HTML
-
Potato (Solanum tuberosum L.), locally known as "patatas" is widely cultivated in 130 countries worldwide including the Philippines. According to Fernald (1970) and Spooner & Knapp (2013), Solanum species comprise more than 1000 species. Potato follows rice and wheat as one of the most important food crop for human consumption. The nutritional composition of potato has major impacts on population health such as the presence of vitamins and minerals thereby preventing malnutrition and slows down the accumulation of chronic diseases because of its antioxidant content (Camire et al. 2009).
However, this staple food can be infected by post-harvest diseases caused by fungi such as dry rot, caused by several species of Fusarium causing great loss in crop production. Accordance to the earlier reports, there are 13 Fusarium species considered as causal agents of dry rot of potatoes worldwide (Cullen et al. 2005) and the most frequent and devastating are F. sambucinum, F. solani and F. oxysporum (Li 1992, Ye & Wang 1994, Peng & Zhu 2008, Hay et al. 2018). Infection starts when the pathogen enters the tuber, causing rotting out of the left, and once the pathogens penetrated the tuber skin, it begins to develop internally into the tissue (Wharton et al. 2007).
To control fungal diseases, chemical fungicides are extensively used in agriculture but can cause problems such as environmental pollution and deterioration of human health (Gomathi & Ambikapathy 2011). Consequently, studies reveal that a fungi can be used as a biocontrol agent if interactions between other fungi can be determined (Duffy et al. 2003). Moreover, biological control agents have specific advantages over synthetic fungicides. It was proven to have effective control of pathogens and reduces pests with the use of natural enemies (Brimmer & Boland 2003, Adebola & Amadi 2010). Hence, the present study was conducted to isolate and identify molds associated with dry rot of potato. Interactions between the identified molds were carried out to determine the species of fungi that have potential as bio control agent.
-
Potato tubers showing diseased symptoms such as dark depressions on the surface, wrinkled skin in concentric rings, necrotic areas from light to dark brown or black in color and rotted cavities (Wharton 2015) were randomly selected and obtained from 5 traders of La Trinidad, Benguet Vegetable Trading Post. Tubers were kept in proper labeled paper bags and immediately transported to laboratory for isolation.
Isolation of fungal species
-
Collected potato tubers were rinsed with tap water to remove the dust and adhering debris. Then, the infected part of the sample was cut from the healthy part using sterilized blades under aseptic conditions. Infected part were then rinsed with sterile distilled water for 1 minute. Then, infected potato parts were then pulverized using a blender. Ten grams from the pulverized sample were added with 90 ml sterilized distilled water and was regarded as 10-1. Six test tubes were prepared having 9 ml of sterilized distilled water each and were labeled from 10-2 to 10-7. One ml from 10-1 dilution was transferred into 10-2 dilution and was shaken. Then, one ml from 10-2 was transferred to 10-3 dilution. This procedure was done in which one ml was being transferred from one dilution to another up to 10-7 dilution. Then, one ml of sample from 10-1, 10-3, 10-5 and 10-7 were poured on sterilized petri plate separately and pour plated with approximately 20 ml previously sterilized Potato Dextrose Agar (PDA). Triplicate was made for each plates. It was incubated at room temperature in alternating light and dark conditions for 4-5 days or until sufficient mycelial growth was available for isolation.
Purification of fungal isolates
-
Distinct fungal colonies were purified into pure culture using three-point inoculation technique. Using a sterilized needle, mycelia were isolated from the mixed culture into a previously plated PDA. The plated isolates were incubated for 4-7 days at room temperature. Isolates were only considered as pure culture when colonies show consistent growth characteristics. Pure culture of the fungal isolates were deposited at the Biodiversity Conservation Laboratory, Department of Biological Sciences, College of Science, Central Luzon State University, Science City of Munoz, Nueva Ecija, Philippines, 3120. Facesoffungi numbers were registered as mentioned in Jayasiri et al. (2015).
Collection of infected potato with dry rot disease
-
Three-point inoculation was done in previously prepared plated PDA to observe the cultural characteristics of the six isolates. The re-growth, size and pigmentation of fungi were observed and recorded.
