-
Flower sex differentiation is a special organogenesis phenomenon in certain plants, characterized by the occurrence of stamens or pistils in unisexual flowers with functional stamens or functional carpels[1]. A single flower can be male, female, or bisexual. A single plant, however, can be any of seven modes of sexuality: (1) androecious, in which the plant has only male flowers; (2) gynoecious, in which the plant has only female flowers; (3) hermaphroditic, in which the plant has only bisexual flowers; (4) monoecious, in which the plant has both male and female flowers; (5) andromonoecious, in which the plant has both male and bisexual flowers; (6) gynomonoecious, in which the plant has both female and bisexual flowers; and (7) trimonoecious, in which the plant has male, female, and bisexual flowers[2]. In nature, there are approximately 240,000 species of flowering plants, of which 7% are monoecious and 6% are dioecious[3,4]. Sex chromosomes and sex-determination genes located in the sex-determination region (SDR) are the genetic basis of sex determination in dioecious plants. Cloning sex-determination genes is the key to elucidating the genetic mechanism of sex determination in plants, and dioecious plants with sex chromosomes provide ideal materials for studying the mechanism of sex determination.
Horticultural plants supply healthy fruits and vegetables in our daily lives. Many horticultural plants are sexually reproducing, and the ratio of their male to female flowers is closely related to yield and other important agronomic traits. As most of these horticultural plants are cultivated for the purpose of obtaining fruits or seeds, it is necessary to maintain high numbers of female flowers to improve fruit setting. Because of this, identification of key sex determinants, the root cause of sex differences, is of great significance for breeding and yield increase. Horticultural plants, particularly perennial fruit trees, have diverse types of sexuality and complex sex-determination mechanisms. Their sexuality is often affected by exogenous factors, yielding great challenges to the exploration of molecular mechanisms underlying their sex determination. With the rapid development of molecular biology, genetics and genomics, and high-throughput sequencing technologies, sex-determination genes and sex-determination regions have been identified and functionally characterized for some horticultural plants. For a quick and thorough understanding of the molecular mechanism of flower sex determination in plants, we summarize recent research in three types of horticultural crops: fruits, melons and vegetables. These findings provide crucial information and reference for molecular marker-assisted breeding and high-yield production of sexually reproducing plants.
-
Fruit plants are an important source of vitamins and inorganic salts, and contain a variety of biologically active substances. To date, only persimmons (Diospyros lotus) and kiwifruits (genus Actinidia) have had their sex determinants uncovered with their functions verified experimentally. Some other fruit crops, including grape (Vitis vinifera), papaya (Carica papaya), date palm (Phoenix dactylifera), and strawberry (Fragaria virginiana), are being investigated in order to identify their sex-determination regions and candidate sex-determination genes.
Persimmon
-
Persimmons originated in China and have been cultivated for > 1,000 years. In 2014, Japanese scholars identified the genes determining the sex of persimmon plants using diploid D. lotus[5]. Diploid persimmons are dioecious and either androecious (chromosomes XX) or gynoecious (chromosomes XY)[6]. Through de novo whole-genome sequencing and transcriptome approaches, the researchers identified a Y-specific non-coding gene OGI expressed in male flowers[5]. OGI produces male-specific 21-nt small RNAs that specifically target its homologous gene MeGI, a class I homeodomain transcription factor (HD-ZIP) (Fig. 1a)[5]. MeGI is expressed at high levels in the buds and flowers of female plants, sterilizing the androecia and promoting the formation of female flowers (Fig. 1a)[5]. In male flowers, small RNA produced by OGI on the Y chromosome triggers transitive and persistent small RNA production from MeGI, which in turn represses mRNA production of MeGI and allows for the normal development of stamens and the formation of male flowers (Fig. 1a)[5]. Two years later, Akagi et al.[7] identified an epigenetic mechanism regulating sex determination in hexaploid persimmons (D. kaki). In hexaploid persimmons, plants containing Y chromosomes have both female and male flowers. OGI expression is undetectable in developing male flowers, potentially due to the presence of a 268 bp short interspersed nuclear element (SINE)-like insertion in the OGI promoter region of the Y chromosome. However, in male flowers, methylation of the MeGI promoter can activate MeGI small RNA (smMeGI) production, which represses MeGI expression and in turns results in male flower development[7]. Occasional sex reversal from male to female may originate from spontaneous demethylation of the MeGI promoter[7]. Since methylation can be accumulated and reset, the sex of the flowers on the offspring of hexaploid persimmons can change[7]. Hexaploid persimmons, then, have more flexible flower sex determination than do diploid persimmons because of the involvement of DNA methylation. In 2020, Akagi et al.[8] found that MeGI (Chr.13) was derived from its homologous gene Sister of MeGI (SiMeGI) (Chr.4) through whole-genome duplication, suggesting that MeGI acquires a new function as a repressor of stamen development, while SiMeGI still maintains the original function. After that, segmental duplication events produced OGI, the regulator of the MeGI gene, on the Y chromosome, thus completing the path to dioecy[8]. The discovery of the OGI-MeGI system in persimmons provides a good reference for studying other dioecious plants.
