-
Industrial progress is crucial for global economic development. However, industrial evolution has created a serious problem of pollution worldwide. Almost every industry contributes to environmental pollution. Organic pollutants like dyes and pesticides that originate from the effluents of various industries like textiles, printing, paper, cosmetics, etc. have emerged as the most serious environmental problems during the last decade[1−4]. Water pollution is one of the four major types of pollution, alongside air pollution, noise pollution, and radiation pollution. The poor quality of water is one of the environmental and socioeconomic challenges that contribute to deteriorating human capital and poor health outcomes[4−6]. Wastewater contaminated with these pollutants have been treated with various physical techniques (for example adsorption, osmosis, etc), and chemical techniques (for example chemical oxidation and photocatalysis). The adsorption of pollutants on various adsorbents is a common technique for the treatment of contaminated water, but it is not effective in the complete removal of the pollutants[7,8]. Alternately, photocatalysis, which is an eco-friendly, simple, and promising technique, is a more attractive technique for the breakdown of toxic organic substances in an aqueous medium. The breakdown of toxic molecules using semiconductor materials as photocatalysts under the irradiation of sunlight is the most acceptable protocol[9,10]. The photocatalytic breakdown of toxic organic molecules is a type of advanced oxidation process (AOP) that consists of a complete breakdown of organic molecules by oxidation with hydroxyl radicals (•OH)[11,12].
ZnO and TiO2 are the two most widely studied semiconductors in photocatalysis due to their exceptional catalytic activities, photo sensitivities, low cost, and physical, chemical, and catalytic stabilities[13−15]. ZnO has higher catalytic properties than TiO2 due to its unique characteristics such as wide energy difference in VB and CB (3.37 eV, the band gap), significant binding energy of exciton (60 meV), and significant mobility of electrons (300 cm2·V−1·s−1).[16,17] However, high band gap energy and fast recombination of excitons suppress the visible-light-assisted catalytic applications of ZnO. Hence, the visible light cannot be harvested by ZnO. Since visible light is naturally abundant (sunlight consists of about 40% visible light), therefore a photocatalyst capable of harvesting visible light for practical applications is needed. Thus, various strategies such as the development of composites and doping of metal or non-metal atoms are adopted to enhance the photocatalytic performance of ZnO under visible light[18−21]. The fabrication of composite material by a combination of carbon-based substances, like graphene oxide (GO), with ZnO is an effective strategy for developing visible-light-driven photocatalysts with an extended photo response and reduced exciton recombination. Graphene oxide is a thin honeycomb-like, two-dimensional carbon-based substance, in which the hybridization form of C is sp2. Graphene oxide is characterized by outstanding electrical and mechanical properties. Being an outstanding electron acceptor and conducting material, graphene oxide effectively transfers the electrons generated by the absorption of photons and ultimately suppresses the recombination of excitons[22−24]. Due to its two-dimensional planar structure and greater specific surface area, graphene oxide supports various substances to become attached to it. As a result, the pollutant molecules readily adsorb on the surface of the composite photocatalyst[25,26]. Hence, the composite formed by the hybridization of graphene oxide with ZnO is expected to be a photocatalyst with extraordinary catalytic properties for the breakdown of toxic organic molecules due to the unique optical and electrical properties of graphene oxide. This study reports ZnO-graphene oxide (ZnO-GO) as an efficient photocatalyst for the degradation of organic dyes under natural sunlight. The Sandalfix orange P3R and Sandalfix Turq. blue PG dyes were used as model dye pollutants. Both dyes are reactive in nature. Reactive dyes are the dyes that form covalent bonds by reaction with fibers. These dyes are highly water-soluble and are commonly used for dyeing polyamide, protein, and cellulose fibers. These dyes are widely used in the textile industry. Consequently, Sandalfix orange P3R and Sandalfix Turq. blue PG were selected as representative reactive dyes for this study.
-
Graphite powder (Hebei Yunai New Material Technology Co., Ltd, China), sodium hydroxide (Merck, Germany), hydrogen peroxide (Scharlab, Spain), sodium nitrate (Merck, Germany), sulfuric acid, potassium permanganate, polyvinyl alcohol, and zinc acetate (Scharlau, Germany) were used as precursor materials.
Synthesis and characterization of ZnO-GO
-
In the first step, GO was synthesized as follows. First, 4 g graphite and 2 g sodium nitrate were added to sulfuric acid (100 mL). The suspension was kept under stirring for 30 min. Then, 14 g potassium permanganate was mixed in, and the mixture was kept under stirring for 5 h. As a result, a thick paste was formed. Then a yellowish suspension was formed by the addition of distilled water (100 mL) to a paste. The reaction was terminated by adding 40 mL of hydrogen peroxide, after which graphene oxide (GO) was collected.
In the second step, ZnO was synthesized by the precipitation method. Typically, the pH of a solution of zinc acetate was raised to 10 by dropwise addition of 0.1 M sodium hydroxide solution. The precipitate of zinc hydroxide formed was washed to remove the remaining reactants and followed by drying at 100 °C for 12 h. Then, it was calcined at 500 °C for 3 h to obtain ZnO.
In the third step, ZnO-GO was synthesized. For this purpose, 1 g as prepared GO and ZnO were suspended in 20 mL distilled water in separate beakers. Both suspensions were ultrasonicated and then mixed. Seventy mg polyvinyl alcohol was also added in mixed suspension and ultrasonicated again. The fabricated ZnO-GO was then filtered, washed, and dried.
The as-prepared ZnO-GO was characterized by advanced instrumental techniques including XRD, DR-UV-vis spectroscopy, surface area measurement, FTIR, and TEM.
Photocatalytic activity
-
Photocatalysis of ZnO-GO was explored by performing photodegradation of the selected dyes. A UV–visible spectrophotometer was utilized to estimate the catalytic properties of ZnO-GO. A total of 0.1g ZnO-GO was suspended in a 50 mL solution containing a known amount of either or both dyes in a beaker. The solution was then swirled for half an hour under the dark magnetically. After that, a reaction mixture sample was separated for analysis by a UV-visible spectrophotometer. Then, the suspension was irradiation with sunlight while stirring continuously. Reaction mixture samples were separated and analyzed after a time interval.
-
The impregnation of ZnO nanomaterials on the surface of a GO nanosheet is due to the interactions between ZnO and hydroxyl functional groups on GO. Furthermore, there is a delocalization of π electrons in GO resulting in a delocalized π bond. The interactions of unpaired π electrons and Zn2+ result in Zn-O-C linkages. Hence, ZnO-GO is formed due to the affinity of GO towards Zn2+ producing active sites for the attachment of ZnO on GO. All the above-mentioned factors also play a crucial role in the generation and separation of charge carriers[27,28].
