-
Unlike the unicellular gametes produced directly by sporophytic tissues in animals, angiosperms generate multicellular gametophytes that give rise to gametes. In the anther, microspore mother cells undergo meiosis followed by mitosis to form pollen grains, which are the male gametophytes comprising two sperm cells (the male gametes), and one vegetative cell. The female gametophyte, or embryo sac, in most angiosperms contains two synergid cells, two gamete cells (the egg cell and the central cell), and three antipodal cells[1]. Unlike the motile sperm of other land plants and animals, sperm cells in angiosperms are immobile and rely on siphonogamy, a process in which the pollen tube transports the two sperm cells into the embryo sac[2]. Once released, one sperm cell fuses with the egg cell to form the zygote, which develops into the embryo, while the other fuses with the central cell to create the triploid endosperm, a process known as double fertilization[3,4]. Following fertilization, the zygote undergoes genome activation, polarity establishment, cell division, and differentiation, which leads to embryo development and patterning of the seedling’s body plan[5].
The fusion of the sperm cell with the egg cell or central cell integrates genetic information from both parents, combining parental genomes and gene expression products deposited in the cytoplasm. In the offspring, genes that are specifically expressed in sperm or egg cells/central cells are referred to as gamete-delivered parent-of-origin genes[6]. These genes can be classified into two categories: imprinted genes and those whose transcripts are delivered by gametes during fertilization[7]. Parent-of-origin genes are important in the gamete quality and parental influences on the development of embryo and endosperm[8]. However, our understanding of how parent-of-origin genes regulate the development of the offspring remains limited. It is known that paternal factors play a key role in early zygote activation and division. For example, in Arabidopsis, the interleukin-1 receptor-associated kinase (IRAK)/Pelle-like kinase gene SHORT SUSPENSOR (SSP) is transcribed in sperm cells and translated in the zygote to activate a MAPK cascade, which determines the cell fate of the basal daughter cell[9,10]. In rice, BABY BOOM1 (OsBBM1) transcripts are delivered from sperm cells to the egg cell to initiate zygotic transcription of OsBBM1, which is essential for early embryogenesis[11]. Additionally, paternal genes also control the endosperm proliferation[12,13].
Recently, a study by Cheng et al. reported that two plant sperm-delivered genes regulate the cell differentiation of the hypophysis, the upper-most cell of the suspensor in Arabidopsis, which is the root founder cell and the founder cell of the root stem cell niche [14]. By analyzing the transczriptomes of sperm cells, egg cells, zygotes, and embryos at different developmental stages, the authors identified two key parent-of-origin transcription factor genes, Transcriptional Repressor of EIN3-dependent Ethylene-response 1 (TREE1) and DUO1-Activated Zinc finger 3 (DAZ3). The fusion proteins TREE1-GFP and DAZ3-GFP, driven by their respective endogenous promoters, first appeared in sperm cells at the early tricellular pollen stage and increased in mature pollen and pollen tubes, while remained undetectable in female gametes. After fertilization, TREE1-GFP and DAZ3-GFP were observed in the zygote and rapidly diminished, disappearing completely by the octant embryo stage — a typical behavior of gamete-delivered paternal genes. Reciprocal crosses between wild type and TREE1-GFP/DAZ3-GFP transgenic lines revealed that GFP signals were present in early embryos only when TREE1-GFP or DAZ3-GFP plants were used as the pollen donor. The tree1 daz3 mutant showed normal pollen development, fertilization, embryogenesis, and cell differentiation of the apical and basal cells of the two-celled embryo, while the cell division pattern of the hypophysis was altered at the early globular stage. Reciprocal crosses showed that this defect appeared only in hybrid embryos where tree1 daz3 was used as the male parent. Ectopic expression of TREE1 and DAZ3 in the central cell of the tree1 daz3 mutant failed to rescue this hypophysis defect. These lines of evidence suggest a novel mechanism through which gamete-delivered parent-of-origin genes influence embryonic organ formation.
