-
Vegetables, derived from various plant parts such as roots, stems, leaves, flowers, fruits, or seeds, serve as edible components or food ingredients. From a botanical standpoint, a plant's reproductive structure, specifically its flower, produces fruits and classifies the other parts of the plant as vegetables. However, vegetables are usually classified based on their cooking usage or cultural significance, thereby permitting the utilization of any part of the plant. Crops of significant economic value yield immature fruits resembling vegetables, such as cucumber, chili, eggplant, tomato, squash, and bell pepper.
Chilling temperatures are an environmental experience that induces stress in plant cells. Exposure to excessively low temperatures but above the freezing points for a while during growth or after harvest leads to physiological disturbances in cold-sensitive plant cells, causing chilling damage and death in tropical plants of various vegetable species. The physiological disorder or damage is typically called 'chilling injury' (CI). Tropical fresh produce, including vegetables, are susceptible to low temperatures. This review aims to comprehensively analyze the mechanisms, localization, and structural composition of the membranes found in both CI-tolerant and sensitive cultivars. This will examine the defensive mechanisms employed by these cultivars and the various triggers that initiate these mechanisms.
-
The tropical region exhibits a tropical climate, rendering it conducive to cultivating various vegetables and fruits. These crops thrive in hot climates on both sides of the equator, where the climate is either tropical equatorial or tropical. The temperature at which fresh vegetables and fruits are stored determines their shelf life. The quality of harvested plant parts undergoes rapid changes when exposed to room temperature. After a week of harvesting, tropical horticultural crops generally experience a significant decline in quality attributes, with losses associated with significant reductions in fresh weight and an increase in decay of up to 90%[1]. Due to reduced respiration rates and metabolisms, the lower the storage temperature, the longer the storage life. However, inappropriately low temperatures have not maintained the quality and can lead to CI, which tropical produce is susceptible to if temperatures are consistently below 13 °C. Eaks & Morris[2] noticed that cucumbers stored at various low temperatures from 0 to 30 °C showed that fruit held at non-chilling temperatures between 13 and 30 °C exhibited a continually declining rate of normal respiration patterns, whereas fruit held at 0 and 5 °C deviated from the patterns of unchilled fruit. According to existing research, tropical vegetables, and fruits are more susceptible to low-temperature stress than their temperate counterparts.
-
CI presents a range of symptoms that vary depending on the commodities affected. These symptoms include color shading, surface pitting, surface browning, and water-soaking. Additionally, CI affects the inner appearance, causing symptoms such as water succulence, internal browning (IB), and flesh translucence or jelly-like consistency[1]. Exposing the CI vegetables and fruits to room temperature triggers the manifestation of the symptoms listed in Table 1. Some vegetables and fruits, such as papaya, mango, gourd, tomato, cucumber, and okra, exhibit contrasting symptoms of CI between their exocarp and mesocarp due to the adhesive nature of the flesh and peel derived from the ovary wall. When stored at low temperatures, cucumbers exhibit distinct visual characteristics such as a water-soaked patch and subsequent surface pitting collapse. The mesocarp of the cucumber exhibits a translucent coloration, whereas the exocarp darkens and turns green. The variation in the response of vegetables and fruits to CI, specifically between the peel and pulp[3] is depicted in Fig. 1.
Table 1. Critical temperature, CI response and symptoms of some tropical horticultural commodities.
Horticultural
commodityCritical temperature
( °C)CI response
(+)Symptoms Ref. External Internal Banana 13−15 + Skin-darkening Poor ripening [6] Mango 13−15 + Senescent spotting, brown skin Poor ripening [7] Papaya 13 + + Surface pitting Water-soaking [8] Pineapple - Queen 11−13 + ++ Pink bract of fruitlets Internal browning [9] - Smooth cayenne 13 + Non-response Internal browning Ripe tomato 14 + Surface pitting Excessive softening, water soaking, aroma loss [10] Asparagus 0−2 + Dull, grey-green, limp tips [11,12] Bean 7 + Pitting and russeting [11] Eggplant 7 ++ Surface scald, Seed blackening [11] Gourd 10−13 ++ Surface pitting and browning Seed browning [3,11,12] Cucumber 8−10 ++ Surface pitting Water soaking [11,12] Zucchini 0 + Surface pitting [12] Summer squash 5−10 + Surface pitting and fungal rot Breakdown [13] Gac fruit 10−13 + Fruit shriveling, water soaking peel Water leathery like mesocarp and aril [14] Chili 7 + Surface pitting Placenta and seed browning [15] Okra 8−10 + Surface pitting Seed browning [16,17] Chinese kale 4−8 + Water soaking peel [18] Sweet basil 12 ++ Leaf browning, necrosis, decay, and leaf abscission [19] Holy basil 12 ++ Browning spots and water soaking leaves [20] Lemon basil 12 ++ Leaf blackening [5] (+) degree of susceptibility to CI. 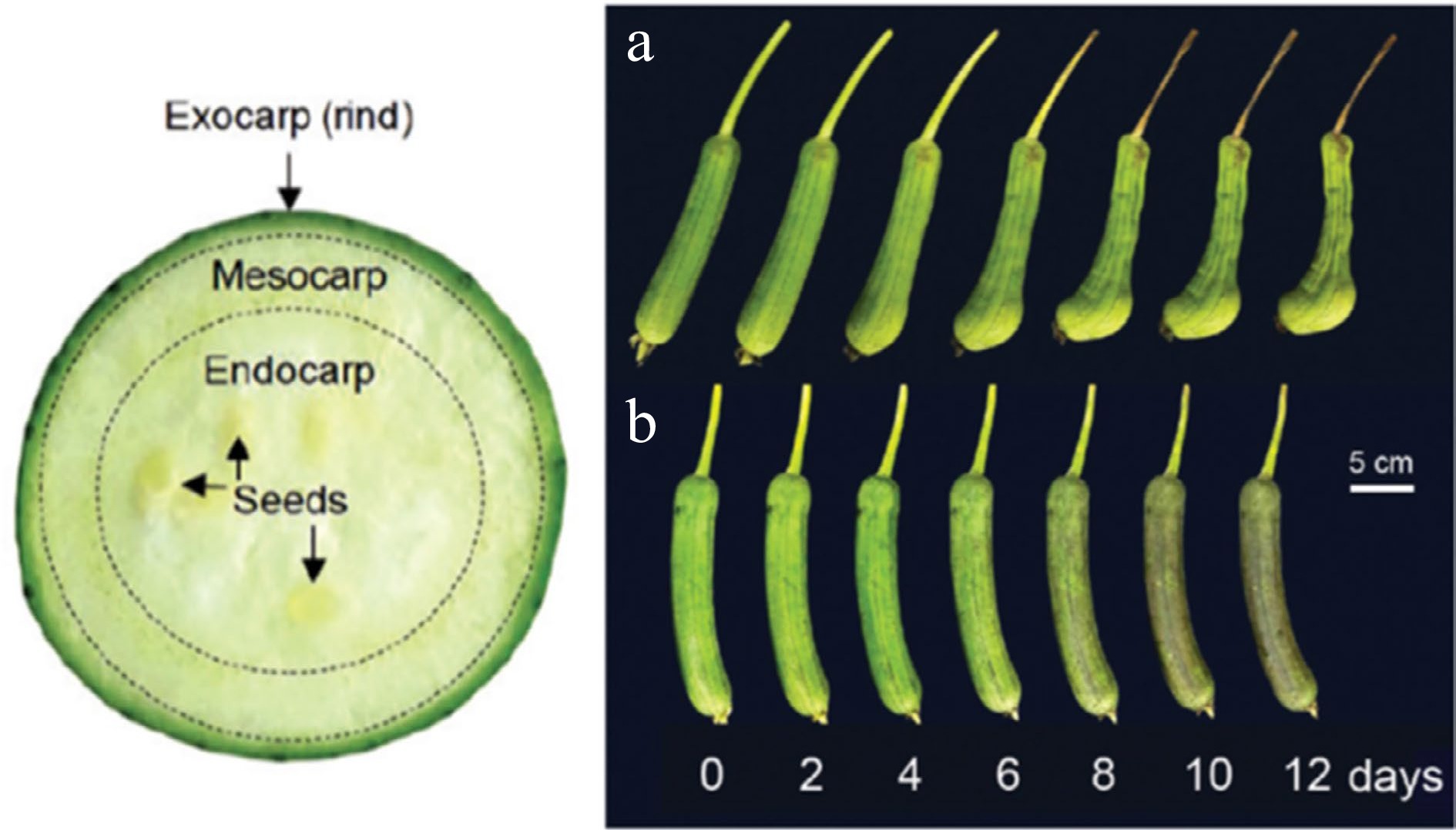
Figure 1.
