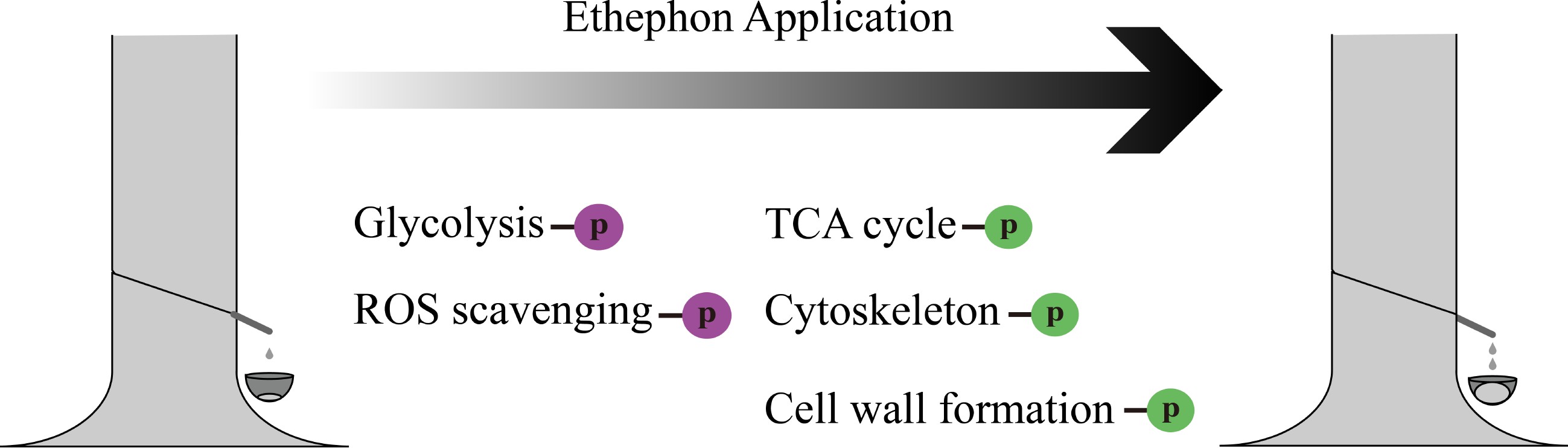
-
Post translational modification (PTM) is one of the important ways to influence protein activity, expression, and interactions[1, 2]. Over the past few decades, more than 400 types of PTMs have been discovered, including phosphorylation, methylation, acetylation, glycosylation, ubiquitination, etc.[3]. Among them, phosphorylation is one of the most widely studied[4, 5]. Phosphorylation is a reversible modification process. The phosphate group is added to the protein by a protein kinase and removed by a protein phosphatase[6]. In eukaryotes, approximately one-third of proteins undergo phosphorylation modification[7]. In the past, it was difficult to identify phosphorylated proteins. However, recently, large-scale protein phosphorylation analyses have been performed with the development of mass spectrometry technology. In wheat, Vu et al. identified 5,178 and 7,023 phosphorylated sites in leaf and spikelet, respectively[8]. In Lotus japonicus, Zhang et al. identified 2,957 phosphopeptides during the various stages of nodule development[9].
Natural rubber (NR) is vital to human society due to its excellent properties, including resilience, elasticity, abrasion, and impact resistance[10]. More than 2,500 plants can produce NR, including Eucommia ulmoides, Hevea brasiliensis, Taraxacum kok-saghyz, Parthenium argentatum, etc[11]. Among them, Hevea brasiliensis (termed as rubber tree) is the only commercialized species, providing more than 98% of the total NR production worldwide[12]. The laticifers located in the inner bark of rubber tree are the sites for NR biosynthesis and storage[13]. The latex is essentially the cytoplasm of laticifer cells, which contains 30%−40% NR. In production, latex is collected by regularly tapping the rubber tree bark. As one of the vital phytohormones, ethylene plays an important role in regulating plant growth and development[14,15]. In the rubber tree, application of ethephon (an ethylene releaser) can notably increase NR production per tapping by prolonging the duration of latex flow[16]. The significant increase in rubber production has attracted the attention of many researchers, and a wealth of information have been obtained. At the transcriptional level, transcriptome analysis suggested that the genes related to glycolysis are up-regulated while the genes related to cell wall formation are down-regulated[17]. At the protein level, proteins associated with sugar metabolism and rubber particle aggregation are influenced by ethephon treatment[18,19]. In 2015, Wang et al. performed a proteomics analysis of ethylene-stimulated rubber latex and identified 59 differential phosphoprotein by using two-dimensional differential in-gel electrophoresis combined with Pro-Q Diamond dye[20]. Here, we explore a high-resolution phosphoproteomic profiling of latex upon ethephon application by using the Tandem Mass Tag (TMT) approach, attempting to uncover the underlying mechanisms for the significant increase in latex production.
-
Eighteen regularly tapped rubber trees of the Hevea brasiliensis clone CATAS7-33-97 were grown at the Experimental Farm of the Chinese Academy of Tropical Agricultural Sciences. To avoid the influence of endogenous hormones produced by tapping, all the selected trees were rested for 20 d. Of these, nine rested trees were treated with 0.5% ethephon on the 1st, 3rd, and 5th days before tapping, and the remaining trees were used as control. Each treatment consisted of three individual trees as biological replicates. All the selected trees were tapped with a half spiral pattern, every 3 d (S/2, d/3). The latex samples were dropped into an equal volume of extracting buffer (0.4 M mannitol, 0.02 M Tris-HCl, 2 mM PMSF, 2 X phosphatase inhibitor cocktail), and subsequently stored at −80 °C for protein extraction. The latex production was measured using a graduated cylinder after latex flow completely stopped, and the dry rubber content was determined according to the method published previously[21].
