-
Named in honor of the distinguished physician to Emperor Augustus, Antonio Musa, Musa spp., colloquially referred to as the banana, stands as a genus of monocotyledonous arborescent herbaceous perennial flowering plants within the Musaceae family, situated within the taxonomical order Zingiberales[1, 2]. Bananas are predominantly cultivated in equatorial and subtropical regions across the globe, spanning from the Southeast Asia-New Guinea territory to select segments of the South Pacific and West Africa[3]. This fruit is a fundamental dietary staple for many populations and exhibits year-round availability. Noteworthy for its widespread global production, trade, and consumption, the bananas that fill the shelves of supermarkets in the European Union, the United States, China, Russia, and Japan are primarily sourced from Central and South America, as well as the Philippines[4].
Banana fruits are sources of vital minerals and phytochemical compounds, rendering them nutritionally significant. At the same time, the cultivation of banana plants offers economic opportunities, particularly in resource-constrained local communities. Within the Musaceae family, banana plants represent monoecious perennial herbaceous specimens, characterized by a stature spanning 10 to 30 feet[5] Their basal, unbranched, spiral leaves are conspicuous, with certain individuals exhibiting spiral-arranged leaf sheaths. The pseudostem, sympodial in nature, is subterranean and undergoes senescence following the fruiting phase[3]. The epigynous and ebracteate flowers yield nectar from sepal nectaries, while the elongated fruit, classified as a berry, harbors endospermic seeds, typically disseminated through avian and chiropteran vectors in their natural habitat[2]. Presently, the Musa genus encompasses approximately 70 recognized species, offering diverse applications, including ornamental use, utilization of plant components as animal feed, and the consumption of fruit as a reliable source of essential vitamins and minerals. Moreover, globally distributed banana plantations, particularly in developing nations, serve as stable sources of income for local communities and integral components of export-oriented agricultural establishments.
Banana production confronts different challenges, both biotic. Plant stressors adversely affect fruit output, influencing yield and, consequently, growers' financial returns. Within the realm of biotic stressors, fungi occupy a significant presence and are frequently implicated in various plant diseases[6]. The most formidable menace to the banana industry is the Panama wilt of the Fusarium wilt Tropical Race 4 (TR), a fungus that causes wilting and death of the plant, making Sigatoka disease a significant threat to banana production worldwide. Global banana exports, excluding plantains, achieved a record-breaking production level of 20.2 million tons in 2019 with two major producers, namely Ecuador and the Philippines. In 2019, worldwide banana export volumes encompassed approximately 18.9 million tons[7]. However, Panama disease has caused the destruction of over 15,000 hectares of Cavendish bananas in the Davao region, which serves as the primary cultivation area for the Philippines' export-grade banana production[8]. The Sigatoka leaf complex leads to diminished photosynthetic function of the leaves and, in severe cases, defoliation. As a result, the infected plant will have a reduced fruit bunch yield and produce fruits that are smaller and prematurely ripened[9]. The disease has the potential to reduce yields by as much as 90%. It can trigger premature ripening, which poses a significant challenge for a fruit typically harvested unripe and subsequently subjected to artificial ripening during transportation or within controlled industrial greenhouses[10, 11] making it a prominent disease worldwide.
This paper aims to provide a comprehensive overview of the Sigatoka leaf spot complex, elucidating the associated fungal pathogens, factors influencing disease prevalence and dissemination, and strategies for its effective management. This information augments the current comprehension of the causative agents responsible for this disease and delineates practices that can be employed to mitigate its impact and introduce effective and sustainable disease control methodologies.
-
Bananas, like any other food crops grown worldwide, are not exempt from being susceptible to several diseases, which pose a serious threat to the industry. Frequently reported foliar diseases include the Sigatoka leaf spot complex.
Pseudocercospora (family: Mycosphaerellaceae; order: Mycosphaerellales) is a genus of ascomycetous fungi with cosmopolitan species usually growing under a wide range of climatic conditions and occurring on a wide variety of plant hosts as either phytopathogens, endophytes, saprobes, or biological control of weeds[12,13]. This genus has previously been associated with the anamorphic or asexual state of the genus Mycosphaerella (e.g., the anamorph P. fijiensis for the teleomorph M. fijiensis) but was now recognized as a holomorphic genus in compliance with the recent modification of the International Code of Nomenclature for algae, fungi, and plants (ICN) in 2011 during the annual Botanical Congress in Australia[14]. As an important pathogenic fungus of plants, some of the diseases caused by Pseudocercospora spp. are P. vitis which causes leaf blights and premature defoliation on grapes (Vitis sp.), P. graseola as the causal agent for angular leaf spots of common bean (Phaseolus vulgaris), as well as the P. ulei of the Para rubber tree (Hevea brasiliensis) which affects the natural rubber production due to the South American Leaf Blight (SALB) disease[15].
The Sigatoka leaf spot complex, all of which have appeared in the center of the diversity of bananas − the Southeast Asian-Australasian region, are associated with the three phylogenetically distinct Pseudocercospora (anamorph; teleomorph Mycosphaerella) species which previously followed a dual nomenclature system. These are the P. fijiensis (causal agent of black Sigatoka or black leaf streak), P. musae (causal agent of yellow Sigatoka), and P. eumusae (causal agent of Eumusae leaf spot) that follows a hemibiotrophic life cycle with similar, often confounded, disease progression and are believed to have host range that is only specific to bananas. Furthermore, they originated from the same common ancestor as the result of a multilocus DNA analysis revealed that the three form a monophyletic group[16]. The black Sigatoka is considered the most aggressive and economically significant fungal disease among the three that causes a serious threat to global food security, and P. musae is the least harmful[3, 14, 17, 18].
