-
'Ruaner' pear (Pyrus ussuriensis Maxim.) is the primary cold-tolerant pear cultivar grown in the plateau regions of western China, with a minimum temperature threshold of −45 to −52 °C. It is hardy, unlike other Pyrus ussuriensis varieties, such as 'Jingbai'[1], and 'Nanguo' pears[2], and not eaten at harvest. However, pears can be softened after harvest or kept in the cold after being exposed to the warm temperature. Furthermore, the 'Ruaner' pear loses firmness rapidly and decays easily in storage and retail, making it a particularly perishable fruit[3]. A recent study showed that the lifespan of 'Ruaner' pears was only 29 d when the fruit were harvested at 22.5 N and placed at 8 °C under regular-air conditions[3]. On the other hand, little information is accessible for local packers to use 'Ruaner' pears at a relatively low temperature (i.e., 0 °C). Depending on the growers' and pear industries' goals, they wish to increase pear consumption and extend the storage life of pears with less deteriorated and attractive eating-quality fruit after long-term storage and shelf. Therefore, this study's primary goal was to assess the development of decay and quality attributes of 'Ruaner' pears following cold storage (0 °C) and being subjected to 20 °C.
It is well-recognized that fruit maturity has a greater impact on the pears' quality after storage[4−6]. If fruit were harvested too early, the immature pears with a small size, low sugar level and lack of juice and flavor would reduce the growers' return and destroy the consumers' purchasing desire; additionally, these pears were more prone to friction discoloration and shriveling after handling and storage[7,8]. If harvested too late, these over-mature pears will develop high rates of punctures, bruises, and decay during handling and storage; furthermore, the rapid quality deterioration will curtail the pears' life[6,9]. In practice, the 'Ruaner' pears maturity standards in our locality use a combined index of date (from Sep. 25 to Oct. 8) and skin color (light yellow). Unfortunately, the lifespan of those harvested pears was no more than 40 d; furthermore, this recommendation caused a high rate of decay during cold storage and at retail. Therefore, pears would have a lengthy storage life and comparatively low incidences of postharvest disorders when picked at optimum maturity. Based on this issue from packers, this study's second objective was to investigate whether there was another reasonable indicator (i.e., flesh firmness) to harvest 'Ruaner' pears by reducing fruit quality deterioration and minimizing loss.
Generally, the rapid development of postharvest disorders coincides with the onset of fruit senescence in pears[10,11]. Fortunately, the antioxidants (i.e., phenolics, flavonoids, carotenoids, and indigoids) and the level of antioxidant capacity produced in pears can postpone the onset of postharvest disorders and senescence by reducing oxidative stress[12,13]. Additionally, before consumption, retaining high antioxidant properties in fruit after storage would provide great benefits for human health[14,15]. Therefore, this study's final objective was to assess the changes in antioxidants and antioxidant capacity of 'Ruaner' pears affected by maturity after cold storage and shelf. The goal was to give an important message to growers and packers to harvest the 'Ruaner' pears at an appropriate maturity with low incidences of disorders but high quality as well as antioxidant properties after over-longer storage and shelf life.
-
'Ruaner' trees were selected from an orchard located in Shanping Village, Guide County, Hainan Tibetan Autonomous Prefecture, Qinghai, China (36°1'43'' N, 101°19'50'' E, elevation 2,237 m, and annual rainfall 251−559 mm). The 13-year-old trees were grafted onto the 'Duli' rootstock. There was a 6.0 m × 8.0 m space between the rows and lines. In accordance with standards, fertilizers, herbicides, and pesticides were applied to each tree.
Pears were hand-harvested three times on Sep. 8 (harvest maturity 1 [HM1], flesh firmness [FF] at 68.22 ± 4.31 N), Sep. 18 (HM2, FF at 57.12 ± 3.96 N), and Sep. 28 (HM3, FF at 39.14 ± 3.01 N), 2021. After selecting fruits without any obvious damage or fungal infection, they were packaged in plastic boxes with 90 fruits each, using polyethylene (PE) bags (0.80 m × 0.80 m) with 12 holes of 8-mm diameter. Fruit were stored in a regular-air cold room (0 °C) with a relative humidity (RH) of 90%−94%. In all, there were three groups, including HM1, HM2, and HM3 fruit; each group had three replications per group, and two boxes as a replicate. After 0, 15, 30, 45, 60, 90, and 120 d of cold storage, a ripening test involved transferring two boxes per replicate, from each group to 20 °C. After 1, 4, 8, and 12 d at 20 °C, the decay, FF, hue angle, soluble solids content (SSC), titratable acidity (TA), sensory score of textural quality, antioxidants, and antioxidant capacity were measured. Flesh tissue was quickly removed and frozen in liquid nitrogen (N2). After grounding with liquid N2, the samples were stored at −80 °C for subsequent analysis. Due to harvest maturity having effects on the development of decay and the quality of 'Ruaner' pears, pears were harvested on Sep. 16 during the 2022 growing season when the FF of pears dropped to 58.19 ± 4.05 N; unfortunately, no data were collected due to COVID. In the 2023 growing season, 'Ruaner' pears were harvested on Sep. 17 when FF was 57.88 ± 3.68 N; the decay incidence, FF, hue angle, and titratable acidity were collected after 60, 90, and 120 d of storage at 0 °C plus a 12-d shelf period at 20 °C under the same storage conditions as 2021 (Supplementary Table S1).
Evaluation of decay incidence
-
During a ripening period, 30 pears from each group per replicate were assessed at each evaluation date and shown as a percentage of fruit that has any skin tissue pathological lesion. Decay incidence (%) = the number of fruit with decay/total number of fruit (30) × 100.