Protein extraction and digestion
-
Latex proteins were extracted using a modified phenol/acetone extraction method[22]. Briefly, the thawed latex samples were mixed with an equal volume of tris-saturated phenol. After shaking for 10 min, the samples were centrifuged at 18,000 rpm for 45 min at 4 °C. The phenol phases were collected and then mixed in 4-fold volumes of ice-cold 10% (v/v) NH4AC in acetone, and stored overnight at −20 °C. The protein pellets were collected by centrifugation at 12,000 rpm for 20 min at 4 °C and solubilized in 1 X PBS buffer with phosphatase inhibitor. The digestion was performed using trypsin with a ratio of 1:50 (w/w) at 37 °C overnight. The peptide segments were desalinated with Strata X C18 and vacuum freeze-dried.
Tandem Mass Tag (TMT) labeling and phosphopeptide enrichment
-
The peptide segments were dissolved in 0.5 M TEAB and labeled according to the manufacturer of TMT Kit. The TiO2 beads were added and slowly shaking for 2 h. The beads were collected by centrifugation at 5,000 rpm for 1 min and washed with washing buffer three times. Finally, the phosphopeptides were collected by eluted using elution buffer and vacuum freeze-dried.
Mass spectrometry analysis
-
The peptide segments were separated by an ultra-high performance liquid chromatography system and injected into an NSI ion source for ionization, followed by analysis by Q Active HF-X mass spectrometry. The ion source voltage was set to 2.0 kV, and the peptide parent ions and their secondary fragments were detected and analyzed using high-resolution Orbitrap. The scanning range of the primary mass spectrometry was set to 350−1,600 m/z, and the scanning resolution was set to 60,000. The scanning range of the secondary mass spectrometry was fixed at a starting point of 100 m/z, and the resolution of the secondary scanning was set to 30,000.
Phosphoproteins identification
-
The secondary mass spectrometry data were retrieved using Maxquant (v1.5.2.8) and searched against the rubber tree clone CATAS8-79 database[23]. Trypsin was selected for the enzyme. The tolerance for mass error of the primary parent ion in First search and Main search was set to 20 ppm and 5 ppm, respectively. The tolerance for mass error of the secondary fragment ion was 0.02 Da. The false positive rate (FDR) for phosphoproteins identification was set to 1%. The differential enriched phosphoproteins (DEPs) were identified based on the threshold of fold change (FC) ≥ 1.3 (upregulated) or ≤ 0.769 (downregulated) and p-value ≤ 0.05.
Bioinformatics analysis
-
For motif enrichment analysis, the sequence of 13 amino acids (including the modified sites and six upstream/downstream sites) were analyzed using MoMo software. For Gene Ontology (GO) analysis, the Blast2GO program was used to get GO annotation based on molecular function, biological process, and cellular component.
Statistical analysis
-
Statistical analysis was performed by GraphPad Prism (version 8.0) by analysis of variance (ANOVA) based on t-test. Standard error indicates three independent biological replicates. Asterisks indicate significant differences (* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001).
-
We determined the latex production and dry rubber content of latex from three successive tappings treated with ethephon at different time points: 1 d, 3d and 5 d. Results showed that although there was no difference in rubber content of latex, there was a significant change in latex production (Fig. 1a, b). Compared to 1 d and 5 d, the latex production notably increased at 3 d after treatment, reached 4.12-fold, 6.51-fold, and 5.59-fold at three successive tappings. The latex of 3 d after treatment at tapping No.1 (as well as relative control) were selected for further phosphoproteomic analysis.
