-
The plant cuticle is an extracellular hydrophobic layer that covers the aerial portions of all land plants, providing protection against uncontrolled water loss; therefore, it is one of the key adaptations in plants to external environmental stresses[1−3]. The cuticle is composed of polymer cutin and cuticular wax[3]. Cutin is physically associated with the underlying polysaccharide cell wall, with which it has overlapping functions. Cutin contributes to the biomechanical properties of the cuticle as well as to pathogen resistance[4−6], whereas cuticular waxes are mainly responsible for limiting non-stomatal water loss from leaves[7]. Cuticular waxes, including amorphous intracuticular wax, are embedded in the cutin polymer and epicuticular wax crystals that cover the outer plant surface. Epicuticular wax is exposed on the outermost surface of plant organs and plays a crucial role in plant adaptation to the environment[2]. The chemical composition of cuticular waxes is complex. They consist of a mixture of very-long-chain fatty acids (chain lengths ranging from C20 to C34), their derivatives (alkanes, primary and secondary alcohols, aldehydes, ketones, wax esters, and triterpenoids), and flavonoids[2]. Composition analysis demonstrated a distinct chemical composition between the intracuticular and epicuticular wax[8−10]. For example, epicuticular wax on the leaves of Ligustrum vulgare mainly comprises very-long-chain aliphatic compound classes, whereas intracuticular wax comprises triterpenoids, ursolic acid, and oleanolic acid[10]. Furthermore, different wax components form different types of wax crystals. Based on the micromorphology of three-dimensional crystals observed by scanning electron microscopy (SEM), wax is classified into 23 types[11]. Together, these studies suggest that cuticular waxes have complex chemical compositions and crystal morphology. However, the exact implications of this variation in wax composition and crystal structure on the biological functions of the cuticle are poorly understood.
Cuticular wax serves the essential function of limiting non-stomatal water loss[12]. Our previous study showed that a higher accumulation of epicuticular wax crystals better limits non-stomatal water loss in Dianthus spiculifolius under drought conditions[13]. The present study aimed to further define the crystal type and chemical components of cuticular wax on the leaves of the wild type and a mutant of D. spiculifolius with high wax content and to identify the role of cuticular wax in non-stomatal water loss control. Moreover, the corresponding cuticular wax characteristics were analyzed and compared in three other Dianthus species distributed across different geographical regions, D. caryophyllus, D. gratianopolitanus, and D. carthusianorum[14−17]. These Dianthus plants are important ornamental plants, and they are widely used in greening and cutting flowers in different regions of China. In cultivated production, we found that they exhibited different leaf color and drought resistance. The accumulation of cuticular wax can not only affect plant leaf color but also mainly limit non-stomatal water loss[13], which is one of the key adaptations of plant drought resistance. Here, we investigated and compared the characteristics of cuticular wax on the leaves of five Dianthus plants in order to understand their contribution to non-stomatal water loss and drought resistance. SEM, cryo-SEM, and gas chromatography-mass spectrometry (GC-MS) analyses revealed details of the crystal micromorphology and chemical composition of cuticular wax on the leaves of the five Dianthus plants. Our analyses suggest that non-stomatal water loss may be more effectively controlled through accurate regulation of cuticular wax components to further enhance drought tolerance in plants.
-
The leaf phenotypes of the five Dianthus plants were highly similar, except for their color, length and width (Fig. 1a, b). Our previous study confirmed that the 'greyish-green' leaf phenotype of D. spiculifolius (HW) compared to that of the wild-type (WT) is because of increased cuticular wax[13]. To confirm whether leaf color differences are related to cuticular wax deposition, the leaf surface of five Dianthus plants was observed by SEM. After treatment based on the common sample fixation method, leaf samples were fixed in glutaraldehyde (2.5%) and then dehydrated with alcohol, and the leaf surfaces were observed. The SEM images show irregular platelet-shaped wax crystals densely distributed on the adaxial leaf surface of D. spiculifolius (WT), D. spiculifolius (HW), and D. carthusianorum, whereas the wax crystals on the leaves of D. caryophyllus and D. gratianopolitanus displayed the karst cave pattern (Fig. 2a). This karst cave-like pattern appeared to be an artefact of dissolution of wax crystals. Therefore, we repeated the observations of the wax crystal morphology using two other fixation methods: leaf samples were dried at a high temperature (40 °C) or at room temperature. In both methods, rodlet-shaped wax crystals were observed on the adaxial leaf surface of D. caryophyllus, D. gratianopolitanus, D. spiculifolius (WT), and D. spiculifolius (HW), but irregular and platelet-shaped crystals on the leaf surface of D. carthusianorum (Fig. 2b, c). Wax crystal micromorphology on the abaxial leaf surfaces of the five Dianthus plants was the same as that on the adaxial leaf surface under the three sample preparation methods (Supplemental Fig. S1a−c).