Characterization of ZnO-GO
-
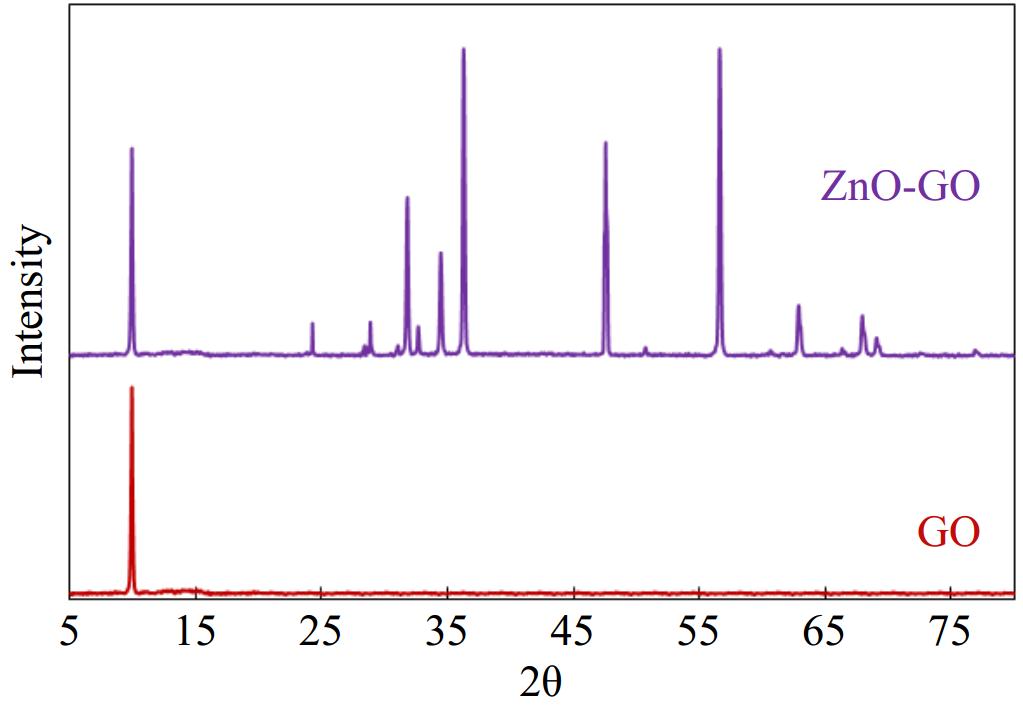
The phase purity and crystallinity of a sample can be estimated by XRD analysis. Therefore, we performed the XRD analysis of the as-prepared ZnO-GO sample. The results of XRD analyses are given in Fig. 1. It can be observed that XRD patterns show sharp and well-resolved peaks which confirm the crystallinity of the prepared material. A peak was observed in XRD of GO at 10.4° which is a characteristic peak of GO. This peak has been indexed to the (002) plane of GO[29,30]. Similarly, several sharp peaks can be noticed in the XRD pattern of ZnO-GO which confirms the crystalline nature of the prepared sample. These peaks include peaks at 2 theta 10.4°, 31.6°, 34.2°, 36.2°, 47.4°, 56.4°, 62.5°, and 67.9°. The peaks at 2 theta 10.4° have been indexed to the (002) plane of GO. The remaining peaks observed in XRD of ZnO-GO have been assigned to (002), (100), (101), (102), (103), (110), and (200) planes of ZnO, respectively[31−35].
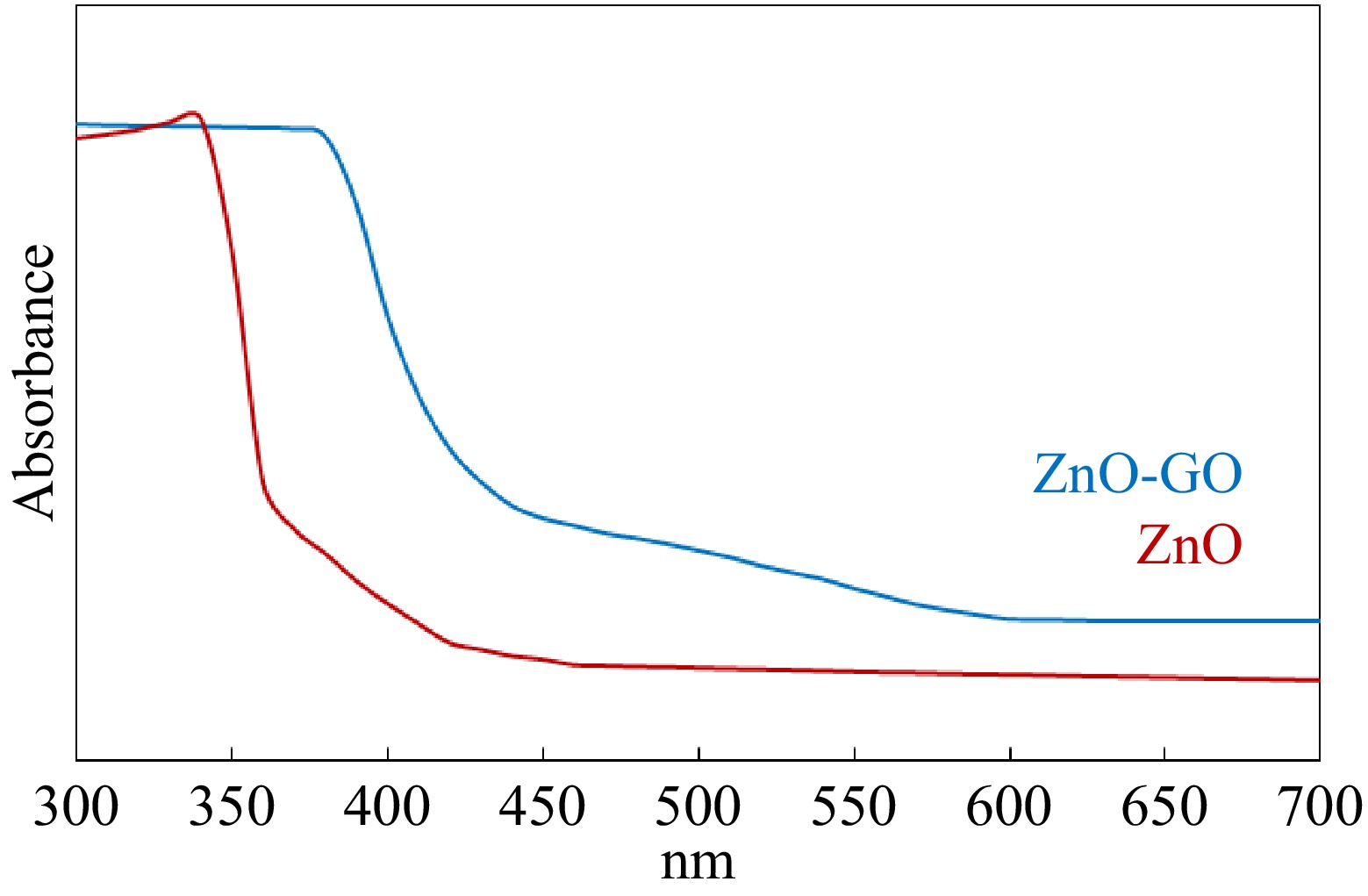
The band gap of ZnO-GO was investigated by DR-UV-visible spectroscopy. Figure 2 shows the UV-visible spectra of ZnO and ZnO-GO. It can be noticed that the formation of ZnO-GO shifts the absorption edge towards the longer wavelength side. Using Eqn (1), the band gap energy of ZnO and ZnO-GO was calculated as 3.37 and 2.91 eV, respectively. The wavelength in nm for ZnO and ZnO-GO was determined using the concept of extrapolation of the sharply rising portion and horizontal portion of the curves[36−38]. The wavelength of intersection was determined as 368 and 426 nm for ZnO and ZnO-GO, respectively. As the bad gap energy of ZnO-GO (2.91 eV) corresponds to the visible region of the radiation spectrum, therefore it can be used as a visible-light-driven photocatalyst for environmental applications. The excitation of ZnO-GO by absorption of visible light is due to the dye-like photosensitizer behavior of GO in ZnO-GO[23,39,40]. Hence, we used ZnO-GO as a photocatalyst for the photodegradation of dyes in this study.