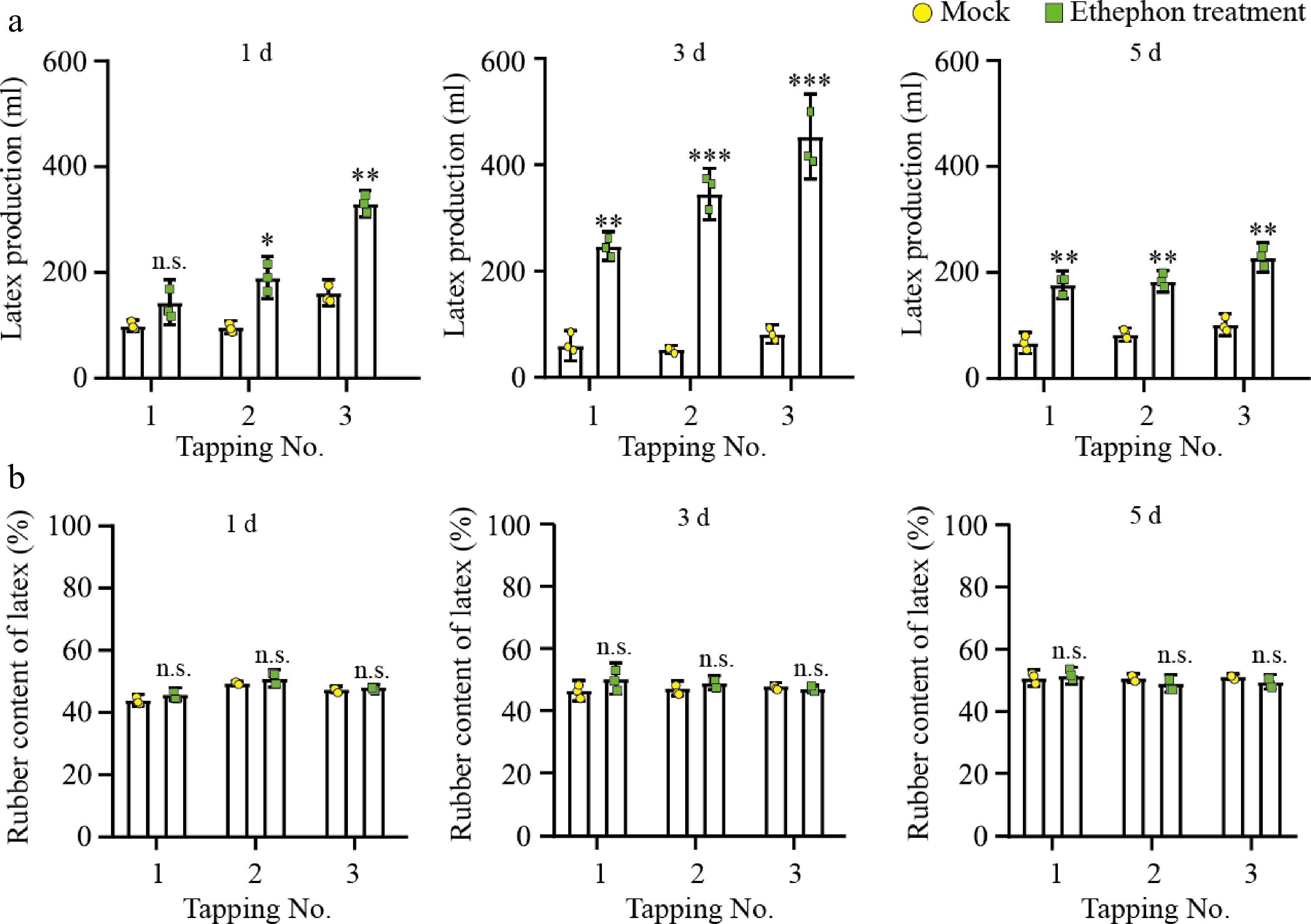
Figure 1.
(a) Latex production and (b) rubber content from ethephon-treated rubber trees. Significant difference was indicated by the asterisks above the bars (n.s. represents no significance, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001). 1 d, 3 d, 5 d represent 0.5% ethephon treatment on the 1st, 3rd, and 5th days before tapping. Tapping No. represents tapping number.
Overview of latex phosphoproteomic profiling
-
Using the TMT approach, a total of 5,548 phosphorylation sites originating from 2,380 proteins were identified (Fig. 2a). Among them, 87.53% (4,856/5,548) of the sites were from phosphoserine (pSer), 10.11% (561/5,548) were from phosphothreonine (pThr), 2.36% (131/5,548) were from phosphotyrosine (pTyr) (Fig. 2b). Among the 2,380 phosphoproteins, 1,210 (50.84%) contained only one phosphorylated site, while the remaining 1,170 (49.16%) contained more than two phosphorylated sites. An uncharacterized protein (encoded by Hb07g045150.5) had the most, with 33 phosphorylated sites.
Under the threshold of fold change (FC) ≥ 1.3 (upregulated) or ≤ 0.769 (downregulated) and p-value ≤ 0.05, 389 differential enriched phosphoproteins (DEPs) were identified, including 246 up-regulation and 143 down-regulation respectively (Fig. 2c). Phosphorylation motifs enrichment analysis showed nine motifs enriched in up-regulation ([pSP], [pSPR], [SxxpS], [PxpSP], [pSPK], [LxKxpS], [RxxpS], [GpS], [KxxpS]), while three motifs enriched in up-regulation ([RxxpSP], [pSP], [SxxpS]) (Fig. 2d).