Figure 1.
Phenotypes of (a) plants and (b) leaves of five Dianthus plants: D. caryophyllus, D. gratianopolitanus, D. spiculifolius (WT), D. spiculifolius (HW), and D. carthusianorum. WT: wild type; HW: high wax content. Bar = 1 cm (b).
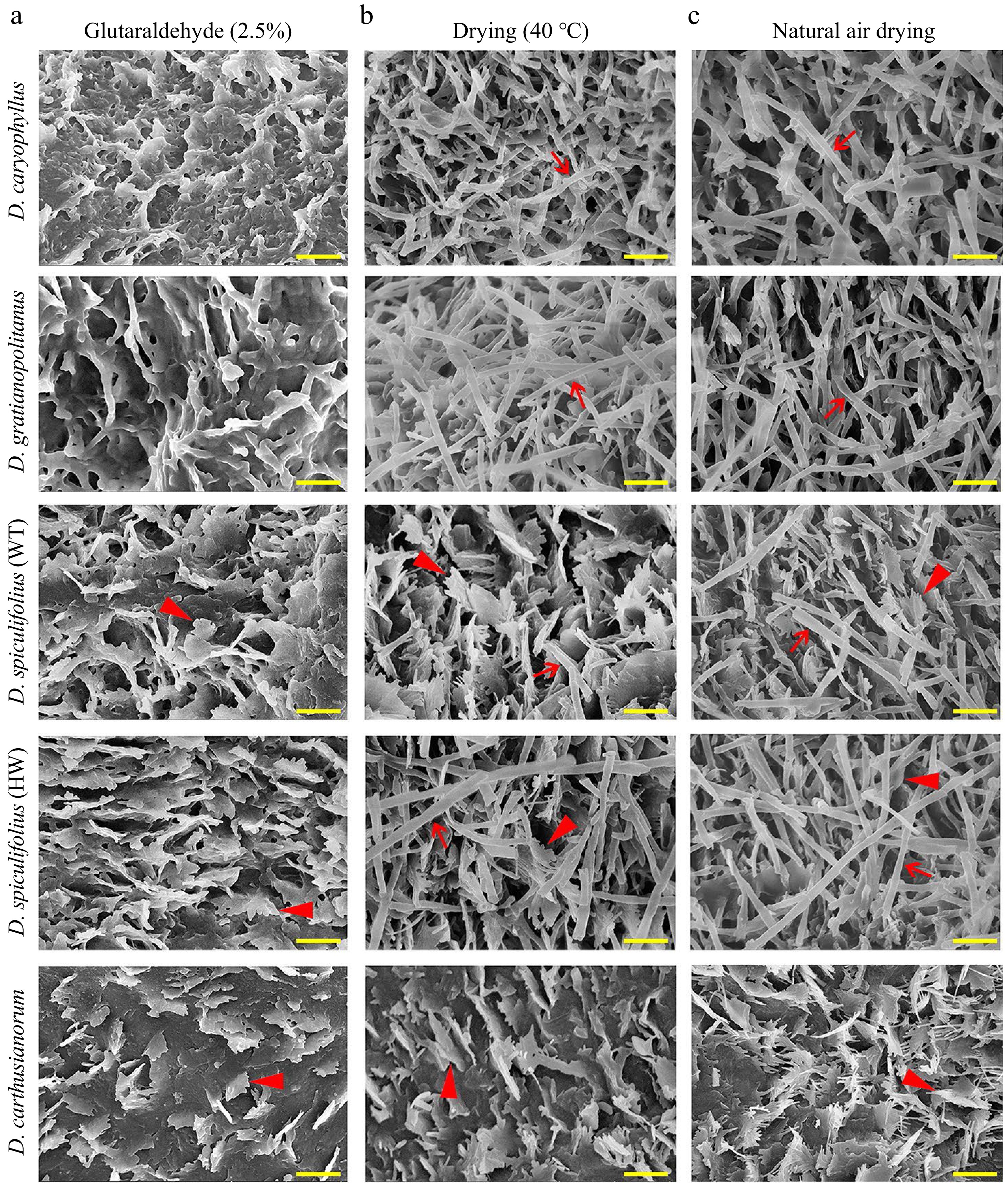
Figure 2.