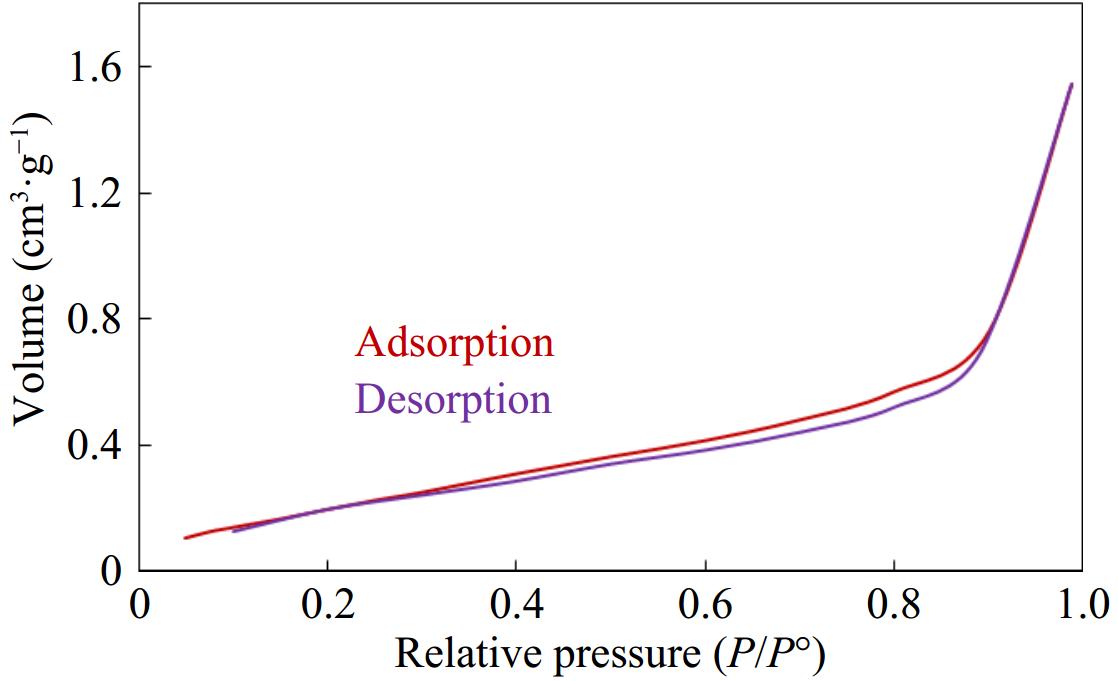
BandgapEnergy(eV)=1240nm (1) The surface area was determined by studying the adsorption-desorption of nitrogen gas on ZnO-GO. Figure 3 shows the behavior of adsorption and desorption of nitrogen on ZnO-GO. The adsorption-desorption isotherm observed as given in Fig. 3 is a type IV isotherm according to the IUPAC classification of isotherms. The hysteresis loop formed by adsorption-desorption of nitrogen is classified as H3 type. The observed type of isotherm and hysteresis loop shows the mesoporous and non-homogeneous nature of the ZnO-GO. The BET surface area was measured to be 56.5 m²·g−1 for ZnO-GO, which is approximately three times greater than pure ZnO (17.4 m²·g−1)[41−43].
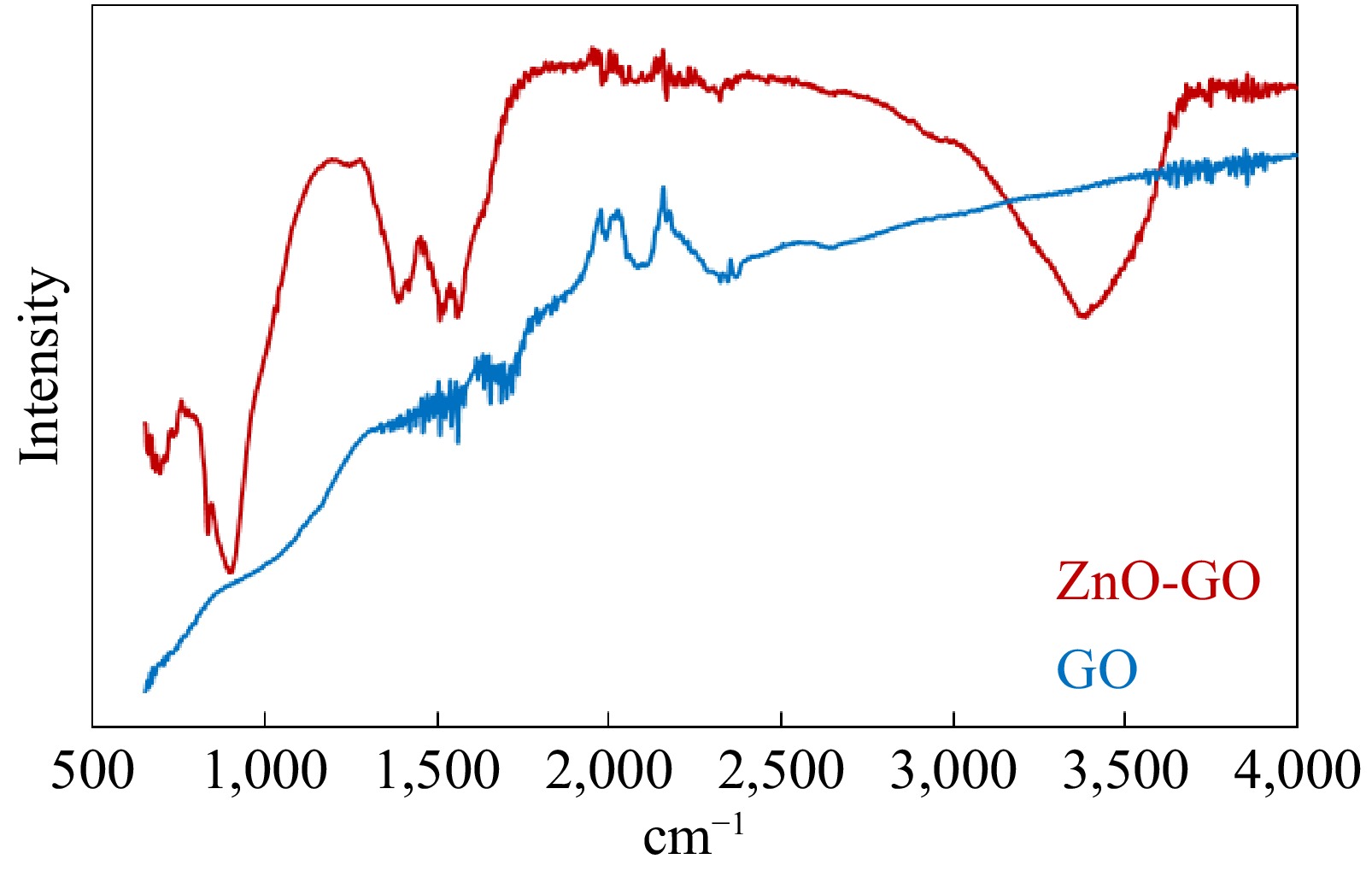
The functional groups associated with GO and ZnO-GO were estimated by FTIR analyses of the samples. Figure 4 depicts the results of FTIR analyses. The absorption bands at 555, 670, and 774 cm−1 observed in the FTIR spectrum of ZnO-GO are representative peaks of Zn-O linkage. The bands at ~1,055 and 1,723 cm−1 represent the C-O-C and C=O linkages. Similarly, the peaks at ~1,400 and ~1,600 cm−1 are associated with O-H or C-OH and hydroxyl groups of H2O, respectively[41,44−47]. Zhou et al.[48] have assigned the bands at 1,078 and 3,432 cm−1 to the bending and stretching vibrations of the hydroxyl group.