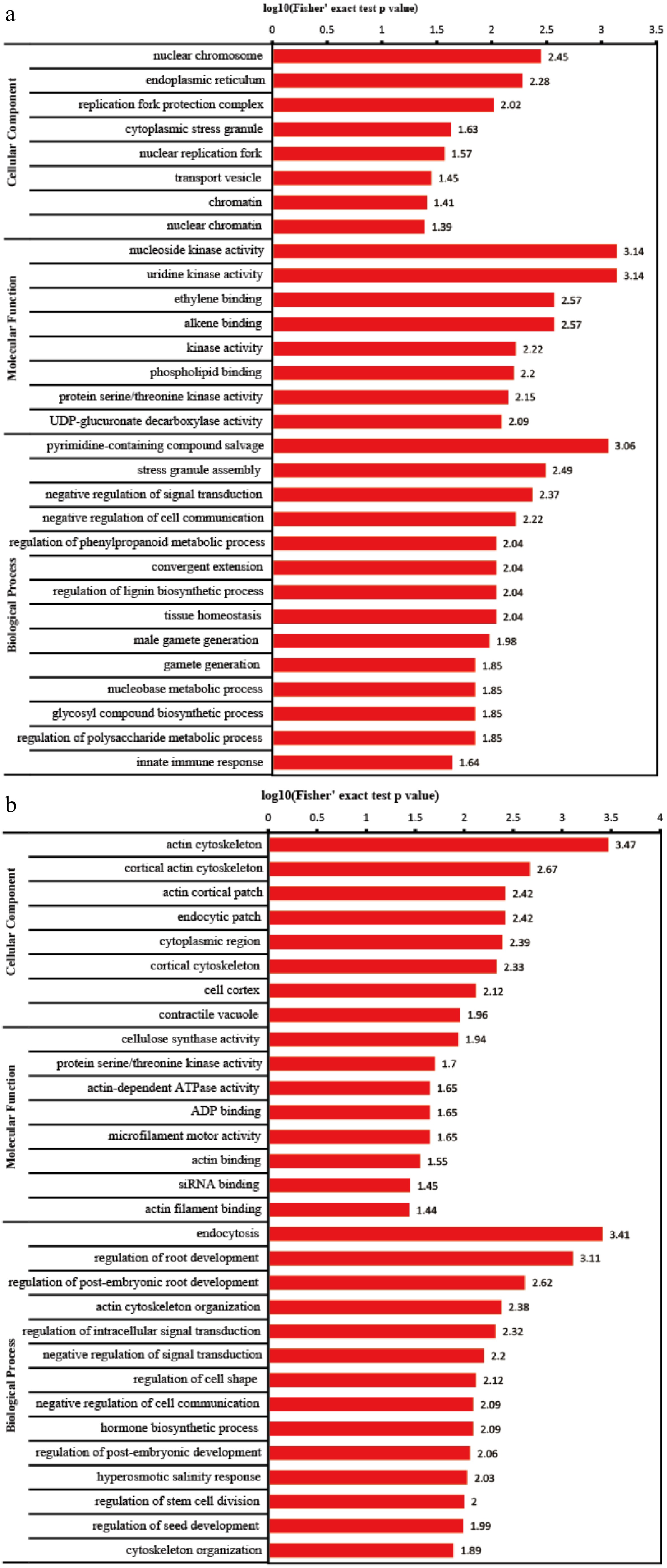
We then analyzed the enriched GO terms in both up-regulation and down-regulation (Fig. 3a, b). The numbers of terms for cell components, molecular functions and biological processes were 8, 8, and 14, respectively. For cell components, the terms enriched in up-regulation mainly related to DNA replication, such as 'nuclear chromosome', 'nuclear chromatin', 'chromatin', 'nuclear replication fork', 'replication fork protection complex', but it mainly related to cytoskeleton in down-regulation, such as 'actin cytoskeleton', 'cortical actin cytoskeleton', 'actin cortical patch', 'cortical cytoskeleton'. For molecular functions, the terms enriched in up-regulation mainly related to kinase and ethylene signal, such as 'nucleoside kinase activity', 'uridine kinase activity', 'kinase activity', 'protein serine/threonine kinase activity', 'ethylene binding', but it mainly related to cytoskeleton in down-regulation, such as 'actin-dependent ATPase activity', 'microfilament motor activity', 'actin binding', 'actin filament binding'. For biological processes, the terms enriched in up-regulation mainly related to secondary metabolism and carbohydrate metabolism, such