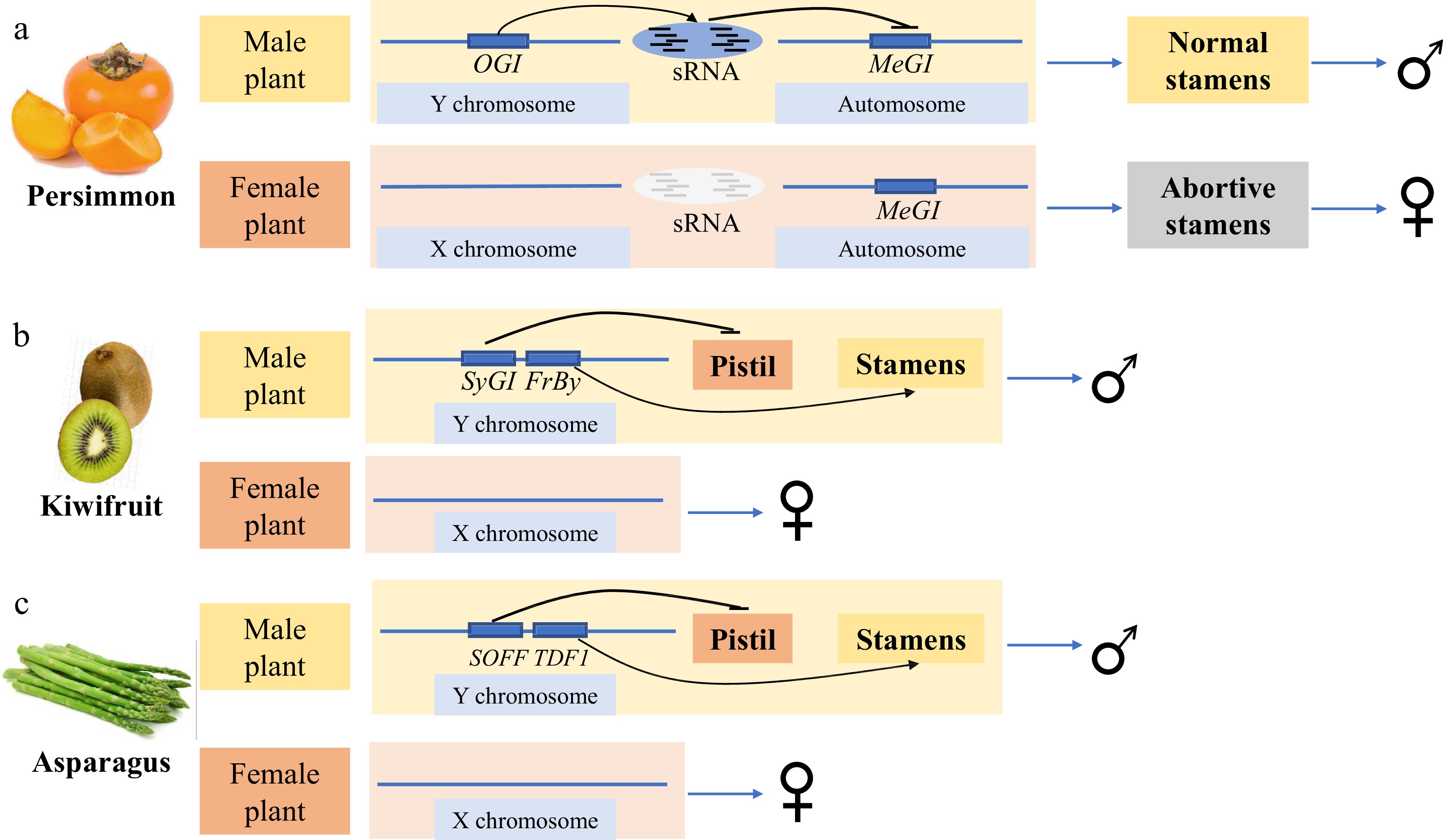
Figure 1.
Horticultural crops with known sex-determining genes. (a) The sex-determining genes of persimmon. (b) The sex-determining genes of kiwifruit. (c) The sex-determining genes of asparagus.
Kiwifruit
-
Kiwifruit, a fruit consumed worldwide, is native to China and belongs to the genus Actinidia of Actinidiaceae. Most Actinidia species are dioecious with XY males and XX females[9]. Akagi et al.[9,10] identified a female sterility factor, Shy Girl (SyGI), and a male promoter gene, Friendly Boy (FrBy), as sex determinants of kiwifruit. Both genes are encoded in the male-specific region of the Y chromosome (MSY). SyGI is a type-C cytokinin response regulator that is specifically expressed in the rudimentary carpel in developing male flowers (Fig. 1b)[9]. By inhibiting the development of the carpel in male flowers, SyGI acts as a dominant suppressor in female fertility[9]. FrBy exhibits strong expression in tapetal cells during the early stages of anther development[10]. Gene-editing and complementation analyses in Arabidopsis thaliana and Nicotiana tabacum indicated that FrBy acts on male maintenance independently of the SyGI gene[10]. Hermaphrodite kiwifruit can be formed through FrBy gene expression in female kiwifruit[10]. This clearly shows that SyGI and FrBy on the Y chromosome act independently as the female suppressor SuF and the male promotor M in kiwifruit (Fig. 1b)[10].