Evaluations of FF, skin color, SSC, TA, and sensory score of textural quality
-
At each evaluation date, the FF of ten pears from each group per replicate was measured using a digital fruit firmness tester (model GY-4, Yueqing Handpi Instrument Co., Zhejiang, China) with a 3.5-mm probe that penetrated 10 mm. After a peeler removed a 1-mm peel from each fruit, the measurement was taken once on opposite sides of the equator, and the maximum force was recorded. The data were expressed as Newton (N) and averaged per ten pears. Following the determination of FF, a colorimeter (model CR-400, Konica Minolta, Osaka, Japan) was used to measure each fruit's skin color at two locations on opposite sides of the equator. The hue angle was calculated according to that described by McGuire[16]. Following the determination of skin color, flesh tissue (100 g) from ten pears from each group per replicate was juiced for 180 s using a juicer (model FPP226, De'Longhi Appliances (Shanghai) Co., Ltd., Shanghai, China). Then, a digital refractometer (model PAL-1, Atago, Tokyo, Japan) was used to measure the SSC of the juice; an automatic titrator (model G20, Mettler Toledo, Greifensee, Switzerland) was used to measure the TA by titrating 10 mL of the juice to pH 8.1 with 100 mM NaOH; the data were expressed as a percentage of malic acid equivalents. On a 5-point hedonic scale, the sensory quality (melting/mealy texture score) was assessed[17]. Fruit classified as moderately or extremely firm (i.e., underripe pears) or moderately or very mealy flesh texture (i.e., overripe pears) were rated as 2, or 1, respectively, while fruit classified as strongly, moderately, or slightly melting texture were rated as 5, 4, or 3. Before the first evaluation session, ten taste testers were oriented on scale anchor points and definitions. Commercially acceptable was defined as an average score of three or above. One fruit slice from each replicate of the five pears was sampled by each panelist.
Determination of total phenolics (TP) and total flavonoids (TF)
-
Ten pears per replicate, each containing 2 g of ground pulp sample, were extracted using 5 mL of ethanol/acetone (7/3, v/v) at 4 °C for 1 h, then centrifuged at 8,000 g for 10 min at 4 °C. For subsequent evaluations, the supernatants were collected and kept at −20 °C.
The method for measuring TP content was as described by Du et al.[18]. A 10-mL glass tube was filled with a 0.05 mL aliquot of the extract, 5.05 mL of distilled water, and 0.3 mL of 0.25 M Folin-Ciocalteu reagent. Following 5 min of mixing at 20 °C, 0.6 mL of 1 M Na2CO3 was added, and the mixture was then allowed to incubate in the dark at 20 °C for 2 h. A spectrophotometer (model T6, Purkinje General Instrument Co., Beijing, China) was used to measure absorbance at 765 nm. Gallic acid was used to create a standard calibration curve, and the data were presented as mg·kg−1 on a fresh weight basis.
The method for measuring TF content was as described by Du et al.[18]. A 10-mL glass tube was filled with a 0.15 mL aliquot of the extract, 3.55 mL of 30% (v/v) ethanol, 0.15 mL of 0.5 M NaNO2, and 0.15 mL of 0.3 M AlCl3. Following 5 min of mixing at 20 °C, a spectrophotometer was used to measure absorbance at 506 nm. Rutin was used to create a standard calibration curve, and the data were presented as mg·kg−1 on a fresh weight basis.
Determination of the ability to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals and ferric-reducing antioxidant power (FRAP)
-
The method for measuring DPPH free radical scavenging capacity was as described by Du et al.[18]. A 10-mL glass tube was filled with a 25 μL aliquot of the extract, 0.975 mL of ethanol/acetone (7/3, v/v), and 2 mL of 62.5 μM DPPH in methanol. Following 30 min of incubating in the dark at 37 °C, a spectrophotometer was used to measure absorbance at 517 nm. Trolox was used to create a standard calibration curve, and the data were presented as mg·kg−1 on a fresh weight basis.
The method for measuring FRAP was as described by Du et al.[18]. Stock solutions included 2.5 mL of 10 mM 2,4,6-tripyridyl-S-triazine (TPTZ) solution in 40 mM HCl, 25 mL of 0.3 M acetate buffer (pH 3.6), and 2.5 mL of 20 mM FeCl3. A 10-mL glass tube was filled with a 25 μL aliquot of the extract, 0.975 mL of ethanol/acetone (7/3, v/v), and a 2 mL fresh working solution. Following 10 min of incubation in the dark at 37 °C, a spectrophotometer was used to measure absorbance at 593 nm. Trolox was used to create a standard calibration curve and the data were presented as mg·kg−1 on a fresh weight basis.
Statistical analysis
-
The designs of the experiments were entirely random. The data were evaluated using Fisher's protected least significant difference (LSD) test at p < 0.05, along with a one-way analysis of variance (ANOVA). Using Pearson's correlation coefficient, correlation analysis was performed on the decay, FF, hue angle, SSC, TA, sensory score of textural quality, TP, TF, and FRAP in HM1, HM2, and HM3 fruit. Using the Origin Pro Software (version 2021, OriginLab Corporation, Northampton, MA, USA), the data on decay, hue angle, and TA of HM1, HM2, and HM3 fruit were displayed in a three-dimensional scatter plot to determine the ideal director for pear growers to utilize 'Ruaner' pears.
-
For HM1 fruit, no decay was observed after 0, 15, 30, and 45 d of cold storage plus 1−12, 1−8, 1−8, and 1−4 d at a ripening test, respectively (Fig. 1). While the HM1 pears showed 1% and 2% of fruit with decay after 15 and 30 d of cold storage and 12 d at shelf, respectively. Following 45 d of cold storage plus 8 d at shelf, the HM1 pears developed 3% of fruit with decay; this increased to 10% on day 12. Following 60, 90, and 120 d of cold storage, an increased rate of decay was observed in HM1 fruit over the entire shelf period. Notably, extending the cold storage period from 60 to 120 d resulted in a rapid increase in decay incidence in HM1 fruit during a 12-d shelf period.