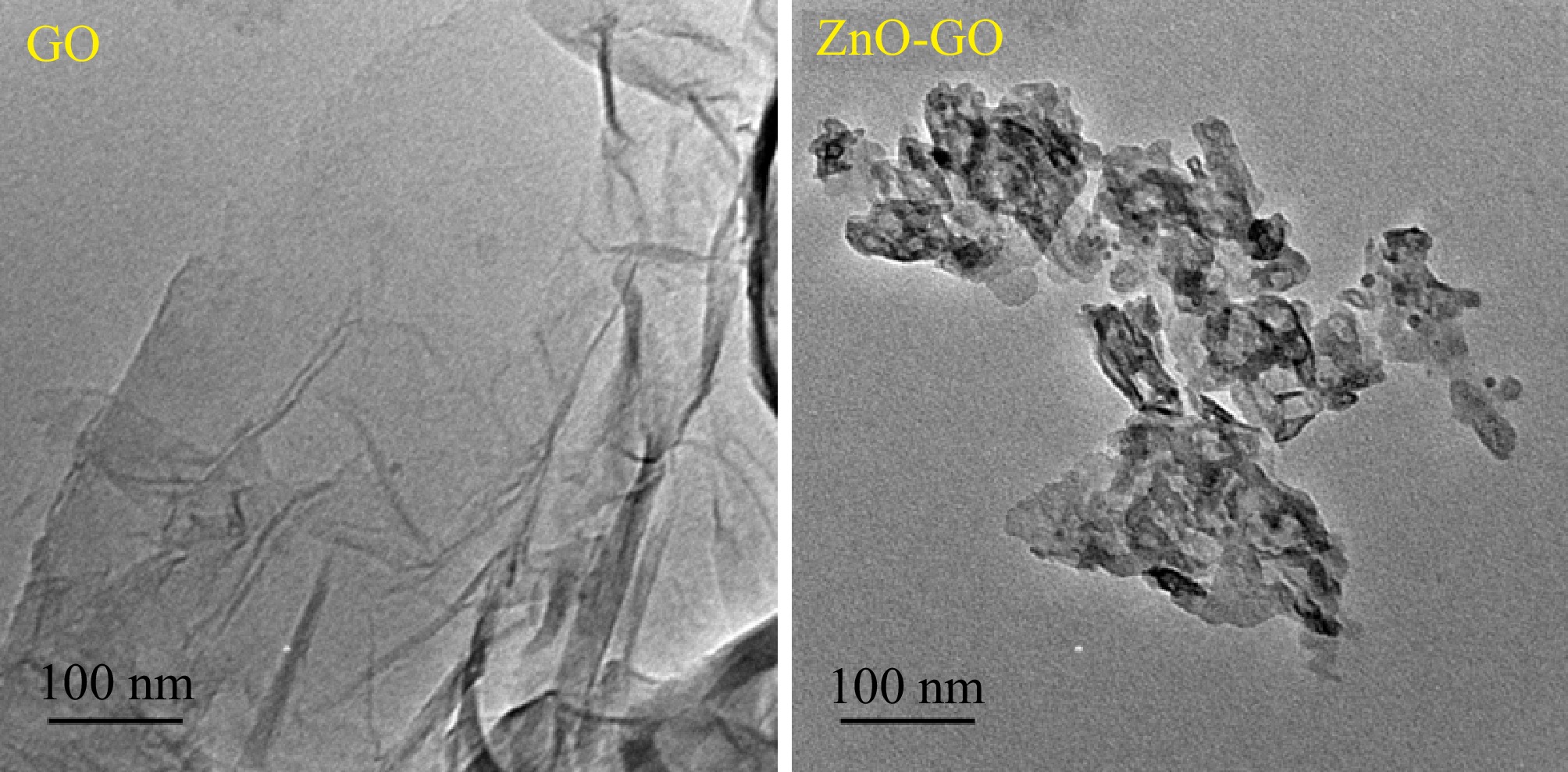
The shape and morphology of the GO and ZnO-GO were analyzed by TEM analysis. For this purpose, the JEM 2100 JEOL transmission electron microscope was used. Figure 5 shows the obtained results. Considering the TEM of GO given in Fig. 5, it is concluded that the prepared GO is characterized by a significant level of translucency. The TEM indicates that the morphology and shape of the prepared GO is crumpled, and flat-flake-like in nature. The TEM indicates that GO exists in the form of thin sheets. It confirms the excellent exfoliation during the fabrication of GO. Furthermore, the wrinkles and darker regions observed in TEM of GO confirm the stacking and folding of the thin sheets. The TEM of ZnO-GO (Fig. 5) reveals the homogeneous and independent distribution of ZnO on thin sheets of GO. The deposition of the ZnO particles on the sheets of GO is evident by the dark regions in the micrograph of ZnO-GO[49−52].