Grapevine
-
Grapevine is a long-lived perennial plant and is one of the most important fruit crops in the world. Wild grapevine (V. vinifera ssp. sylvestris) is dioecious with XY chromosomes, while cultivated grapevine (V. vinifera ssp. vinifera) has reverted to hermaphroditism[11]. The evolution of hermaphroditism was key in the domestication of cultivated grapevine, reflecting the influence of artificial selection pressure on plant sex differentiation[12]. Sex determination in grapevine is proposed to be controlled by a major locus containing three alleles, including male gene M, female gene F, and hermaphroditic gene H, and the dominant epistasis between alleles is M > H > F[13]. Various genetic studies have shown that a region across ~150 kb on chromosome 2 is the sex determination region of grapevine[12−16]. By analyzing 20 Vitis SDR haplotypes and the associated gene expression data, Massonnet et al.[16] identified a recessive allele of VviINP1 as the candidate gene for male sterility, and the M allele of VviYABBY3 as the gene for female sterility. In contrast, Badouin et al.[14] suggested that the upregulation of VviAPT3 on the Y chromosome causes female sterility, and the downregulation of VviAPT3 on the Yh chromosome may trigger reversal to hermaphroditism, thus VviAPT3 is the candidate gene for female sterility. Zou et al.[12] reported results consistent with VviINP1 and VviYABBY3 being the genes determining male sterility and female sterility, respectively, after investigating the pattern of sex-linked SNPs and chromosome painting of these SNPs coupled with allele-specific transcriptome analysis (Table 1). The above results are based primarily on comparative genomic analyses, and should be verified further using biological experiments.
Table 1. The sex-related gene with known function in horticultural crops.
Horticulture
plantsGene name Organs with high expression level Gene function Persimmon OGI Male flowers Promoting the development of stamens MeGI Buds and flowers of female Promoting the formation of female flowers Kiwifruit SyGI The rudimentary carpel of developing male flowers Inhibiting the development of carpel in male flowers FrBy Tapetal cells during early stage of anther development Male maintenance Grapevine VviINP1 Female flowers The candidate gene for male sterility VviYABBY3 Male flowers The candidate gene for female sterility Cucumber CsACS1G (F) Early stages of flower buds development Promoting the formation of female flowers CsACS11 (A) Phloem cells connected to flowers programmed to become female Promoting carpel development CsWIP1 Male flowers Inhibiting carpel development and promoting male flower development CsACS2 (M) Flower buds at different stages of development Inhibiting stamen development CsACO2 Carpel primordia Promoting carpel development Melon CmASC11 Vascular bundles of female flowers Promoting carpel development in female flower CmWIP1(g) The carpel primordia of male flowers Leading to carpel abortion and promoting stamen development CmACS-7(a) The carpel primordia of female and hermaphrodite flowers at the early stages Inhibiting stamens development Watermelon ClWIP1(gy) Carpel primordia Leading to carpel primordia abortion ClACS7 Carpel primordia of flower buds that are determined to develop into full carpels Promoting the development of carpels CitACS4(a) Carpel primordia in pistillate flowers Inhibiting of stamen development Pumpkin CpWEI Undefined Inducing female flower development in undifferentiated asexual buds Asparagus SOFF Flower buds in supermale (weakly) Suppressing female organogenesis aspTDF1 Anther tapetum Promoting anther development Papaya
-
Papaya is major fruit crop growing in tropical and subtropical regions, and is a trioecious species with male, female, and hermaphrodite flowers on separate plants[17]. The sex determination system in papaya is particularly intriguing, not only because it has three sex types, but because it shows frequent sex reversal caused by environmental factors[18]. Genetically, papaya sex determination is controlled by three chromosomes; in addition to the X and Y chromosomes, papaya has a unique sex chromosome Yh, such that the genotype of males is XY, females is XX, and hermaphrodites is XYh[18]. In 2008, scientists found that DNA methylation and heterochromatinization played an important role in the early stage of evolution of the papaya Y chromosome, and proposed that Yh chromosomes were likely present at the initial stage of sex differentiation[19]. The male-specific region of the Y chromosome (MSY), which determines male flower development, was estimated to account for only 10% of the Y chromosome[20]. The hermaphrodite-specific region of Yh (HSY) determines hermaphrodite flower development[21]. In 2015, the HSY and MSY sequences were aligned and their sequence similarity was estimated to be 99.60%[22]. Further analysis showed that the Yh chromosome originated from the Y chromosome and arose approximately 4,000 years ago, well after plant domestication in Mesoamerica (> 6,200 years ago), but coinciding with the rise of Maya civilization[22]. Therefore, it was speculated that the evolution of the Yh chromosome resulted from the domestication of papaya[22]. Both MSY and HSY are approximately 8.1 Mb from the LG1 (Linkage Group 1) centromere, and recombination with the X chromosome is suppressed[20,21]. Urasaki et al.[17] found a MADS-box transcription factor specific to the Y and Yh chromosomes which is expressed only in male and bisexual plants. The MADS-box gene encodes a protein with 85% similarity to the Short Vegetative Phase (SVP) protein in Arabidopsis, a well-known transcriptional regulator of flowering time. By comparing the sequences of MSY and HSY, it was found that the SVP-like gene (CpSVPL) on the Y chromosome encodes a complete protein, but on the Yh chromosome encodes an incomplete protein because of transposon insertion[23]. Furthermore, CpSVPL had one SNP associated with the three sex genotypes, and was highly expressed in the male and female sterile flowers (abnormal hermaphrodite flowers), which lacked the fourth whorl structure[24]. These results suggest that the SVP-like gene is a candidate gene for sex determination in papaya, but further analysis, including gene knockout in the male or overexpression in the female plants, is required to definitively determine its role.