Internal structure of immature sponge gourd fruit (left) and visual appearance of immature sponge gourd fruit stored at (a) 25 °C and (b) 5 °C[3].
Mature leaves of sweet basil (Ocimum basilicum L.) stored at 4 °C developed a severity of CI browning symptoms (Fig. 2) with higher levels of polyphenol oxidase (PPO) and lipoxygenase (LOX) activities, resulting in an increase in malondialdehyde (MDA) content compared to the young leaves[4]. Wongsheree et al.[5] reported that mature lemon basil (Ocimum × citriodourum) leaves, having higher LOX, suffered more severe CI than young leaves when stored at 4 °C.

Figure 2.
Chilling injury symptoms generated on young leaves of holy basil (upper right) and sweet basil (below right) during 4 °C storage, compared to non-chilled leaves (left).
CI closely relates to the assessment of fruit maturity. Mango, papaya, banana, pineapple, and corn, ranging from immature to mature stages, possess the potential to serve as vegetables for food ingredients. Green bananas are susceptible to low temperatures. This sensitivity manifests itself as surface pitting and browning on the banana peel. When the fruit is returned to its normal temperature, the ripening process is complete. Blackheart as an internal browning (IB) is a form of CI in the field when maturing in winter. Meanwhile, IB can develop in the core and the surrounding flesh of pineapple fruit stored at 10−15 °C for a week in 'Queen' and 2 weeks in 'Smooth Cayenne' group. The observation of enhanced pink coloration on the upper surface of crown leaves and in the grooves of fruitlets suggests a correlation between the external appearance of pineapple fruit and the production of IB[11]. Cultivars '73−50' and 'Gold' were explicitly developed to reduce IB vulnerability in Hawaii, USA. Although the 'Gold' pineapple fruit is more resistant to IB, it is more susceptible to fruit rot than the 'Smooth Cayenne'[21].
-
Cucumber, gourd, mango, and banana are examples of fruits that have separate peel and flesh. Certain types of fruit flesh, such as aril developed from parts of the seed (integument or funiculus), exhibit a distinct response to low temperatures compared to the peel. In many fruits, the peel developed from the ovary wall typically signifies indications of CI before in the flesh.
Basils' CI symptoms were generally characterized by leaf blackening and brown spots on the leaves (Fig. 2). The symptoms began with the dysfunction of oil glands on lemon basil leaves, which progressed to a blackening mark[22]. Several lines of sweet basil produce volatile phenylpropane oils (eugenol and chavicol derivatives) that accumulate in the leaves in specialized structures known as peltate and capitate glands. The peltate glands serve as phenylpropene class defense tissues[23]. The dysfunction of these oil glands could lead to imbalanced intermediates that cause visual browning spots. CI can be induced inside the fruits of some vegetables, such as eggplant, guard, and chili. When stored at low temperatures, the seeds' IB and discoloration initially occur when the exocarps appear normal. In gac fruit, storage at 4 °C induces the tissue collapse of the mesocarp and aril[14].
Reactive oxygen species (ROS) as CI triggers
-
Various physiological stresses can produce ROS in different forms, as outlined in Table 2. Low-temperature exposure triggers redox reactions that generate oxygen species (O2·−)[24]. Oxygen radicals are commonly produced through electron transport chains during photosynthesis and respiration. The presence of a dysfunctional electron in ROS can efficiently cause damage to macromolecules such as proteins, lipids, and DNA/RNA. The membrane-lipid bilayer of cells and organelles represents a pivotal piece of evidence. Reactive oxygen species are conveyed across cellular boundaries via hydrogen peroxide (H2O2) with the assistance of superoxide dismutase (SOD), which encompasses both iron-dependent SOD (Fe-SOD) and manganese-dependent SOD (Mn-SOD). Hydrogen peroxide is transported to neighboring cells and acts as a signal transduction molecule. Nevertheless, H2O2 is unstable and undergoes the Fenton reaction, which converts it into a highly reactive hydroxyl radical (HO·−) and a hydroxide ion (HO−). This reaction has the potential to degrade the integrity of membrane lipids, resulting in detrimental alterations in fluidity and permeability.
Table 2. General ROS produced in plant cells under low temperature storage.