Photocatalytic activity
-
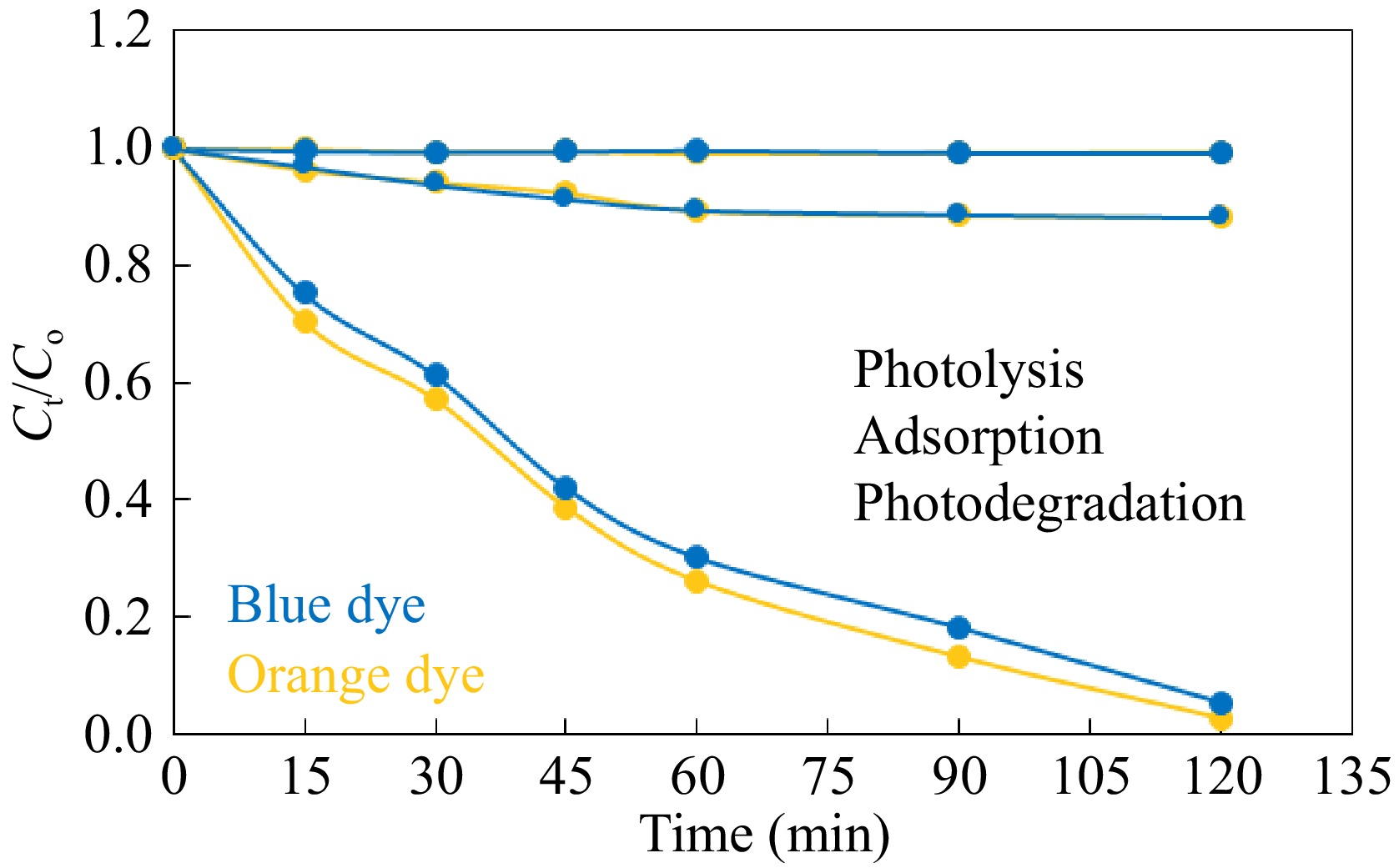
Sandalfix orange P3R and/or Sandalfix Turq. blue PG dye were utilized as representative pollutants for the study of the catalytic activity of developed photocatalysts. Detailed information about these dyes is given in Table 1. A 50 mL solution of Sandal-fix orange P3R and/or Sandalfix Turq. blue PG dye (100 mg·L−1) was used as model wastewater for the study of catalytic activity. As degradation of dye due to photolysis may interfere with the photocatalytic efficiency of ZnO-GO, therefore the contribution of photocatalysis in degradation of Sandal-fix orange P3R and Sandalfix Turq. blue PG dye was explored in the first step. It was accomplished by stirring the dye solution under sunlight irradiation for 2 h. The analysis of samples taken at different time intervals did not show any change in concentration solutions (Fig. 6). It confirmed that there is no degradation of Sandal-fix orange P3R and Sandalfix Turq. blue PG dye due to photolysis in this study. In addition, the contribution of sorption in the removal of dye was also explored. It was carried out by stirring the dye solution containing 0.1 g ZnO-GO under dark conditions. A ~10% decrease in the concentration of Sandal-fix orange P3R and Sandal-fix Turq. blue PG dye was observed due to adsorption on the surface of the ZnO-GO (Fig. 6). Then, the catalytic eradication of dyes in the presence of a ZnO-GO catalyst was investigated. This investigation was accomplished by stirring the dye solution containing 0.1 g ZnO-GO under natural sunlight irradiation. The extent of photodegradation was followed by regular measurement of the absorbance of dye solution at respective wavelengths of maximum absorbance. Since the absorbance is proportional to concentration, therefore the removal of dyes was expressed as a plot of time vs Ct/Co as given in Fig. 6. More than 95% of each dye (100 mg·L−1, 50 mL) was degraded during 120 min of reaction duration. Hence, it is confirmed that ZnO-GO can be used as an active catalyst for the sunlight-assisted photodegradation of Sandal-fix orange P3R and Sandal-fix Turq. blue PG dyes.
Table 1. Detailed information about the selected dyes.
Name Sandalfix orange P3R Sandalfix Turq. blue PG Color Orange Blue Class Reactive Reactive CI number Reactive orange 12 Reactive blue 21 λmax 485 nm 414 nm Molecular weight 793 g·mol−1 1,079.6 g·mol−1 Molecular formula C21H14ClN8Na3O10S3 C40H25CuN9O14S5 Molecular structure 


Figure 6.
Treatment of the selected dyes with ZnO-GO. Reaction conditions: 50 mL solution having concentration 100 mg·L−1 0.1 g ZnO-GO were stirred at 40 °C.
The recyclability of ZnO-GO in photodegradation of Sandalfix orange P3R and Sandalfix Turq. blue PG dyes were also investigated. The spent ZnO-GO used in photodegradation was collected and washed using ethanol and water. After drying, the spent ZnO-GO was re-used under similar experimental conditions. More than 83% of 100 mg·L−1 (50 mL) of each dye was degraded during 120 min of reaction duration. Hence, the fabricated ZnO-GO is a stable and active catalyst for the photodegradation of dyes.