Other fruit plants
-
Sex determination has been investigated in many other fruit plants, but specific sex-determination genes have not been fully identified. In 2019, researchers decoded the red bayberry (Morella rubra) genome by combining genome sequencing and transcriptome sequencing, and confirmed that its sex determination followed the ZW model[25]. In this system, the sex is determined by the genotype of the egg cell, and males are homogametic (ZZ) and females are heterogametic (ZW)[25]. A 59 kb female-specific region (FSR) located on distal end of pseudochromosome 8, which contains abundant transposable element and seven putative genes, was identified and cloned. Further transcriptome data revealed that four female-specific genes (MrCPS2, MrASP2, MrSAUR2, and MrFT2) in the FSR region were expressed only in female flower buds. MrCPS2 and MrASP2 were most highly expressed during the female flower initiating stage, whereas MrSAUR2 and MrFT2 were most highly expressed during the flower primordium formation period. This demonstrated that MrCPS2 and MrASP2 were key initiating factors[25].
The date palm (Phoenix dactylifera L.) is one of the most economically important crops in North Africa, the Middle East, and South Asia[26]. The date palm is dioecious, with separate male and female trees. The sex determination region was found in the approximately 5–13 Mb region of the long arm of LG12[26,27]. Torres et al.[28] sequenced the genomes of 15 female and 13 male Phoenix trees representing all 14 species, and found that only four genes contained sequences conserved in all Phoenix males. Among them, CYP703 and GPAT3 are male-specific genes and are critical for male flower development, while a LOG-like gene appears translocated into the Y-linked region and may play a role in suppressing female flowers. The remaining gene encodes a cytidine deaminase and was not expressed in male or female flowers. The role of these genes in date palm and other plants of Phoenix requires further investigation.
The female sex chromosome in strawberry is ZW, and the male sex chromosome is ZZ. In 2013, Tennessen et al.[29] identified the sex-determination region of the gynodioecious diploid wild strawberry (Fragaria vesca ssp. bracteate), and found that male-sterile genes were located in a gene-dense 338 kb region of chromosome 4. Then, in 2015, Ashman et al.[30] found a new sex-determination region in a 1.769 Mb region on chromosome 6. In the absence of the MS allele at the LG4 locus (identified by Tennessen et al.[29]), a dominant R allele located at the novel locus fine-mapped on LG6 can restore fertility[30]. In 2018, a 13 kb female-specific fragment which is a conserved, mobile W-specific SDR was identified in wild North American octoploid strawberries (Fragaria)[31]. The SDR could be translocated repeatedly, increasing the size of the female-specific hemizygous sequence on the W sex chromosome and revealing a new potential mechanism for expansion and diversification of incipient sex chromosomes[31].
-
Gourds belong to the Cucurbitaceae family, which includes cucumbers (Cucumis sativus), melons (Cucumis melo), watermelons (Citrullus lanatus), bitter melons (Momordica Charantia), pumpkins (genus Cucurbita), etc. To date, there are many studies investigating sex determination in these melons, and they represent the most well-understood plant sex determination systems.
Cucumber
-
Cucumber is a widely cultivated crop, and its edible part is the fruit formed from female flowers. Therefore, the female flower rate of cucumber is an important yield characteristic. Cucumbers are diploid and have seven pairs of chromosomes. They are trioecious, with male, female, and hermaphrodite flowers on the same or different plants[32]. In the 1960s and 1970s, researchers discovered three alleles (M/m, F/f, and A/a) controlling sex determination in cucumber[33−35] The F (female) gene partially dominates the all-female phenotype and promotes the early appearance of female flowers. The M (andromonoecious) gene shows complete dominance and inhibits stamen development, while recessive homozygous mm controls the occurrence of bisexual flowers. The A (androecious) gene shows complete dominance and promotes the development of carpels, while recessive homozygous aa represents the all-male phenotype. The F gene is dominantly epistatic to the a gene. Scientists have cloned the f genes (CsACS1)[36], F genes (CsACS1G)[36], M genes (CsACS2)[37,38], and A genes (CsACS11)[39,40], and found that they are all involved in the biosynthesis pathway of the plant hormone ethylene. M, F, and A are members of the aminocyclopropane-1-carboxylic acid synthase (ACS) gene families, and all encode ACC synthase.
In 1997, Trebitsh et al.[36] identified the gene CsACS1, which is linked to the F locus in ff cucumbers, and has been shown to encode ACS and be involved in ethylene biosynthesis. FF cucumbers have an additional copy of CsACS1, and that is CsACS1G. CsACS1G is a duplication of CsACS1 but with a recombinant distal promoter that may contribute to expression of gynoecy[41]. Due to the structural variation of the genome, ACS1G in all-female plants show a different promoter and expression pattern than does ACS1 in monoecious plants, and the extra CsACS1G increases ethylene content and accelerates the formation of female flowers[42]. ACS1G is expressed in the early stages of flower bud development, and functions with CsACO2 to generate an ethylene burst and establish a dominant pathway for female flower development[42]. This bypasses the need for ACS11 to produce ethylene, and may explain why the F gene is epistatic to the a gene[42].
Prior to the discovery of the F gene, Rosa[38] discovered a dominant locus controlling the development of unisexual/bisexual flowers in cucumber and named it the M gene. In 2009, the M gene (CsACS2) was cloned and shown to encode an ACS[37]. CsACS2 was examined in cucumber flower buds at different stages of development[43]. CsACS2-mediated ethylene biosynthesis in individual flower buds is associated with the differentiation and development of female flowers[43]. Subsequent studies showed that both CsACS2 and CsACS1G genes could induce ethylene production in cucumber, and ethylene treatment could induce the expression of CsACS2[44]. Because CsACS1G is expressed earlier than CsACS2, CsACS1G may produce ethylene to activate the expression of CsACS2. Constant CsACS2 transcription may continuously promote ethylene synthesis via positive feedback, leading to continuous arrest of stamen development during the development of female flowers[44]. F and M genes together regulate the production of unisexual or bisexual flowers.
