.
-
Goji berries are becoming more popular due to their significant medicinal and nutraceutical value. It has been learned through literature goji berries native to South and North America became naturalized in northwest China, where domestication led to the emergence of a significant number of potential landraces. It is necessary to investigate the genetic potential of other Lycium species, notably those from the New World and Africa, with a stronger emphasis on fruit characteristics and important chemical compounds, such as zeaxanthin and antioxidants[18]. As predicted, new cultivars with more desirable features, including leaf-vegetable-oriented cultivars of goji berries, could be generated for industrial production using other species[66]. The canvas of major breeding goals should be expanded to disease and pest resistance, valuable chemical constituents, leaf-related traits, early flowering, plant architecture, and geographical adaptability, instead of fruit- and yield-related traits. However, by focusing on particular genes linked to pest or disease resistance and genes of interest, both conventional and modern breeding techniques such as hybridization, marker-assisted breeding, and CRISPR/Cas9 gene editing tools offer precise ways to enhance resistance corresponding to the desired traits[18,67]. Research must concentrate on the long fruit, thick flesh, hard skin, sweet and excellent flavors, and other characteristics of the fresh-fruit goji berry industry. Owing to the scarcity of resources, the genetic composition of cross-pollinated perennial goji berry plants is extremely complex[18]. Currently, the most commercialized varieties of Chinese goji berries are the Ningxia and black-fruited varieties, with Ningxia goji berry cultivars being the most extensively grown. These varieties lack genetic diversity and have a single genetic base, making it difficult to produce improved goji berry variants. The genetic backgrounds of many resources are unclear, and there are few comprehensive studies on the genetic mechanisms underlying the biological characteristics of these resources. These limitations hinder the development and utilization of specific resources and breeding of new goji berry varieties. Nonetheless, it is imperative to enhance the recognition and evaluation of goji berry germplasm resources and broaden theoretical research on the genetics of goji berries[20,39]. Resources from the germplasm of the goji berry plant serve as both the raw material for germplasm production and the source of genes required for further genetic improvement. To reduce the detrimental effects on local goji berry germplasm resources, building projects, such as new and renovated farmland, water conservation, and transportation, the distribution region, especially the existing sample stations, should be dynamically monitored. Increasing public understanding of science, lessening the severity of naturally scattered population picking, and maintaining the population's relative stability[39]. The extent of resource collection is limited to the national level, and vast categories of domestic wolfberry germplasm resources have been acquired in great quantities. The introduction and collection of foreign wolfberry resources have occurred relatively infrequently. Ex situ preservation is the main technique used to preserve resources, whereas in situ preservation and seed preservation techniques are practically non-existent. Resource availability and quality boost resource banks create comprehensive and extensive resource archives, identify and analyze morphology and molecular biology, and define taxonomic status and genetic history. Develop a comprehensive germplasm resource nursery to lay the groundwork for the preservation, study, advancement, and use of germplasm resources. The multigeneration asexual reproduction of clones of elite varieties such as Ningqi-1 and Ningqi-7 degrades the outstanding qualities of the improved varieties and reduces yield; thus, the improved varieties must be immediately rejuvenated and purified[18]. To satisfy the demands of the ongoing development of traditional Chinese medicine for human health, new varieties of L. barbarum will be generated with high yields, excellent quality, climate resilience, and adaptation to processing or mechanized operations. Fundamental studies on the collection and preservation of goji berry resources, classification and identification, resource chemistry, and resource physiology are limited in resource-based research owing to a lack of professional knowledge. Currently, in vitro preservation is the primary method for resource preservation. More resource collection necessitates the use of in vivo methods and seed preservation, both of which require specific facilities[18,39].
In light of this, the collection of domestic and exotic goji germplasm resources should be strengthened, particularly through international cooperation, which can expand goji berry resources and significantly accelerate their innovation and utilization. Establishing protection points or protected areas in resource distribution areas, strictly forbidding illegal and excessive digging of wild goji berry plants, encouraging artificial domestication and seedling breeding of wild goji berries, and creating regulations for the protection of wild goji berries are some of the key aspects that can be employed[39]. Ex situ protection should be employed for goji berries that are threatened by extinction or whose habitats have been destroyed. This can be achieved by seed propagation and digging live plants in the field. Goji berry resource innovation and use should be accelerated and expanded by strengthening the identification methods and evaluation of resources using distant hybridization to produce new germplasms, collecting and processing data based on the characteristics of resources to create databases, and establishing a shared technology platform for a variety of resource uses[39,41]. The genetic material required to investigate genetic traits should be created using high-purity inbred lines, hybridization, and backcross offspring. Additionally, understanding the biological aspects of goji berry reproduction is essential, as plants primarily pass on genetic information to the next generation through reproduction[20,56]. The goji leaf, sometimes referred to as 'Tianjing grass', is a prominent medicinal plant, useful vegetable, and great tea, encouraging the development of goji leaves for use as a vegetable and medication[66]. L. chinense, a wild type goji berry with bigger, oblong leaves, might be regarded as a vegetable species of the Lycium genus, as well as Ningqicai-1 and Ningqi-9 leaf vegetable-oriented varieties of goji berries successfully cultivated in Ningxia province[18]. Interestingly, significant changes in leaf size were observed among the F1 individuals of interspecific populations, particularly in terms of leaf length, diameter, and area, which could be important crossing materials for generating vegetable-oriented cultivars of goji berries[26,29].