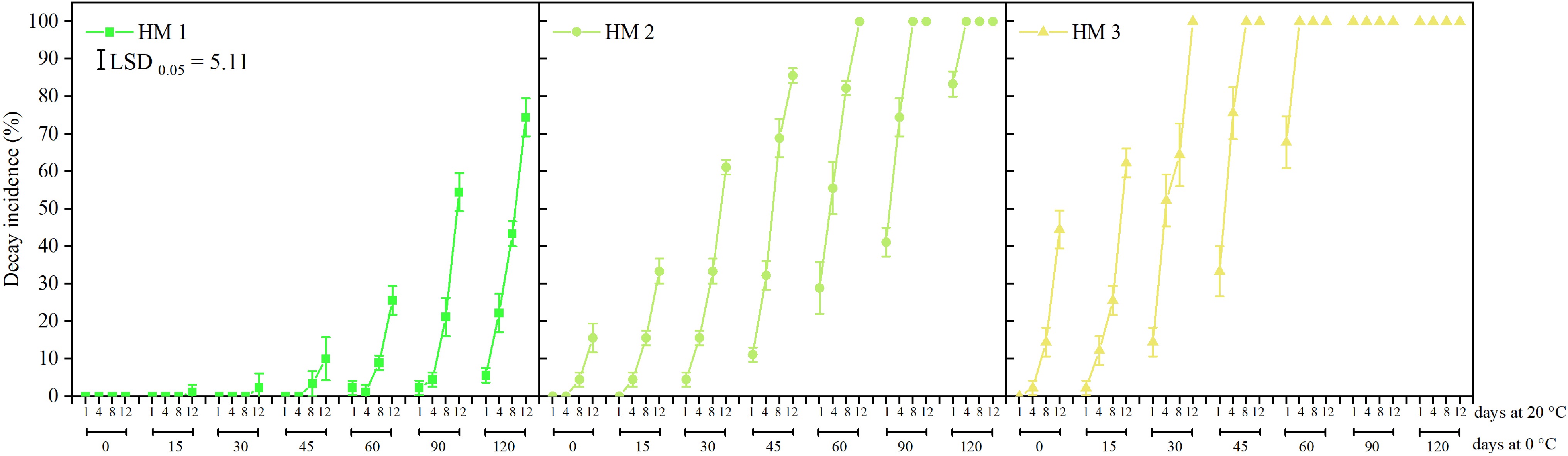
Figure 1.
Decay incidence of 'Ruaner' pears with three harvest maturities (HM1, HM2, and HM3) after 0, 15, 30, 45, 60, 90, and 120 d of cold storage (0 °C) plus 1, 4, 8, and 12 d at shelf (20 °C). Values are presented as the means ± standard deviation (SD).
For HM2 fruit, no decay was observed after 0 and 15 d of cold storage and 1−4 and 1 d at shelf, respectively (Fig. 1). However, HM2 fruit earlier developed decay compared to the HM1 fruit; furthermore, these pears first expressed decay after harvest plus 8 d at 20 °C, and the rate of decay elevated to 16% on day 12. Worse than the HM1 fruit, extending the cold storage period of HM2 pears significantly increased decay incidence during shelf life. Notably, 100% decay was observed after 60, 90, and 120 d of cold storage and 12, 8−12, and 4−12 d at shelf, respectively.
For HM3 fruit, no decay was observed after harvest plus 1 day at shelf (Fig. 1). The increase in decay incidence of HM3 pears paralleled the extended cold storage and shelf periods. Compared to the HM1 and HM2 pears, the HM3 fruit had earlier developed 100% decay. For example, HM3 fruit displayed 100% decay after 30, 45, 60, 90, and 120 d of cold storage plus 12, 8−12, 4−12, 1−12, and 1−12 d at 20 °C, respectively.
Effect of harvest maturity on quality attributes of 'Ruaner' pears after storage and during shelf
-
Due to 100% decay observed in HM2 and HM3 fruit after over-longer storage and shelf life, no results of FF, hue angle, SSC, TA, sensory score of textural quality, antioxidants, or antioxidant capacity were shown in subsequent evaluations. Regardless of harvest maturity, all 'Ruaner' pears softened, yellowed, and developed a melting texture after moving from cold storage and subjecting to 20 °C (Fig. 2a, b & e). Prolonging the cold storage period for three harvest maturities of 'Ruaner' pears gradually resulted in the pears losing their capacity to soften; as a result, these pears failed to develop a normal softening behavior with melting texture. Regardless of storage condition, more mature fruit (i.e., HM3) had a relatively higher SSC relative to the less mature pears (i.e., HM1) (Fig. 2c). Additionally, the SSC of HM1, HM2, and HM3 fruit displayed increased trends during 0−120 d of cold storage at 0 °C, while no significant difference was observed for SSC during shelf life. After HM1, HM2, and HM3 pears were harvested, no difference was found for TA during 1−12 d of shelf life (Fig. 2d). However, following 15−120 d of cold storage at 0 °C, extending shelf life induced a fast decrease in TA of either HM pears, especially in HM3 pears.