Morphological characteristics
-
In a petri plate with moistened tissue paper, a clean glass slide was placed on top of a v-shaped foil and was sterilized. Agar block approximately 1 cm-thick was placed on the left of the slide. Fungal species were inoculated on the agar block and covered with sterilized cover slip. Inoculated slides were incubated for 3-5 days at room temperature. After incubation, morphological characteristics were observed under a compound microscope.
Molecular identification through sequencing
-
Seven-day old fungal isolates cultured on test tubes with PDA medium was sent to Philippine Genome Center in Quezon City, Manila for DNA extraction and sequencing following their standard procedures and protocols. In which DNA extraction was done by CTAB method using universal ITS1 (5'- TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') primers with 58'C annealing temperature for polymerase chain reaction. Capillary sequencing was carried out on the ABI 3730xl DNA Analyzer using a 50cm 96-capillary array, POP7TM Polymer, and 3730xl Data Collection Software v3.1 and base calling was done on Sequencing Analysis Software v5.4. Trimming and assembling of sequences were done using Codon Code Aligner V8.0.2
In vitro interaction of identified fungi
-
Two fungi were inoculated using one-point technique at the opposite sides of the previously plated PDA and then allowed to ramify at room temperature for seven days. After incubation, colony growth was observed and their interaction was determined (Waing et al. 2015).
Identification of in vitro interaction
-
Interactions of fungi were described as antagonism and mutual antagonism. Fungal isolates were classified as victim or aggressor in all antagonism tested (Dix & Webster 1995). Mutual inhibition or mutual slight inhibition is determined if there is mutual antagonism between fungi. In mutual inhibition, the fungus approached each other until almost contacted and a demarcation line of more than 2 mm was visible while mutual slight inhibition shows a distance of 0.1 to 2mm between two fungal colonies (Fakhrunnisa et al. 2006).
Interfungal parasitic relationship
-
The procedure for interfungal parasitic relationship was adopted from Matroudi et al. (2009) with minor modifications, in which the slide culture technique determined the antagonistic interaction between fungal species. In a petri plate with moistened tissue paper, a clean glass slide was placed on v-shaped foil and was sterilized. The inocula of the two fungi were placed on both ends measuring 1 cm apart of sterilized glass slide separately. One end of the slide was coated with approximately 5.0 mm-thick layer of PDA while the other end was kept free for handling. The inoculated slides were incubated at room temperature for 3-5 days. After incubation, presence of coil formation and penetration structures, or wall disintegration where both hyphae meet were observed microscopically.
Cultural characteristics
-
A total of six species of fungi were identified from infected potato with dry rot disease. The external part of diseased potato was seen (Fig. 1A) having dark depression on the surface with large lesions and the skin was dry and wrinkled (Fig. 1B). The internal diseased part of the potato was observed (Fig. 1C) with mycelial clumps of varying color from white to yellow to pink.
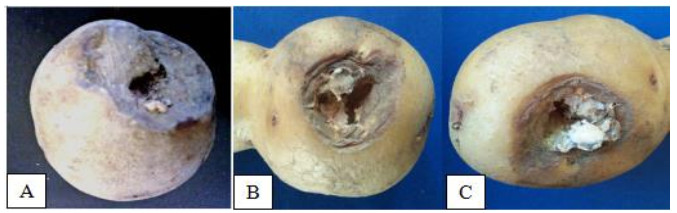
Figure 1. Observed symptoms of dry rot in samples collected in La Trinidad. A Benguet showing surface dark depression. B wrinkled and dry skin. C mycelial clumps in dead skin.