Scanning electron microscopy (SEM) images of cuticular wax crystals on the adaxial leaf surface of five Dianthus plants obtained by three leaf sample fixation methods. (a) Cuticular wax morphology of leaf samples fixed with glutaraldehyde (2.5%) and then dehydrated with alcohol. (b) Cuticular wax morphology of leaf samples dried at 40 °C. (c) Cuticular wax morphology of leaf samples air-dried at room temperature. Red arrows indicate rodlet-shaped wax crystals, and red arrowheads indicate irregular platelet-shaped wax crystals. Bar = 1 µm. WT, wild type; HW, high wax content.
Furthermore, cryo-SEM observations confirmed that rodlet-shaped wax crystals on the leaves of the four Dianthus plants were actually tubular cuticular wax (Fig. 3a−d), and only irregular, platelet-shaped wax was present on the leaves of D. carthusianorum (Fig. 3e). These results confirmed that glutaraldehyde fixation and alcohol dehydration treatment caused the disappearance of tubular wax on the leaf surfaces of the four Dianthus plants, and air drying at high temperature (40 °C) or room temperature is an appropriate method of sample preparation. In summary, the characteristics of leaf cuticular waxes of the five Dianthus plants are as follows: (1) the cuticular wax on the leaves of D. caryophyllus and D. gratianopolitanus is mainly composed of tubular wax; (2) the cuticular wax on the leaves of D. spiculifolius (WT and HW) is mainly composed of irregular platelet-shaped and tubular wax; and (3) the cuticular wax on the leaves of D. carthusianorum is composed of only irregular platelet-shaped wax.
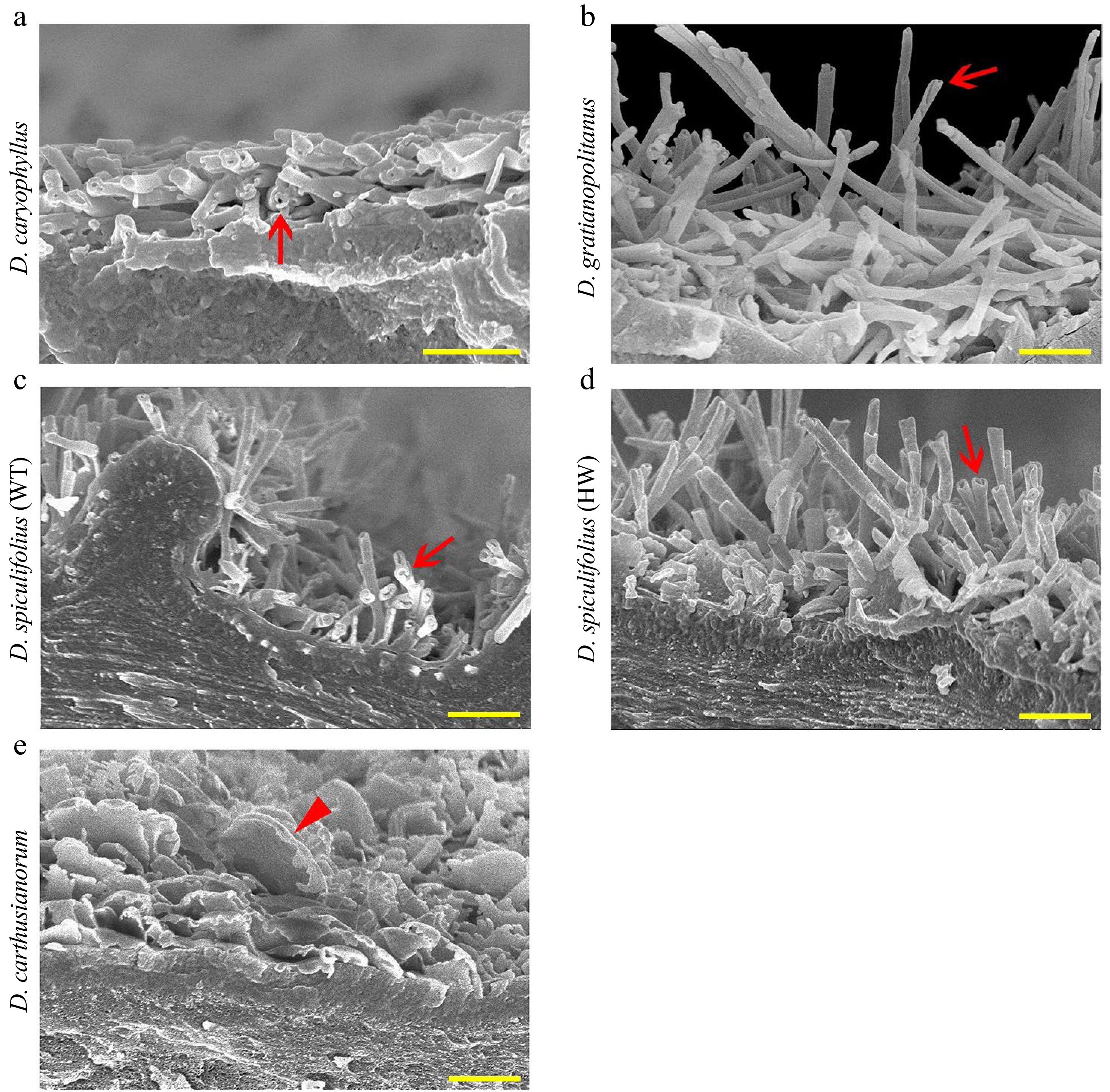
Figure 3.