Black Sigatoka or Black Leaf Streak (BLS)
-
First described in Fiji in 1963 and a common disease of bananas in warmer environments[19], the black Sigatoka or Black Leaf Streak (BLS) is the most recognized foliar fungal disease of bananas due to its ability to infect a wider range of banana cultivars among the three, constraining the banana production globally due to its deteriorating effects not just on the leaf portion of the plant but also to the fruit[16]. Caused by the P. fijiensis, the progress of the disease follows a six-stage symptom development in accordance to Fouré in 1987[3]: (1) minute whitish or yellowish specks observed on the underside of the leaf which then turn into 1 mm long rusty brown specks that are invisible in translucent light; (2) observable reddish-brown to dark brown streaks on the underside that are 2−5 mm in length that are now visible in translucent light, with the corresponding upper portion of the leaf turning brown to black; (3) dark brown streaks become longer, reaching about 20−30 mm in length and the fusion of streaks to form leaf necrosis under favorable climatic conditions and inoculum potential; (4) streaks that did not merge form a spot, with brown coloration on the underside and black coloration on the upper side of the leaf; (5) the spot will turn elliptical and both sides of the leaf will have black coloration that is usually surrounded by a yellow halo with a flattened center; and (6) drying of the center of the spot which then turns into a fading whitish to clear gray with a distinct black border that will remain visible after the leaf has died and dried (Fig. 1).
Due to its short life cycle, high-volume sporulation, and capacity for sexual reproduction, P. fijiensis is considered a high-risk pathogen with a maximal effect on the banana crop yield and fruit quality[20]. Both the conidia (asexual spores) and ascospores (sexual spores) infect the leaf stomata and play a role in the spread of the disease − the conidia are transmitted through rainwater and are essential for the local spread of the disease; ascospores are the primary method for disease spread through wind and water splashes over long distances[3, 19].
Yellow Sigatoka
-
Another fungal disease of banana leaves commonly occurring in cooler and drier banana-growing environments and at higher altitudes[3, 21] and the first of the Sigatoka leaf spot disease complex to be recorded in Java, Indonesia in 1902, in the Philippines in 1921, and in Africa in 1938[3,17], the Yellow Sigatoka with P. musae as its causative agent was the first most important foliar fungal disease of the banana plant until the discovery and replacement of the more virulent and competitive Black Sigatoka. The effects and symptom development of this disease have similarities with the Black Sigatoka such as the necrosis and premature death of leaf tissues as well as premature and uneven ripening of the fruit which has a higher risk potential for postharvest infection, except for the progress of the coloration of the specks to streaks to spots[3]. The progression of the disease begins with the appearance of narrow, light green specks of about 1 mm in height on the upper surface of the leaf. The specks then elongate into streaks running parallel to the leaf veins that are much longer and wider, usually several millimeters long and 1 mm in width. Change in the coloration of the streak from light green to rusty red or rusty brown, and its lateral expansion to become elliptical are observed in Stage 3 of the disease progression. This is then followed by Stage 4 wherein a more defined elliptical streak is formed together with young spots with a sunken, dark brown center. The streaks are usually surrounded by a yellow halo and the production of conidiophores and conidia. The last stage of the disease is Stage 5 with a gray, dried-out center and a pronounced darker brown halo or margin which creates a defined ring that is still visible even after the death of leaf tissues[3, 20] (Fig. 1).
Moreover, a film of dew or rainwater can induce conidiophore formation and ascospore germination. The conidia or the asexual spore are transmitted to adjacent leaves of the same plant or the neighboring plants via rainwater or wind. Factors such as favorable environmental temperature and the susceptibility of the banana cultivars can trigger the intensity of the disease in plantations, with the Yellow Sigatoka specks appearing on younger and younger leaves emerging from the pseudostem[3].
Eumusae leaf spot
-
Finally, the third fungal disease associated with the Sigatoka leaf spot complex is the Eumusae leaf spot with P. eumusae as its causative agent. This disease has been particularly dominant in Asian countries since its first discovery in Southern and Southeast Asia in the 1990s. It is known to infect banana cultivars in Sri Lanka and Thailand which are known to be resistant to P. fijiensis and P. musae[16]. The symptom progression of the disease is similar to that of the Black and Yellow Sigatoka. The initial obvious symptom is a small yellow speck that broadens and lengthens into light yellow streaks that expand into a spot and darken into dark brown. The development of a dark brown border will be observed as the center of the spot turns gray. Additionally, the density of the infection affects the appearance of the symptom on the leaf in the later stages of the disease: a low infection density will create an individual mature spot that is ovoid with the surrounding chlorotic halo; a high infection density will produce a coalescence on the developing lesions and the surrounding leaf tissues becoming necrotic with a yellow margin as a border[3].
-
The Sigatoka leaf spot complex is a significant threat to banana production worldwide. Black Sigatoka for instance, in a study by Strobl & Mohan[22], was reported to have significantly increased in occurrence and distribution over the years. At the start, the disease was present in only 4.4% of the affected areas, but it rose steadily to over 53% by 2016. The disease was mainly present in the United States (Hawaii) and small parts of Asia and the South Pacific in 1961, had spread more widely across Asia and began to appear in Africa by 1980, and spread to the South American Continent and the Caribbean, as well as expanded more into Africa by 1999. The socio-economic impact of the Sigatoka disease complex is much higher in small farming communities in sub-Saharan Africa, Southeast Asia, and Latin America that depend almost exclusively on the banana crop for their survival[22]. In a review by Henderson et al., the worldwide distribution of Yellow Sigatoka was said to be first recorded in Java and later in the Sigatoka Valley in 1912 on the island of Viti Levu, Fiji as hypothesized by Stover (1962)[23,24]. Later on, the disease was observed in Australian banana plantations in 1924 and made its way to Surinam and Trinidad in 1934. By 1937, it had disseminated to encompass a majority of the Caribbean, Central America, Colombia, and Venezuela. In a relatively short period, the disease extended its presence to neighboring countries, with Brazil (1944), Peru (1946), and Ecuador (1952) becoming affected. Yellow Sigatoka first appeared in Africa in 1938, with Uganda as its initial detection point. Herbarium specimens indicated that Sigatoka was present in Sri Lanka in 1919 and the Philippines in 1921[23, 24]. Presently, Yellow Sigatoka is regarded as having a worldwide distribution, although it has not been recorded in the Canary Islands, Egypt, or Israel[25]. The Eumusae leaf spot was first reported from southern and southeast Asia in the late 1990s[26]. Surveys in India and Southeast Asia suggest that the disease is widespread and significant[27].
Given the challenge of distinguishing M. eumusae from M. fijiensis and M. musicola, it is assumed that the actual extent of this distribution is considerably wider than what is presently documented.