as 'regulation of phenylpropanoid metabolic process', 'regulation of lignin biosynthetic process', 'glycosyl compound biosynthetic process', 'regulation of polysaccharide metabolic process', but it mainly related to development and cytoskeleton in down-regulation, such as 'regulation of root development', 'regulation of post-embryonic root development', 'regulation of post-embryonic development', 'regulation of stem cell division', 'regulation of seed development', 'actin cytoskeleton organization', 'cytoskeleton organization'.
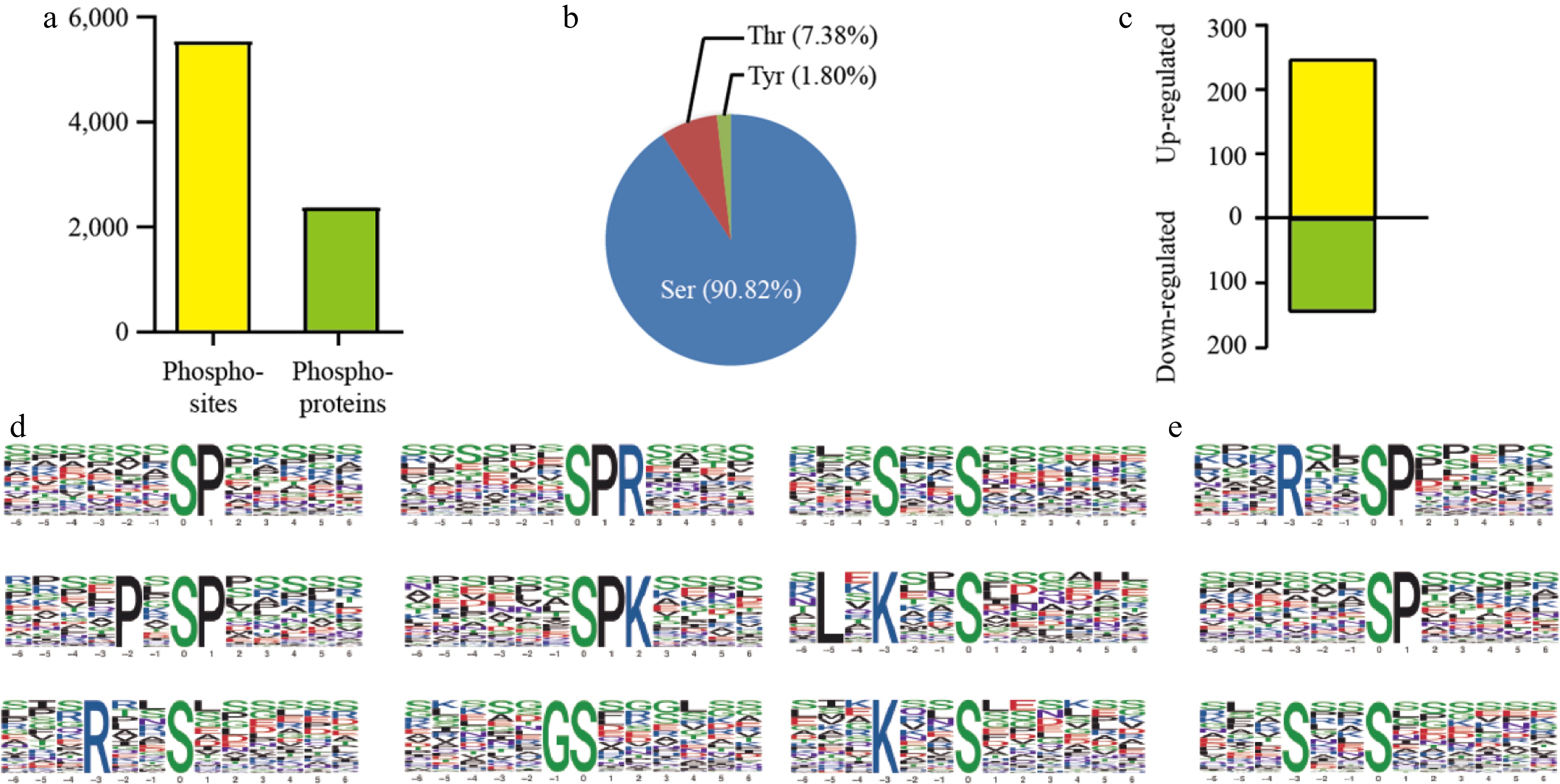
Figure 3.
Mass spectrometry analysis of phosphoproteins. (a) Statistical results of phosphoproteome identification. (b) Distribution of phosphorylated amino acids. (c) Differential enriched phosphoproteins identification. (d) Motif analysis of 246 upregulated phosphopeptides. (e) Motif analysis of 143 downregulated phosphopeptides.
DEPs related to carbohydrate metabolism and IPP pathway
-
In rubber tree, activated carbohydrate metabolism provides not only energy but also carbon skeletons for organic compound formation, including NR. Acetyl-CoA generated by glycolysis is the starting point of mevalonate (MVA) pathway, which