Using genetic methods, Kubicki[40] discovered a recessive gene (A) that controls the all-male phenotype and can enhance male flower development. Boualem et al.[39] cloned the male gene A (Csa2G353460) in cucumber via positional cloning, and named it CsACS11 because it could encode ACS. CsACS11 is expressed in phloem cells connected to flowers programmed to become female, and drives normal carpel development by producing ethylene[39]. A single non-synonymous nucleotide deletion at the D843 position within exon 3 of CsACS11 led to a premature stop codon, and this missense mutation of CsACS11 led to male plants (aa)[39].
In addition to the three key major genes encoding ACC synthase (CsACS1G, CsACS2, and CsACS11), an ACC oxidase gene (CsACO2) that converts ACC into ethylene and a transcription factor (CsWIP1) were also identified and cloned[45], and also play a key role in regulating flower sex in cucumber. CsACO2 is expressed at carpel primordia, providing sufficient ethylene required for appropriate CsACS2 expression, and CsACO2 mutation leads to androecy[45]. CsWIP1 is repressed by CsACS11 and acts as a carpel inhibitor to control the coexistence of male and female flowers in monoecious plants[45]. CsWIP1 can bind directly to CsACO2 promoter and inhibit its expression, leading to aborted pistils. CsWIP1 further inhibits CsACS2 expression, while CsACS2 inhibits stamen development[45]. These results strongly suggest that ethylene plays a central role in cucumber sex determination and provide important theoretical reference for cucumber production and breeding.
The model of sex determination in cucumber can be summarized as follows (Fig. 2a, Table 1): CsACS1G (F) is the epistatic control gene of CsACS11 (A). CsACS11, CsWIP1, and CsACS2 (M) act upstream, intermediately, and downstream of the sex-determination pathway, respectively. CsACS2 inhibits the stamen of female flowers, while CsWIP1 inhibits the carpel. CsACS1G and CsACS11 produce endogenous ethylene synergistically with CsACO2 at different flowering stages, and endogenous ethylene inhibits CsWIP1 expression. CsWIP1 can inhibit carpel development by repressing the transcription of CsACO2, and can suppress CsACS2 expression, which in turn inhibits stamen development. The interaction of these genes gives rise to the various flower sexes of cucumber plants[39,46]
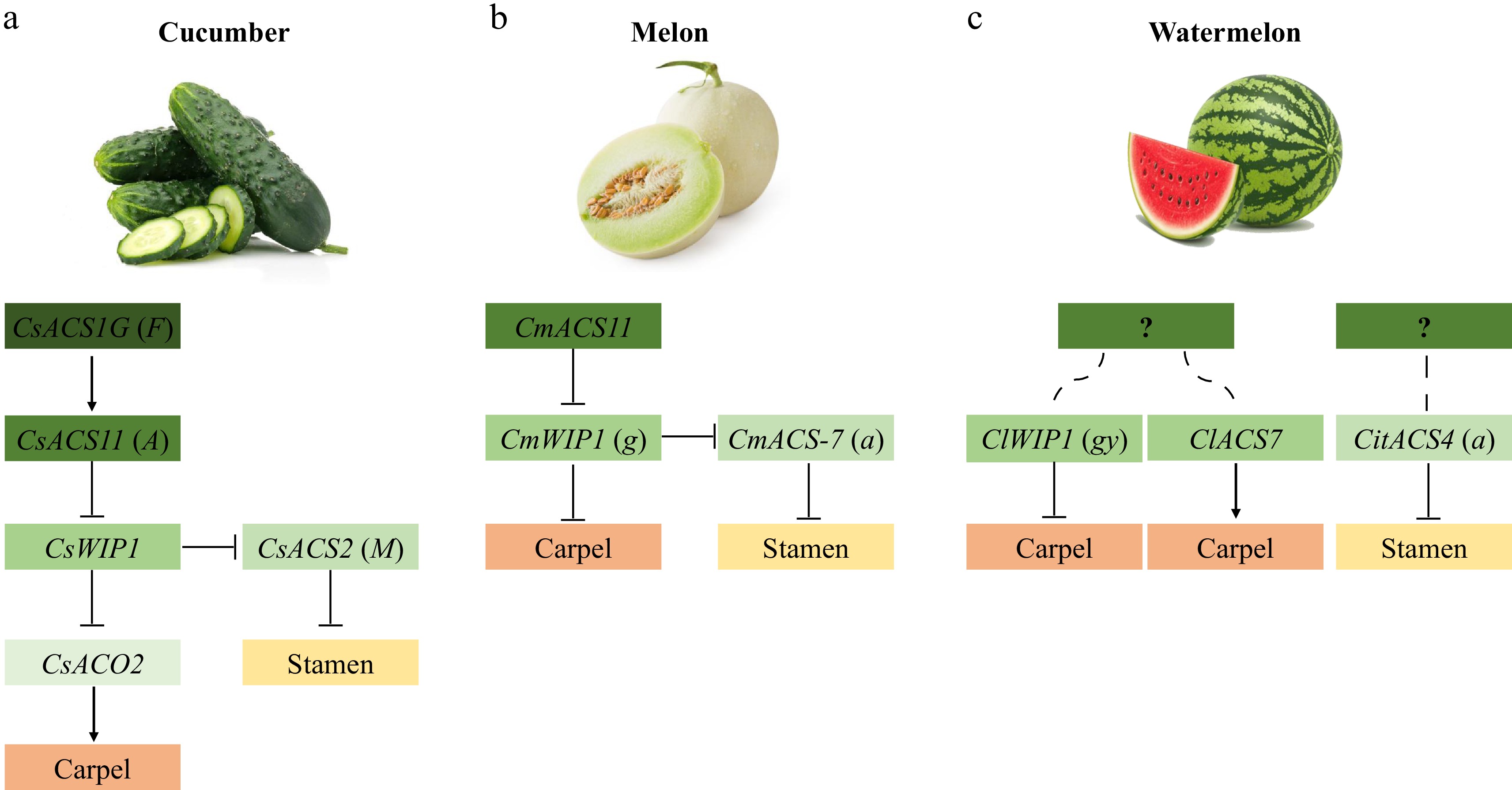
Figure 2.