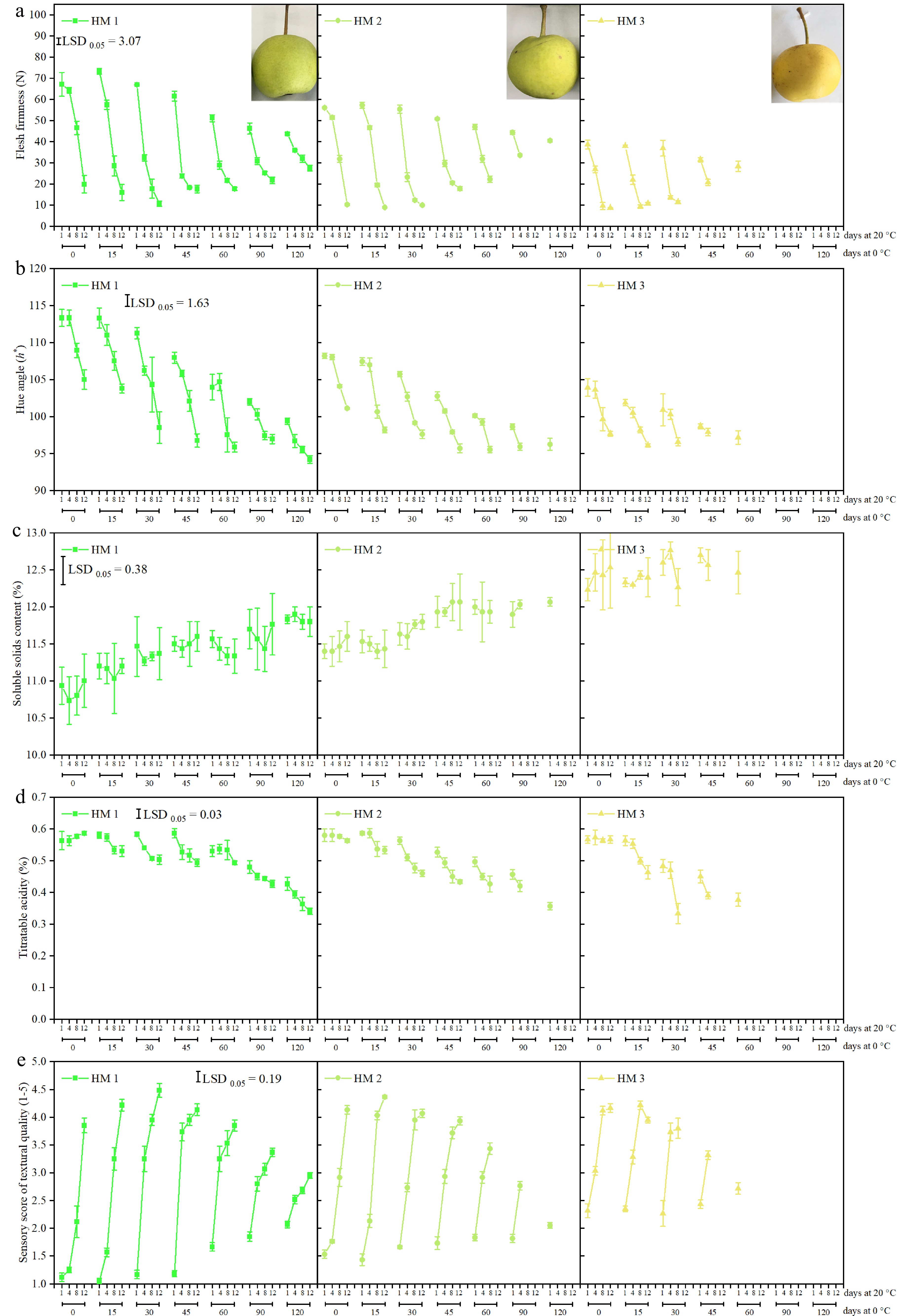
Figure 2.
(a) Flesh firmness (FF), (b) hue angle, (c) soluble solids content (SSC), (d) titratable acidity (TA), and (e) sensory score of textural quality of 'Ruaner' pears with three harvest maturities (HM1, HM2, and HM3) after 0, 15, 30, 45, 60, 90, and 120 d of cold storage (0 °C) plus 1, 4, 8, and 12 d at shelf (20 °C). Values are presented as the means ± SD.
Effect of harvest maturity on antioxidants of 'Ruaner' pears after storage and during shelf
-
For HM1 fruit, decreased TP and TF were observed after 0−60 d of storage at 0 °C plus a 12-d shelf, while no significant difference was found during shelf after 90 or 120 d of storage at 0 °C (Fig. 3a & b). Compared to HM1, more mature pears had lower TP and TF; furthermore, they were more susceptible to losing TP and lower TF after storage and during shelf. Notably, following 90 and 120 d of storage at 0 °C, no difference was observed in TP or TF in HM1 fruit during a 12-d shelf storage. Similar results were observed in HM2 fruit when the pears were subjected to 0 °C for 90 d plus 1−4 d at shelf.
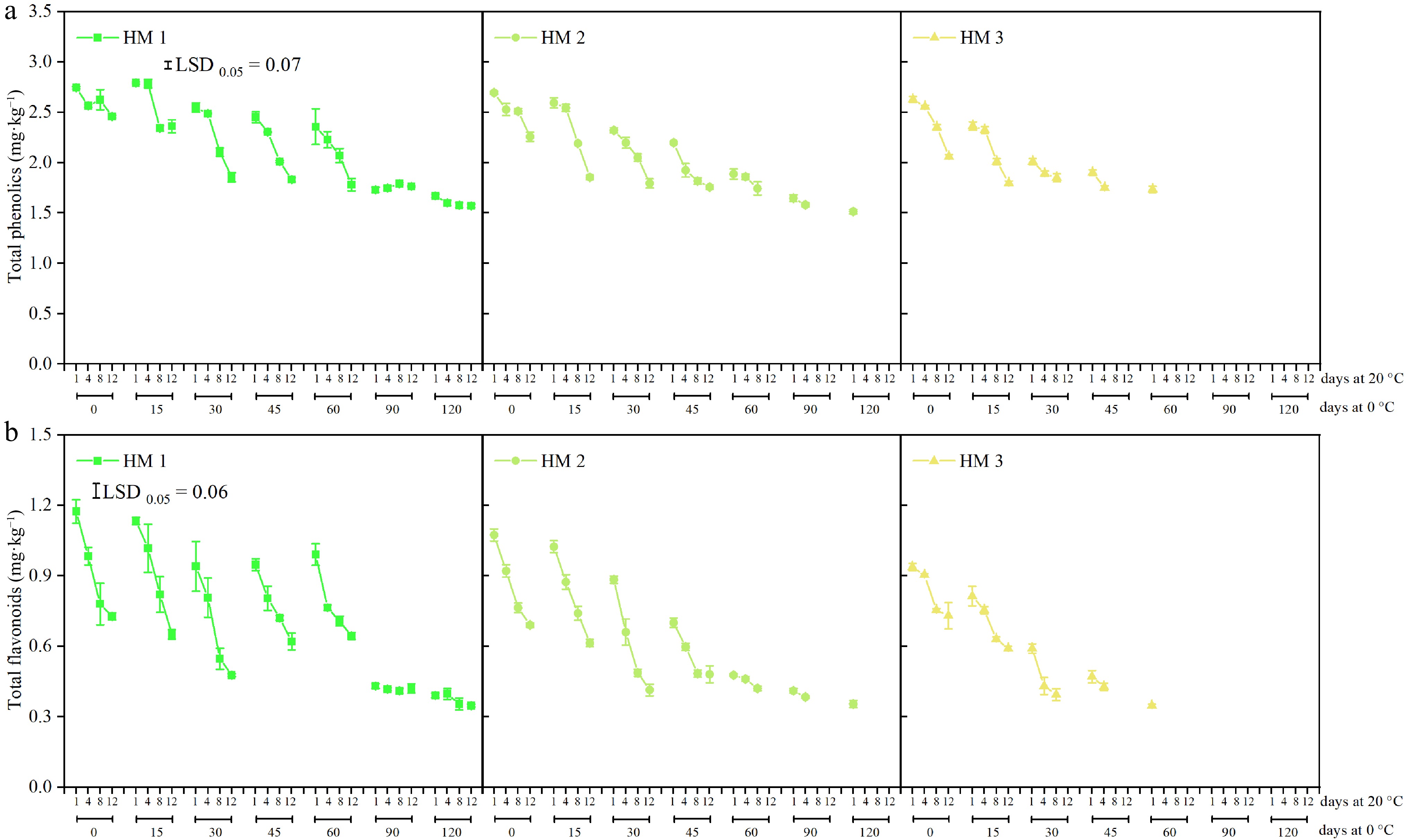
Figure 3.