Comparison of photocatalytic activity
-
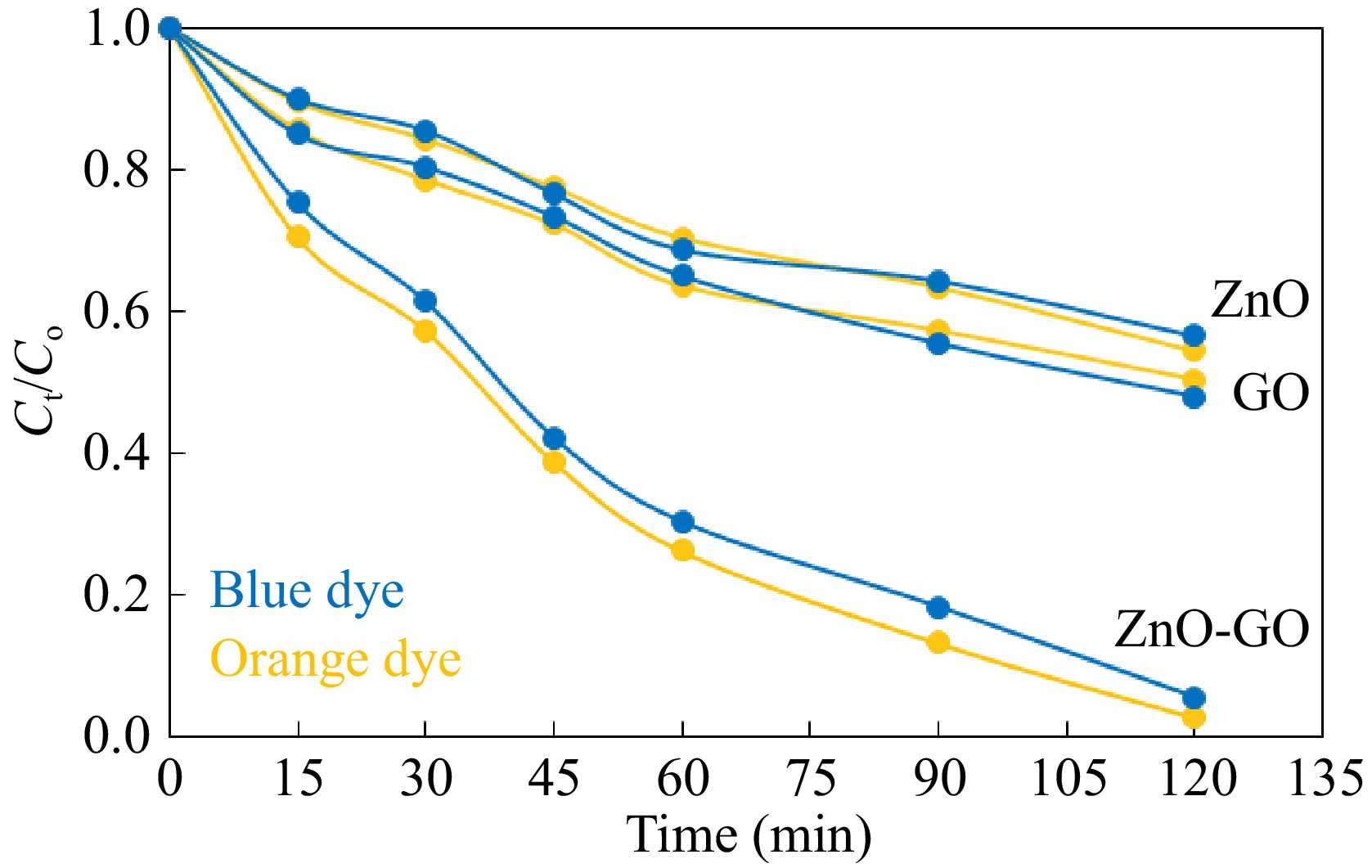
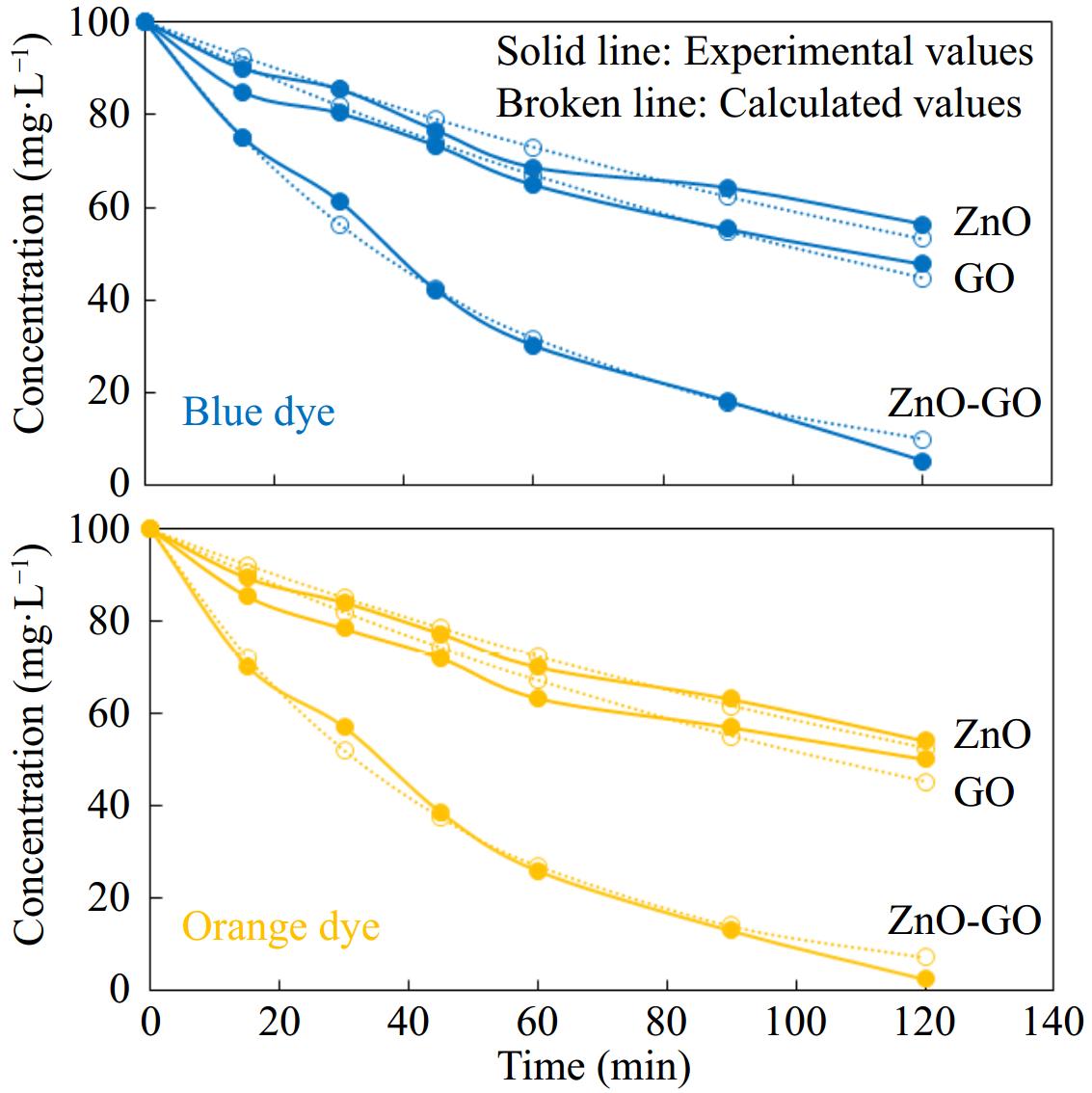
The effect of the formation of composite between ZnO and GO on the photodegradation of dyes was also explored. This study was conducted through photodegradation experiments using ZnO, GO, and ZnO-GO as separate components. The ZnO-GO composite showed much higher catalytic performance in the photodegradation of Sandal-fix orange P3R and Sandal-fix Turq. blue PG dyes than pristine ZnO and GO. The comparison of the catalytic performance of GO, ZnO, and ZnO-GO is given in Fig. 7. The catalytic activity of ZnO-GO was 2.2 and 1.9 folds than the activity of ZnO and GO, respectively. The higher catalytic activity of ZnO-GO is attributed to the efficient transfer of the photo-induced electrons through graphene sheets.

Figure 7.
Treatment of the selected dyes with ZnO, GO, and ZnO-GO photocatalyst. Reaction conditions: 50 mL solution having concentration 100 mg·L−1 0.1 g each catalyst in separate experiments were stirred at 40 °C.