Form Name Form Name O2·− Superoxide radical 1O2 Singlet oxygen OH· Hydroxy radical H2O2 Hydrogen peroxide RO· Alkoxyl radical ROO· Alkylperoxyl radical ROOH Alkylhydroperoxide ClO− Hypochlorite ion Fe5+O Periferryl ion Fe4+O Ferryl ion NO· Nitric oxide Cellular localization of CI
-
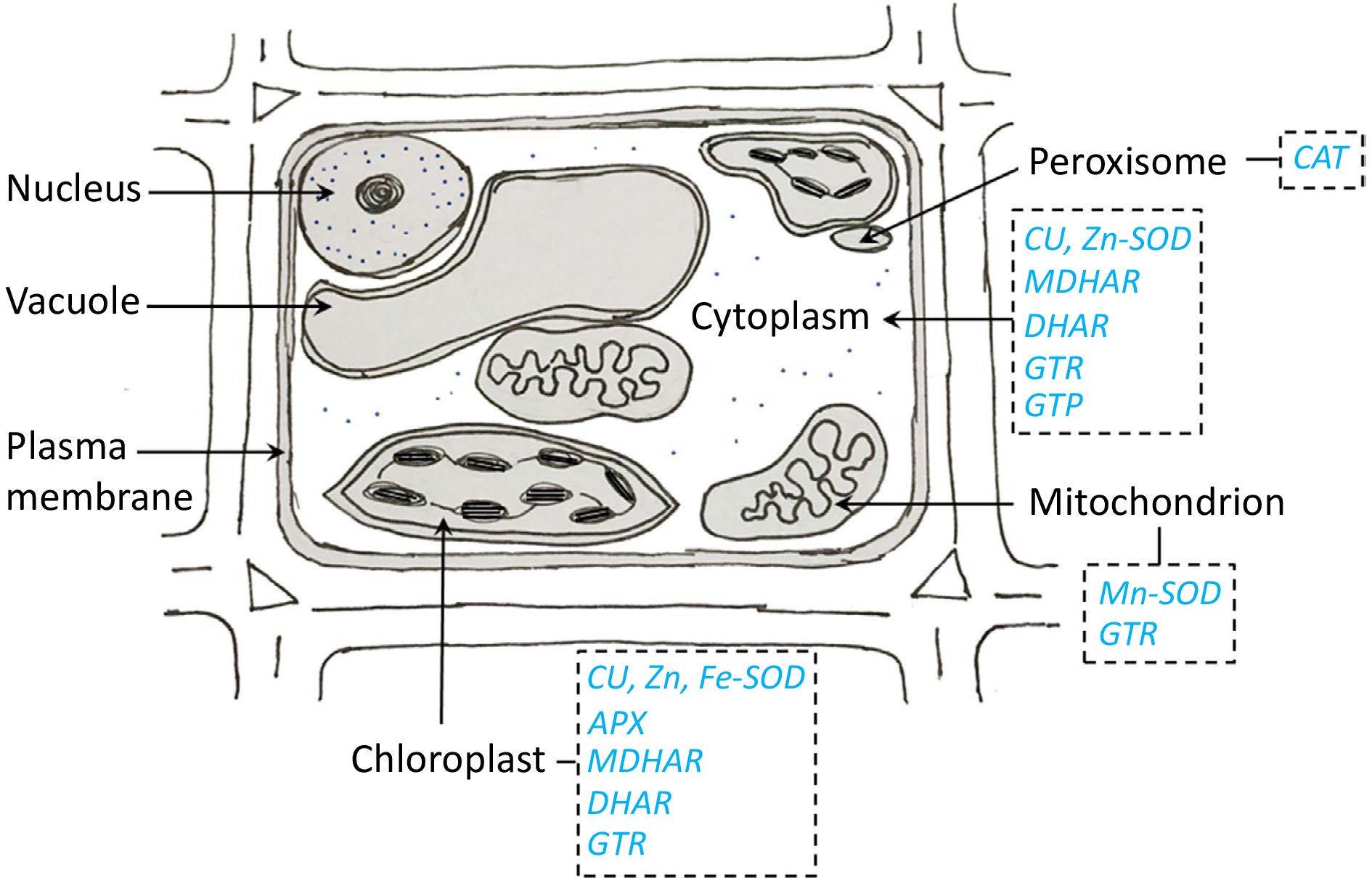
CI causes an impairment of membrane function, resulting in an elevation in permeability. The cellular membrane consists of phospholipid bilayers, wherein hydrophilic heads are oriented toward the solute, and hydrophobic tails are oriented toward each other. This phenomenon complicates the transportation of the solute. The saturation or unsaturation of fatty acids in the membrane component is a determining factor. Fatty acids react differently at different low temperatures. Plants possess defensive mechanisms in the form of antioxidants, which serve the purpose of scavenging free radicals through the presence of various enzymes found in different cellular compartments. The cytosol contains enzymes such as Cu-SOD, Zn-SOD, monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GTR), and glutathione peroxidase (GTP). Mn-SOD and GTR are found in the mitochondria, while Cu-SOD, Zn-SOD, Fe-SOD, ascorbate peroxidase (APX), MDHAR, DHAR, and GTR are present in the chloroplast. Lastly, catalase (CAT) is found on the peroxisome (Fig. 3).
Tropical vegetables and fruits are more susceptible to cold temperatures than temperate ones. CI was previously believed to occur through a membrane phase transition, specifically from a semi-liquid state to a solid state. However, the current phase transition results in an exceedingly high amount of energy that is unattainable to produce within living cells. Conversely, cellular injury leads to the production of CI, which is evidenced by the release of electrolytes. Consequently, research has been conducted to examine the etiology of membrane injury.
-
Cold stress response leads to alterations in the plasma membrane's structure, composition, and functions[25]. The more unsaturated fatty acids (UFA) there are in membrane lipids, the lower the temperature at which they transition from one form to another and the greater their fluidity. This is because saturated acyl chains pack tightly together. Polyunsaturated fatty acids (PUFA) reduce packing density by incorporating cis-double bonds into the acyl chains, preventing the membrane from transitioning from a liquid crystalline to a solid-gel state[26]. Typically, the tolerant plant has a higher concentration of 18:2 and 18:3 fatty acids compared to 18:0 fatty acids (Fig. 4). Cytomembrane destabilization, which results from a decrease in fatty acid desaturation, primarily causes the symptoms of CI in bell peppers[27].
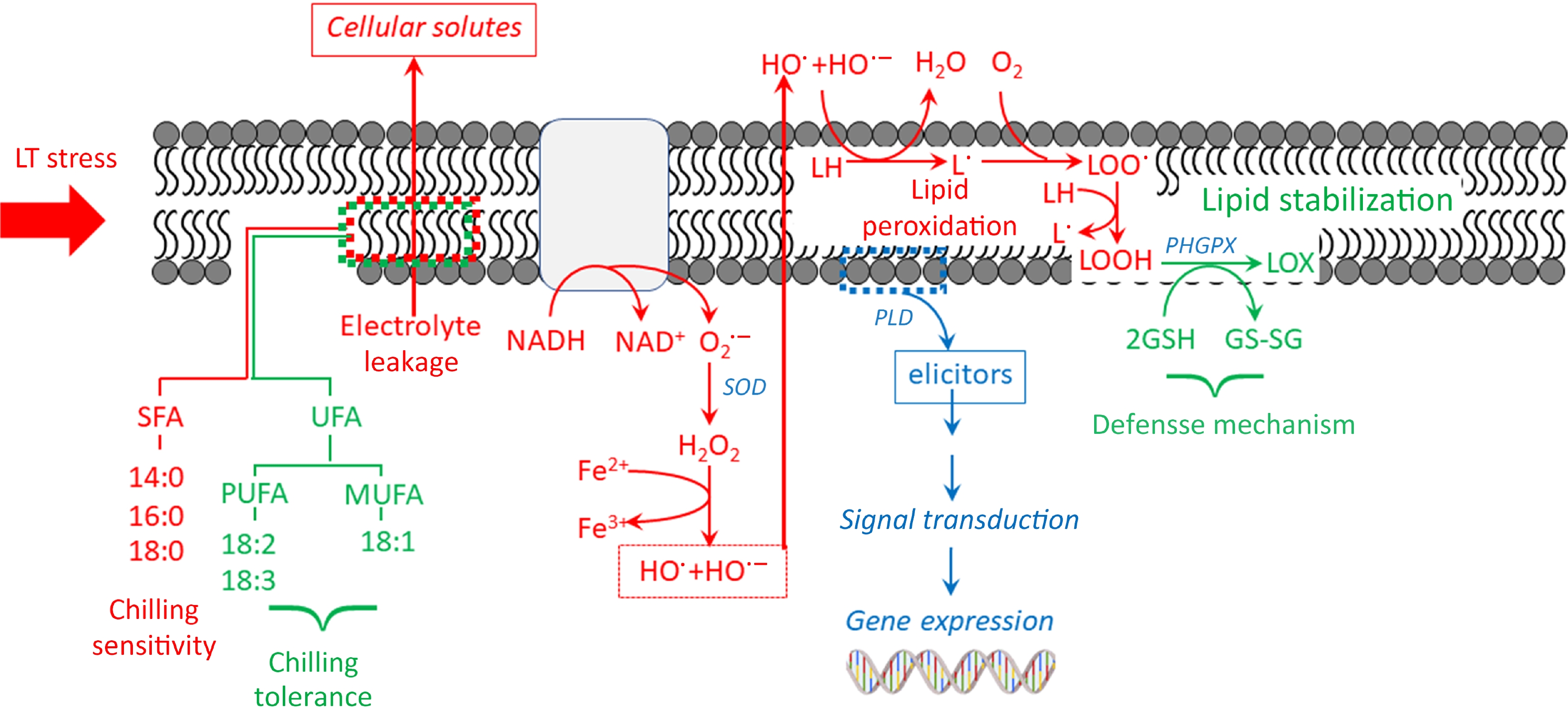
Figure 4.