Cultural characteristics
-
The cultural characteristics of isolated fungi described on the 7th day of incubation were as follows: A. flavus colonies (Fig. 2A) formed fastidious whitish mycelium at the margin and yellow green color at the left on obverse side of the plate. Mycelium at reverse side was dirty white in appearance with light brown color at the left with a diameter of 48.80 mm. Colonies of A. fumigatus (Fig. 2B) attained 58.18 mm in diameter. On its surface and margin, it appears blue green in color with white fluffy mycelium. Reverse side was dark blue to pinkish in appearance with light brown color at the left. The colonies of A. niger (Fig. 2C) in PDA plate produce white spreading mycelium at the margin with powdery pale yellow to black color mycelium on the left in both sides of the agar plate. Colony growth measures 51.15 mm. The colonies of F. oxysporum (Fig. 2D) were peach in color with velvetly aerial mycelium. Reversed side of the plate was yellowish to orange with dark violet mycelium on its left. Colony diameter was 57.29 mm. The colonies of F. solani (Fig. 2E) in obverse side of the plate were seen creamy white in appearance with cottony mycelium. Reversed side appears yellowish to pale brown mycelium, and colony diameter measures 46.87 mm. M. velutinosus (Fig. 2F) colonies at obverse side of the plate was seen powdery gray in color and white on the left. Reverse side of PDA plate show pale yellow in the left with white concentric rings. Colony diameter was 61.48 mm.
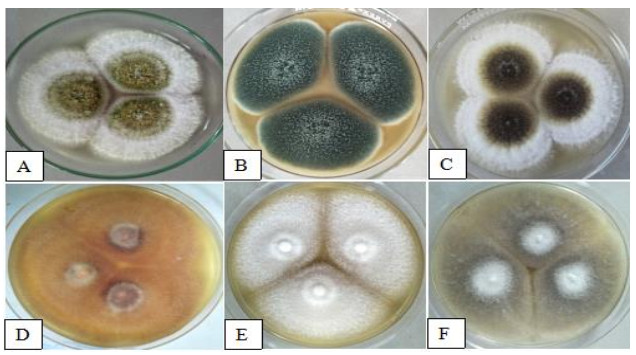
Figure 2. Cultural characteristics represented by A A. flavus. B A. fumigatus. C A. niger. D F. oxysporum. E F. solani. F M. velutinosus.
Morphological characteristics
-
Observed morphological characteristics of the isolated fungi were described on the 7th day incubation were as follows: The stipes of A. flavus (Fig. 3A) appears rough and light brown, while the conidium was rough and have spherical surface. The conidia of A. fumigatus (Fig. 3B) is non septated and have globose shape with rough edges, conidiophores appears hyaline. Conidiophores of A. niger (Fig. 3C) are branched with lumped phialides. The conidiophores of F. oxysporum (Fig. 3D) were long with fusiform conidia, hypha is hyaline with branch monophialides. F. solani (Fig. 3E) shows narrow macro conidia, and long and branch monophialides. M. velutinosus (Fig. 3F) have globose sporangiospores, and formed septated hyphae.
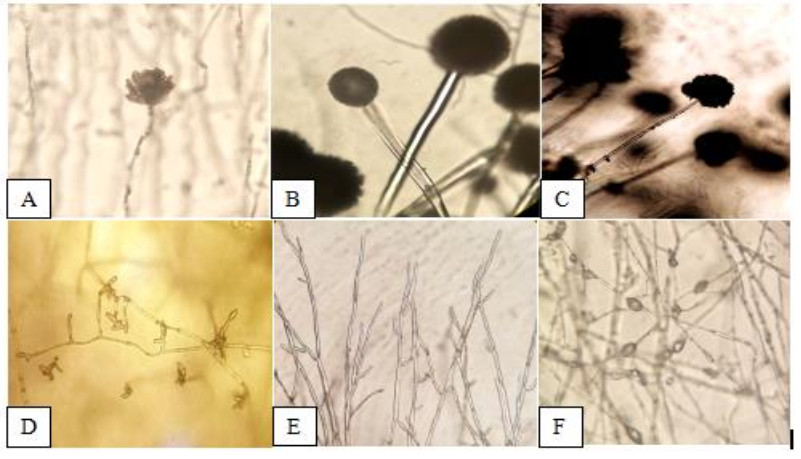
Figure 3. Morphological characteristics represented by A A. flavus. B A. fumigatus. C A. niger.D F. oxysporum. E F. solani. F M. velutinosus (400X).