Model of sex determination in gourds. (a) Model of sex determination in cucumber. (b) Model of sex determination in muskmelon. (c) Model of sex determination in watermelon.
Melon
-
Melon is an economically important plant with edible sweet and fleshy fruit. The sex-determination pattern of melon is similar to that of cucumber. Melon sexual forms are primarily controlled by three recessive genes (a, g, and m)[47−49]. The a gene controls the presence of andromonoecy, the g gene controls the presence of gynomonoecy, and the m gene controls the presence of gynoecy[47]. In 2008, Boualem et al.[50] cloned the a gene by constructing high-resolution genetic and physical maps. The a gene encodes ACC synthase, which is designated as CmACS-7 based on its homology to the ACS-7 gene (At4g26200) in Arabidopsis. CmACS-7 is expressed in the carpel primordia of female and hermaphroditic flowers during the early stage, when flowers are not morphologically distinguishable[50]. CmACS-7–mediated ethylene production in the carpel primordia inhibits stamen development in female flowers, and the loss of CmACS-7 can relieve this inhibition, resulting in bisexual flowers and leading to the conversion of monoecy to andromonoecy[50]. The pistil of bisexual flowers develops normally when CmACS-7 is inhibited, indicating that CmACS-7 is not required for carpel development[50]. Martin et al.[51] used this method to clone the g gene, which encodes a C2H2 zinc-finger transcription factor of the WIP protein subfamily, and named this clone CmWIP1. CmWIP1 is primarily expressed in the carpel primordia of male flowers, leading to carpel abortion and male flower formation. The insertion of a transposon Gyno-hAT is required for the induction and maintenance of CmWIP1 DNA methylation in monoecy, resulting in gynoecy. In addition, CmWIP1 indirectly promotes stamen development by inhibiting CmACS-7 expression, suggesting that CmACS-7 and CmWIP1 interact to control the development of unisexual and bisexual flowers in melon. Boualem et al.[39] cloned CmACS11 in melon, which controls the coexistence of male and female flowers in monoecy by repressing CmWIP1 expression, based on the ACS11 gene in cucumber. When CmACS11 is inactivated, CmWIP1 is expressed normally, resulting in the conversion of monoecy to dioecy. This indicates that CmWIP1 inhibits stamen development in pistil primordia and is necessary for carpel abortion in male flowers.
CmACS11 acts the most upstream of the sex-determination pathway in melon (Fig. 2b, Table 1). CmACS11 inhibits the expression of CmWIP1 (g), which disinhibits CmWIP1 on carpel development and leads to the formation of female flowers. CmWIP1 acts upstream of CmACS-7 (a) and is epistatic to CmACS-7 in the sex-determination pathway. CmWIP1 expression not only arrests carpel development, but represses CmACS-7 expression, allowing stamens to develop and male flowers to form. The absence of CmWIP1 enables CmACS-7 to inhibit stamen development, resulting in female flower development. When CmWIP1 and CmACS-7 are simultaneously inactivated, both carpels and stamens are able to develop, resulting in the formation of bisexual flowers.
Watermelon
-
Watermelon (Cucurbitaceae) is one of the top five most consumed fresh fruits worldwide. As early as the end of 1920s, Rosa[38] discovered that andromonoecy is a recessive characteristic. Later, in 1945, Poole & Grimball[52] confirmed that a recessive gene controlled andromonoecy in watermelon. It was not until 2007 that the first natural gynoecious watermelon mutant was found and reported[53]. Ji et al.[54] showed that watermelon sex determination is determined by three recessive alleles; andromonoecious (a), gynoecious (gy), and trimonoecious (tm). a is epistatic to tm. A locus encoding ACS has been identified and designated CitACS4[55−57]. CitACS4 is expressed predominantly in carpel primordia in pistillate flowers and results in andromonoecy in watermelon[56,57]. A missense mutation in a very conserved residue of CitACS4 (C364W) that co-segregates with the andromonoecious phenotype can reduce CitACS4 activity, leading to reduced ethylene production in the floral buds and the conversion of female into hermaphrodite flowers, and therefore of monoecy into andromonoecy[56,57]. In addition, an orthologue of CmWIP1 in melon, ClWIP1 (Cla0088537), was identified as the gy gene in watermelon[58]. The ClWIP1 gene is interrupted by a chromosome translocation breakpoint in a spontaneous gynoecious watermelon mutant[58]. Gynoecious watermelon lines can be obtained via gene-editing of ClWIP1 in monoecious plants[58]. ClWIP1 is expressed predominantly in the carpel primordia and is involved in the abortion of carpel primordia in early flower development[58]. Boualem et al.[55] carried out cloning and functional verification of the ClACS7 gene in watermelon, and demonstrated that ClACS7, a homolog of CmACS-7/CsACS2, co-segregated with the A locus. The enzymatic activity of ClACS7 leads to the development of female flowers in monoecious watermelon, and decreased enzymatic activity produces bisexual flowers, indicating that the silencing of the ClACS7 gene led to the conversion of female flowers into male flowers. ClACS7 is highly expressed in the carpel primordia of flower buds that are programmed to develop into full carpels and acts as a carpel promoter, but not in male flowers[55]. These results act as an important scientific foundation for using these key genes to improve yield and seed production efficiency.