Proposed mechanisms of membrane lipid components related to CI sensitivity and tolerance under low temperature.
After 35 d at 1 °C, the tolerant loquat variety 'Qinzhong' showed reduced levels of O2·−, H2O2, and LOX activity. However, it did have higher membrane lipid unsaturation, and increased levels of SOD and CAT enzymes than the sensitive variety 'Fuyang'[28]. Furthermore, after 180 d of fruit storage at 2 and 5 °C followed by an additional 2 d at 20 °C, the peel of tolerance 'Sanguinello' blood oranges exhibited the highest levels of UFA and UFA/SFA ratio and the lowest levels of SFA. On the other hand, the 'Moro' variety, which is sensitive to cold, displayed the highest levels of SFA, the lowest levels of UFA, and UFA/SFA ratio[29]. The physiological response of CI results in cell membrane disruption due to changes in the lipid composition. The membrane structure and antioxidant profile of sensitive and tolerant plum cultivars were examined during storage on CI at low temperatures. Because lipids are the primary component of the membrane, different types of fatty acids are responsible for CI. The sensitive CI variety contains more linoleic acids (C18:2) than oleic acid (C18:1), whereas the tolerant cultivar has a higher proportion of oleic acid than linoleic acid. This contradicts the pattern observed in other plants. Nevertheless, in contrast to antioxidants, sensitive cultivars exhibit elevated glutathione levels, whereas tolerance cultivars have elevated ascorbic acid levels. Therefore, it suggests that ascorbic acid and the central antioxidant system primarily contribute to the ability to withstand low temperatures[30]. Furthermore, chilling injury at 4 °C correlates with a decrease in ascorbic acid content, as well as SOD and CAT activity, leading to peel browning in 'Chok Anan' mango fruit[31]. The fatty acid composition of mature and young lemon basil leaves, on the other hand, was linoleic (C18:2) and linolenic acid (C18:3). Young leaves, which had more chilling sensitivity, contained more linoleic acid, but levels of linolenic acid and palmitoleic acid (C16:1) were lower. However, the ratio of UFA and SFA was the same in young and mature leaves, and the increase in ratio tended to be similar in both throughout the storage period at 4 °C[5].
When compared to less sensitive varieties, CI-sensitive sweet basil has higher levels of free fatty acids (FFA) C16:0 and lower levels of C18:1 in the phospholipid bilayers of its chloroplasts[32]. Arabidopsis plants possess fatty acid desaturases (FAD), which decrease the saturation level of lipids found in cell membranes when exposed to low temperatures. This enhances the plant's ability to withstand the adverse effects of low temperatures[33]. The presence of IB in peaches stored at 0 °C was reduced by applying a pressure of 10−20 kPa. Hypobaric treatments suppressed the increase in membrane fluidity, thereby preserving mitochondrial activity and preventing mitochondrial dysfunction. Additionally, these treatments triggered the ascorbate-glutathione (AsA-GSH) cycle[34]. According to Liu et al.[35], acclimatized mulberry seedlings exposed to chilling stress at 3 °C showed increased APX activity.
The plasma membrane initially detects cold temperatures and experiences phase transitions when exposed to low-temperature stress. The membrane undergoes a gradual transition from a liquid crystal to a solid crystalline state, which has a detrimental impact on its structure and function. Plant cells adapt to low temperatures by modifying their physiological metabolism to cope with short-term cold stress. Failure to do so can result in cellular injuries, leading to a condition called chilling-induced injury. Initial responses to cold stress identify changes in the cell membrane, which subsequently lead to an increase in membrane permeability and impairment. Phospholipases and LOX partially contribute to the breakdown and peroxidation of lipid membranes, which primarily cause membrane injury. Phospholipase D (PLD) breaks down glycerophospholipids into phosphatidic acid (PA). This can change the shape of the cell membrane and stop it from working normally (Fig. 4). Additionally, at low temperatures, phospholipase A1 (PLA1), phospholipase A2 (PLA2), and phospholipase C (PLC) can induce the breakdown of glycerophospholipids, resulting in irreversible membrane damage. Membrane lipid peroxidation is a significant consequence of membrane damage. This is evidenced by a decrease in unsaturation in membrane phospholipids and an increase in malonaldehyde (MDA) levels. LOX catalyzes the oxidation of the carbon-carbon double bond found in UFAs like linoleic and linolenic acids. As a result, the fluidity of the cell membrane is reduced. ROS catalyzes the oxidation of phospholipids and UFAs, resulting in an excess of MDA, which causes significant harm to the structure and function of the membrane. To alleviate CI in pepper fruit, it is critical to scavenge excess ROS and inhibit membrane lipid peroxidation. Enzymatic and non-enzymatic antioxidant systems regulate the ROS metabolisms in plants, working together to maintain a dynamic equilibrium and safeguard against oxidative stress. SOD, peroxidase (POD), CAT, and APX are the primary antioxidant enzymes in plants[5, 8, 16].
'Queen' pineapples exhibit a high susceptibility to IB[36,37], as indicated in Table 1. The IB links the vascular bundles of fruitlets, which exhibit the highest levels of polyphenol oxidase, hydrogen peroxide, and phenolic compounds[36]. The IB coincides with the simultaneous deterioration of the phloem tissue. The CI-sensitive cultivars have a lower proportion of sclerenchyma fibers in the tissue. In addition, the cell wall of fruitlet tissues exhibiting IB displays a collapsed structure, potentially indicating the loss of certain constituent elements[38]. Ultrastructural changes in the cell wall matrix between the normal and CI flesh surrounding the core are depicted in Fig. 5.