Factors influencing the incidence of Sigatoka leaf spot complex
Environmental conditions
-
After its first reported observation in Fiji in 1963, Black Sigatoka (or BLS) has replaced Yellow Sigatoka as the predominant foliar fungal disease of bananas. As Burt, and also Jones mentioned, it was favorable for the disease to occur in areas of high relative humidity and a tropical temperature pattern, typically in the lowlands. This condition best induces ascospore germination, the primary means of disease spread and infection on the leaf tissue[3, 21]. In the Philippines, a tropical country, the disease is highly suitable and can persist under natural rainfall and irrigation scenarios, except for high-altitude areas such as in Mountain Province[28]. Furthermore, the expansion of the leaf streak and production of ascospore in the coastal tropics was higher with Black Sigatoka than with Yellow Sigatoka - probably an advantage since water is needed for the spread and infection of the disease[3]. Recent studies have shown that Black Sigatoka can thrive at higher altitudes already, in Costa Rica at 900−1,500 m above sea level, at 1,600 m above sea level in Colombia, and in Uganda and Tanzania as high as 1,800 m above sea level; an expanding adaptation range of the pathogen which threatens banana cultivars present at high altitudes and the possible gradual displacement of P. musae[17].
Meanwhile, the Yellow Sigatoka is more prevalent at cooler temperatures and higher altitudes, usually at 1,350 m above sea level[17]. In a study conducted by Khan et al.[29], in Bangladesh on the severity of the disease on banana suckers, results revealed that the highest incidence and severity occurred at a temperature of 27.18 C, rainfall of 54.80 mm, and relative humidity of 79.83%. On the other hand, the lowest incidence and severity were reported for temperature, rainfall, and relative humidity of 17.38 C, 14.75 mm, and 77.25%, respectively. Further understanding of the result presented pointed out the importance of leaf wetness and relative humidity on the symptom appearance and infection, having similar concerns with Black Sigatoka[29]. While ascospore germination and growth as a source of inoculum are greatly observed during the rainy season, there is a conversing article by Freitas et al., in Brazil wherein slower progress on the disease development was observed during the rainy season and higher progress during the dry season; possible reasons may be due to longer incubation periods, spore latency, and higher rates of leaf initiation during the rainy season, and a lower leaf initiation rate during the dry season[30].
Due to the limitation on published articles, there is no written information about the environmental climatic conditions affecting the incidence of Eumusae leaf spot. However, since the coexistence of the same lesion can happen for the three phytopathogenic species or the misidentification of P. eumusae to P. fijiensis and P. musae, therefore, suggesting a more comprehensive report on the epidemiology of the pathogens in major banana-growing regions of the world[31].
Variety of banana cultivars
-
Infecting a wider range of banana cultivars in major banana-growing regions of the world compared to P. musae, P. fijiensis possesses more considerable damage to banana plantations and thus affects global food security[16]. The Cavendish (e.g., 'Dwarf Cavendish', 'Gran Naine', 'Williams' and 'Robusta) and Plantain subgroups of banana cultivars are known to be highly susceptible to Black Sigatoka, although local isolates of the pathogen were observed to have varying levels of pathogenicity or aggressiveness; such as in Papua New Guinea, Honduras, and Nigeria. For example, the Obino I'Ewai' (AAB, Plantain Subgroup) is a susceptible cultivar, and the Calcutta 4 (AAw accession) or the Musa acuminata ssp. Burmannica, the Pisang Lilin' (AA), and 'Tuu Gia' (AA) are highly resistant cultivars. In addition to that the Pisang Mas' (AA, Sucrier subgroup) with moderate partial resistance, and the 'Pisang Ceylan' (AAB, Mysore subgroup) and 'Fougamou' (ABB, Pisang Awak subgroup) have a strong partial resistance[3].
In Uganda and Tanzania, the most common banana varieties of AAA (EAHB-cooking) and AAAB (FHIA hybrids, FHIA 17 and FHIA 23) were the most susceptible. However, breakdown of resistance was also observed on the highly resistant cultivar of Yangambi KM5 (AAA), the first reported case in the variety[17] as a result of modifying the nutritional status of plant and/or some epidemiological factors that are linked to agricultural practices, and the 'Paka' (AA), FHIA 18 (AAAB), and the Jamaican-bred tetraploid 'T8' (AAAA) in East Africa after exposure to Black Sigatoka for eight years. This was probably due to the failure of single-gene conditioning resistance[3].
In India, the presence and disease severity of the Yellow Sigatoka is highest among the cultivars of the important Cavendish (AAA) group, particularly the 'Grand Naine', 'Robusta', 'Gros Michel', 'Lakatan', 'Pisang Susu' and Ney Poovan (AB) group, that are categorized as "Susceptible" and "Partial Resistance" to Black Sigatoka, respectively[3,32]. M. schizocarpa, M. balbisiana and M. acuminata ssp. malaccensis, microcarpa, siamea, and truncata are wild varieties of Musa under the Eumusae section; among the varieties mentioned, only the M. siamea is susceptible while the rest are highly resistant to the P. musae. Numerous cultivars were observed to be highly resistant to P. musae, such as all the cultivars in the Australimusa section (M. jackeyi, M. textilis, M. maclayi ssp. ailuluai, M. maclayi ssp. maclayi var. maclayi, M. peekelii ssp. peekelii, and M. peekelii ssp. angustigemma), M. ornata of the Rhodochlamys, 'Pisang Lilin', 'Pisang Tongat', 'Paka' and 'Tuu Gia' of the 'Pisang Lilin' subgroup (AA), 'Yangambi KM5', Plantains subgroup (AAB) near sea level, and the 'Bluggoe', 'Pisang Awak', 'Kluai Teparot' of the ABB, among others. On the other hand, 'Sucrier' and 'Inarnibal' (AA), and SH-3142 and SH-3362 (diploids) together with the 'Goldfinger' / 'FHIA-01 (AAAB; tetraploid) of the Honduran breeding programs were evaluated as highly susceptible[3].