produces isopentenyl pyrophosphate (IPP), the direct precursor of natural rubber. Here, we detected one sugar transporter protein (SWEET), two glycolysis related enzymes (PFK and F2KP), one MVA pathway rate-limiting enzyme (HMGR) that were up-regulated, and two tricarboxylic acid cycle related enzymes (CS and MDH) that were down-regulated (Fig. 4).
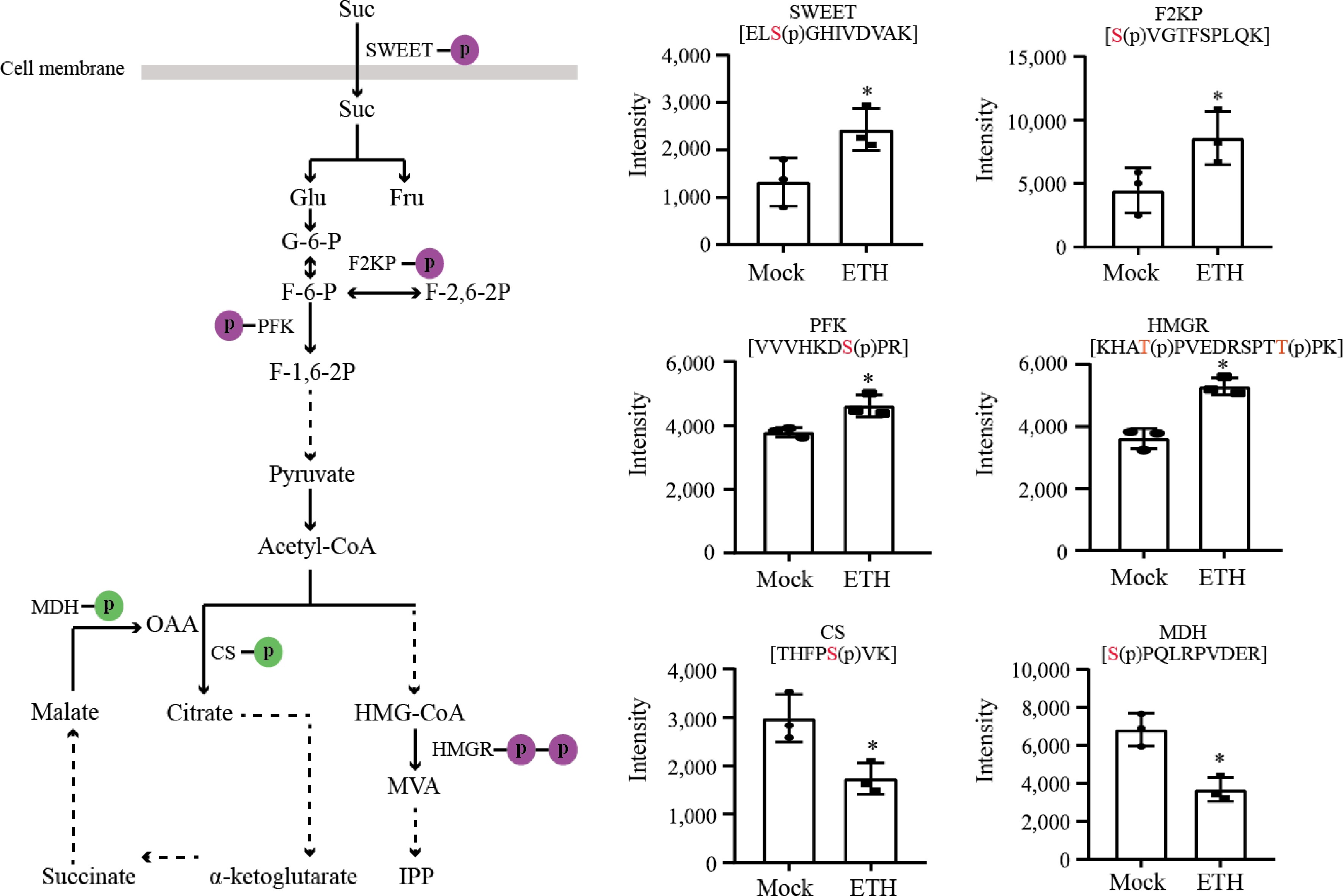
Figure 4.
Protein phosphorylation related to carbohydrate metabolism and IPP pathway. The purple circles represent phosphorylation and the green circles represent dephosphorylation. PFK, phosphofructokinase; F2KP, fructose-2,6-bisphosphatase; CS, citrate synthase; MDH, malate dehydrogenase; HMGR, hydroxymethylglutaryl-CoA reductase.
DEPs related to latex flow
-
Several factors, including turgor pressure caused by cell wall, agglutination formation, and the ROS scavenging system, can influence latex flow upon tapping. We detected three cell wall related proteins (CESA1, CESA3, 4CL) and three agglutination formation related proteins (Myosin-15, Myosin-17, Gluc) that were down-regulated, while one ROS scavenging system member (SOD) was up-regulated (Fig. 5).
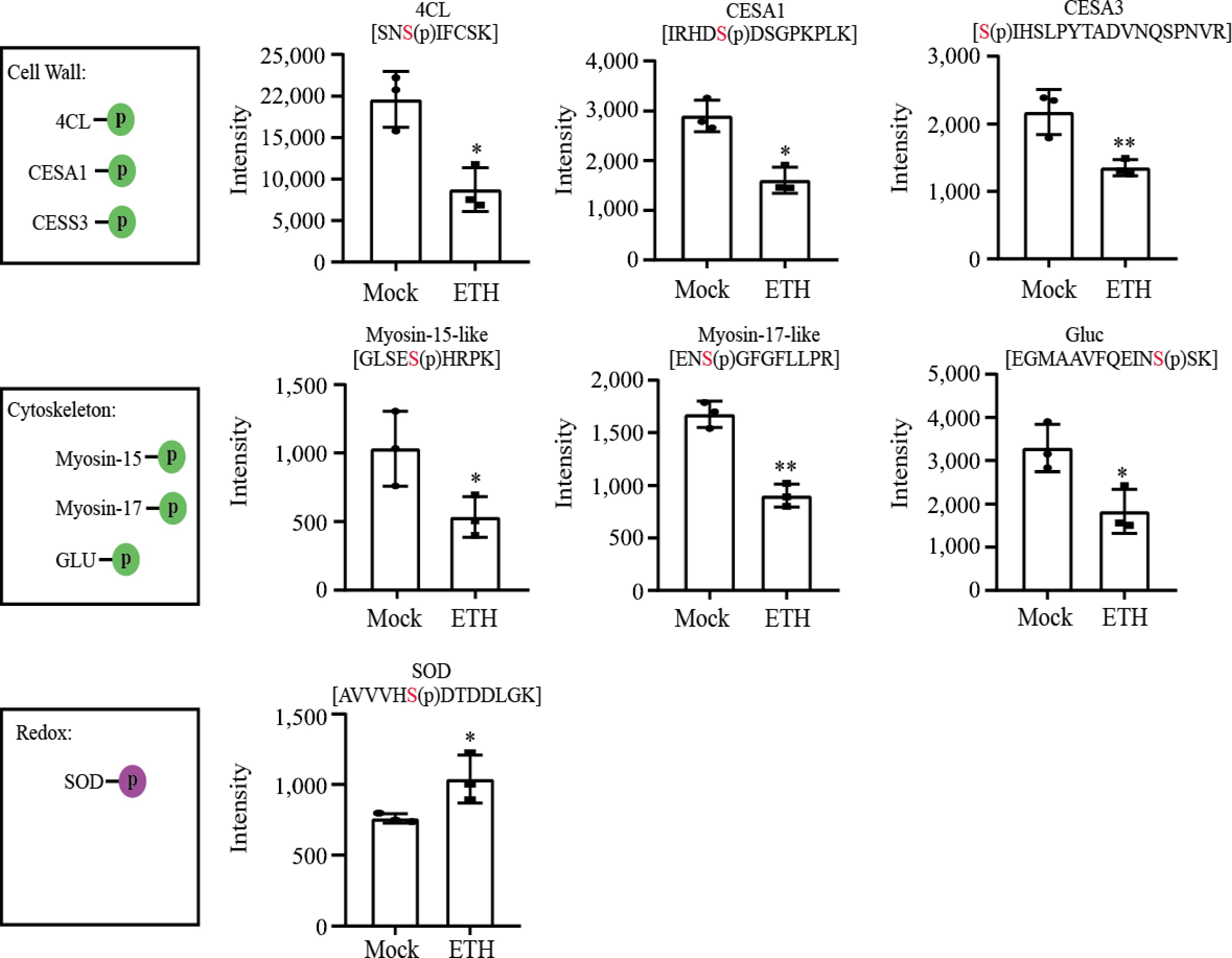
Figure 5.