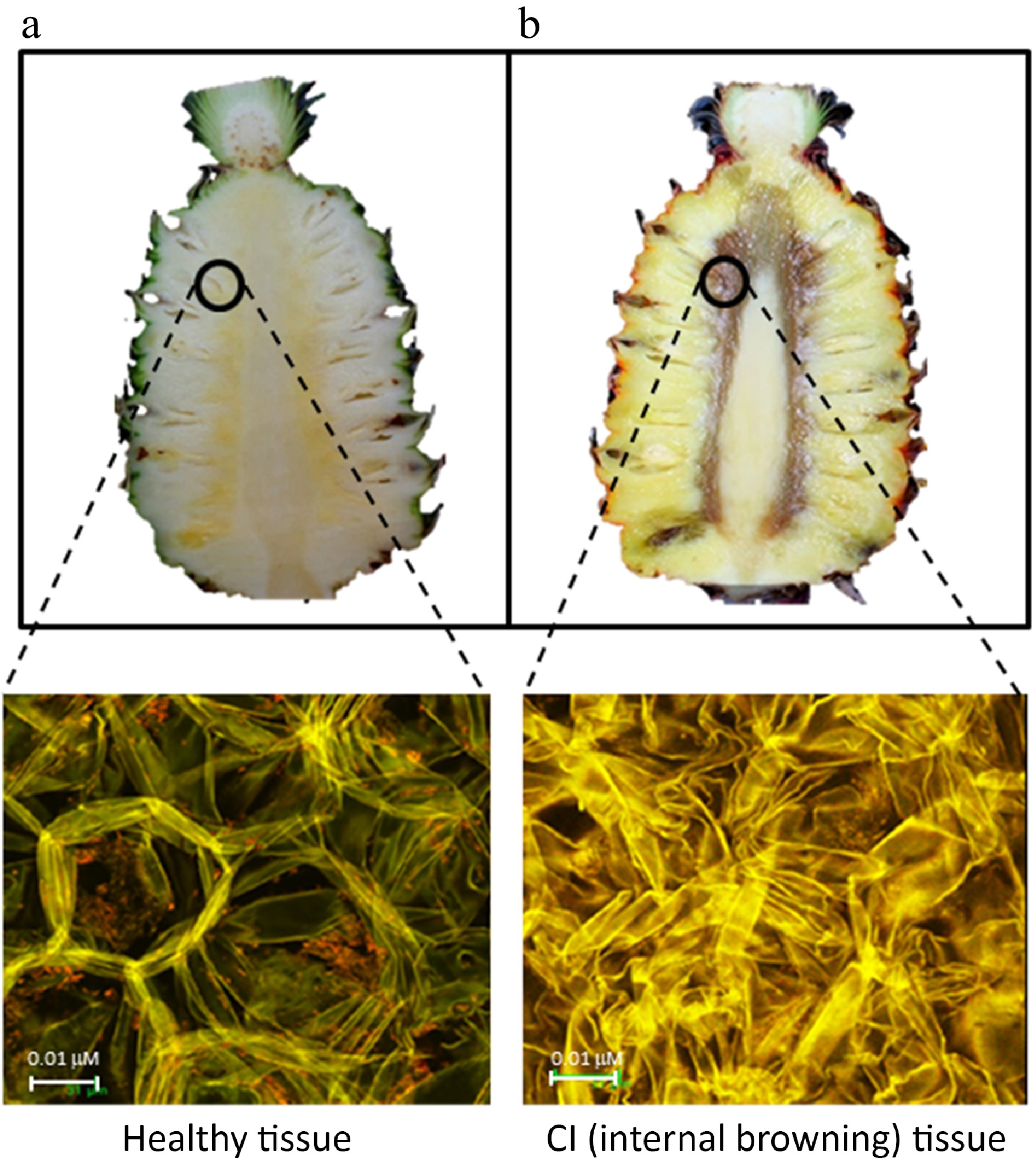
Figure 5.
Long section fruit-cut of Queen pineapple (upper) (a) on the harvesting day and (b) fruit stored at 13 °C for 14 d generating internal browning. The flesh tissues of a fruitlet adjacent to the core (in the circle) of fruit cross-sectioned and photographed under a confocal microscope (below) (sourced from Dr. Panipa Youryon with permission).
CI initiation and development in lipid membrane damage
-
Living cells consistently generate free radicals. Redox reactions produce free radicals, which the cell scavenges to eliminate. Because free radicals have a brief half-life, the cell can efficiently eliminate them and sustain its survival. However, plant parts are obtained from the parent plants, which are stressed due to water and food deprivation, physical injury, and extreme temperatures. Stress at such low temperatures can decelerate or accelerate certain metabolic processes, such as respiration, which encompasses oxidative phosphorylation, which generates ROS[39].
Nevertheless, there are highly effective scavenging mechanisms in place, such as the ascorbate-glutathione system. APX, an enzyme present in the cytosol, plastids, and mitochondria, utilizes ascorbic acid and H2O2 to eliminate ROS. ROS are produced by Redox, which results from electron transport in mitochondria and chloroplasts. NAD(P)H oxidase, which generates O2·− and is located on the plasma membrane, becomes dysfunctional due to low-temperature stress. When exposed to fungal elicitors, superoxide can be generated within the plasma membrane of plant cells, which is responsible for triggering the hypersensitive response observed in certain plants[40]. The ROS are transformed into a non-reactive free radical of H2O2. These intermediates are then converted into water in the peroxisome by CAT action. Alternatively, the enzyme POD can use them as a precursor to synthesize the secondary cell wall. A compromised or inadequate ROS defense will cause the cell to accumulate a high concentration of H2O2. While H2O2 is, initially, a non-reactive form of ROS, it can be converted into OH− via the Fenton reaction by the action of Fe2+. This conversion leads to continuous fatty acid peroxidation in the lipid membrane, resulting in damage[24] (Fig. 6).
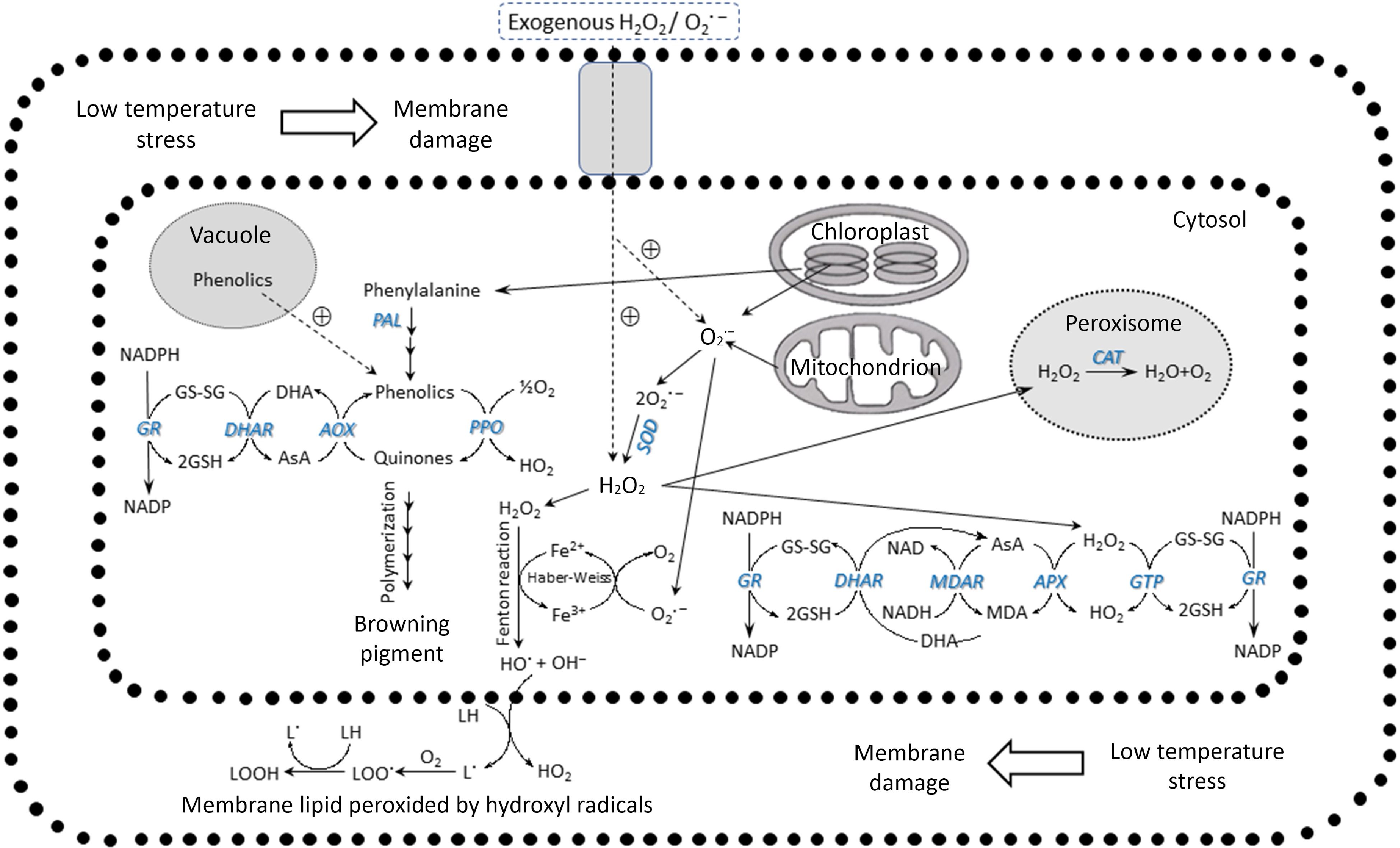
Figure 6.