Finally, for the Eumusae leaf spot with the P. eumusae as the causative agent, the Cavendish subgroup (AAA) was seen to possess the Eumusae-like leaf symptoms, but with varying levels of susceptibility; Williams' are considered highly susceptible, while the 'Petite Naine' (AAA), 'French Clair' (AAB), 'GCTCV-119' (somaclonal variant AAA from 'Giant Cavendish') and 'FHIA-18' (bred AAAB Pome-type hybrid) were evaluated to be susceptible. Conversely, 'Pisang Ceylan' (AAB, Mysore subgroup) and 'FHIA-21' (bred AAAB Plantain-type hybrid) are resistant, and 'Yangami KM5' (AAA, Ibota subgroup) is highly resistant; it was worth noting however that the Mysore subgroup in India and Sri Lanka was labeled as susceptible. Furthermore, a disease severity comparison was created in Nigeria between P. fijiensis and P. eumusae with the following list of banana cultivars: 'Dwarf Valery' (AAA, Cavendish subgroup), 'Agbagba' (AAB, Plantain subgroup) and the 'Calcutta' (AAw). Results have revealed that the severity of the disease was higher for the three cultivars inoculated with P. eumusae mycelial suspension than those with P. fijiensis[29].
Identification of Pseudocercospora species
-
Disease progression and the appearance of the symptoms were similar and confounded for the three fungal diseases, a problem often encountered by researchers in the quest for accurate identification of the Pseudocercospora species. Exacerbating the problems of confounding symptoms, there are challenges in culturing these pathogens in an artificial media in the laboratory. Despite their preferred minor environmental conditions, coexistence in the same lesion is observed among the pathogens[33].
For pathogen recognition, focusing on the development of specific molecular-based diagnostic tools should be considered in addition to morphological characterization. The development of an enzyme-linked immunosorbent assay (ELISA) for the detection of P. fijiensis was an essential tool for disease forecasting and management strategies as it can detect the pathogen as early as two weeks before the appearance of symptoms. However, the limiting factor of the tool is the identification at the specific species level of the target genus, Pseudocercospora. Although the development of Polymerase Chain Reaction - Restriction Fragment Length Polymorphism (PCR-RFLP) and microsatellite markers became important, they are still unsuitable as diagnostic markers. To address this problem, the first PCR-based diagnostic assay designed for the internal transcribed spacer (ITS1) region of the fungal rDNA with species-specific primers capable of differentiating P. fijiensis and P. musae was generated[14, 23] as well as confirmation of the presence of P. eumusae on the collected local isolates in India[33].
In a study on the Sigatoka leaf spot disease observed in South Africa, the combined Polymerase Chain Reaction (PCR) with species-specific primers, sequencing of the ITS region of isolated South African pathogens, and morphological studies verified the existence of P. musae (or M. musicola) which was previously identified as either the M. fijiensis and M. eumusae species[34]. Another study used the conventional PCR method in India and found Eumusae leaf spot as the dominant leaf spot disease in the country. PCR amplification of the target ITS-rDNA region using the combination of specific primer and sequencing confirmed the presence of the P. eumusae causing the Eumusae leaf spot in the agroclimatic regions of India, which was thought to be from Black Sigatoka or Yellow Sigatoka because of the appearance of Sigatoka-like leaf spots on infected leaf tissues[33] .
Another rapid, cost-effective, and accurate method of identifying pathogens of infectious diseases is the Loop-Mediated Isothermal Amplification (LAMP) method. LAMP has been used for the detection of fungal pathogens in bananas, particularly P. eumusae. The result of the study conducted by Thangavelu & Devi showed a positive reaction to the LAMP method in the rapid detection of target pathogen P. eumusae even at a concentration of 1 ρg/µl, hence showing a higher sensitivity as compared to a conventional PCR method[35].
Another method applied in the identification of infected leaf tissues of a banana plant is characterized by a visual analysis of an imaging system at the early stage of disease progression - the hyperspectral image analysis that allows the nondestructive hyperspectral scanning of the infected leaf that is still intact on the plant. Classified leaves as either infected or non-infected were further verified using the leave-one-out cross-validation (LOOCV) method which garnered a 98% classification accuracy[36]. This offers a promising technique for the early management or treatment plan as well as preventive measures for small or large-scale banana plantations in the country and worldwide.
Strategies for disease control and management
Cultural practices
-
Cultural method of disease control and management involves key agricultural sanitation practices such as leaf pruning or the removal of necrotic leaf tissues with a difference in the pruning cycle or stage of the disease progression, such as the controlled defoliation or early pruning of leaves that has shown to improved disease control in its implementation in Costa and the application of 10% urea solution which can reduce the inoculum levels and promote faster decomposition of leaf tissues. Good soil fertility and adequate nutrient supply with balanced soil minerals (i.e., potassium, nitrogen, magnesium, boron, and iron) improve the general plant health, therefore being able to show lower disease severity in comparison to banana cultivars thriving in poorer soils. Since relative humidity is an important environmental factor for the spread and infection of the ascospores and conidia, reduction in the relative humidity within the plantation by efficient drainage system (i.e., drip irrigation system or under-canopy), periodic weed control, and preventing the overlapping of leaves among others, became an important method for disease management of Sigatoka leaf spot complex[3].
Agricultural remote sensing tools such as Unmanned Aerial Vehicles (UAVs) and Machine Learning (ML) tools gradually became important remote data processing tools due to their efficiency and high speed during the four-stage decision-making: identification, classification, quantification, and prediction. UAV technology is powered with high spatial resolution RGB images. At the same time, ML is responsible for interpreting and generating results from obtained data in the field - a new way of disease estimation that can help mitigate crop losses from fungal pathogens[37].
Generally, cultural practices are sustainable and cost-effective strategies for controlling the spread of the disease. However, cultural practices alone may not be sufficient to control the spread of the disease, require significant labor and time investment, and may not be effective in areas with high rainfall and humidity, which are ideal for disease development.
Chemical applications
-
Chemical application in preventing the spread or mitigating the severity of the Sigatoka leaf spot complex in major banana-producing countries relies heavily on the utilization of both the systemic (curatives) and contact (protectants) fungicides as part of the integrated disease management strategies in cooperation with agricultural sanitation practices; from the first usage of Bordeaux mixture (suspension of copper sulfate, hydrated lime, and water) in the mid-1930s against Yellow Sigatoka and its conidial production until 1957 where it was replaced by zineb and copper oxychloride in petroleum oil, and the aqueous suspension of dithiocarbamate fungicides and the change in application from less effective spraying in the ground to aerial spraying by aircraft and helicopters. The Mancozeb, the most popular compound of the protectant dithiocarbamate fungicides, is a complex of zinc and maneb and a profungicide of the Ethylene Bisdithiocarbamates (EBDCs) class of compounds; exposure to water allows the release of the Ethylene Bisisothiocyanate Sulfide (EBIS) - an active toxicant that inhibits the biochemical processes within the cell cytoplasm and mitochondria of the fungi. Mancozeb formulations both exist as water solutions and oil-water emulsions. However, it became apparent that instead of water solution, protectant fungicides are more effective when applied as an oil-in-water emulsion. Another protectant fungicide is Chlorothalonil (chloronitrile group) with low water solubility, favoring its fungitoxic activity. After replacing the Yellow Sigatoka as the predominant fungal pathogenic disease of bananas, various fungicides were developed to lessen the devastating effects of the more virulent Black Sigatoka. In the early 1970s, Benomyl (i.e., in oil or oil-water emulsion) was the first systemic fungicide of the benzimidazole group to exist. It was in widespread use to regulate the disease severity of both Black and Yellow Sigatoka[3].
Although the application of fungicide in oil-water allowed a significant reduction in the crop loss from the pathogen, it should be taken into account that when oil accumulates on the surface of the leaf, it can reduce the rate of leaf growth since it interferes with gas exchange and photosynthesis, therefore reducing banana yield. Usage of oil should be based on the severity of the disease, environmental climatic conditions, and the presence or absence of the fungicide-resistant pathogen population in the area. In cases of protectant fungicides, since most infections of the Sigatoka leaf spot complex occur on the lower surface of the plant during the unfurling of new leaves, their effectiveness should also be related to how well they can cover the lower leaf surface of the infected plant for the inhibition of spore germination and penetration of the pathogens[19].
Systemic fungicides, despite attributes such as better absorption and penetration to the tissues, and less susceptibility to being washed away by rainwater or water-induced mechanisms, can still have a potential risk for resistance development in the pathogen population[19]. Because the benzimidazole group of fungicides can induce resistance to P. fijiensis 2−3 years after the first usage, as observed in Honduras, Western Samoa, countries in Central America, and the Philippines, the development of other fungicides that can induce negative cross-resistance to the pathogen is considered. The Diethofencarb (N-phenyl carbamates group) fungicide which was approved to be utilized against Black Sigatoka in 2016 is capable of providing reasonable control of the pathogen even when there is a high resistance of local P. fiiensis isolates with benzimidazole, but should be applied with caution due to risk of having isolates that can develop both resistance to benzimidazole resistance, and insensitivity toward diethofencarb. The fenpropimorph and tridemorph (Morpholine group), spiroxamine (spiro-ketalamine chemical group), and fenpropidin (piperidine chemical group) of systemic fungicides which inhibits ergosterol - an important component of fungal cell membrane - are also utilized extensively in Central America with the morpholine group showing no field reports about the resistance of the population of P. fijiensis[3].
Chemical application is an effective strategy for controlling the spread of the Sigatoka complex but the levels of fungicide treatment used to control the disease are not sustainable in the long term. The frequent application of fungicides can have a significant socioeconomic impact that includes both environmental and human health hazards. Therefore, other control solutions such as cultural practices, biological control, and resistant cultivars are desperately needed to provide additional control.
Biological control
-
The quest for alternative methods to chemical control of the Sigatoka leaf spot complex has been in increasing demand for years due to the following reasons: chemical applications of fungicides have a risk for the development of resistance among the population of pathogens, the increase in the number of chemical applications/year which increases the cost of disease control, and the environmental impact of fungicides, particularly the protective ones in human health and to aquatic organisms, the EBDCs(e.g., mancozeb and its degradation product) and chlorothalonil, respectively[3].
In Brazil, the genus Trichoderma of the family Hypocreaceae which has previously been utilized as a biological agent of many plant pathogens, showed a promising result in its inhibition against P. fijiensis in the laboratory that is similar to the fungicide Azoxystrobin. The same genus was also applied in banana plantations of Northern Queensland and showed antagonistic interactions with banana leaf pathogens[38,39]. Moreover, this genus can also be applied with fungicides and shows bio-stimulant effects on 'Williams' (Cavendish subgroup; AAA) cultivars[3]. The bacterial biological control, B. amyloliquefaciens and Serenade ASO from the B. subtilis also sustained in vitro inhibition of colonial growth for Black Sigatoka and leaf spot caused by Cordana musae, although differs in the period for control[9]. Furthermore, Serratia marcescens, a Gram-negative bacterium, exhibits a synergistic antifungal effect of prodigiosin (PGN) and chitinolytic enzymes produced by the strain, thereby discouraging the germ tube growth of the M. fijiensis (sexual morph) ascospores but not its germination[3,40]. Failure to impede ascospore germination was also observed in the Streptomyces galilaeus strain, although there is still inhibition in the elongation of the germ tube and mycelial growth.
A mixture of foliar substrates, such as the mineral-based solution, barley flour, and urea, or chitin, glucan, urea, and calcium nitrate in combination with milk and molasses under field conditions increases chitinolytic and glucanolytic bacteria on banana leaves, and reduces the applications of fungicides, respectively. Plant extracts and vegetable oils as a potential source of natural fungicides, were observed to have high inhibitory effects that are comparable with the effects induced by the fungicide benomyl on ascospore germination and mycelial growth in vitro[3]. Currently, biological agents of control are still under evaluation in the laboratory and have not been subjected to large-scale management of the virulent Black Sigatoka disease.
Biological control methods have been extensively employed to control many plant pathogens. Biological control agents are environmentally friendly and have a low risk of adverse impacts on human health. They can be used in combination with other control methods such as cultural practices, chemical applications, and the use of resistant cultivars to provide additional control, and can be specific to the target pathogen, reducing the impact on non-target organisms. However, biological control agents may not be effective in all situations and may require specific environmental conditions to be effective). Additionally, the specificity of biological control agents is dependent on the host breadth of the natural enemy, which may limit their effectiveness[41], and may require significant research and development to identify and optimize effective agents[42].
Use of resistant banana cultivars
-
Since 1922, several breeding programs around the world have been focusing on developing Musa spp. that are capable of protection or resistance to fungal diseases caused by the Pseudocercospora sp.[31] to improve banana programs which are related to attaining food security on a global scale with the rising number of human population annually. Moreover, evaluating the reaction of Musa spp. in the germplasm to the Sigatoka leaf spot complex enables the possibility of coning those with higher resistance, replacing susceptible cultivars, or being a potential parental material in banana breeding programs[3]. Published reports, however, solely focused on the more aggressive Black Sigatoka disease among the Sigatoka leaf spot complex.
The 'Chato' (Bluggoe subgroup), 'Pelipita', and 'Saba' of the ABB cooking subgroup became substitutes for the susceptible local Plantain group in Central America. The 'FHIA-20' and 'FHIA-21'(AAAB bred French plantain-like hybrids) of the Dominican Republic and the 'FHIA- 17', 'FHIA-20', 'FHIA-21, and 'FHIA25' of Jamaica were widely accepted in the local market and consumers. In addition to that, Brazil's Brazilian Agricultural Research Corporation (EMBRAPA) developed and released resistant AAAB hybrids from its breeding program, namely the Pacovan-types 'Japira', 'Vitória', 'Pacovan Ken' and 'BRS Preciosa', and the Prata-types 'BRS Garantida' and 'Caprichosa' together with the Prata-type hybrid 'BRS Platina' and the Pome-type hybrids 'BRS Tropical' and 'BRS Princesa' that are partially resistant. Lastly are the resistant clones 'Saba' (ABB) and 'Mysore' (AAB) that were both grown in the Federated States of Micronesia and the former spreading to the Marshall Islands, as well as the 'Bluggoe' (ABB) and 'Rokua Mairana' ('Kalapua'-like ABB clone) of Tokelau and the northern Cook Islands in the south. In Australia, the 'Mysore' and the bred hybrid 'T8' (AAAA) were also utilized to reduce inoculum levels thus inhibiting the advances made by the Black Sigatoka in regions of Queensland[3]. In East Africa, the International Institute of Tropical Agriculture (IITA) and the National Agricultural Research Organisation (NARO) breeding programs in Uganda developed NARITA cultivars – triploid hybrids from the cross of the highly susceptible 'Matooke' (Mutika-Lujugira' subgroup, AAA) cultivars, and highly resistant 'Calcutta 4' (Aaw) to the Black Sigatoka disease.
Disease development and severity were then evaluated in the presence of environmental factors together with the local isolates of P. fijiensis. Among the 25 NARITA hybrids observed, there is an overall higher resistance in comparison to the local susceptible cultivar of Mbwazirume (AAA) except for NARITA 10, 12, 13, 15, and 18, and a difference in the cultivar response based on the environmental factor of the growth site, host genotype, and the pathogen profile. As a result, only seven NARITA hybrids have a good level of Black Sigatoka resistance, namely the NARITA 2, 4, 6, 7, 14, 21, 23 that were deployed in different agricultural sites where they best possess the resistance[43,44]. Other resistant banana cultivars that were developed by IITA and are currently being grown in Cameroon, Ghana, Ivory Coast, Nigeria, and Uganda were the PITA 14, 21, and 23 together with BITA 3[44].
Several studies have proven that resistant cultivars are an effective strategy for controlling the spread of the disease[45−47]. It is a promising long-term solution for Sigatoka control in export bananas. It can reduce the need for fungicide applications, which can have a significant socioeconomic impact that includes both environmental and human health hazards. However, the availability of resistant cultivars is limited. The development of resistant cultivars is a long-term process that requires significant research and development[45]. Moreover, the lack of genetic diversity in the current commercial banana crop is a risk factor for the development of new diseases[48]. Apart from the response of the developed resistant banana cultivars to the pathogens of the Sigatoka leaf spot complex, the palatability and the quality of cooking banana that suits the taste of local consumers and as an export quality should also be taken into consideration, knowing that bananas are a widely-consumed food crop.
The Sigatoka complex is a group of fungal diseases that affect banana plants. There is no single method by which this disease can be directly eradicated but the combination of these strategies is recommended for effective disease control and management of the Sigatoka complex. Cultural practices and the use of resistant cultivars are sustainable and cost-effective strategies, while chemical applications and biological control can be used in conjunction with these strategies to provide additional control. The effectiveness of disease control and management strategies for the Sigatoka complex can vary depending on the specific conditions in a given banana plantation. This further emphasizes the need for integrated disease management that combines several approaches.
-
The control and management of the Sigatoka complex in bananas faces several challenges. From this review, seven major aspects of the control and management of the Sigatoka complex are identified (Fig. 2).
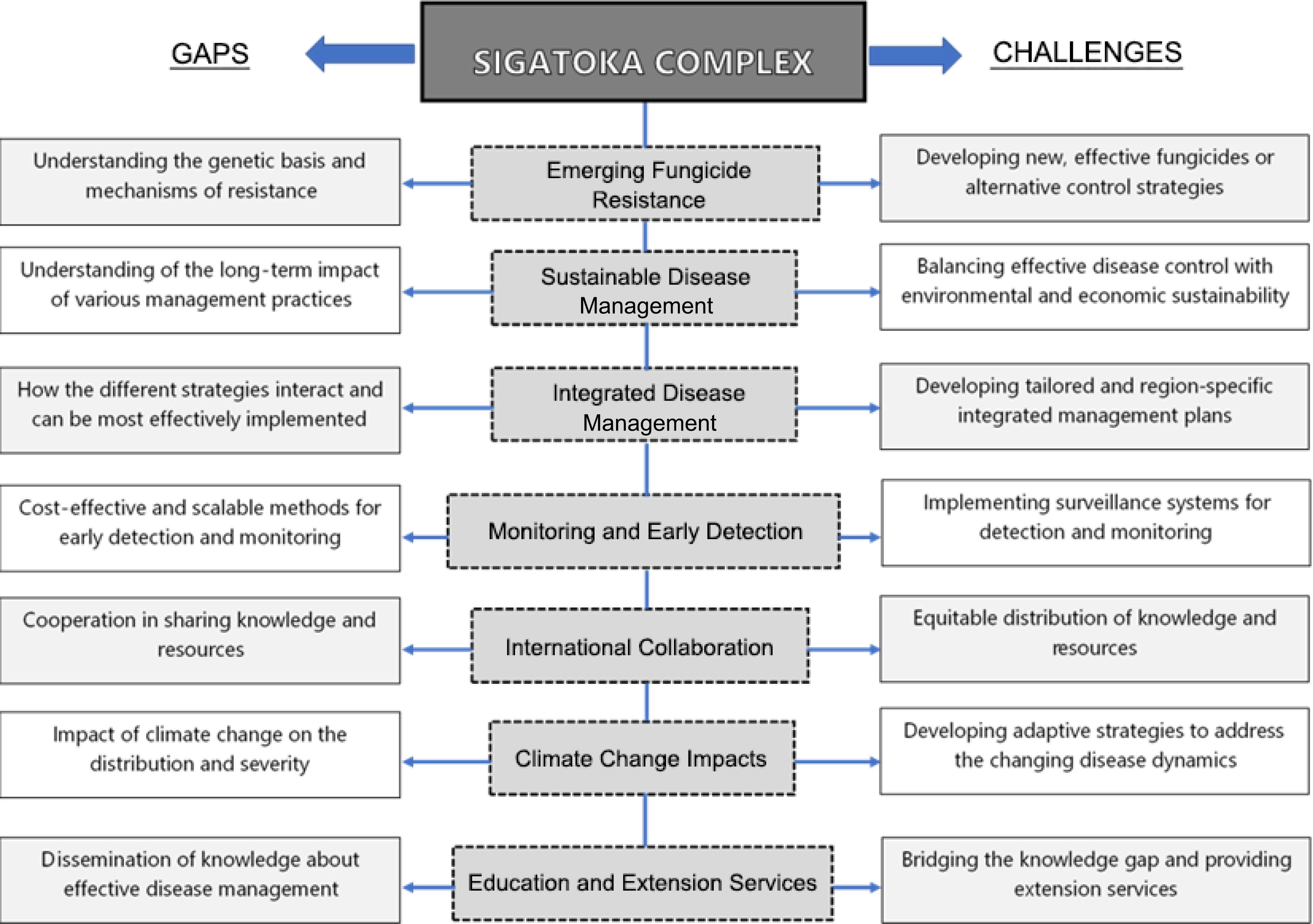
Figure 2.
Simplified schematic model showing aspects of the control and management of the Sigatoka complex that need to be addressed and further studied.
Firstly, the emerging fungicide resistance in the Sigatoka complex pathogens poses a significant challenge, as it limits the effectiveness of chemical control methods. A relevant study by Oliveira et al. showed evidence of resistance to QoI fungicides in contemporary populations of Mycosphaerella fijiensis, M. musicola and M. thailandica from banana plantations in Southeastern Brazil. Several studies have reported that the heavy use of fungicides as the primary means of disease management has resulted in resistance to several fungicide classes, reducing efficacy through the development of fungicide-resistant strains and posing significant socioeconomic impact that includes both environmental and human health hazards[49]. Understanding the genetic basis of resistance and the mechanisms by which it develops is a crucial aspect of devising strategies to combat this issue[48]. Likewise, developing new fungicides with different modes of action or exploring alternative control measures like biological agents continues to be relevant[19, 50,51].
Another aspect is the timely detection of Sigatoka complex pathogens. Just like any other crop disease, early detection is crucial to prevent disease outbreaks. Several studies have explored several methods such as the use of sensitive SPR immunosensors[52], molecular techniques like real-time PCR (RT-PCR)[53], and spectroscopic and imaging techniques[54]. The development of more rapid methods such as biosensors for Pseudocercospora spp. continues to be an area of challenge. Developing cost-effective, scalable, and reliable methods for early detection is of great relevance. Remote sensing technologies, smartphone applications, and citizen science initiatives may offer innovative solutions for real-time monitoring and early warning systems and need to be explored further.
As climate change influences the distribution and severity of plant diseases, early detection and understanding of these impacts on the Sigatoka complex is vital. A warming world may create new disease hotspots and alter the disease dynamics. Bebber demonstrated that the impact of climate change has rendered the study area more hospitable for pathogens. Consequently, the risk of infection has surged by a median of 44.2% across banana-producing regions in Latin America and the Caribbean since the 1960s. This underscores the urgency of devising adaptive measures, such as adjusting planting schedules or introducing banana cultivars resilient to climate change[55]. Similar studies affirmed such findings where climatic factors were identified as pivotal in influencing the consequences of the Black Sigatoka Leaf Disease (BSLD) on banana cultivation and that there is a correlation between the suitability of climate conditions for BSLD and the export ratings indicating the disease's impact[22,28]. Climate change therefore has the potential to complicate the control of the Sigatoka complex and studies that explore these effects such as altering disease dynamics, increasing disease pressure, influencing fungicide resistance, and impacting the interaction between banana plants and pathogens should be areas of concern.
Implementing sustainable disease management is a critically significant strategy. However, achieving sustainability in disease management is a complex problem due to the trade-off between effective control and environmental preservation. There's a need for comprehensive research on the long-term effects of various management practices, including their impact on soil health, biodiversity, and the overall ecosystem. Striking the right balance between disease control and sustainable agricultural practices is essential for the future of banana production. This emphasizes the need for integrated disease management. Its implementation involves harmonizing multiple strategies, but the most effective combinations may vary by region. Researchers and practitioners should focus on tailoring integrated approaches to local conditions and addressing region-specific challenges. This calls for a more localized, adaptive approach to disease management.
In addition, effective disease control often requires international cooperation for information sharing and resource allocation. The banana industry is global, with production and trade spanning multiple countries. However, ensuring equitable distribution of knowledge and resources can be a significant challenge, as countries have varying capabilities and interests in disease management[51]. Establishing frameworks for equitable collaboration is essential. Moreover, agricultural trade could have a significant role in disseminating the disease between nations[22]. Despite strict import regulations, diseases can spread over long distances due to favorable climatic conditions, requiring countries to prepare for potential disease outbreaks. Hence, countries should prepare for the probable incursion of a disease outbreak and it is a continuing challenge to strengthen this coordination among nations.