In summary, in watermelon (Fig. 2c, Table 1), CitACS4 (a) acts as an inhibitor of stamen development by affecting the production of ethylene. ClWIP1 (gy) accelerates the abortion of carpel primordia, whereas ClACS7 is highly expressed in the carpel primordia and promotes the development of carpels. Upstream regulators of these three genes have not been characterized, and the link between these genes is not yet understood.
Pumpkin
-
Pumpkin (genus Cucurbita) is another annual trailing herb of the Cucurbitaceae family. Research investigating sex determination in pumpkin is lagging behind that of other fruits. Kubicki[59] found that strong maleness is controlled by a single recessive gene a, while the genotype AA or Aa can code for male or hermaphroditic flowers. Since the extreme male phenotype of the Vegetable Spaghetti (Veg) inbred line cosegregates with a weak ethylene-insensitive phenotype, the extreme male phenotype may be the result of a single mutation in a major gene that regulates ethylene perception or response. This gene was designated Cucurbita pepo WEAK ETHYLENE INSENSITIVE (CpWEI) and is considered the main controller of the extreme male phenotype[60]. Subsequently, Manzano et al.[61] cloned and identified two CONSTITUTIVE TRIPLE RESPONSE1 (CTR1) homologues from C. pepo, CpCTR1 and CpCTR2, which are key in the ethylene signal transduction pathway. CpCTR1 and CpCTR2 expression is higher in male floral organs, explaining the lower ethylene sensitivity of male relative to female flowers. Manzano et al.[62] further found that the transition from male to female C. pepo flowers was regulated by genes related to ethylene synthesis, perception, and signaling. Ethylene receptor CpETRs and CTR-like genes in the apex of plants act as negative regulators in the transition from male to female flowers during the early stages of plant development.
In 2016, Shan[63] found that subgynoecious C. maxima is controlled by a single recessive nuclear gene. Yang[64] confirmed that strong femaleness in C. maxima is controlled by a single recessive gene mapped to a 35.2 kb region of chromosome 2 that contains a Pentatricopeptide Repeat-containing family (PPR) gene, which may be a candidate gene. In 2018, Sun et al.[65] mapped the strong female gene of C. maxima in an interval of 48 kb on chromosome 2 containing 29 coding genes, and the gene Cma3_0000021, associated with gibberellin signal transduction, was predicted to be the candidate gene. There are two SNPs within this gene sequence between strong female and normal plants, and these result in premature termination of protein coding. Furthermore, Cma3_0000021 is predominantly expressed in the female flowers[66]. However, due to the presence of many unknown protein-encoding genes in the mapping region, further fine-mapping and functional validation of candidate female genes is necessary for C. maxima.
Bitter melon
-
In bitter melon, the male flowers appear earlier and in higher numbers than the female flowers, however, all-female bitter melon plants occur in nature. Ram et al.[67] proposed that gynoecious bitter melon is controlled by a pair of recessive genes, but other studies suggested that the whole female bitter melon is controlled by a single recessive gene, gy-1[68]. Matsumura et al.[69] found a marker GTFL-1 linked to the gynoecious locus at a distance of 5.46 cM, and in 2018, two flanking markers on LG12 at a distance of 3.04 cM were identified for the gynoecious (gy-1) locus[70]. These results lay a foundation for the mapping of whole-female gene(s) in bitter melon. The production of various sexual forms of bitter gourd is highly influenced by hormones such as ethylene. Wang et al.[71] found that there were only two amino acid differences among the ACS genes (99% homology) of whole-female bitter melon and common bitter melon. Mc-ACS4 may be regulated by ethylene, which may be related to sex differentiation of whole female bitter melon.
In conclusion, ethylene plays a key role in sex determination in melons of the Cucurbitaceae family. Gynoecy is an advantageous trait for hybrid seed production under spatial isolation, because it avoids tedious artificial emasculation and pollination.
-
Vegetables are essential parts of our daily diets, and are the main source of vitamins and minerals needed by the human body. There is not yet much progress in our understanding of the mechanisms of sex determination in vegetables such as garden asparagus (Asparagus officinalis L.) and spinach (Spinacia oleracea L.).