ROS induced by low temperature and the enzymatic scavenging systems (modified from Noichinda et al.[24]).
Adding phenylalanine to tomato plants makes them more resistant to CI by protecting cell membranes, as shown by lower electrolyte leakage and MDA accumulation. This demonstrates the involvement of ROS in CI. Phenylalanine stimulates the functions of SOD, CAT, APX, and GR, which decrease the buildup of ROS and H2O2. Increased accumulation of phenols and flavonoids, facilitated by PAL, is responsible for the higher content of ascorbic acid. An increased activity of the methylenetetrahydrofolate dehydrogenase (MTHFD) enzyme in tomatoes, caused by external applications of phenylalanine, could activate the plant's ROS removal system[41]. Cooling has a substantial impact on the rate of O2·− production, H2O2 content, and SOD and APX activities in chloroplasts and mitochondria. SOD and APX levels are higher in chloroplasts than in mitochondria due to the increased presence of membrane-bound Fe-SOD and APX[42].
Gourd peel and mesocarp respond differently to CI (Fig. 7), primarily attributed to generating free radicals under low-temperature stress. A variety of antioxidants, including ascorbic acid, phenolic compounds, and vitamins found in the fruit parts, neutralize these free radicals. Therefore, the positioning of CI is influenced by multiple regulatory factors. Sweet basil leaves stored at 4 °C exhibited CI and decreased polyphenols and antioxidant capacity, compared to those stored at 12 °C[43]. CI Okra pods show water soaking and CI surface pitting (Fig. 8a). When compared to the normal okra surface stored at 4 °C (Fig. 8b), a CI area shows collapsed cells of the pod epidermis, obviously on the surface pitting (Fig. 8c). The data are shown in H2O2 and MDA, and weak scavenging systems in chilled okra pods[16].
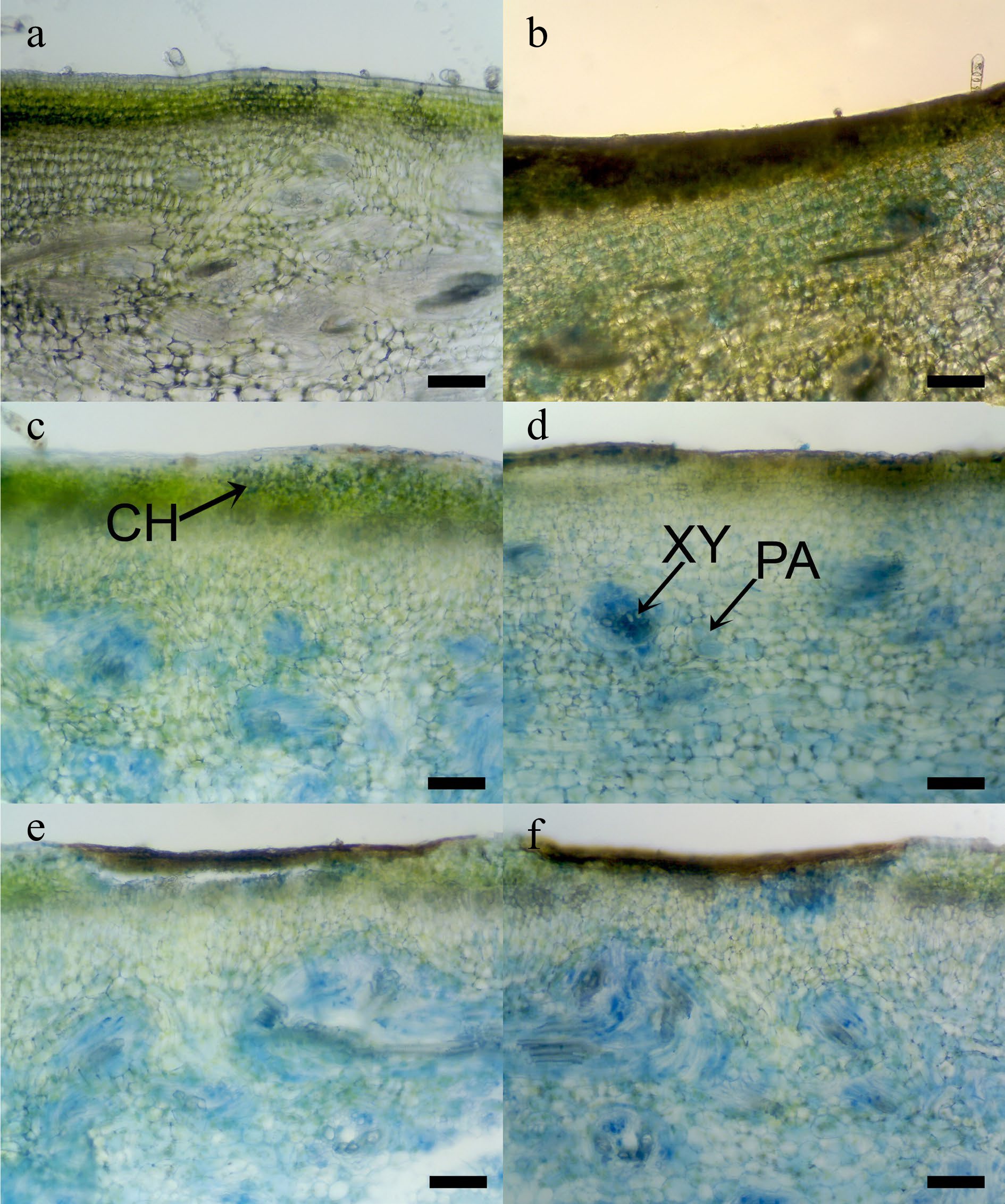
Figure 7.
Photomicrographs of internal chilling injuries (CI) symptoms in immature sponge gourd tissue after Evans blue dye staining, (a)−(f ) were 2, 4, 6, 8, 10, and 12 d during storage at 5 °C. Arrow pointed Evans blue dye staining area: CH = chlorenchyma, PA = parenchyma, and XY = xylem vessel. (Scale bar = 100 μm)[3].