Cryo-SEM images of cuticular wax crystals on the adaxial leaf surface of five Dianthus plants. (a) D. caryophyllus, (b) D. gratianopolitanus, (c) D. spiculifolius (wild-type, WT), (d) D. spiculifolius (high wax mutant, HW), (e) D. carthusianorum. Red arrows indicate tubular wax crystals, and red arrowhead indicates irregular platelet-shaped wax crystals. Bar = 1 µm. WT, wild type; HW, high wax content.
Non-stomatal water loss in the leaves of five Dianthus plants
-
Cuticular wax serves the essential function of limiting non-stomatal water loss[12]. Therefore, we measured and compared the cuticle permeability, non-stomatal water loss and chlorophyll (Chl) leaching rates in the leaves of the five Dianthus plants. The toluidine blue-O staining assay showed that D. carthusianorum leaves were all stained the quickest, while the leaves of the other four Dianthus plants were only slightly stained (Fig. 4a), indicating that D. carthusianorum leaves have higher cuticle permeability. The lowest rate of non-stomatal water loss was detected in D. spiculifolius (HW) leaves, followed by that in D. gratianopolitanus, D. spiculifolius (WT), and D. caryophyllus leaves; the fastest rate of non-stomatal water loss was measured in D. carthusianorum leaves (Fig. 4b). Cuticular wax accumulation affects the rate of Chl leaching[12]. The rate of Chl leaching was the slowest in D. spiculifolius (HW) leaves and the fastest in D. carthusianorum leaves, whereas the rate was similar in the leaves of the other three Dianthus plants (Fig. 4c). This result is similar to that of non-stomatal water loss. These results suggest that different wax components (tubular and irregular platelet-shaped wax) may contribute differently in limiting non-stomatal water loss in the leaves of the five Dianthus plants.
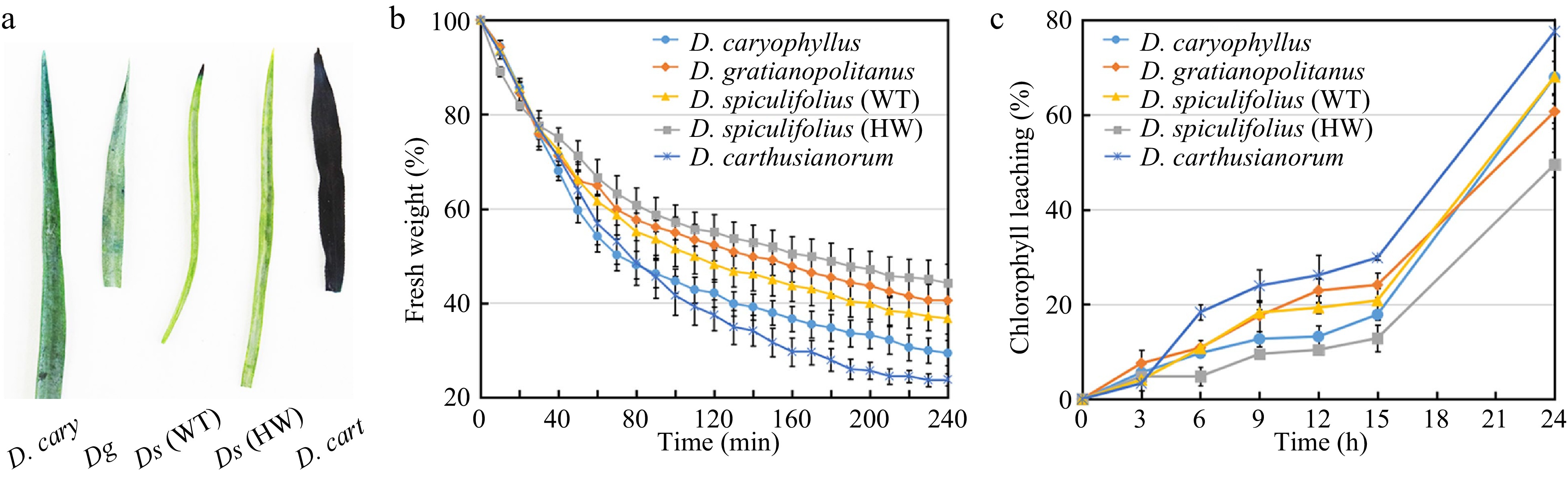
Figure 4.