Lastly, effective knowledge transfer and education are essential components of disease management. Small-scale and resource-poor banana growers often lack access to information and extension services, making it challenging for them to implement best practices. Bridging this knowledge gap and extending support to these stakeholders is essential for the equitable and sustainable management of the Sigatoka Complex. Several studies have also expounded on this aspect such as in understanding the differences in knowledge and perceptions of Black Sigatoka between farmers and scientists. It is known that while most farmers could see the disease's symptoms on plantain leaves, they didn't fully understand the disease itself or how it spreads. Many farmers associate lower crop yields with the disease but rarely do they employ disease control methods. The study also highlighted that agricultural research and advisory systems need to be more involved in helping farmers manage Black Sigatoka effectively. Therefore, future research should focus on developing new and innovative ways to transfer knowledge and educate farmers and other stakeholders on Sigatoka disease management[56].
Addressing knowledge gaps and challenges requires collaborative efforts between researchers, industry stakeholders, government agencies, and international organizations. It is crucial to prioritize research and extension efforts that promote sustainable and effective disease management strategies for the global banana industry while considering the ecological and economic implications.
-
The Sigatoka Leaf Spot Complex encompasses three major fungal diseases – black Sigatoka, yellow Sigatoka, and Eumusae leaf spot—posing a significant threat to banana plants worldwide. These pathogens include leaf streaks, spots, and necrosis, leading to decreased photosynthesis, defoliation, and compromised fruit quality. Notably, black Sigatoka is the most destructive, affecting a wide array of banana cultivars, while yellow Sigatoka occurs in cooler regions and Eumusae leaf spot predominantly in regions in Asia. The diseases spread through wind, rain, and ascospores, severely impacting banana yield and overall quality. Particularly, Black Sigatoka (BLS), thrives in high humidity and tropical temperatures. While traditionally found in lowlands, recent studies reveal its adaptation to higher altitudes, posing a threat to high-altitude banana cultivars. The variety of banana cultivars' susceptibility to diseases varies, with certain subgroups, such as Cavendish and Plantain, being highly vulnerable. Molecular approaches like PCR-RFLP, specific primers, and Loop-Mediated Isothermal Amplification (LAMP) are the recent tools for accurate pathogen identification, alongside imaging systems like hyperspectral analysis for early disease detection. The strategies for controlling and managing diseases in banana crops include cultural practices that encompass measures like leaf pruning, controlled defoliation, and urea solution application to enhance disease control. Soil fertility and a balanced nutrient supply contribute to better plant health. Reduction of humidity through drainage systems aids disease management while agricultural remote sensing tools and machine learning aid decision-making. Chemical applications involve fungicides, including systemic and contact types, but should be used carefully due to potential environmental impact. Biological control methods like Trichoderma and bacterial agents show promise and natural fungicides from plant extracts are being explored. The use of resistant banana cultivars is essential, with various resistant hybrids developed globally to combat diseases like Black Sigatoka, considering both disease resistance and palatability for consumers.
The control and management of the Sigatoka Complex in bananas presents several challenges. Seven key aspects are identified. Fungicide resistance in Sigatoka pathogens limits the effectiveness of chemical control, emphasizing the need to understand resistance mechanisms and explore alternative control measures. Timely detection is vital, with various detection methods, but the development of rapid and cost-effective techniques remains a challenge. Climate change affects disease severity and distribution, making adaptive measures like adjusted planting schedules and resilient cultivars necessary. Balancing effective disease control with environmental preservation is complex, requiring research into sustainable disease management's long-term effects. Integrated approaches must be tailored to local conditions. International collaboration is crucial, but equitable knowledge and resource distribution pose challenges. Effective knowledge transfer and education are essential, particularly for small-scale and resource-poor farmers, underscoring the need for innovative educational methods in Sigatoka disease management.
-
The authors confirm contribution to the paper as follows: study conception and design: Esguera J, Balendres MA, Paguntalan D; data collection: Esguera J, Balendres MA, Paguntalan D; analysis and interpretation of results: Esguera J, Balendres MA, Paguntalan D; draft manuscript preparation: Balendres MA, Paguntalan D. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors acknowledge the invaluable support of the Office of the Municipal Agrarian Reform of Valderrama Antique for their assistance during the field visit for this study.
-
The authors declare that they have no conflict of interest.
-
Received 11 August 2023; Accepted 3 January 2024; Published online 17 January 2024
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Esguera JG, Balendres MA, Paguntalan DP. 2024. Overview of the Sigatoka leaf spot complex in banana and its current management. Tropical Plants 3: e002 doi: 10.48130/tp-0024-0001
Overview of the Sigatoka leaf spot complex in banana and its current management
- Received: 11 August 2023
- Revised: 30 October 2023
- Accepted: 03 January 2024
- Published online: 17 January 2024
Abstract: Banana (Musa spp.) holds immense significance on both global and local scales as a source of nutrition, economic stability, environmental stability, and cultural significance. However, plant diseases have greatly impacted the overall production and yield of banana plantations, specifically, diseases caused by fungi. This paper explores the Sigatoka leaf spot complex, its associated fungi, factors that influence disease incidence and spread, and management measures of the disease. The Black Sigatoka is identified as the greatest threat due to its wide-ranging impact on banana cultivars. Further endangering banana production is disease dissemination through wind, rain, and ascospores. Here we emphasizee the significance of in-depth comprehension of disease characteristics and progression stages, crucial for devising effective management strategies and safeguarding sustainable banana farming. This paper presents knowledge gaps and challenges in the control and management of the Sigatoka complex. This includes emerging fungicide resistance, sustainable disease management, integrated disease management, monitoring and early detection, international collaboration, climate change impacts, and education and extension services. To fill knowledge gaps and overcome challenges, collaboration among researchers, industry players, government bodies, and international organizations is essential, prioritizing research and outreach to advance sustainable disease management for the global banana industry, mindful of ecological and economic consequences.
-
Key words:
- Pseudocercospora /
- Banana disease /
- Fungal plant pathogen /
- Musa species /
- Tropical plant