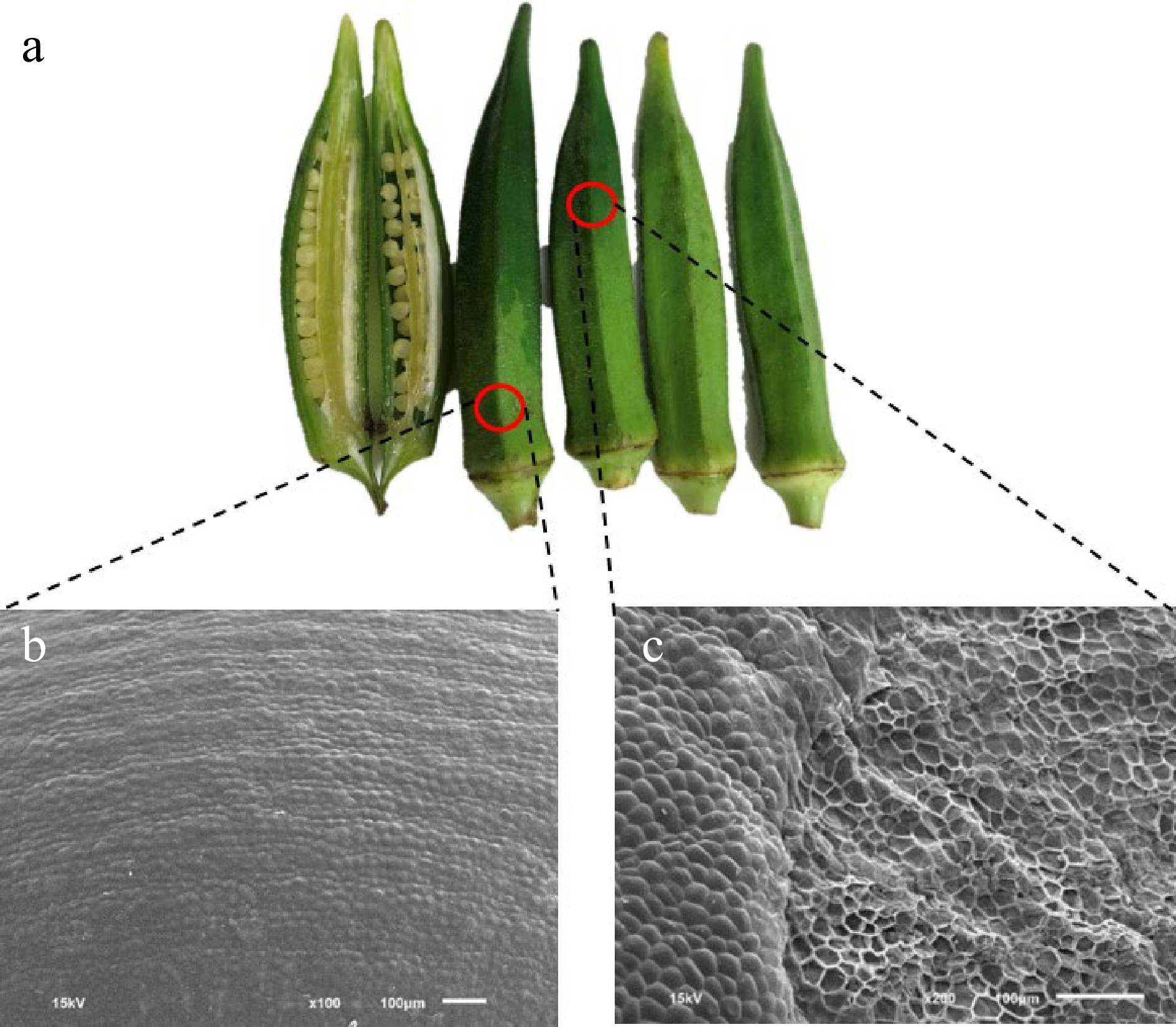
Figure 8.
CI appearances of (a) okra pods and (b) SEM pictures of the peel stored at 4 °C for 12 d at normal surface at 100× and at (c) the collapsed surface at 200× (sourced from Dr. Surisa Phornvillay with permission).
Lemon basil leaves (Ocimum × citriodourum) exhibited visible CI within 24 h at 4 °C. Elevated electrolyte leakage in the chilling leaves indicated damage to the organelle membranes. The leaf blackening caused 80% electrolyte leakage. After 24 h of chilling, the C14:0 SFA disappeared, while the UFA C16:1, C18:1, C18:2, and C18:3 increased. Mature leaves had higher CI, possibly due to decreased CAT activity[5]. The findings of Boonsiri et al.[44] align with the notion that young hot pepper fruit (Capsicum annuum L.) exhibited more pronounced seed browning when stored at a temperature of 5 °C in comparison to mature fruit. Severe browning showed a positive correlation with elevated levels of saturated fatty acids, decreased unsaturated fatty acids, and increased TBA and LOX activity levels.
-
The generation of CI in fresh produce occurs through two mechanisms: the structural integrity of the cell membrane, which is influenced by the ratio of PUFAs (C18:1, C18:2, C18:3) to SFAs, and the oxidation of membrane lipids by ROS. Plants employ diverse defensive mechanisms against ROS. The generation of cellular injury depending on low-temperature levels, crop types, and storage duration is primarily attributed to the imbalance between increasing ROS and decreasing scavenging systems, especially antioxidants such as ascorbic acid content (Fig. 9). Chilling temperatures at the lower critical point can interfere with the activity of respiratory enzymes that reduce ROS and LOX. However, the presence of CI in produce can occur due to low temperatures, which trigger LOX to oxidize lipid peroxidation, resulting in more severe symptoms. During cold stress, it is crucial to maintain the integrity of the membrane by modifying the composition of membrane lipids to effectively deal with the stress. Plants have the ability to remodel the ultrastructure of unsaturated fatty acids after a short period of chilling in response to infrequent temperature fluctuations[45].