Asparagus
-
Asparagus is a typical dioecious plant with unique evolutionarily primitive homotypic X and Y chromosomes. In 2017, Harkess et al.[72] reported a high-coverage (93.7%) full-male genome sequence of garden asparagus, identified a non-recombining SDR covering approximately 1 Mb on the 132.4 Mb Y chromosome, and proposed Suppressor of female function (SOFF) and DEFECTIVE IN TAPETUM DEVELOPMENT AND FUNCTION 1 (aspTDF1) as the female suppressor and male promoter gene, respectively (Fig. 1c). γ-ray induced deletion of the entire SDR on the Y chromosome results in a male-to-female conversion, suggesting that the SDR contains all the genes necessary for normal male flower development[72]. Harkess et al.[73] then compared the genome sequences of XX double haploids with YY double haploids, and found that hemizygosity underlies the loss of recombination between the genes suppressing female organogenesis (SOFF) and promoting male function (aspTDF1). Neither aspTDF1 nor SOFF was expressed in female XX. In contrast, in supermale YY, aspTDF1 was expressed in anther tapetum cells and SOFF was weakly expressed in flower buds. A premature stop codon in aspTDF1 in neuters obtained via EMS mutagenesis of XY male plants causes complete anther abortion, verifying the function of the aspTDF1 gene in promoting anther development[73]. These results directly show that SOFF dominantly suppresses female organogenesis and aspTDF1 promotes anther development. These two linked genes determine the sex of asparagus (Fig. 1c).
Spinach
-
Spinach (Spinacia oleracea L., 2n = 12) belongs to the subfamily Amaranthaceae, which consists of typical dioecious plants with a small number of hermaphrodites. It has the XY type sex determination system and is an ideal plant for studying mechanisms of sex determination. Qian
et al.[74] marked six sex-linked gene groups using SLAF combined with BSA (Bulked Segregation Analysis) and high-density genetic maps, and the sex-determination genes were located at 66.98–69.72 cM and 75.48–92.96 cM of LG 4. In 2021, researchers identified an approximately 21 kb male-specific region (MSR) on chromosome 4 of spinach based on resequencing data from (five female and five male plants)[75]. A KASP marker, SponR, developed from a SNP closely linked to the MSR, co-segregated with the sex-determination gene in the population of 958 individuals[75]. Moreover, inhibition of GA production and proteasome activity resulted in feminization of male flowers. West and Golenberg[76] identified a DELLA family transcription factor gene, GIBBERELLIC ACID INSENSITIVE (SpGAI), whose expression in female inflorescences was two-fold higher than in male inflorescences. Reducing the expression of SpGAI in female to male levels masculinized female flowers[76]. These results indicate that SpGAI may be the feminizing factor in spinach. -
In recent years great progress has been made in our understanding of flower sex determination. With the rapid development of genetic and genomic technologies, key sex-determination genes have been discovered in a few economically important horticultural plants. In view of this progress, research on the following topics will be of significance in the future:
Development of model plant systems for the study of flower sex differentiation. The mechanism of sex differentiation in plants is complex. Although the community has made great progress in understanding this mechanism, plants from different families or lineages seem to have evolved different strategies and different genes associated with distinct reproductive pathways. For instance, the kiwifruit, persimmon, and asparagus all have different sex determination systems. It is difficult to dissect the underlying codes, especially for perennial fruit trees, which take years to flower for the first time. Identifying model plants to study flower sex formation is a good way to circumvent this obstacle. These model plants should be closely related (from the same lineage), have similar flower sex types and a relatively short life cycle, be easy to genetically manipulate, etc.
The application of deep sequencing technologies and comparative genomics. With the rapid development of these techniques, it has become easier to execute comparative studies among close species. Lower cost of deep sequencing and higher efficiency of bioinformatics analysis will accelerate genome sequencing and make it feasible to sequence a large number of genomes of closely related species or plants and explore their similarities and differences at the genome level. This will benefit our understanding of the evolution and diversification of flower sex determination systems in plants.
Phytohormone networks. Hormone signaling is one of the key factors in the regulation of sex differentiation in horticultural plants (e.g., ethylene and its crosstalk with gibberellins in Cucurbitaceae). It is generally believed that ethylene promotes female flower development and GA stimulates male flower development. Investigating various hormone signals and their crosstalk in flower sex determination is an exploratory area of great significance.
Once sex determining genes and associated regulatory networks are uncovered, design and development of plants with unisexual or bisexual flowers will become feasible and of practical value. In this way, cross pollination of self-fertilizing plants can be used to avoid inbreeding depression, and bisexual flowers can ensure sufficient fertility and yield for many monoecious and diecious plants.
This work was supported by the National Key Research and Developmental Program of China (#2018YFD1000104), the National Natural Science Foundation of China (#32072547), and the Special Support Program of Guangdong Province (#2019TX05N193).
-
The authors declare that they have no conflict of interest.
- Copyright: 2022 by the author(s). Exclusive Licensee Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zheng J, Xia R. 2022. Flower development and sex determination in horticultural crops. Fruit Research 2:9 doi: 10.48130/FruRes-2022-0009
Flower development and sex determination in horticultural crops
- Received: 21 November 2021
- Accepted: 16 June 2022
- Published online: 29 June 2022
Abstract: Horticultural crops are extremely valuable due to their high nutritional value, and fruits, in particular, provide indispensable vitamins and minerals. Fruit yield of edible crops is closely related to the number of flowers, which are often unisexual. The mechanism of sex differentiation in plants with unisexuality is complex, and research investigating this mechanism is in great demand. Sex determinants were first discovered in Cucurbitaceae (e.g., cucumber, melon, watermelon), and in recent years, with the rapid development of deep sequencing technologies and genomics, they have also been deciphered in some dioecious plants (e.g., persimmon, kiwifruit, asparagus). This has deepened our understanding of the evolution and diversification of sexual reproductive systems. This review summarizes recent research investigating flower sex-determination genes and their working networks, focusing on horticultural crops. Perspectives on future research in flower sex differentiation are also discussed.