Molecular identification
-
The identities of fungal organisms isolated from the S. tuberosum infected with dry rot disease were confirmed through amplification and sequencing of the Internal Transcribed Spacer region (ITS) region using ITS1 and ITS4 primers. BLAST analysis showed the identities of fungal isolates, namely Aspergillus flavus FoF 09597 (100%), Aspergillus fumigatus FoF 10086 (100%), Aspergillus niger FoF 10087 (99.82%), Fusarium oxysporum FoF 03824 (95.06%) Fusarium solani FoF 01873 (100%) and Mucor velutinosus FoF 10088 (96.45%) as shown in Table 1.
Table 1. Identities of the identified fungi with Facaesoffungi number using BLAST with NCBI Genbank Accession number.
Isolate No. FacesofFungi Number Species E-Value Identify Accession 1 FoF 09597 Aspergillus flavus 0.0 100.00% MN511747.1 2 FoF 10086 Aspergillus fumigatus 0.0 100.00% MF540309.1 3 FoF 10087 Aspergillus niger 0.0 99.82% KY607770.1 4 FoF 03824 Fusarium oxysporum 0.0 95.06% MG372014.1 5 FoF 01873 Fusarium solani 0.0 100.00% MN202790.1 6 FoF 10088 Mucor velutinosus 0.0 96.45% KY203942.1 Antagonistic interaction and interfungal parasitic relationship
-
Meanwhile, antagonistic interaction was observed in all fungal isolates (Table 2). Antagonism in all fungal isolates was identified as aggressor or victim. The observed interfungal parasitic relationships were hyphal penetration, hyphal coiling, hyphal denaturation, hyphal branching and lysed cells, all observed under the compound microscope (Figs 4-7) (Waing et al. 2015).
Table 2. Antagonistic interaction of isolated fungi in potato tuber infected with dry rot disease.
Interacting Fungi Interaction M. velutinosus(+) A. flavus(-) Antagonism M. velutinosus(+) A. fumigatus(-) Antagonism M. velutinosus(+) F. solani(-) Antagonism M. velutinosus(+) F. oxysporum(-) Antagonism M. velutinosus(-) A. niger(+) Antagonism A. flavus(-) A. fumigatus(+) Antagonism A. flavus(+) F. solani(-) Antagonism A. flavus(-) F. oxysporum(+) Antagonism A. flavus(-) A. niger(+) Antagonism A. fumigatus(-) F. solani(+) Antagonism A. fumigatus(-) F. oxysporum(+) Antagonism A. fumigatus(-) A. niger(+) Antagonism F. solani(-) F. oxysporum(+) Antagonism F. solani(-) A. niger(-) Antagonism F. oxysporum F. oxysporum Antagonism F. oxysporum(+) A. niger(-) Antagonism (+) aggressor, (-) victim 
Figure 4. Antagonistic interaction represented by; A M. velutinosus (left) and A. flavus (right). B hyphal denaturation and lysed cells of A. flavus (400x). C M. velutinosus (left) and A. fumigatus(right). D hyphal denaturation and lysed cells of A. fumigatus (400x). E M. velutinosus (left) and F. solani (right). F hyphal penetration of M. velutinosus to F. solani (400x). G M. velutinosus (left) and F. oxysporum (right). H hyphal penetration of M. velutinosus to F. oxysporum (400x). I M. velutinosus (left) and A. niger (right). J hyphal denaturation and lysed cells of M. velutinosus (400x).

Figure 5. Antagonistic interaction represented by; A A. flavus (left) and A. fumigatus (left). B lysed cells of conidiophores of A. flavus (400x). C A. flavus (left) and F. oxysporum (right). D hyphal coiling of F. oxysporum leading to hyphal denaturation of A. flavus (400x). E A. flavus (left) and A. niger (right). F hyphal denaturation of A. flavus (400x). G A. flavus (left) and F. solani (right). H hyphal denaturation and lysed cells of F. solani (400x).