Mechanism and kinetics of photocatalytic degradation
-
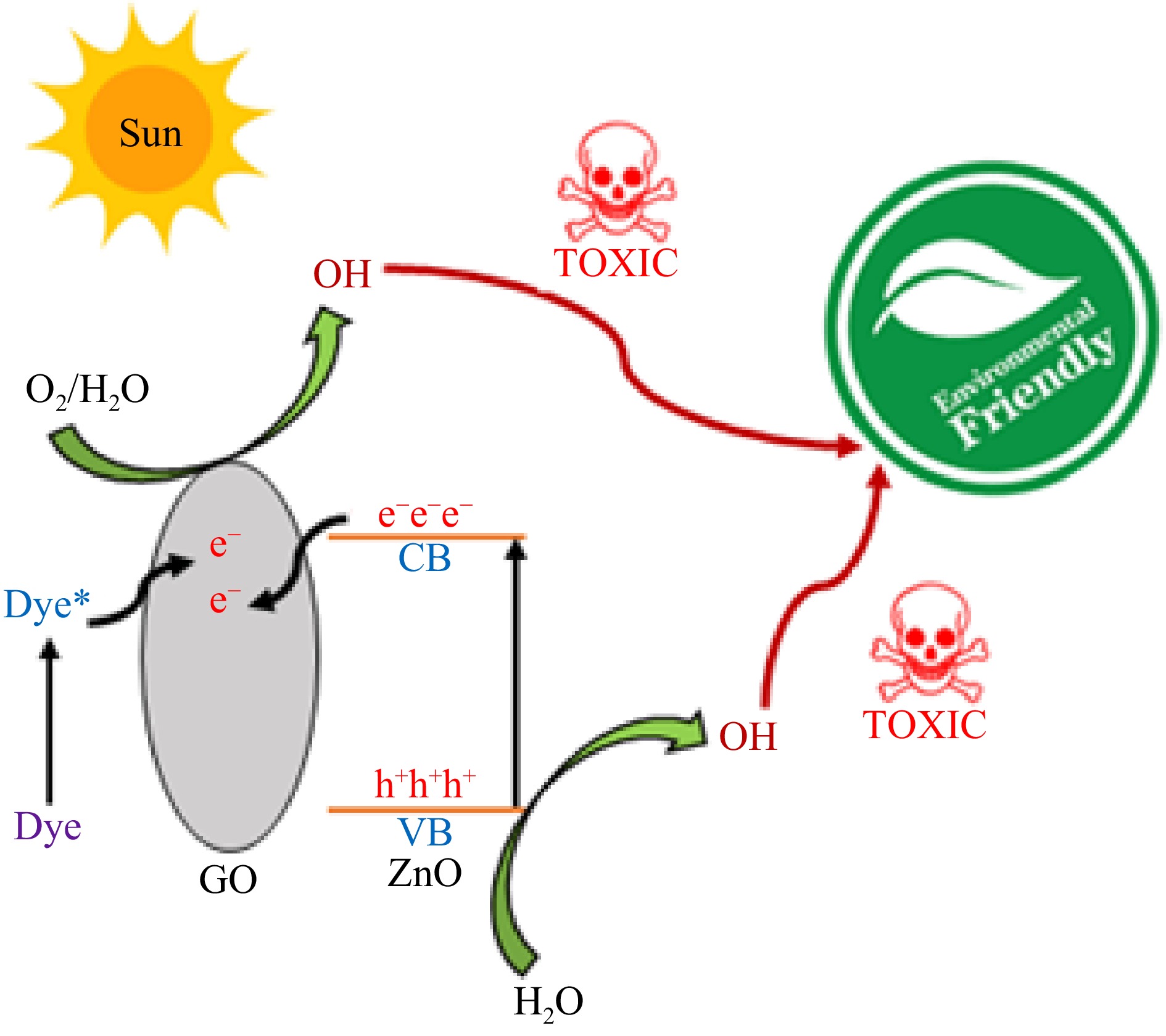
Figure 8 illustrates the proposed mechanism of dye photodegradation in the presence of ZnO-GO. The catalytic efficiency of a photocatalyst depends on the contact between the photocatalyst and dye molecules, the rate of formation of electron-hole pairs, and the separation of electrons and holes. Graphene oxide (GO) has many functional groups including a π-π conjugation system. This π-π conjugation facilitates the efficient contact between dye molecules and ZnO-GO. The irradiation of dye solution containing a photocatalyst causes the excitation of ZnO-GO and dye molecules as well by absorption of photons. The excitation of the photocatalyst creates positive holes and electrons in the valence band and conduction band of ZnO-GO, respectively. Due to electron-electron-accepting nature of GO, the photo-induced electrons flow to the sheet of GO. As a result of the separation of electrons and positive holes, their rate of recombination is reduced. In other words, the recombination of photo-induced charges takes place through GO because its work function (−4.42 eV) is in between the VB and CB of ZnO (−7.25 and −4.05 eV, respectively). Hence, the separation of electrons due to their flow to the GO sheet causes an improvement in the catalytic performance. Similarly, the electrons of excited dye molecules also follow the GO path. Once separated, the positive holes and electrons initiate complex redox reactions and finally generate OH radicals. The OH radicals attack dye molecules and degrade them into environmentally friendly products[53−55]. This whole process can be described in the following reactions (ZG: ZnO-GO; DY: Dye molecules).
The role played by OH radicals was verified by a scavenging experiment using benzoquinone as a scavenger for hydroxyl radicals. A 15% decrease was observed in catalytic performance in the presence of benzoquinone. It shows that OH radicals are involved in the degradation of dyes.
ZG+hv→h+(ZG)+e−(ZG) h+(ZG)+H2O→→OH⋅ e−(ZG)+(O2)ads→→OH⋅ DY+OH⋅→Degradationproducts The proposed mechanism shows that the rate of reaction (r) depends on three factors: 1. Concentration of dye; 2. Adsorbed oxygen; 3. Energy of photon. Hence, we can describe the rate as:
r=kr[DY](O2)adsEPhoton (2) As the reaction is taking place in an open atmosphere and under continuous irradiation, therefore the rate of reaction becomes independent of the last two factors. Hence, we can write as:
r=kr[DY] (3) r=−d[DY]dt=kr[DY] (4) On integration and rearrangement ([DY]o, [DY]t, and kr represent the initial concentration of dyes, concentration at different times, and rate constant, respectively[56].
[DY]t=[DY]oe−krt (5) The above rate expression was used to analyze the degradation data by the non-linear method. Figure 9 shows the fitting of the kinetics model to the experimental data. The rate constants determined are listed in Table 2 which indicate that the rate of reaction over ZnO-GO is 3.3 and 4.1 times higher than the rate of GO and ZnO, respectively.

Figure 9.
Kinetics of photocatalytic degradation of the selected dyes. Reaction conditions: 50 mL (100 mg·L−1) dye solution was treated with 0.1 g catalyst at 40 °C.
Table 2. Rate constants for photocatalytic degradation of the selected dyes.