Genetic advancement of goji breeding
-
Although traditional methods of breeding goji berries are, to some extent, effective, there is a need to advance the use of marker-assisted breeding (MAB) for goji berries to incorporate effective production and development under genetic breeding. Transgenic breeding involves genetic alterations in crop plants. On the other hand, transgenic breeding offers a rapid method for combining genes from various genetic backgrounds[59,68]. The breeding of elite cultivars, as well as the cultivation and preservation of new genetic resources, could thus be facilitated by research on various attributes using MAB. Abundant worldwide resources of Lycium can offer a wide range of germplasm resources for the cultivation and breeding of goji berries[1]. Crossing distinctive plants yields offspring with considerable genetic variants, such as varying fruit colors, flower terminal shoots, and larger fruits with multi-locule formation, compared to typical small goji berries bearing one or two locule numbers, which forms the basis of marker-assisted breeding. The production of new germplasms with advanced breeding objectives may be facilitated by these genetic modifications[68]. Studies on the genetic resources of goji berries have lagged behind those of other Solanaceae crops, such as tomato, potato, and pepper, in terms of systematic and in-depth examination of genomic analysis and a varied range of traits[56]. Genomic regions or quantitative trait loci (QTLs) identified through genotyping and phenotyping offer a wealth of DNA polymorphisms and raise the possibility of selecting elite cultivars coupled with a particular trait of interest[69]. The fruit size of goji berries differs greatly; for example, the fruit of L. barbarum is approximately 2 cm in diameter, with two to three locules in the indigenous cultivars, which is slightly larger than the fruits of L. chinense and L. ruthenicum. Remarkably, substantial variations were observed in the locule number of the Ningqi series cultivars (Ningqi-1, -5), Zhongkeluchuan-1 (ZKLC1), and offspring of the interspecific hybrid F1 population, with an average locule number of 2.564[29] (Fig. 3).

Figure 3.
Red goji berries (L. barbarum var. ZKLC1 and Ningqi-1, 5), black goji berries (L. ruthenicum), and locule number variations in different cultivars and wild germplasms, scale bars = 1 cm.
It is assumed that larger fruit size cultivars with greater locule numbers in the goji berry industry will be a major breakthrough in expanding cultivation and production. Several major QTL/genes are responsible for fruit size variations in tomatoes, including LOCULE NUMBER (LC), FASCIATED (FAS), and CLAVATA3 (CLV3), which increase the locule number, fruit diameter, and eventually fruit size[67,70,71]. Topless/Topless-related (TPL/TPR) proteins are involved in a number of signalling pathways in higher plants, including biotic stress, meristem maintenance, floral induction, hormone-signaling pathways, and circadian oscillator mechanisms[72]. SlTPL1 and SlTPL4 were found to be highly expressed in vegetative tissues (roots, stems, and leaves) as well as in developing flowers (buds and anthesis), but with reduced expression in ripening fruits. In contrast, SlTPL3 expression is constant and high during fruit ripening[72]. SlTPL3 and SlWUS control locule development in tomatoes and SlTPL3 RNAi lines have shown that an increase in carpel number is the primary driver of multiple locule formation[73]. Likewise, mechanical harvesting is challenging because of the indeterminate growth habit of goji berries, with the simultaneous existence of ripe, immature fruits and blooms on the same branch[74]. Goji berry harvesting in China now makes extensive use of labor, which leads to labor-intensive and costly picking. New cultivars with determinate growth habits and synchronous fruiting are needed to overcome the difficulties of mechanical harvesting of goji berries[75]. The tomato self-pruning (SP) homologue gene of centroradialis (CEN) from Antirrhinum, which has determinate growth characteristics, has been found in separate studies[76,77]. Several studies have employed CRISPR/Cas9 gene editing to disrupt the SP gene in tomato and other solanaceous crops, leading to a determinate growth pattern with terminal flowers at the top of the branch[78−80]. Interestingly, significant numbers of flower terminal and non-terminal plants have been collected for transcriptomic data analysis to identify candidate genes for the regulation of terminal and early flowering, and efforts are already underway to knock out SP-like genes to generate determinate growth habits with terminal-flowering shoots under efficient genetic improvement of goji berries (unpublished). Nevertheless, the direct breeding of compatible red goji berry variations, functional characterization of potential genes, construction of genetic maps, and development of molecular markers all contribute to an insightful theoretical and practical foundation.
-
The ultimate goal of resource studies is to hasten variety breeding and enhance the quality and quantity of goji berries. It is becoming increasingly crucial to protect the genetic diversity of goji berries owing to their rising global demand. This diversity must be preserved, and effective conservation efforts combining in situ and ex situ methods are essential to lay the groundwork for subsequent breeding initiatives. Recently, it has been witnessed that goji breeding has made enormous strides in China, the development of breeding technology has advanced significantly. Goji berry has an abundance of resources, and through germplasm innovation and conventional methods like distant hybridization, new germplasms are developed; in addition, the genetic basis for breeding is expanded under next generation sequencing approaches, and the breakthrough in goji breeding is achieved. The value of germplasm resources is becoming increasingly evident in light of the rapid advancement of science and technology, as well as various challenges. The primary objective of agricultural development is to expedite the collection, development, and usage of genetic resources while simultaneously offering technical assistance for speeding up rural rehabilitation. The genomic studies including goji berry domestication and genetic breeding would help in comprehending the potential of goji berry's extensive genetic background and enhancing the current goji berry cultivars including disease resistance, fruit quality and acclimatization using CRISPR-based gene editing system. By adopting these strategies, goji berry cultivation may become more resilient and sustainable, ensuring a steady output and protecting the genetic resources necessary for future breeding endeavors. However, breeders and legislators are required to bridge the gap between traditional methods of resource conservation and state-of-the-art breeding techniques, interdisciplinary support, and the proper utilization of goji berry genetic potential for sustainable agriculture and food security.
-
The authors confirm contribution to the paper as follows: conceptualization, visualization, writing - review & editing: Rehman F, Wang Y, Huang H, Zeng S, Yang C; writing - manuscript preparation: Rehman F; goji germplasm resources data: Zhao J, Zhao Y, Qin K, Rehman F, Wang Y; investigation and formal analysis: Wang Y, Huang H, Rehman F, Zeng S, Yang C; supervision, funding acquisition, project administration: Wang Y. All authors have reviewed the final version and agreed for the submission.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This research was supported by the National Natural Science Foundation of China (Grant No. 32170389), Guangdong Science and Technology Plan Project (Grant No. 2023B1212060046), and the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDA24030502).
-
The authors declare that they have no conflict of interest.
- Supplementary Table S1 Goji berries germplasm resources of all the continents.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Rehman F, Zeng S, Zhao Y, Zhao J, Qin K, et al. 2024. Preservation and innovation of goji berry germplasm resources. Medicinal Plant Biology 3: e022 doi: 10.48130/mpb-0024-0022
Preservation and innovation of goji berry germplasm resources
- Received: 27 July 2024
- Revised: 26 August 2024
- Accepted: 10 September 2024
- Published online: 18 October 2024
Abstract: Goji is a significant edible small-fruited shrub and has been a top-grade Chinese medicinal material since ancient times. In comparison to other crop species, very little information is available on goji as an important medicinal plant in terms of genetic resource conservation and development and germplasm improvement strategies. This comprehensive study illustrates the goji species-rich diversity, geographical classification, and domestic and international germplasm repositories, including their distribution and botanical features, all of which highlight the significance of the genetic architecture of this woody tree plant and provide a thorough understanding of the global distribution of goji berry germplasms, particularly in China, which can further facilitate germplasm collection for goji breeding. We also shed light on how goji berry germplasm has been improved by utilizing conventional and modern cutting-edge technologies, future research directions on the preservation and sustainable use of germplasm resources, and advances in genomic breeding strategies. This review will facilitate the development of novel goji germplasm repositories and support the strategic development of genetic resource management by prioritizing the acquisition of underutilized goji berries and advancing ex situ conservation through the application of contemporary gene bank techniques. Critical investigations have revealed gaps that need to be filled by breeders and researchers, such as the identification of wild germplasm and comprehensive genomic approaches, which could provide a basis for rapid breeding and fully utilize the genetic potential of goji berries to improve agronomic and economic traits.
-
Key words:
- Goji berry /
- Germplasm resources /
- Global repositories /
- TCM /
- In situ /
- Ex situ