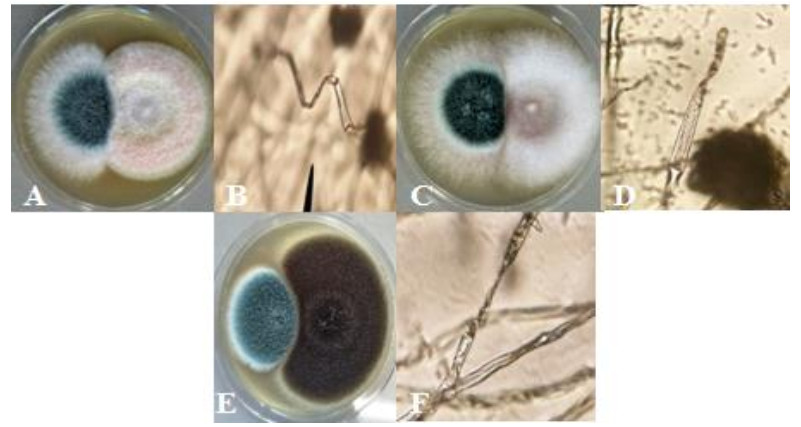
Figure 6. Antagonistic interaction represented by; A A. fumigatus (left) and F. solani (right).B hyphal denaturation of A. fumigatus (400x). CA. fumigatus (left) and F. oxysporum (right). D lysed cells of A. fumigatus (400x). EA. fumigatus (left) and A. niger (right). F lysed cells of A. fumigatus (400x).
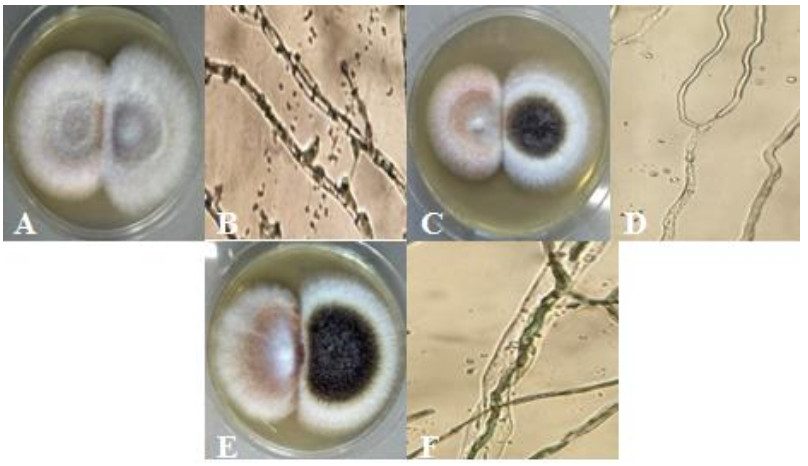
Figure 7. Antagonistic interaction represented by; A F. solani (left) and F. oxysporum (right). B hyphal penetration of F. oxysporum to F. solani (400x). C F. solani (left) and A. niger (right). D hyphal denaturation and lysed cell of F. solani (400x). E F. oxysporum (right) and A. niger (left). F hyphal penetration of F. oxysporum to A. niger (400x).
Observed symptoms of potato dry rot
-
Observed characteristics of infected potato with dry rot disease are similar to the study of Wharton (2015). In addition, wounds caused by unavoidable process of harvesting potato allowed the manifestation of fungal organisms that later on led to dry rot disease (Wharton et al. 2007). The present study has isolated two species of Fusarium, F. solani and F. oxysporum in which based on the study conducted by Stefanczyk et al. (2016), Fusarium species have been associated with potato dry rot. Such, F. sambucinum, F. solani and F. oxysporum are the most frequent and devastating species. In addition, three species of Aspergillus namely: A. flavus, A. fumigatus and A. niger were also isolated. According to the study of Ibrahim & Tambuwal (2015), A. niger, and A. fumigatus were both pathogenic causing rotting of tomato fruit. Meanwhile, A. flavus was previously isolated from Dioscorea spp. rot (Okigbo & Ogbonnaya 2006). Moreover, M. velutinosus is one of the most frequent fungal endophyte identified from the rotted root samples of D. arayalpathra (Premalatha et al. 2014). Fungal epiphytes are found on the surface of the plants. Also, many of them are obligate parasites which penetrated the host plant and prevent the uptake of the nutrients as cited by Hongsanan et al. (2016). These isolated fungal species are confirmed through BLAST analysis. In which based on the previous study of Nagano et al. (2008) the use of ITS primer for fungal identification shows accurate and reliable result. Furthermore, ITS primer is the standard marker for fungal DNA barcoding (Bellemain et al. 2010) and is generally used for the assessment and characterization of fungal communities from environmental samples (George et al. 2019).