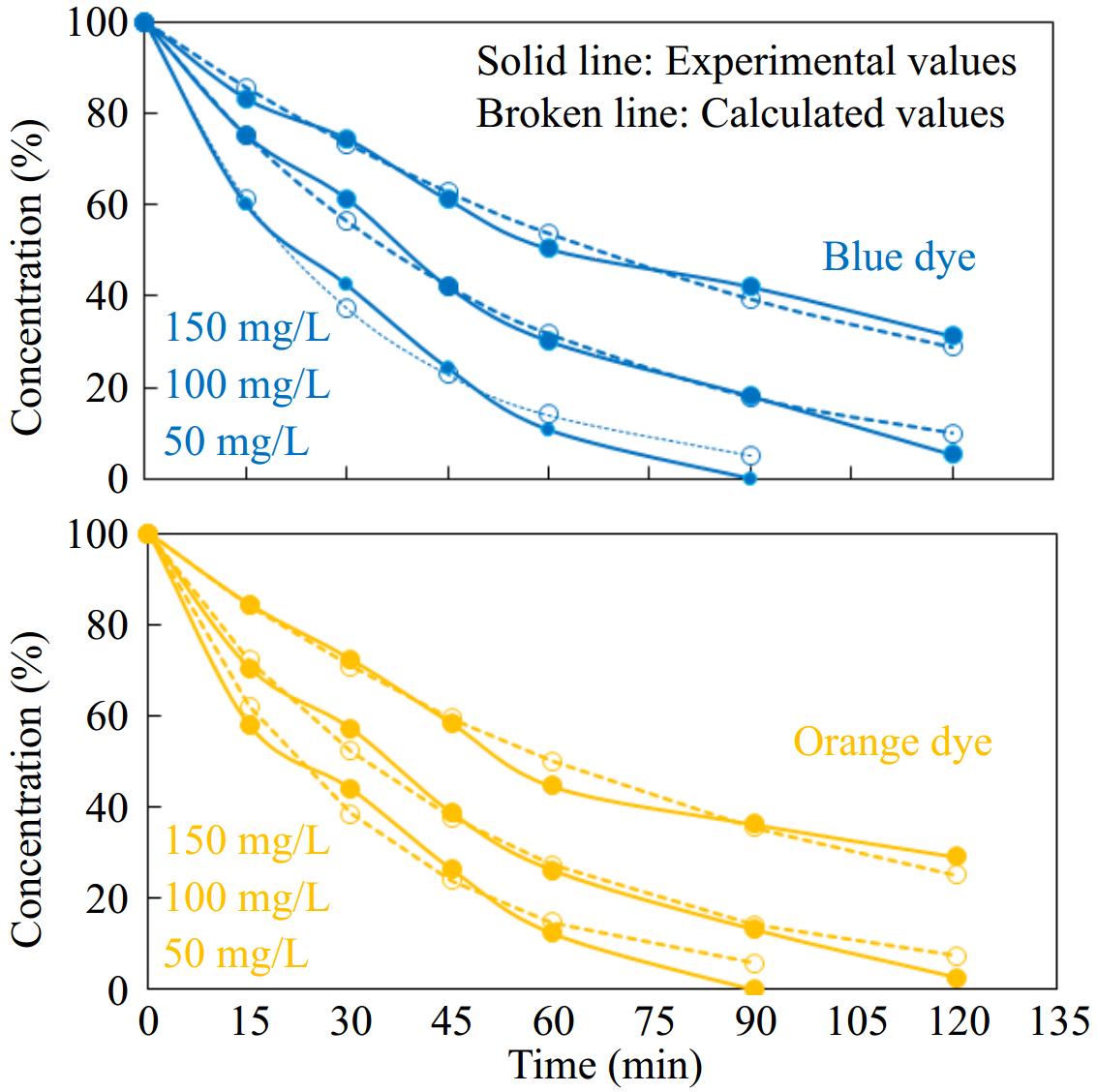
Photocatalyst Sandalfix orange P3R Sandalfix turq. blue PG kr (per min) R2 kr (per min) R2 ZnO 0.0054 0.99 0.0053 0.99 GO 0.0066 0.98 0.0067 0.98 ZnO-GO 0.0217 0.99 0.0191 0.99 The kinetics rate expression given in Eqns (2) and (5) shows that the rate of reaction depends on the concentration of the reactant. Therefore, the effect of the initial concentration of selected dyes on photocatalytic performance was also analyzed. For this purpose, separate photodegradation experiments in the presence of the ZnO-GO catalyst were performed using 50, 100, and 150 mg·L−1 as initial concentrations of each dye. The photocatalytic performance was monitored by measurement of absorbance of the reaction mixture at different time intervals. The obtained photodegradation data was analyzed by a non-linear method of analysis according to the kinetics rate expression given in Eqn (5). The obtained results are given in Fig. 10. The rate constants determined are listed in Table 3. The kinetics expression given in Eqn (3) shows that the rate of reaction is directly proportional to the concentration of the reactant, however, the reverse trend was observed in this study. The data given in Fig. 10 and Table 3 show that the rate of photocatalytic performance decreased with an increase in concentration of Sandalfix orange P3R and Sandalfix Turq. blue PG dyes. The main reason for the decrease in photocatalytic performance is the inhibition of photons to the surface of the catalyst at higher concentrations because the photons are absorbed by dye molecules at higher concentrations. Hence higher concentration of dyes results in blockage of the penetration of sunlight into the surface of the catalyst[57,58].

Figure 10.
Effect of concentration on catalytic activity of ZnO-GO in photodegradation of the selected dyes. Reaction conditions: 50 mL dye solution was treated with 0.1 g ZnO-GO at 40 °C.
Table 3. Rate constants for photocatalytic degradation of the selected dyes with different initial concentrations.
Concentration (mg·L−1) Sandalfix orange P3R Sandalfix Turq. blue PG *Activity (%) kr (per min) *Activity (%) kr (per min) 50 88 0.0319 89 0.0326 100 74 0.0217 70 0.0191 150 55 0.0115 50 0.0103 * Photodegradation after 60 min. -
The ZnO-GO composite was successfully synthesized and characterized using various techniques, including XRD, DR-UV-vis spectroscopy, surface area measurement, FTIR, and TEM. The synthesized ZnO-GO demonstrated exceptional performance in the photodegradation of Sandalfix orange P3R and Sandalfix Turq. blue PG dyes under natural sunlight. The degradation data were analyzed using a first-order kinetic model through a non-linear approach with Solver-Excel software. The rate constants for dye photodegradation using ZnO-GO were 3.3 and 4.1 times higher than those obtained with GO and ZnO, respectively. The superior catalytic performance of ZnO-GO was attributed to the efficient transfer of photoinduced electrons facilitated by the graphene sheets. Additionally, the photocatalytic efficiency was found to be inversely proportional to the initial concentration of the dyes.
The Travel Grant (Ref No. HEC/R&D/TG/212886, Date 23-08-2024) provided by the Higher Education Commission (HEC) of Pakistan for presenting this paper at the 1st International Conference on Civil and Environmental Engineering for Resilient, Smart and Sustainable Solutions organized at the College of Engineering, Prince Mohammad Bin Fahd University, AL-Khobar, Saudi Arabia during November 3-5, 2024, is acknowledged.
-
The authors confirm contribution to the paper as follows: conceptualization, data curation, methodology, project administration, writing - original draft; Saeed M; supervision: Saeed M, Haq A, Akram N; validation: Saeed M, Haq A; formal analysis: Mubarik I, Laksaci H; investigation: Mubarik I, Akram N; resources: Laksaci H, Ashraf MK; writing - review & editing: Saeed M, Ashraf MK. All authors reviewed the results and approved the final version of the manuscript.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Saeed M, Mubarik I, Haq A, Akram N, Laksaci H, et al. 2025. Development of ZnO-graphene oxide composite for enhanced photodegradation of Sandalfix orange P3R and Sandalfix Turq. blue PG dyes under irradiation of sunlight. Progress in Reaction Kinetics and Mechanism 50: e004 doi: 10.48130/prkm-0025-0004











 DownLoad:
DownLoad: