-
Edible mushrooms are a rich source of nutrition as well as income generation for many poor communities. The mushroom industry is quickly gaining the attention of many entrepreneurs, particularly young ones. Due to concerns about health issues, mushrooms are in high demand among the general public, particularly in developed countries, as a medicinal product as well as a health food worldwide[1]. Grey oyster mushroom (Pleurotus sajor caju) is a popular mushroom in the edible group of mushrooms. The cultivation of grey oyster mushrooms requires high humidity of 95%−100% with temperatures between 28 to 30 °C , no exposure to sunlight and a good substrate material of lignocellulose agro-biomass[2]. Oil palm plants are a good source of lignocellulose plants. On the other hand, palm oil-producing countries including Malaysia, Indonesia, Africa, Papua New Guinea, and America face problems due to the vast amount of the plant residue management, especially for oil palm fronds[3]. Based on the economic conditions of using oil palm ago-residues such as oil palm frond and empty fruit bunch to convert mushroom substrates, will especially benefit the rural farmers. The global mushroom market is showing that the demand is projected to rise from 15.25 million tonnes in 2021 to 24.05 million tonnes in 2028 at a CAGR of 6.74% in the forecast period. Hence, the future economic value of mushrooms is predicted to be very high. However, to fulfil the demand of the market, growers need different types of good quality mushroom substrate rather than depending on a single substrate. In Malaysia, the grey oyster is one of the most popular edible mushrooms, mainly cultivated using rubber sawdust substrate. Therefore, mushroom growers often face problems with material supply due to the high demand for rubber sawdust. Besides mushroom cultivation, sawdust is also used for poultry farming; therefore, using oil palm-based residue substrates can be introduced in two factors as waste management and also for a newly formulated substrate.
Mushrooms are a highly perishable vegetable crop. Mushrooms structurally do not consist of cuticles, which influences the high moisture content, and only 10% is fiber content. According to Thakur[4], numerous phytochemicals, enzymes, primary metabolites, and secondary mycometabolites cause sudden degradation, short shelf life, and high postharvest losses (30%–35%). In addition, fresh mushrooms have a short shelf life, which is within 1 to 8 d which will reduce their economic value[4]. After harvest, mushrooms undergo a series of quality degradations, including moisture loss, discolouration, texture changes, off-flavour, and nutrient loss[5]. The moisture content of fresh mushrooms ranges from 85% to 95%[6].
As a result of their high moisture content, mushrooms should be preserved at low temperatures to prevent microbial infection. Mushrooms gradually lose moisture during the postharvest period, which causes ongoing weight reduction. Due to water loss and enzyme activity, postharvest mushrooms' colour shows a browning tendency, impacting customer purchase decisions[5]. Thus, the growers of mushrooms, especially in rural areas, often face postharvest losses. Therefore, our study also focused on easy product conversion from mushrooms.
-
Empty fruit bunch, palm pressed fiber, and oil palm fronds were freely collected from the palm oil plantation at Felda Kemahang, Tanah Merah, Kelantan, Malaysia. Rice straw was purchased from a paddy farmer in the Tumpat, Kelantan area after the harvesting season, and sawdust was purchased from the soil oil mill at Jeli, Kelantan.
Preparation of substrates
-
The substrates, such as the empty fruit bunches (EFP), were shredded into small pieces, and the rice straw was cut into small pieces using a cutter machine. The oil palm fronds were cut into an appropriate size due to the hardwood used and ground using a grinder machine at ATP. After cutting, the substrates such as EFB, PPF, and RS were soaked overnight to reduce any excess water. On the next day, all the substrates were rinsed with clean water and put on the newspaper or plastics to dry for a few days under sunlight. All the dried materials were again ground using a grinding machine to obtain a smooth size (1.00 mm) for ease of mycelia penetration during mycelium colonization.
Preparation of substrate in each polyethylene filling bag
-
All the composition substrates including calcium carbonate, and rice bran as an additional supplement for mycelia growth, were mixed with a mixing machine and took around half an hour to mix well as shown in Table 1. Distilled water was added to retain moisture for mycelia growth, and each treatment was added to least two bottles (9.5 L/bottle). Moisture content should be in the 70 to 75% range. Before filling the substrates into the sawdust bag, the pH reader was checked for each treatment.
Table 1. Substrate composition.
Substrate/
treatmentComposition of substrate T0 (Control) 97.2% SD + 0.8% RB + 2% L T1 (Mixed) 60% RS + 22.2% EFB + 15% PPF + 0.8% RB + 2% L T2 (Mixed) 60% OPF + 22.2% EFB + 15% SD + 0.8% RB + 2% L OPF, Oil Palm Frond; EFB, Empty Fruit Bunch; PPF, Palm Pressed Fibre; SD, Sawdust; RB, Rice bran; L, Lime. The mixed prepared substrates for each treatment were then filled into a polyethylene (PE) bag (9'' × 15''), and the medium was pressed manually by hand to ensure it was as compact as possible, resulting in 500 g/bag. The medium was then closed with a PVC neck set. The blocks were then autoclaved to prevent contamination. After autoclaving, the sterilized substrate bags were put in a mushroom lab to cool. Next, 1-2 g of mushroom spawn were added to the blocks and all the blocks were arranged on the racks by each treatment for incubation in the mushroom lab. After mycelium was fully colonized, all the blocks showed primordia to produce the mushroom fruiting body. The harvested mushroom was recorded for total yield and biological efficiency to determine the best potential substrate mushroom for production. The biological efficiency was conducted based on the following formula:
$ \text {Biological efficiency, BE }({\mathrm{\%}})=\dfrac{\text { Weight of harvest }}{\text { Weight of dry substratte }} \times 100 {\mathrm{\%}} $ Processing of food crackers from grey oyster mushroom
-
Unsold and leftover fresh mushrooms were cleaned and dried in sunlight for 2 d. The dried mushrooms were then ground into powder using a blender at the Food Laboratory (UMK, Malaysia). For the cracker preparation, the ingredients of PH (not stated due to copyright) were boiled to make a mash. A cup of water was transferred to a pot to boil, PH was then slowly added to the boiled water. In the meantime, further boiled water was added, and the ingredient CFH (not stated due to copyright) was slowly added until a rough dough formed in the pot. After mixing the CFH, the dough was transferred to a large plate. Next, another dry ingredient, such as a teaspoon of pepper powder, was added to the dough. Mushroom powder (approximately 10%) was added according to the final weight of 250 g of the dough. The dough was then divided into four to five parts. After 24 h under refrigeration, each dough piece was cut into thin pieces. Next, the crackers were dried using sunlight for at least two days to ensure the crackers were well dried. Finally, then the dried crackers were analysed for proximate analysis and sensory evaluation of participants.
Proximate analysis
-
Proximate analysis is a chemical analysis method to identify food substance nutritional content such as protein, carbohydrates, fat, and fibre[7]. The analysis results were presented as grades in units of %. The proximate analysis had benefits as an assessment of the quality of the food ingredients, especially on the standard of food substances they should contain.
Protein analysis
-
Protein content of the mushroom powder, mushroom cracker and commerical crackers was determined using the Kjeldhal method according to the procedures of AOAC with some modification. This method involves three stages which are digestion, distillation and titration following the procedure of Naher et al.[8]. Crude protein was calculated using the following formulas:
i) Calculate for N2 content:
$ {\mathrm{\%}}\;{\rm o f\; N_{2}} = \dfrac{(T-B)\times N\times 1.4007}{weight\; of\; sample\;\left(g\right)} $ ii) Crude protein = N2% × 6.25
Ash, fat and carbohydrate analysis
-
Two grams of each mushroom cracker, mushroom powder and commercial cracker were weighed and transferred onto clean, dry, and pre-weighed crucibles, respectively. The samples and crucible were kept in a muffle furnace at 550 °C for 6 h. Then, the sample was cooled in a desiccator and weighed. The fat content was determined using the Soxhlet extractor method with some modifications following the procedure of Naher et al.[8].
The ash and fat content for mushroom cracker and powder was calculated using the following formula:
${\rm Percentage\; ({\mathrm{\%}})\; of\; Ash/Fat\; content} = \dfrac{W_1-W_2}{W}\times 100 $ Where: W1 = weight of final cup, W2 = weight of initial cup, W = weight of sample
The formula for the percentage of carbohydrates determined the carbohydrate content of mushroom crackers, mushroom powder, and commercial crackers. The equation below was used to calculate the carbohydrate content:
${\rm Total\; carbohydrate\; {\mathrm{\%}} = 100 - (Moisture + Protein + Ash + Fat)} $ Analysis of mushroom cracker attributes
-
The physical attributes of mushroom crackers were analysed based on texture (crispiness, hardness, cohesiveness), moisture, and colour. The moisture analysis of the mushroom crackers was performed using the moisture analyzer A&D Heat-Drying Moisture Meter MX-50. A moisture analyzer weighed the sample of mushroom crackers. Then, the analyzer heated the sample at 180 ℃ until the sample dried. Lastly, the sample was weighed again, and the result recorded.
The texture analysis of a mushroom cracker was designed to mimic biting person. The Brookfield CT3 Texture Analyzer with TA-MTP fixture was used to run the mushroom cracker test. The chip sample was penetrated using a stainless-steel cylinder probe type TA7 with a trigger load of 5 g and a speed of 5.00 mm/s[9]. The texture analysis parameters were set, and the mushroom cracker and commercial potato cracker (control) were positioned beneath the probe. Texture analyzers concentrate on the mushroom cracker's hardness, breaking strength, and cohesion. The data sample was recorded in triplicate.
The Hunter Lab Colorimeter's optical sensor was placed on top of the mushroom cracker. A colour metre (Konica Minolta CR-400) was used to determine the product's colour for the instrumental measurement[10]. The mushroom cracker and commercial potato cracker (control) samples were tested. Each sample's colour was measured in the CIE L* a* b* colour space, and the results were reported in terms of lightness (L*), redness or red-green (a*), and yellowness or yellow-blue (b*)[11]. The data samples were recorded in triplicate.
Sensory evaluation
-
The method of sensory evaluation was applied by evaluation of four sensory attributes, which are the number of crackers, texture or feel, colour, richness and overall acceptability of the cracker samples using a 4-point hedonic scale, in which the lowest value (1) stands for extreme dislike while the highest value (4) represents an extreme like (Table 2)[12]. Fifty participants were selected to take part in the determination of the sensory evaluation of mushroom crackers. Before tasting a mushroom cracker, plain water was served to participants to neutralize their mouthfeel and the tasting was carried out under good lighting to determine the colour of the crackers.
Table 2. Survey form for the sensory evaluation of mushroom crackers.
Items Extremely
like 4Like 3 Dislike 2 Extremely dislike 1 Number of crackers Cracker in every bite Cracker in 75% chips Cracker in 50% chips < 50% cracker Texture/
feelConsistently crispy and crunchy chewy Chewy middle, crispy edges Crunchy
only and
not crispyLess crunchy and not crispy Colour Even golden brown Brown with pale centre Very brown Burned Richness Edible Less oily Medium oily High oily Statistical analysis
-
All the parameters including total yield of mushrooms, biological efficiency from each treatment, proximate, physical attributes of mushroom crackers as well as sensory assessment information were collected and processed using IBM SPSS version 26 for the statistical analysis. The significance of the differences in the data was determined using an independent t-test, as well as ANOVA analysis. The significant differences in the mean values were determined at the 95% confidence interval level of (p < 0.05).
-
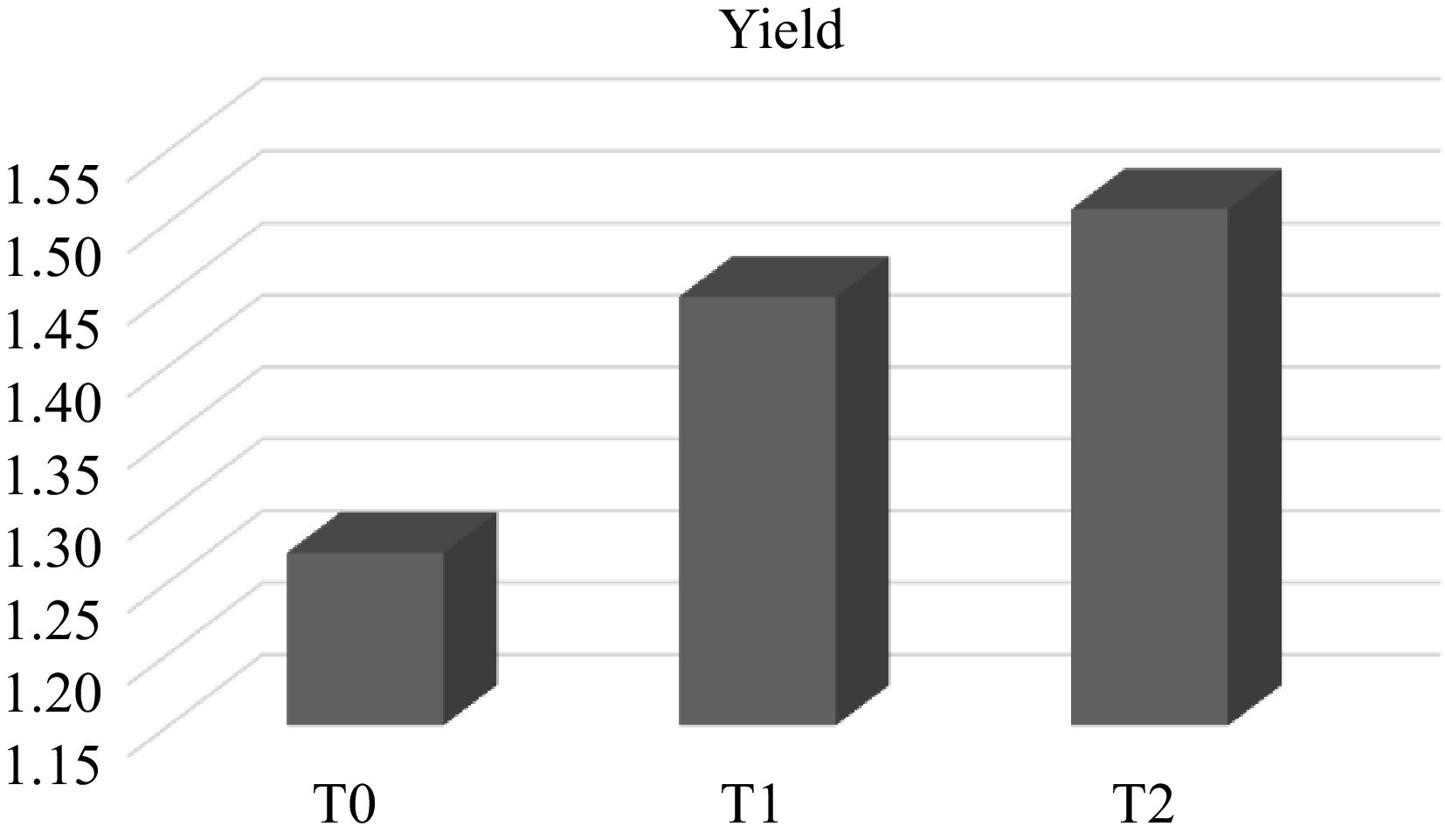
The species of grey oyster (Pleurotus ostreatus) mushroom was cultivated for two months in the mushroom house, at UMK Jeli campus, Malaysia. A total of 45 blocks were cultivated in three different treated substrates in three replicates. The total yield was recorded until the 4th cycle for two months. The results showed (Fig. 1) that oil palm frond majority substrate (T2) produced a higher yield (1.5 kg), while the 2nd highest (1.4 kg) was rice straw substrate (T1) and the lowest (1.2 kg) was sawdust substrate (T0).
For income performance, the individual block preparation cost, yield in four cycles, selling price and net income of each substrate per block are shown in Table 3. T2 was 100 g/block which was higher than T0 and T1. For net income comparison, we make T0 or control a constant of 100% yield, which was compared with T1 and T2 total yield performance. The result showed T2 yield performance was 115% which was 15% higher than T0 (Table 3). The total cost per block also sowed in oil palm form was lowest in T2 as RM 0.60 as compare to T0 and T1. Therefore, the net income of T2 (RM 0.70) was higher than the control (RM 0.38) and T1 (RM 0.53) in Table 3.
Table 3. Income performance of each of the substrate treated mushroom yield.
Products Cost (RM)/block Yield/ block /selling price Yield performance/ income/block T0 (Sawdust commercial/control) 0.74 86.7 g/RM 1.12 100%/RM 0.38 T1 (RS + EFB + PPF) 0.75 93 g/RM 1.2 107% (7% >)/RM 0.53 T2 (OPF + EFB + sawdust) 0.60 100 g/RM 1.30 115% (15% >)/RM 0.70 Proximate analysis of mushroom cracker and commercial potato cracker
-
Proximate analysis was performed to determine the protein, fat, ash, moisture and carbohydrate content. The result shows that there was a significant difference (p < 0.05) in ash, fat and moisture content between commercial potato and mushroom cracker (Table 4). While, no significant difference (p > 0.05) was found in protein and carbohydrate between commercial potato and mushroom cracker (Table 4).
Table 4. Proximate analysis of mushroom cracker and commercial cracker.
Sample Mushroom cracker (1 g) Commercial potato
cracker (1 g)Protein (%) 2.19 ± 0.90 2.22 ± 0.10 Fat (%) 11.335 ± 0.3061 12.8283 ± 0.2475 Ash (%) 2.0567 ± 0.1201 1.4667 ± 0.2566 Moisture (%) 7.8733 ± 0.2219 6.15 ± 0.5 Carbohydrate (%) 76.5467 ± 0.12503 77.3350 ± 0.5327 Colour properties
-
The colour property analysis of mushroom cracker and commercial potato cracker is shown in Table 5. The results found that mushroom crackers had the lowest value (55.89 ± 1.0017) of L* while commercial potato crackers showed higher values (62.8033 ± 0.1721). According to the value, L* indicate lightness. This shows that the colour of the mushroom cracker is darker than the commercial potato cracker because due to dark colour mushroom powder from the grey oyster mushroom. There was a significant difference (p < 0.05) shown in L* between mushroom and commercial potato crackers (Table 4).
Table 5. Independent t-test for colour analysis.
Mushroom cracker Commercial potato cracker Sig.
(2-tailed)Mean S.D. Mean S.D. L* 55.89 1.0017 62.8033 0.1721 0.006 a* 6.75 1.7197 11.7267 0.5258 0.029 b* 18.1667 1.5016 31.55 0.7502 0.001 Next, a* is an indicator for the colour of the crackers to be red or green. The results obtained show that commercial potato crackers are much higher (11.7267 ± 0.5258) than mushroom crackers (6.75 ± 1.7197). This shows that commercial potato crackers have an orange to red colour while mushroom cracker shows less red colour as mushroom crackers have no artificial colour added. Hence, there is a significant different between mushroom crackers and commercial potato crackers which was (p < 0.05) shown in Table 5. Moreover, b* is indicated for yellow or blue colour. The highest b* value indicating yellowness of the sample was observed for the commercial potato cracker (31.55 ± 0.7502). A lower b* was noted in the mushroom cracker (18.1667 ± 1.5016) due to the incorporation of mushroom powder. Due to the mushroom powder's natural brown colour, the mushroom cracker's yellowness was concealed. Consequently, a decreasing trend in b* values was seen as mushroom inclusion increased[13]. Therefore, there is significant difference (p < 0.05) between mushroom crackers and commercial potato crackers.
Texture profile analysis
-
Texture profile analysis (TPA) is used in a wide variety of fields to measure mechanical qualities like hardness, cohesiveness and springiness by repeatedly compressing a sample with a probe at a predetermined rate. The test was equipped using probe TA 7 Knife edge 60 mm W. A number of texture properties were chosen to analyse the crackers such as hardness, cohesiveness and springiness. Table 3 shows the texture properties of mushroom crackers and commercial potato crackers. For the hardness properties, the mushroom cracker (1,117.00 ± 126.74 g) shows a lower value than the commercial potato cracker (2,481.00 ± 115.8836 g). The hardness of the crackers can be determined by the sensory and terminology of hardness is opposite to crispiness. The lower the hardness is, the more crunchiness of the cracker. So, it can be seen that mushroom crackers are much crispier compared to the commercial potato crackers. Customers favour crackers with a high crispiness score, and low hardness will be displayed[14]. The hardness of the cracker is often related to the interaction of ingredients used. Both crackers were significantly different (p > 0.05) in hardness towards each other.
Cohesiveness is a measure of how a cracker withstands deformation. Based on the results in Table 6, mushroom crackers show higher cohesiveness, which indicate about 1.00 ± 0.1473. While, commercial potato crackers indicate 0.1867 ± 0.0851 which is less compared to the mushroom cracker. Low cohesiveness indicates high brittleness or crumbliness of the cracker. Therefore, the higher cohesiveness of the mushroom cracker might be because the proteins in the mushroom cracker formed a three-dimensional cross-linked protein network that could withstand more deformation before breaking[15]. Other than that, the lower the cohesiveness value, the more prone it is to breakage. Therefore, there is no significant difference (p < 0.05) between the cohesiveness of mushroom crackers and commercial potato crackers.
Table 6. Independent t-test for texture properties.
Mushroom cracker Commercial potato cracker Sig.
(2-tailed)Mean S.D. Mean S.D. Hardness (g) 1,117.00 126.74 2481.00 115.8836 0.000 Cohesiveness 1.00 0.1473 0.1867 0.0851 0.003 Springiness (mm) 6.49 0.8972 14.61 9.5103 0.215 In addition, springiness properties refer to how quickly and fully a deforming force is recovered. For springiness, the commercial potato cracker has a higher value (14.61 ± 9.5103 mm) than the mushroom cracker (6.49 ± 0.8972 mm). The higher springiness value is because of the extended storage time as commercial potato crackers have been developed on a large-scale during processing time[16]. So, this would affect the springiness of the cracker. Both crackers showed no significant difference (p > 0.05) in springiness attributes between each other.
Sensory evaluation of mushroom crackers
-
The survey result of the participants' sensory evaluation of mushroom crackers is shown in Table 7. Sensory evaluation of four sensory attributes: the number of crackers consumed, texture or feel, colour, richness, and overall acceptability of the crackers samples, using a 4-point hedonic scale, in which the lowest value (1) stands for 'extreme dislike'. In contrast, the highest value (4) represents 'extremely like'. The percentage of 50 participants determines this sensory evaluation. It was presented that the highest percentage level acceptance of mushroom crackers of 74% are 'extremely like', which means that the respondent eats 100% of the mushroom crackers, whereas another 22% represent 'like', which means they consume 75% of the mushroom crackers while 'dislike' contributed to 4%. Next, for the texture of the mushroom, the largest scale is 'extremely like', contributing 74%.
Table 7. Sensory evaluation of mushroom crackers.
Scale Sensory attribute Acceptable Texture Colour Richness Overall acceptance Extremely like 74% 74% 38% 78% 66% Like 22% 26% 62% 22% 33% Dislike 4% 0 0 0 1% Extremely dislike 0 0 0 0 0 In crackers, the colour attribute symbolizes the exterior colour of the crackers. The highest percentage of mushroom crackers were in the 'like' scale which contributed 62%, meaning that the crackers are brown in colour. Therefore, another 38% voted 'extremely like', which means the crackers are golden brown.
Then for the richness of mushroom crackers, the highest scale percentage was 'extremely like', contributing to 78%, meaning that the mushroom cracker is edible. The participants observed that mushroom crackers could be eaten. Therefore, another 22% vote for the 'like' scale indicates that the mushroom crackers are lower in oil. Finally, the largest scale percentage of overall acceptance is 'extremely like', which contributed up to 66%, while another 33% and 1% are 'like' and 'dislike', respectively.
-
Mushrooms become a high-value food worldwide. Therefore, the demand for mushrooms is increasing day by day. A perfect substrate combination results in good growth of mycelia that helps for profitable production of mushroom fruit bodies in commercial cultivation. This study showed that T2 treatment combinations with 60% OPF (oil palm frond) + 22.2% EFB (empty fruit bunch) + 15% SD (sawdust) + 0.8% RB + 2% L recorded higher (1.5 kg in four cycles) production. While, treatment T1 as 60% RS (rice straw) + 22.2% EFB + 15% PPF + 0.8% RB +2% L) produced 1.3 kg and T0 as 97.2% SD (sawdust) + 0.8% RB + 2% L) produced 1.2 kg mushroom fruit bodies. It is interesting that T2 and T0 material composition is quite similar, as oil palm frond and sawdust mainly contain lignin and cellulose, whereas T1, which is a rice straw substrate, mainly contains cellulose-based material. Physiological attributes for substrate in terms of Carbone (c), Nitrogen (N), minerals, and moisture capacity content play important roles in mycelial development. The material of lignocellulose has less moisture vapour evaporation compared to cellulose-based material[17]. Thus, palm-based substrate maintained moisture in the substrate while with the rice straw substrate, several droplets on the substrate bag can be seen that cause issues for mycelial growth.
The income performance result was also high in oil palm-based substrate (T2). For potential commercial substrates, there is a need to compare its cost price and net income to determine the maximum utilization of the substrate for mushroom production and worth for income generation. In this study, oil palm-based substrate was received free of charge, while sawdust and rice straw were purchased. Hence, oil palm based substrate can be profitable for mushroom farmers as well as a country's economic revenue.
As for nutritional content, there was not much difference in protein content between commercial crackers and mushroom crackers, commercial potato cracker content was slightly higher (2.22% ± 0.10 %) compared to the mushroom crackers (2.19% ± 0.90 %). This is may be due to MSG (monosodium glutamate). MSG contains glutamate, rich in protein[18]. Another study by Bera et al.[19] defined that glutamate from MSG is the most abundant amino acid (the main component of protein). In preparation of mushroom crackers or even decoration time, this study did not use seasoning with MSG. This is because although MSG contains amino acids, it also contains artificial salt. Too much dietary sodium can cause an increase in blood pressure or health issues. Currently, cracker consumers are aware of MSG salt-processed crackers. Cracker lovers are looking for healthy quality ingredients with minimal processing and they should not contain excess salt while the taste should be similar to commercial crackers[2]. On the other hand, mushrooms contain umami flavor, which is the 5th state group that contains natural MSG flavor; therefore, for the preparation of mushroom crackers there is no need for artificial salt. In this study, mushroom crackers were made with very few ingredients, such as rice flour and potato. Conversely, commercial potato crackers usually contain several artificial ingredients that enhance the artificial protein amount. So, it can be noted that mushroom cracker contain completely natural protein. Table 3 shows that fat content in commercial potato crackers is slightly higher (12.8283 ± 0.2475) than in mushroom crackers (11.335 ± 0.3061). The literature suggests that the recommended fat content range is between 10% and 30% fat[20]. Therefore, mushroom crackers and commercial potato crackers are still in the recommended range. In addition, the higher protein content contributions increased in mushroom powder and constricted the starch-lipid interaction, causing a reduction in oil absorption during frying[21]. The ash content in the mushroom cracker proceeded to be higher, which is about 2.0567 ± 0.1201, compared to the commercial cracker (1.4667 ± 0.2566). The relatively high content of ash also shows fiber richness in the food[22]. Therefore, mushroom crackers show that the products are rich in fiber compared to commercial crackers. However, some studies have revealed that the ash content in processed food can be more than 10%, but in natural food, it must be less than 5%[23]. The mushroom crackers in this study are referred to as minimally processed, whereas commercial potato crackers are fully processed food. In recent years, cracker lovers require processed or minimally processed foods[2]. However, the moisture content in mushroom crackers was 7.8733%, which is slightly higher than that of commercial crackers at 6.15%. Higher moisture content is a result of the capacity of fibers and polysaccharides to retain water[24]. If the moisture level is too high (more than 10%), the texture and flavour will suffer, and the shelf life will be shortened[4]. Crackers with a low moisture level (5%) are more prone to breakage, which results in waste. As shown, commercial crackers are in the range of 5 to 10%, and the moisture content of mushroom crackers was also in a range of 5 to 10%, so it can be noted that the production of mushroom crackers is the commercial standard level. For carbohydrate content, there was no significant difference found between commercial crackers (77.3350%) and mushroom crackers (76.5%). Carbohydrates mainly originated from flour and sugar. In the mushroom cracker, no sugar or artificial additives were added except for flour and the mushroom itself. In commercial crackers, besides flour, a few more ingredients, such as sugar or additives, are added to enhance self-life. Mushroom carbohydrates contain good qualities, such as trehalose, xylitol, and sorbitol, which can act as natural additives.
Besides natural sugar, the mushrooms also contain polysaccharides such as glycogen, β-glucan, heteroglycan, and chitin[25]. Among these polysaccharides, β-glucan is one of the dietary fibers that can reduce human blood cholesterol and glucose levels that affect cardiovascular heart disease and diabetes for health[25]. Therefore, it can be noted that mushrooms contain good quality carbohydrates which contribute to the healthy food attribution of mushroom crackers.
As for colors, L* indicates lightness, a* is an indicator of the color of crackers being red or green, and b* indicates yellow or blue color. In all aspects of colour, commercial potato cracker values were higher than mushroom crackers (Table 3). In preparation of mushroom crackers, no artificial colour was added. Texture comparison between mushroom crackers and commercial potato crackers showed no significant difference (Table 3) which showed mushroom crackers as being a potential for commercial standard in taste.
Sensory acceptability of mushroom crackers showed a higher rate of 72% which shows that the mushroom crackers were 'extremely accepted'. Richness means the texture with crispiness which was high at 78%. It means that the participant accepted the texture of the mushroom crackers that are constantly crispy and crunchy. Usually, customers favour crackers with a high crispiness, and low hardness[14]. In terms of colour, 62% showed that mushroom crackers looked to be brown. This is due to the colour of mushroom crackers being brown rather than golden because of the dark colour of mushroom powder from grey oyster mushrooms[26]. Hence, the mushroom powder contributes to the darkening after frying[21]. However, the colour did not affect the overall acceptance, which showed that 66% of participants 'extremely like' the mushroom crackers.
-
Mushroom is a potential agri-food which is referred to as vegetable meat. Since demand for mushroom is increasing dramatically, various types of cultivation substrate are needed to avoid raw material scarcity. The results of this study showed that oil palm plant material-based substrate produced the highest yield of 1.5 kg. The net income performance was 15% highest for oil palm substrate compared to rice straw and sawdust substrate. The food product of mushroom crackers overall acceptance level showed 66% participants accepted mushroom crackers. Therefore it can be concluded that the preparation of oil palm products is cost-effective, and growers can easily adopt them for their income generation, which can influence economic sustainability. The production of mushroom crackers can reduce postharvest losses and open the door for extra income for the growers.
-
The authors confirm contribution to the paper as follows: study conception and design: Naher L; data collection: Mustaffa Bakri NA, Muhammad Sukhri SAN, Nik Raihan NH, Mohd Firdaus Ganga H; analysis and interpretation of results: Md Zain N, Abdul Rahman N; draft manuscript preparation: Naher L, Ch'ng HY, Mokhtar SI. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
The authors would like to acknowledge the Ministry of Finance, grant code R/MOF/A0700/01204A/2020/00724 for providing financial support. We also acknowledge the Universiti Malaysia Kelantan, Jeli Campus for all the laboratory facilities.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Naher L, Mustaffa Bakri NA, Muhammad Sukhri SAN, Nik Hassan NR, Mohd Firdaus Ganga H, et al. 2024. Economical substrate formulation for mushroom cultivation and food production of mushroom crackers to reduce postharvest waste. Studies in Fungi 9: e002 doi: 10.48130/sif-2024-0003
Economical substrate formulation for mushroom cultivation and food production of mushroom crackers to reduce postharvest waste
- Received: 02 September 2023
- Revised: 02 March 2024
- Accepted: 13 March 2024
- Published online: 29 March 2024
Abstract: Agriculture residues of oil palm waste are a big issue for palm oil producing countries. The residues from oil palm fronds are the most crucial to convert wealth. This study focused on oil palm-related agro biomass for mushroom substrate formulation for grey oyster mushroom cultivation. Mushrooms are a highly perishable vegetable that turn into postharvest waste within 4 to 7 d at normal temperatures. Therefore, in this study, the unsold mushroom was converted as a cracker food product to reduce the postharvest losses, especially for small-scale mushroom growers. The agriculture biomass used for substrate preparation is a combination of oil palm frond (OPF), oil palm empty fruit bunch (EFB), palm pressed fiber (PPF), and sawdust (SD). SD as a commercial substrate was used as a control in this study, and rice bran (RB) and lime (L) were used as supplement ingredients for all the treatments. The treatments were according to mixed formulation with the ratio of T0 (control: 97.2% SD, 0.8% RB + 2% L), T1 as a mixed ratio (60% RS + 22.2% EFB + 15% PPF + 0.8% RB + 2% L) and T2 as a mixed ratio (60% OPF + 22.2% EFB + 15% SD + 0.8% RB + 2% L). The total yield in four cycles showed 1.2 kg in T0 (sawdust), 1.4 kg in T1 (majority of rice straw), and 1.5 kg (majority of oil palm frond) in T3 treated substrate. In this study, the oil palm frond was received free of charge as compared to sawdust and rice straw. Therefore, it showed that using the oil palm frond not only gave a high yield of mushrooms at the same time, it was 100 X lower in cost. Next, the unsold yielded mushrooms were used for cracker preparation. The results obtained from this study indicate that mushroom crackers contain fat (11.34%), protein (2.19%), and carbohydrate (76.55%) while being high in moisture (7.87%) and ash (2.06%) compared to commercial potato crackers. Overall acceptance of sensory evaluation towards mushroom crackers showed a high 'extremely like' percentage, contributing about 66%. Thus, this study found that 66% of participants 'extremely liked' the new innovative mushroom crackers. Overall, the results show that oil palm substrate can be an alternative economical substrate for grey oyster mushroom cultivation and food products from mushrooms will be new items in the snack industry.
-
Key words:
- Crackers /
- Cultivation /
- Income generation /
- Mushroom /
- Sustainability