(a) Total phenolics (TP), and (b) flavonoids (TF) of 'Ruaner' pears with three harvest maturities (HM1, HM2, and HM3) after 0, 15, 30, 45, 60, 90, and 120 d of cold storage (0 °C) plus 1, 4, 8, and 12 d at shelf (20 °C). Values are presented as the means ± SD.
Effect of harvest maturity on antioxidant capacity of 'Ruaner' pears after storage and during shelf
-
Comparable to those on TP and TF, HM1, HM2, and HM3 pears showed comparable patterns in their capacity to scavenge DPPH free radicals and FRAP (Fig. 4a & b). After the same periods of cold storage and during shelf, more mature pears were more susceptible to losing their capacity to scavenge DPPH free radicals and FRAP. Following 90 and 120 d of cold storage plus 1−12 d at shelf, after 90 d of cold storage plus 1−4 d at 20 °C, and after 30 and 45 d of cold storage plus 1−8 and 1−4 d at 20 °C, respectively, no differences were found in the fruit's capacity to scavenge DPPH free radicals in HM1, HM2, or HM3 fruit. For FRAP, no difference was observed in HM1 or HM2 pears after 90 and 120 d of storage at 0 °C plus 1−12 d at shelf and after 90 d of storage at 0 °C plus 1−4 d at shelf.
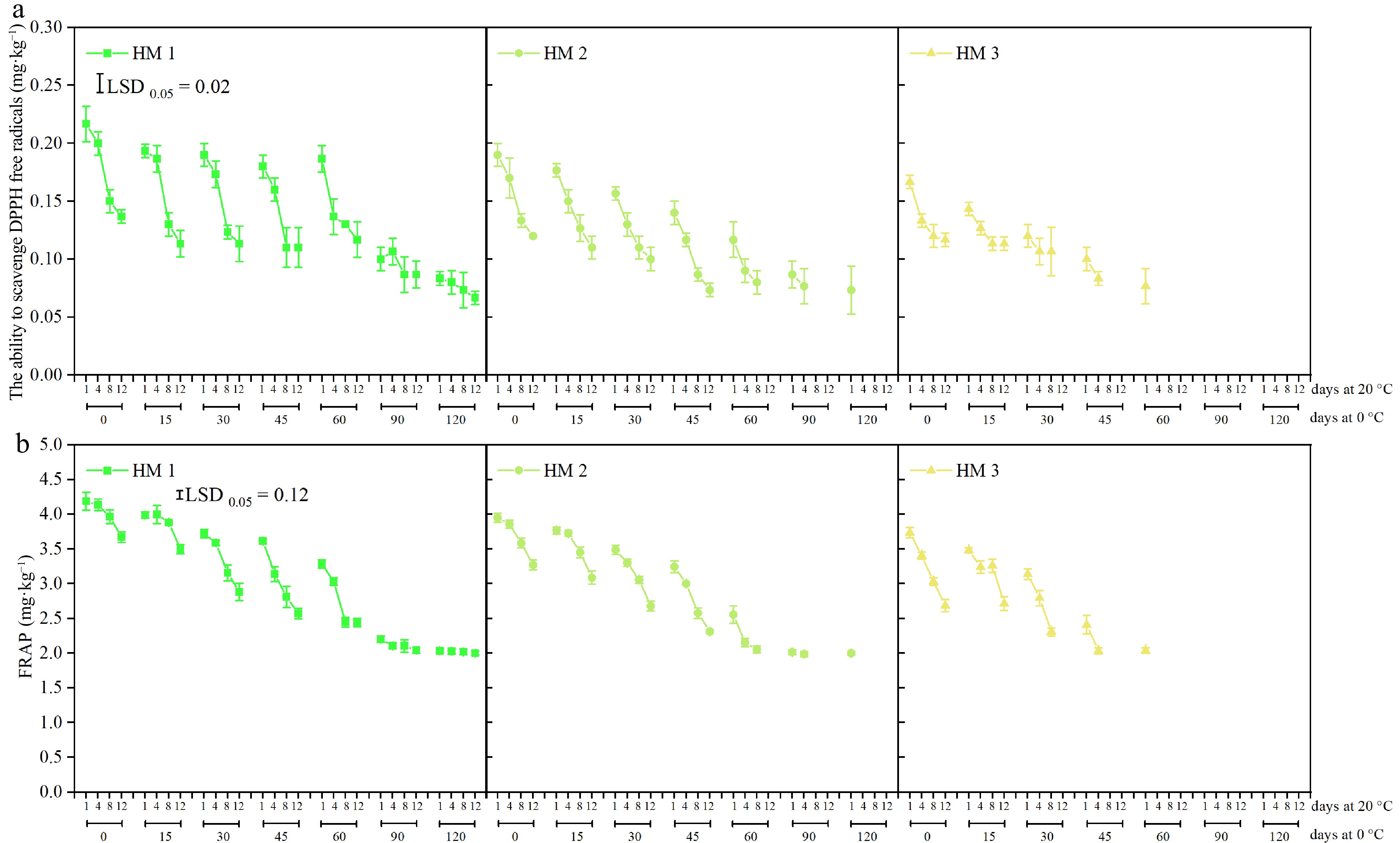
Figure 4.
The ability to (a) scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals, and (b) ferric reducing antioxidant power (FRAP) of 'Ruaner' pears with three harvest maturities (HM1, HM2, and HM3) after 0, 15, 30, 45, 60, 90, and 120 d of cold storage (0 °C) plus 1, 4, 8, and 12 d at shelf (20 °C). Values are presented as the means ± SD.
Major factors influenced the storage and shelf in three HM 'Ruaner' pears
-
Irrespective of harvest maturity, high positive correlation coefficients were found between decay and SSC, decay and SSTQ (sensory score of textural quality), FF and HA (hue angle), FF and TA, FF and TP, FF and TF, FF and DPPH, FF and FRAP, HA and TA, HA and TP, HA and TF, HA and DPPH, HA and FRAP, TA and TP, TA and TF, TA and DPPH, TA and FRAP, TP and TF, TP and DPPH, TP and FRAP, TF and DPPH, TF and FRAP, as well as between DPPH and FRAP (Table 1). By contrast, high negative correlation coefficients were found between decay and FF, decay and HA, decay and TA, decay and TP, decay and TF, decay and DPPH, decay and FRAP, FF and SSC, FF and SSTQ, HA and SSC, HA and SSTQ, SSC and TA, SSC and TP, SSC and TF, SSC and DPPH, SSC and FRAP, SSTQ and TP, SSTQ and TF, SSTQ and DPPH, as well as between SSTQ and FRAP.
Table 1. Correlation analysis of decay, flesh firmness, hue angle, soluble solids content, titratable acidity, sensory score of textural quality, total phenolics, total flavonoids, the ability to scavenge DPPH free radicals, and ferric reducing antioxidant power of 'Ruaner' pears with three harvest maturities (HM1, HM2, and HM3) after 0, 15, 30, 45, 60, 90, and 120 d of storage at 0 °C plus 1, 4, 8, and 12 d at 20 °C.
Decay FF HA SSC TA SSTQ TP TF DPPH FRAP Decay FF −0.408* HA −0.726* 0.707* SSC 0.551* −0.361* −0.594* TA −0.781* 0.342* 0.756* −0.435* SSTQ 0.310* −0.970* −0.593* 0.205 −0.225 TP −0.724* 0.497* 0.890* −0.451* 0.876* −0.391* TF −0.704* 0.557* 0.853* −0.455* 0.869* −0.440* 0.936* DPPH −0.725* 0.631* 0.901* −0.506* 0.811* −0.523* 0.909* 0.943* FRAP −0.711* 0.430* 0.870* −0.489* 0.869* −0.314* 0.942* 0.897* 0.886* FF, flesh firmness; HA, hue angle; SSC, soluble solids content; TA, titratable acidity; SSTQ, sensory score of textural quality; TP, total phenolics; TF, total flavonoids; DPPH, the ability to scavenge DPPH free radicals; FRAP, ferric reducing antioxidant power. * indicates significant p < 0.05. The above results show that high correlation coefficients were observed among decay, TA, hue angle, antioxidants, and antioxidant capacity in all HM fruit. However, it is highly challenging to measure antioxidants and antioxidant capacity in practice for growers to predict the optimum storage and shelf life for 'Ruaner' pears. Additionally, all TP (r = 0.966 in HM1, r = 0.963 in HM2, and r = 0.915 in HM3), TF (r = 0.922 in HM1, r = 0.953 in HM2, and r = 0.958 in HM3), DPPH (r = 0.919 in HM1, r = 0.949 in HM2, and r = 0.877 in HM3), and FRAP (r = 0.935 in HM1, r = 0.966 in HM2, and r = 0.913 in HM3) showed a high positive correlation with TA in all fruit. Therefore, using these significant variables, such as decay, hue angle, and TA, might be effective in helping growers promote the lifespan of 'Ruaner' pears. The polynomial fit analysis and 3D scatter plot of decay, hue angle, and TA in HM1, HM2, and HM3 fruit indicated that fruit with skin color (h*) > 98 and TA > 0.45% would develop a low rate of decay (< 60%) during storage and shelf (Fig. 5a & b); furthermore, these pears maintained relatively higher antioxidants and antioxidant capacity.