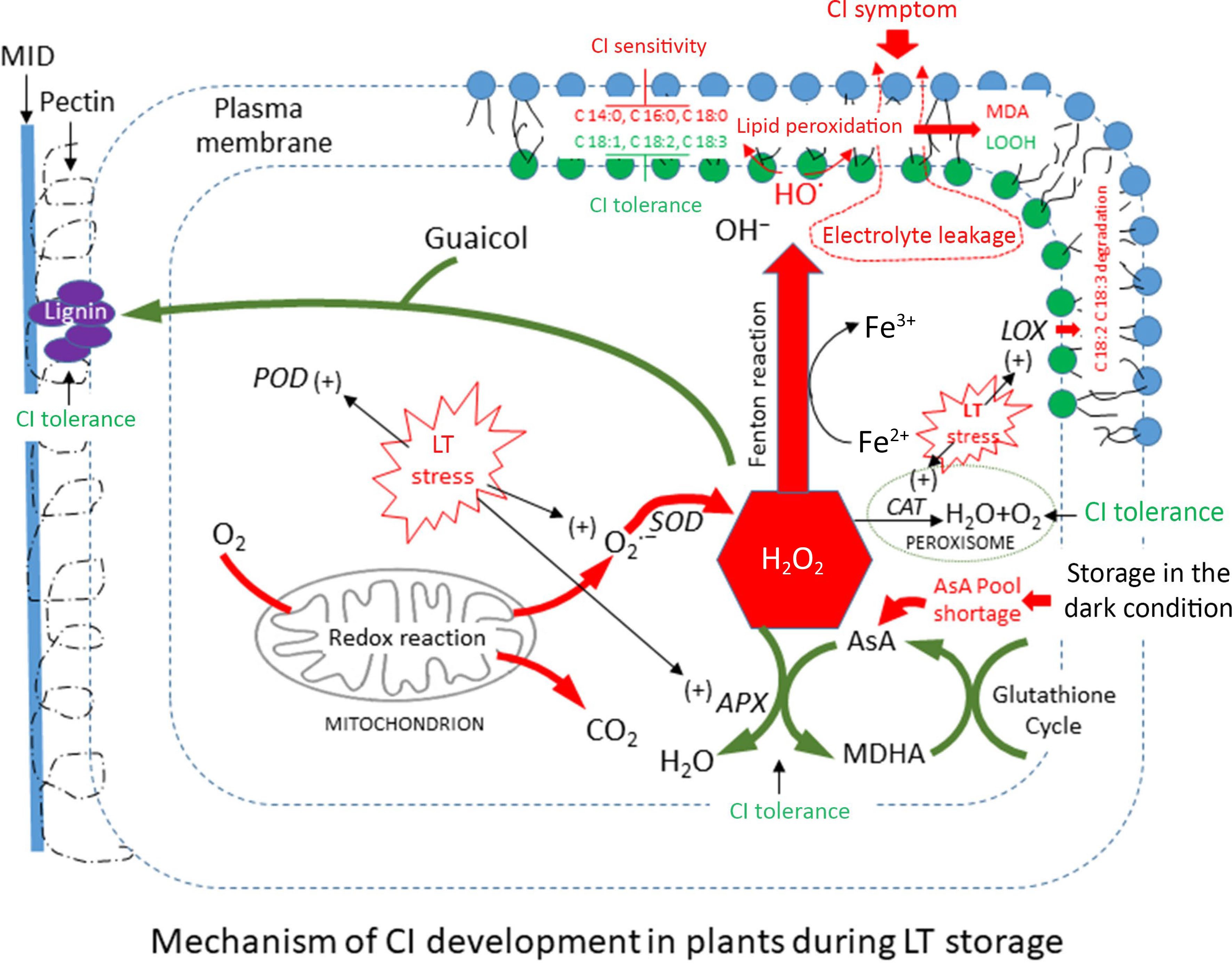
Figure 9.
The proposed chilling injury (CI) sensitivity and tolerance in immature sponge gourd fruit during storage at low temperature conditions: induce [+] and inhibit [–].
There have been studies on preventing cold-induced injuries in low-temperature environments. Plant photosynthesis, especially that of green leafy vegetables, is highly susceptible to inhibition by low temperatures. This is due to a disruption of the ascorbic acid biosynthesis coupling pathway[46]. Intermediate warming is a thermal regulation method for controlling CI. The transfer of chilled plants during the warm afternoon prevented the appearance of visible signs of harm, hampered photosynthesis and transpiration suppression, and decreased leaf osmotic potential[47]. Intermediate warming is commonly used to store plant parts sensitive to low temperatures. A hypothesis suggests that applying temporary heat exposure to chilled tissues can potentially enhance the degradation of toxic substances that accumulate throughout the chilling process or assist in the restoration of depleted components within the tissues.
Additionally, the antioxidant activity of holy basil leaves stored at 7 °C was improved in chemical applications. Pre-treating the leaves with a spray of 5 mM salicylic acid or oxalic acid 24 h before harvest reduced the increase in electrolyte leakage. This treatment also alleviated the occurrence of chilling injuries[48]. Inconsistent with an experiment in a vegetable fruit, okra pods immersed in low concentrations of methyl jasmonate slow down the CI increase at 4 °C by increasing antioxidant activity and reducing MDA accumulation[17, 49]. A pretreatment with 42 °C hot water dipping for 15 min before 4 °C storage can delay peel blackening by 4 days in mature green 'Gros Mitchel' bananas due mainly to lower LOX activity related to detectable membrane degradation[50]. An imbalance in cell elements may cause pineapple IB that peduncle infiltration of a 0.2 M CaCl2 solution a day before storage is the main determinant of IB prevention in 'Queen' pineapple stored above the critical 13 °C[37]. Furthermore, fruit coatings, Sta-Fresh 2952 and 7055 waxes applied to the cultivar 'Paris' pineapple fruit reduce CI and postpone changes in firmness, flesh color, and weight loss[51].
-
Chilling temperatures induce a physiological disorder known as chilling injury (CI), which manifests as surface pitting, water succulency, tissue discolorations, browning, abnormal ripening, and off-flavor. Chilling temperatures below the critical point (in general, below 13 °C for tropical horticultural crops) for a while triggers cellular oxidative stress that damages macromolecules. The significant evidence for severe damage starts with organelle membrane damage by lipid peroxidation and, subsequently, loss of permeability. The balance of free radicals is mismatched with the scavenging by antioxidant systems. CI appears when it passes the reversible phase.
-
The authors confirm contribution to the paper as follows: developing the structural plan, data collection, graphic creation, writing, and manuscript revision: Wongs-Aree C, Noichinda S; contributing ideas and supportive data: Aschariyaphotha W, Palapol Y, Bodhipadma K; theme supervision: Noichinda S. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
We thank Dr. Panipa Youryon from King Mongkut's Institute of Technology Ladkrabang and Dr. Surisa Phornvillay from the University of Malaysia Sarawak for their valuable contributions to certain CI photographs.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wongs-Aree C, Aschariyaphotha W, Palapol Y, Bodhipadma K, Noichinda S. 2024. Structural membrane alterations in tropical horticultural crops under postharvest chilling stress. Vegetable Research 4: e016 doi: 10.48130/vegres-0024-0013
Structural membrane alterations in tropical horticultural crops under postharvest chilling stress
- Received: 09 January 2024
- Revised: 10 April 2024
- Accepted: 15 April 2024
- Published online: 03 June 2024
Abstract: Optimal storage temperatures are essential for preserving vegetables' quality. Tropical plants, meanwhile, have a significant vulnerability to low temperatures, yet the majority of vegetables are farmed within tropical climates. Low temperatures can cause oxidative stress in vegetables, resulting in a condition known as 'chilling injury' (CI). The symptoms may manifest as visible external traits, including color shading, surface pitting, surface browning, and water soaking. Conversely, CI can alter internal changes such as water succulence, internal browning, and flesh translucence. Additionally, CI potentially triggers abnormal metabolic processes, resulting in atypical ripening or the development of an unpleasant odor. Reactive oxygen species (ROS) that occur during oxidative stress at low temperatures first harm the membrane of organelles and subsequently damage macromolecules like proteins, lipids, and DNA/RNA. Therefore, visual signs of cellular damage indicate the advanced stage of damage after membrane transition. The tolerant plant generally contains more polyunsaturated fatty acids (PUFA) than monounsaturated fatty acids (MUFA) in its cellular membrane. All damaging cells display an imbalance between ROS and the scavenging systems, including chemical compounds and enzymatic cycles. Inducing antioxidant systems is essential for preserving the quality of chilled vegetables. Therefore, when the cells reach the advanced stages of CI development, specifically after membrane leakage, they are unable to recover and will progress to the final stage of CI, exhibiting phenotypic CI symptoms.