Protein phosphorylation related to latex flow. The purple circle represents phosphorylation and the green circles represent dephosphorylation. CESA, Cellulose synthase, 4CL, 4-coumarate-CoA ligase; Gluc, glucan endo-1,3-beta-glucosidase; SOD, superoxide dismutase.
Protein kinases and protein phosphatases in DEPs
-
Kinase and phosphatase play important roles in dynamic phosphorylation. In this study, eight protein phosphatases (one PP2C, two tyrosine phosphatases, five Ser/Thr protein phosphatases) and 40 protein kinases (two CDPKs, two CIPKs, two MAPKs, one MAPKK, one MAPKKK, three CKs, two SnRKs, 27 Ser/Thr protein kinases) were detected in DEPs (Supplemental Table S1).
-
Application of ethephon significantly increases latex production per tapping by prolonging the duration of latex flow in rubber tree. Research on the ethephon-enhanced latex production has mainly focused on transcriptomics or proteomics. Protein phosphorylation is one of the important PTM, playing a vital role in regulation of signal transduction, regulating cell proliferation, development, differentiation, and apoptosis. By using two-dimensional differential in-gel electrophoresis combined with Pro-Q Diamond dye, Wang et al. detect more than 200 protein spots and 59 phosphoproteins with differential enrichment upon ethylene treatment[20]. In the present study, we identified 2,380 phosphoproteins by using the TMT approach. The number of phosphoproteins increased 10-folds compared to the previous study, suggesting the high throughput of TMT technology than that of 2-DE gel. Additionally, the phosphorylated rubber elongation factor/small rubber particle proteins reported previously are not detected in this study. This may be attributed to the normal concentration (0.5%) ethephon used here, while the high concentration (3%) ethephon used by Wang et al.[20].
Hundreds of DEPs upon ethephon treatment make it possible to globally analyze. Motif enrichment analysis of up-/down-regulation detected several conserved motifs. It is well-known that [pSP] is targeted by MAPKs and [RxxpS] is targeted by CDPK and SnRK[24,25]. Here, we show [RxxpS] is one of the most enriched motifs in up-regulation, corresponding to the two CDPKs and two SnRKs detected in up-regulation. In addition, [pSP] is one of the most enriched motifs in both up-regulation and down-regulation, suggesting the complex MAPK signal network in latex of rubber tree.
Application of ethephon activates glycolysis and inhibits TCA cycle
-
Carbohydrate metabolism, such as sucrose metabolism and glycolysis, not only supplies energy but also generates a carbon skeleton for the formation of organic compounds including rubber. SWEET protein is a new class of sucrose transporters that function as bidirectional uniporters/facilitators and facilitate the diffusion of sucrose across cell membranes along a concentration gradient[26]. In Arabidopsis, rapid phosphorylation of SWEET11/12 enhances the sucrose transport activity upon drought and abscisic acid treatments[27]. Here, we notice that SWEET is phosphorylated upon ethephon treatment, speculating that it may be contributing to the sucrose transport into laticifer cell. Acetyl-CoA generated from glycolysis is an important carbon skeleton. It enters TCA cycle to produce energy or MVA pathway to produce IPP for rubber biosynthesis[28]. PFK is one of the rate-limiting enzymes in glycolysis. Phosphorylation of PFK results in increased sensitivity to ATP inhibition and stronger binding of ATP to the inhibitory site of the enzyme[29]. In the TCA cycle, oxaloacetate is the rate-limiting factor. It can produce citric acid by CS and be regenerated by MDH catalyzing malate[30]. Here we show that PFK is up-regulated while both CS and MDH are down-regulated. HMGR is a key rate-limiting enzyme in the MVA pathway by controlling the carbon source entry[31]. Here, we detect that it is up-regulated upon ethephon treatment. In summary, the activation of sucrose transport and glycolysis and the inhibition of TCA cycle ensure sufficient IPP for the MVA pathway.
Application of ethephon decreases the resistance of latex flow
-
The duration of latex flow is influenced by various factors, such as laticifer turgor pressure, plug formation at the end of severed laticifer, and latex redox[32]. Turgor pressure is the initial driving force for latex flow after tapping. The properties of laticifer cell wall are closely related to turgor pressure. Lignin and cellulose are the two major components of cell wall. CESA is the key enzyme for cellulose biosynthesis. In Arabidopsis, CPK32 can interacts with both CESA1 and CESA3, and specifically phosphorylates CESA3 on T672 residue in vitro. Overexpressing functionally defective CPK32 variant and phospho-dead mutation of CESA3 led to decreased motility of CSCs and reduced crystalline cellulose content in etiolated seedlings[33]. Here we show CESA1 and CESA3, as well as 4-CL (a key enzyme for lignin formation), reduce the level of phosphorylation, suggesting that ethephon treatment may increase turgor pressure by reducing lignin and cellulose content in laticifer. The formation and accumulation of protein-networks at the end of the laticifer's wounded site is the direct result of the cessation of latex flow after tapping[34]. Cytoskeleton proteins, such as actin and myosin, play a key role in the formation of protein network. In Physarum polycephalum, phosphorylated myosin shows a significant increase in activity[35]. Here we notice the reduction of phosphorylation level in both myosin-13 and myosin-15, hinting the formation of protein networks is affected upon ethephon treatment, which may contribute to the prolonging the duration of latex flow. The balance of ROS generation and scavenging in laticifer cells is an important factor for latex exploitation. High levels of ROS cause lutoids break, subsequently triggering the aggregation of latex[36]. SOD is one of the important ROS scavenging enzymes. In 2001, Csar et al. reported that phosphorylation of Cu/Zn-SOD actives the G-CSF-mediated biological responses granulocyte[37]. The increase in phosphorylation level of SOD upon ethephon treatment may reduce the level of ROS in latex, which benefits to latex exploitation after tapping. Finally, the application of ethephon decreases the resistance of latex flow caused by cell wall and protein network, resulting in the prolonging the duration of latex flow.
-
In this study, 2,380 phosphoproteins were identified in latex using TMT technology, and 389 phosphoproteins with differential enrichment were detected upon ethephon treatment. We speculate that the phosphorylation of key enzymes such as SWEET, F2KP, PFK, HMGR, SOD, while the dephosphorylation of key enzymes such as CS, MDH, 4CL, CESA, Myosin, Gluc, may play an important role in rubber yield increase upon ethephon treatment.
-
The authors confirm contribution to the paper as follows: study conception and design: Chao J, Tian W; ethephon treatment and latex collection: Yang S, Chao J; protein extraction: Chao J, Deng X; data analysis: Yang S, Du X; draft manuscript preparation: Chao J. All authors reviewed the results and approved the final version of the manuscript.
-
The mass spectrometry phosphoproteomics data have been deposited to the Integrated Proteome Resources (iProX) with an accession: IPX0004047002. All data generated or analyzed during this study are included in this published article.
The research was supported by the National Natural Science Foundation of China (31700601), the Hainan Provincial Science and Technology Special Fund (ZDYF2022XDNY252), and the Earmarked Fund for China Agriculture Research System (CARS-33-YZ1).
-
The authors declare that they have no conflict of interest. Jinquan Chao is the Editorial Board member of Tropical Plants who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
-
accompanies this paper at (https://www.maxapress.com/article/doi/10.48130/tp-0024-0002)
-
Received 16 November 2023; Accepted 2 January 2024; Published online 15 January 2024
-
2,380 phosphoproteins are identified in latex by using Tandem Mass Tag (TMT) approach.
389 phosphoproteins show differential enrichment upon ethephon treatment.
The phosphorylation of glycolysis and the dephosphorylation of cell wall metabolism contribute to ethephon stimulated latex production in rubber tree.
-
# Authors contributed equally: Shuguang Yang, Xiaoyu Du
- Supplemental Table S1 The phosphorlated sites of phosphoproteins with differential enrichment upon ethephon treatment.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Yang S, Du X, Deng X, Tian W, Chao J. 2024. Comparative phosphoproteome analyses provide new insight into ethephon stimulated latex yield in rubber tree. Tropical Plants 3: e001 doi: 10.48130/tp-0024-0002
Comparative phosphoproteome analyses provide new insight into ethephon stimulated latex yield in rubber tree
- Received: 16 November 2023
- Revised: 28 December 2023
- Accepted: 02 January 2024
- Published online: 15 January 2024
Abstract: Phosphorylation is one of the important post translational modifications that influences protein activity, expression, and interactions. Natural rubber (NR) is an important industrial raw material, mainly obtained from rubber tree. In production, the application of ethephon can effectively increase NR yield. In the present study, we investigated the phosphoproteomic profiling of latex upon ethephon treatment, by using the Tandem Mass Tag (TMT) approach. A total of 5,548 phosphorylation sites on 2,380 proteins were identified. Of which, 389 phosphoproteins showed differential enrichment under the threshold of fold change (FC) ≥ 1.3 (upregulated) or ≤ 0.769 (downregulated) and p-value ≤ 0.05. Gene Ontology (GO) enrichment analysis showed pathways such as glycolysis and reactive oxygen species (ROS) scavenging were activated, while processes such as tricarboxylic acid (TCA) cycle, cell wall biosynthesis, and cytoskeleton formation were inhibited upon ethephon treatment. The results may provide novel insights into the mechanism underlying ethephon-induced rubber latex production in rubber tree.
-
Key words:
- Ethephon /
- Rubber tree /
- Latex /
- Tandem Mass Tag /
- Phosphoproteomics