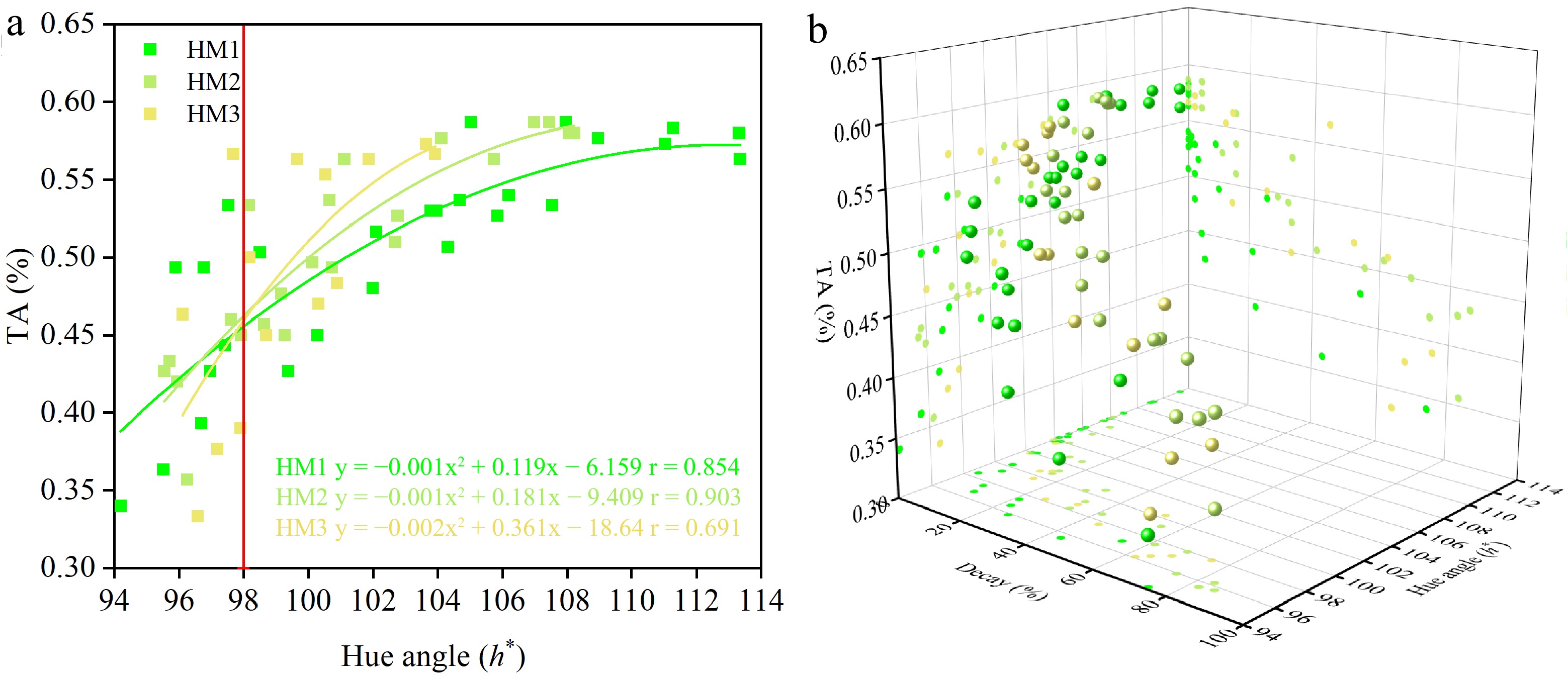
Figure 5.
Changes between (a) titratable acidity (TA) and hue angle, and (b) three-dimensional (3D) scatter plot of decay, hue angle, and TA in 'Ruaner' pears with three harvest maturities (HM1, HM2, and HM3) after 0, 15, 30, 45, 60, 90, and 120 d of cold storage (0 °C) plus 1, 4, 8, and 12 d at shelf (20 °C).
-
For Pyrus ussuriensis Maxim., decay is a major postharvest fruit loss in the supply chain from the farm to the table[3,19]. Furthermore, both retailers and consumers have no tolerance for rotten pears at retail. From an appearance standpoint, the decay occurring in more European pears (Pyrus communis L.) cultivars during storage was strongly associated with decreasing flesh firmness at harvest[6,20,21]. Similar to the present results, more mature pears, such as HM3 fruit, rotted earlier and developed a higher rate of decay incidence during storage and shelf compared to HM1 and HM2 pears, confirming that harvest maturity in 'Ruaner' pears was attributed to the development of decay (Fig. 1). One possible explanation for this phenomenon was that more pectic polyuronides (i.e., homogalacturonan, rahamnogalacturonan-I, and rhamnogalacturonan-II) were degraded in the over-mature pears than the optimum-mature and immature fruit[6,17,22,23], and the solubilization and depolymerization of pectin polyuronides combined with a high SSC in pears could be used as a carbon source by fungi[24]. Although more mature pears showed a bigger size[4], results from the current study corroborated the present practice in local packers that the recommendation for harvesting 'Ruaner' pears should be at a maturity level of > 57 N; this maturity level resulted in a low rate of decay during storage at 0 °C and a long storage lifespan of up to 90 d. To verify this recommendation, pears were harvested at 57.88 ± 3.68 N in 2023 and then evaluated for decay incidence and storage quality attributes during storage and shelf life (Supplementary Table S1); results further confirmed that these pears with FF > 57 N had as long as 90 d of storage life at 0 °C and a 4-d shelf life.
When pears were harvested on Oct. 4, the FF of 'Ruaner' pears dropped from 22.5 N to 9.6 N after 29 d of storage at 8 °C[3]. However, increasing FF at harvest and reducing storage temperature to 0 °C in this study provided greater benefits in reducing FF and extending the lifespan of pears during storage (Fig. 2a). For example, the HM3 pears harvested at 38.62 N (Sep. 28) dropped to 28.35 N after 60 d of cold storage. While the HM1 pears harvested at 67.12 N (Sep. 8) dropped to 43.74 N after 120 d of cold storage. However, the fundamental issue for packers is to lengthen pear storage life without losing qualities such as FF, high levels of sugar and acid, and a dark green color[4,25,26]. Regardless of harvest maturity, 'Ruaner' pears were more susceptible to yellowing and TA loss, with great sensitivity to fungi infection and producing a low-texture sensory characteristic after long-term cold storage and shelf testing (Fig. 2). Furthermore, significant negative correlations were found between decay and hue angle and between decay and TA (Table 1), confirming that susceptibility to yellowing, FF and TA losses, and decay were intrinsic characteristics of 'Ruaner' pears. Therefore, controlling fruit maturity at harvest and maintaining low-temperature storage conditions seem to be implicated in the fast degradation of the quality in 'Ruaner' pears[4,6,7,26,27].
Impact of harvest maturity on the antioxidant properties of 'Ruaner' pears
-
It is well known that increased postharvest disorders are a response to reduced antioxidant systems (including antioxidant enzymes, antioxidants, and antioxidant capacity), which might be affected by harvest maturity[28−30]. In sweet cherries, fruit with high maturation (harvested 4 d after the commercial harvest date) had greater phenolics, total antioxidant activity, and anthocyanins than the other two early harvest maturity cherries after 16 d of storage[31]. In contrast, both enzymatic and non-enzymatic mechanisms that catabolize reactive oxygen species (ROS) decreased after storage when the harvest of pear fruit was delayed[28]. The present results support this notion in 'Ruaner', when pears were harvested at low FF (i.e., HM3), low levels of four antioxidant properties with a high decay incidence were observed (Figs 1, 3 & 4) after storage and during shelf. Furthermore, strong negative correlations were observed between decay and four antioxidant properties in all HM pears (Table 1), indicating that both high maturity and long-term storage curtailed the 'Ruaner' pears' ability to scavenge the ROS; as a result, the high accumulation of ROS caused damage to the cells, early triggering or aggravating the development of fungi infection. Unfortunately, these pears with low levels of four antioxidant properties and high incidences of disorders would be rejected by retailers or sent back to packers because they are unmarketable to consumers[32]. Furthermore, the traditional consumption of 'Ruaner' pears by locals resulted in the delay in harvesting 'Ruaner' pears[33]. For example, the local consumers preferred to consume the yellowing 'Ruaner' pears due to the high level of sugar and soft texture; however, the FF of these pears was below 24 N (Dong & Zhi, unpublished data), where the maturity of these pears was lower than the HM3 fruit in this study.
Regardless of harvest maturity, the strong negative correlations between the decay and hue angle, decay and TA, decay and four antioxidant properties (TP, TF, DPPH, and FRAP) were shown in this study (Table 1). Notably, strong positive correlations were found among hue angle, TA, antioxidants, and antioxidant capacity, suggesting that analyzing the variables of hue angle and TA might help determine the development of decay and levels of antioxidants and antioxidant capacity in 'Ruaner' pears during storage and shelf. As shown in Fig. 5, the polynomial fit analysis and 3D scatter plot showed that when 'Ruaner' pears developed a hue angle > 98 and accumulated TA > 0.45%, the decay incident could be controlled below 60% after storage and during shelf testing. Similar results were observed in 2023 (Supplementary Table S1). Therefore, skin color and TA may be two critical indexes beyond maturity based on FF to determine the development of decay and antioxidant levels in stored 'Ruaner' pears.
-
The current study's findings showed that the storage life of HM1, HM2, and HM3 pears might be increased from 60 to 120 d by storing them at 0 °C. Furthermore, 'Ruaner' pears with maturity (as indicated by FF) > 57 N effectively reduced fruit susceptibility to decay, maintained storage attributes (i.e., FF, green color, and TA) and desirable eating quality, and suppressed the losses in TP, TF, the ability to scavenge DPPH free radicals, and FRAP. Additionally, regardless of the harvest maturity, the development of decay in 'Ruaner' pears was strongly associated with the losses in hue angle, TA, antioxidants, and antioxidant capacity; positive correlations were observed among hue angle, TA, antioxidants, and antioxidant capacity. For optimum utilization of 'Ruaner' pears, harvesting fruit with hue angle > 98 and accumulated TA > 0.45%, the decay of these pears could be controlled after cold storage and during shelf life.
We are grateful to the Construction Project for Innovation Platform of Qinghai Province – Laboratory for Research and Utilization of Qinghai – Tibetan Plateau Germplasm Resources (2024) for financial support.
-
The authors confirm contribution to the paper as follows: methodology, visualization: Zhi H, Wang L; software, formal analysis, writing - original draft: Zhi H, Wang L, Jiang L; validation, investigation, resources, writing - review & editing, supervision, project administration: Dong Y. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Huanhuan Zhi, Lei Wang
- Supplementary Table S1 Decay incidence, flesh firmness (FF), hue angle, and titratable acidity (TA) of 'Ruaner' pears after 60, 90, and 120 d of cold storage at 0 °C plus 1, 4, 8, and 12 d at 20 °C in 2023.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhi H, Wang L, Jiang L, Dong Y. 2024. Harvest maturity in relation to decay, quality attributes, and antioxidant properties of 'Ruaner' pears (Pyrus ussuriensis) after cold storage and at shelf. Technology in Horticulture 4: e030 doi: 10.48130/tihort-0024-0028
Harvest maturity in relation to decay, quality attributes, and antioxidant properties of 'Ruaner' pears (Pyrus ussuriensis) after cold storage and at shelf
- Received: 16 August 2024
- Revised: 01 October 2024
- Accepted: 10 October 2024
- Published online: 02 December 2024
Abstract: 'Ruaner' pears (Pyrus ussuriensis Maxim.) develop rapid decay and have a short storage life due in part to improper harvest maturity and storage conditions. This study aimed to assess whether harvest maturities (HM) affected the development of decay, quality attributes, and antioxidant properties (including antioxidants and antioxidant capacity) in 'Ruaner' pears following cold storage (0 °C) and being subjected to 20 °C; furthermore, the major factors affecting the development of decay were investigated when storing these pears. Results showed that, compared to the pears with maturity (flesh firmness, FF) at 57.12 (HM2) and 68.22 (HM1) N at harvest, the high-maturity fruit (39.14 N, HM3) had a shorter storage life and were more susceptible to decay with poor storage and eating quality attributes and low antioxidant levels and antioxidant capacity during storage and shelf life. Additionally, high negative correlations were observed between decay and skin color (hue angle), decay and titratable acidity (TA), and decay and four antioxidant properties in all pears, while strong positive correlations were observed among hue angle, TA, and four antioxidant properties. When pears developed a hue angle > 98 and TA > 0.45%, the decay could be controlled below 60% during storage and shelf life. Overall, 'Ruaner' pears with FF > 57 N had a long storage life, with high levels of storage quality characteristics, antioxidants, and antioxidant capacity, as well as a low rate of decay following 90 d or more of storage at 0 °C.
-
Key words:
- Pyrus ussuriensis Maxim. /
- Harvest maturity /
- Decay /
- Quality /
- Antioxidants /
- Antioxidant capacity