Comparison of cuticle permeability, non-stomatal water loss and chlorophyll leaching rates in leaves of five Dianthus plants. (a) Five Dianthus leaves were immersed for 24 h in 0.05% toluidine blue-O and then rinsed with water. (b) The rate of non-stomatal water loss from Dianthus leaves at 38 °C for 240 min. Water loss rate was expressed as a percentage of fresh weight at each individual time point versus initial fresh weight. Error bars represent standard error (SE) (n = 3). (c) The rate of chlorophyll leaching from Dianthus leaves within 24 h. Chlorophyll leaching rate was expressed as a percentage of extracted chlorophyll content at each individual time point versus chlorophyll content at 48 h after initial immersion. Error bars represent SE (n = 3). WT, wild type; HW, high wax content.
Cuticular wax composition on the leaves of five Dianthus plants
-
The cuticular waxes on the leaves of the five Dianthus plants were extracted using CH3Cl, and their composition was analyzed using GC-MS (Supplemental Fig. S2). Nineteen cuticular wax components were detected, which were mainly classified into fatty acids, alkanes, aldehydes, alcohols, ketones, and esters (Table 1). Quantitative analysis showed that the total wax load in D. spiculifolius (HW) leaves was the highest (9.84 ± 1.51 µg/cm2), followed by D. spiculifolius (WT) (7.09 ± 1.35 µg/cm2), D. gratianopolitanus (6.85 ± 1.20 µg/cm2), D. caryophyllus (5.47 ± 1.33 µg/cm2), while D. carthusianorum was the lowest (4.72 ± 0.95 µg/cm2) (Table 1). Further analysis revealed that D. caryophyllus and D. gratianopolitanus leaves were highly similar in the composition and proportion of the main wax components, which included hentriacontane (30.6% and 20.81%), heptacosane (27.08% and 36.99%), heneicosane (3.66% and 3.28%), eicosane (7.91% and 7.62%), eicosanal (20.46% and 13.59%), and tricosane-2,4-dione (6.33% and 13.85%) (Fig. 5a, b). However, the wax compositions of the leaves of D. spiculifolius (WT), D. spiculifolius (HW), and D. carthusianorum were similar, and they included 1-octacosanol (60.96%, 40.58%, and 48.09%), hentriacontane (13.57%, 21.07%, and 19.52%), heptacosane (14.8%, 13.25%, and 15.44%), eicosanal (9.6%, 20.4%, and 12.55%), and tricosane-2,4-dione (0.05%, 2.98%, and 0.19%) (Fig. 5c−e). Studies have shown that tubular epicuticular wax is formed mainly of β-diketones[11,18,19]. In the present study, only one β-diketone component (tricosane-2,4-dione) was identified, and it had a relatively high proportion and load in the total cuticular wax of D. caryophyllus and D. gratianopolitanus leaves (Fig. 5; Table 1). These results indicate that the tubular cuticular wax on Dianthus leaves may be mainly formed of tricosane-2,4-dione. In contrast, the loads (0.293 ± 0.105 µg/cm2) of tricosane-2,4-dione in D. spiculifolius (HW) leaves was higher than that (0.003 ± 0.001 µg/cm2) in D. spiculifolius (WT) (Fig. 5c, d; Table 1), indicating that the HW leaves have more tubular wax than the WT leaves. Furthermore, SEM images showed the tubular wax being deposited above the irregular platelet-shaped wax, thus, we speculated that the tubular wax was the epicuticular wax, while the irregular platelet-shaped wax was the intracellular wax. In addition, comparative analysis revealed that 1-octacosanol (C28) is the main wax component in D. spiculifolius (WT), D. spiculifolius (HW), and D. carthusianorum leaves, but is almost absent in the leaves of D. caryophyllus and D. gratianopolitanus. Moreover, SEM observations showed large amounts of irregular platelet-shaped wax crystals deposited on D. spiculifolius (WT), D. spiculifolius (HW), and D. carthusianorum leaves but not on the leaves of D. caryophyllus and D. gratianopolitanus. This suggests that 1-octacosanol (C28) may be the main component responsible for the formation of irregular platelet-shaped waxes. In Pisum sativum, Hordeum vulgare, and Nepenthes alata, hexacosanol (C26) and triacontanal (C30) can form platelet-shaped wax[20,21].