The interaction study revealed antagonism between all identified fungi. Hyphal denaturation, hyphal coiling, hyphal penetration and lysed cells were the observed antagonistic interaction of hypha. In which, M. velutinosus showed the ability to penetrate the host hyphae of the F. solani and F. oxysporum which are considered as causative agents of potato dry rot. In microbial communities, there are different relationships and interactions characterized by mutualism to antagonism and parasitism (Duffy et al. 2003). These interactions happen when nutrients and space are limited (Arya & Perello 2010). Fungi that affects the other by harming them by means of suppressing the growth of other or killing them is mycoparasitism or hyperparasites (Jeffries 1995, Kumar & Gouda 2018). Its behavior which was observed in previous studies includes hyphal penetration, hyphal coiling, hyphal branching, hyphal denaturation, cell lysis, spore production, and barrier formation (Dubey & Dwivedi 1986, El-Debaiky 2017). As a result of these interactions, the host hyphae were vacuolated, shrank, collapsed, and disintegrated. Moreover, at the interface region of colonies, certain fungi were observed having irregular branched hyphae, cracked hyphae, ruptured cell wall, and dead cells at the left of antagonism line (Chet et al. 1981, Xing et al. 2005). The ability of mycoparasites to penetrate the host cell after coiling can lead to cell lysis because of the enzymatic interaction. Wherein, certain fungus penetrates internally and uses direct enzymatic lysis of hyphal cells. Hyphal coiling incorporated by means of hock between the hyphae and lysed cells can lead to fusion. In addition, hyphal cell wall lysis leads to penetration of the hyphae for obtaining nourishment to the host by means of haustoria (Dubey & Dwivede 1986, El-Debaiky 2017). However, certain host fungi counter attack by forming resting bodies. Also, host hypha accumulates cytoplasm to stop the infiltration of antagonist inside the cell. These host defense act as a barrier and survival measures against destruction in adverse condition and the over expression of pyridoxal reductase is a result of stress condition of a certain host fungi (Dubey & Dwivede 1986, Chamoun & Jabaji 2011, El-Debaiky 2017). According to the studies of Barnette (1963) and El-Debaiky (2017), the degree of damage by the parasites to the host cells depends to the resistance of host species. These studies show that certain parasites have direct contact to the host because of the absence of diffusible antibiotics which caused severe damage. In addition, some antagonist can produce spore inside the host hypha depending to the nutrition from the host fungi. Furthermore, according to Verma et al. (2007), there are several modes of actions that mycoparasitism exhibited which includes competition in space and nutrient, production of antibiosis and plant defense system induction. One evidence of this mode was observed by Waing et al. (2015) in which, the presence of zone of inhibition was observed when the two fungi are paired which indicates the production of antibiotics.
Emerging fungal diseases in various agricultural crops due to rapid growth of new pathogens that are fungicide resistant posed a significant threat in agricultural productivity and risk to global food security (Fones et al. 2020). An example of which is the fungal disease known as Septoria tritici blotch (STB) that resulted to

- Copyright: © 2021 by the author(s). This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| GAD Carreon, EE Gandalera, KGD Waing. 2021. Molecular identification and in vitro interaction of molds associated with dry rot of potato (Solanum tuberosum L.) collected in La Trinidad, Benguet, Philippines. Studies in Fungi 6(1):315−326 doi: 10.5943/sif/6/1/22 |