Considering the much lower proportion (about 1.5%) of abnormal hypophysis cell division in tree1 daz3 mutants, and the previous reports that damaged hypophysis partially regenerates through the jasmonate (JA) signaling[15], the authors applied the JA biosynthesis inhibitor DIECA to in vitro cultured tree1 daz3 zygotes. This treatment led to an increase in the proportion of abnormal hypophysis cell divisions in tree1 daz3 to about 15%. Additionally, when using the JA biosynthesis and signaling mutants, opr3 (opda reductase 3) and coi1-2 (coronatine insensitive 1), as female parents crossed with tree1 daz3 as the male parent, the proportion of abnormal hypophysis cell division significantly increased. These results suggest that a JA-mediated hypophysis cell recovery system is active in tree1 daz3 mutant ovules, accounting for the low proportion of abnormal hypophysis in the mutant. In tree1 daz3 embryos, the abnormal division planes in the hypophysis, resulting in altered morphology of the derived sister cells. Further observations using WOX5 and DR5 markers demonstrated that the hypophysis cell fate and the root stem cell niche establishment were altered in tree1 daz3, which was associated with abnormal auxin distribution. These findings suggest that TREE1 and DAZ3 regulate hypophysis specification and proper root stem cell niche establishment by modulating cell division orientation and auxin response[14].
Moreover, the authors identified RKD2 (RWP-RK domain-containing transcription factor 2) as a downstream gene regulated by TREE1 and DAZ3. RKD2 is a transcription factor involved in the embryo sac development and maintenance of egg cell fate[16]. RKD2 is exclusively expressed in the egg cell but rapidly declines after fertilization due to direct transcriptional suppression by the binding of TREE1 and DAZ3 proteins to its promoter sequence. Failure to repress RKD2 expression in the zygote caused irregular cell divisions, altered cell fate in hypophysis cells, and abnormal suspensor divisions, resulting in the formation of embryo-like structures and other detrimental effects. These findings suggest that after being transmitted into the egg cell, TREE1 and DAZ3 inhibit RKD2 expression to prevent its harmful effects on the subsequent embryo development[14].
Finally, the researchers found that the loss of function of TREE1 and DAZ3 significantly reduces the plant's capacity of root regeneration, indicating an active role of these genes in root stem cell regeneration. Ectopic expression of TREE1 and DAZ3 in the embryo failed to rescue the defective stem cell niche development or restore root stem cell niche regeneration in the tree1 daz3 mutant. However, ectopic expression of TREE1 and DAZ3 in the egg cell successfully rescued the embryonic defect[14], highlighting the critical role of sperm-derived TREE1 and DAZ3 in the zygote. In conclusion, the study by Cheng et al.[14] uncovered a novel mechanism by which plant parent-of-origin genes regulate post-fertilization embryonic development and organ formation . These genes are introduced into the egg cell after fertilization, where they suppress the expression of egg-expressed genes, which is detrimental to the following embryo development and organ formation (Fig. 1).
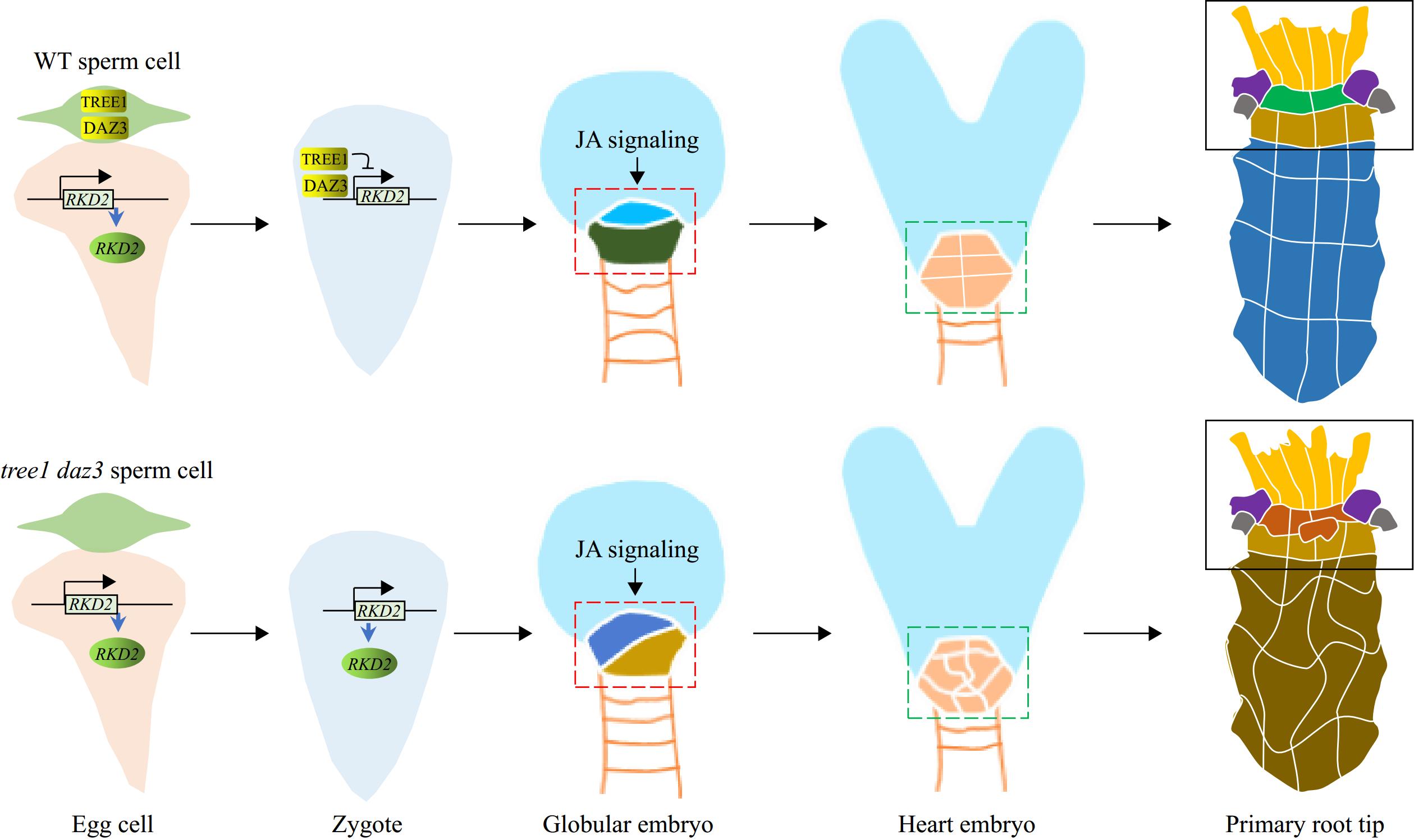
Figure 1.
A diagrammatic working model of the TREE1/DAZ3-controlled development of embryonic hypothesis and root meristem. The sperm-cell-delivered TREE1 and DAZ3 proteins bind to the promoter region of RKD2, a transcription factor expressed in the egg cell, to suppress its transcription during subsequent embryonic development. This suppression ensures proper cell division and differentiation in the root lineage from the early stages of hypophysis formation. During this process, jasmonate (JA) signaling genetically works in concert with TREE1 and DAZ3 to maintain the correct development of the root lineage. In the tree1 daz3 double mutant, however, the continued expression of RKD2 disrupts this process. Although JA signaling remains intact and can partially compensate for the loss of TREE1 and DAZ3, it is insufficient to fully counteract the harmful effects caused by the loss of these proteins. The region outlined with dashed lines represents the root lineage.
While it has long been known that numerous parent-of-origin genes exist in plants, their biological functions and significance have remained largely elusive. This study provides a compelling example, demonstrating that interactions between parent-of-origin genes are crucial for shaping the development of specific organs in offspring. Moreover, it highlights an intricate interplay between male and female parent-of-origin genes, showing that their interactions include both gene activation and suppression. This research offers new insights into fertilization and may inspire further investigation into the role of parent-of-origin genes. Future research into the regulatory mechanisms of gamete quality and parental effects on organ formation is expected to become a new hotspot in the field of plant developmental biology. Investigating these congenital regulatory mechanisms will help deepen our understanding of the molecular processes underlying plant development and crop hybrid breeding.
HTML
-
The authors confirm contribution to the paper as follows: draft manuscript preparation: Chen SY, Li HJ. Both authors reviewed the results and approved the final version of the manuscript.
-
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
This work was supported by the National Natural Science Foundation of China (32425009, 32170343), CAS Project for Young Scientists in Basic Research (Grant No. YSBR-078), and the National Key Research and Development Program of China (2022YFF1003500).
-
The authors declare that they have no conflict of interest. Hong-Ju Li is the Editorial Board member of Seed Biology who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Hainan Yazhou Bay Seed Laboratory. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Chen SY, Li HJ. 2024. Paternal protection of offspring organogenesis. Seed Biology 3:e016 doi: 10.48130/seedbio-0024-0015 |