Table 1. Quantification of multiple cuticular wax components identified from the leaves of five Dianthus plants.
Classification Name Chemical formula Wax load (µg/cm2) Ds (HW) SE (n = 3) Ds (WT) SE (n = 3) Dcart SE (n = 3) Dg SE (n = 3) Dcary SE (n = 3) Fatty acids Oleic acid C18H34O2 0.0117 0.0033 0.0038 0.0013 0.0045 0.0009 0.0100 0.0062 0.0112 0.0080 Erucic acid C22H42O2 0.0011 0.0002 0.0006 0.0002 0.0010 0.0001 0.0033 0.0021 0.0028 0.0019 Alkanes Heptadecane, 2-methyl- C18H38 0.0007 0.0002 0.0004 0.0004 0.0009 0.0007 0.0036 0.0020 0.0026 0.0011 Eicosane C20H42 0.0404 0.0404 0.0314 0.0075 0.1133 0.0500 0.5224 0.1524 0.4323 0.1792 Heneicosane C21H44 0.0273 0.0061 0.0138 0.0033 0.0356 0.0118 0.2248 0.0643 0.2821 0.2118 Heptacosane C27H56 1.3036 0.3190 1.0488 0.2382 0.7294 0.1948 2.5350 0.2712 1.4806 0.1268 2-methyloctacosane C29H60 0.0053 0.0014 0.0012 0.0004 0.0009 0.0000 0.0292 0.0227 0.0043 0.0021 Hentriacontane C31H64 2.0727 0.4401 0.9618 0.2272 0.9219 0.0980 1.4263 0.1561 1.6730 0.2989 Dotriacontane C32H66 0 0 0.0011 0.0010 0.0013 0.0006 0 0 0.0032 0.0041 Hexatriacontane C36H74 0.0043 0.0022 0.0031 0.0018 0.0045 0.0019 0.0095 0.0048 0.0311 0.0216 Aldehydes 13-Octadecenal, (Z)- C18H34O 0.0139 0.0058 0.0019 0.0004 0.0017 0.0006 0.0243 0.0193 0.0044 0.0029 Octadecanal C18H36O 0.0100 0.0043 0.0033 0.0004 0.0027 0.0003 0.0061 0.0046 0.0056 0.0027 Eicosanal C20H40O 2.0066 0.2791 0.6801 0.1167 0.5929 0.1364 0.9309 0.2463 1.1186 0.2585 Alcohols 1-Octacosanol C28H58O 3.9916 0.2895 4.3199 0.7506 2.2719 0.4369 0.0000 0.0000 0.0000 0.0000 Ketones 2-Nonadecanone C19H38O 0.0418 0.0085 0.0091 0.0022 0.0160 0.0039 0.0900 0.0633 0.0308 0.0389 Tricosane-2,4-dione C23H44O2 0.2934 0.1054 0.0033 0.0007 0.0092 0.0040 0.9492 0.1375 0.3463 0.1357 Esters Myristic acid isobutyl ester C18H36O2 0.0012 0.0004 0 0 0 0 0.0291 0.0205 0 0 Hexadecanoic acid, 15-methyl-, methyl ester C18H36O2 0.0048 0.0020 0.0009 0.0002 0.0016 0.0003 0.0015 0.0009 0.0058 0.0021 Octadecanoic acid, phenylmethyl ester C25H42O2 0.0047 0.0011 0.0018 0.0004 0.0147 0.0056 0.0572 0.0291 0.0332 0.0337 Total wax 9.8352 1.5093 7.0865 1.3530 4.7240 0.9468 6.8525 1.2031 5.4679 1.3302 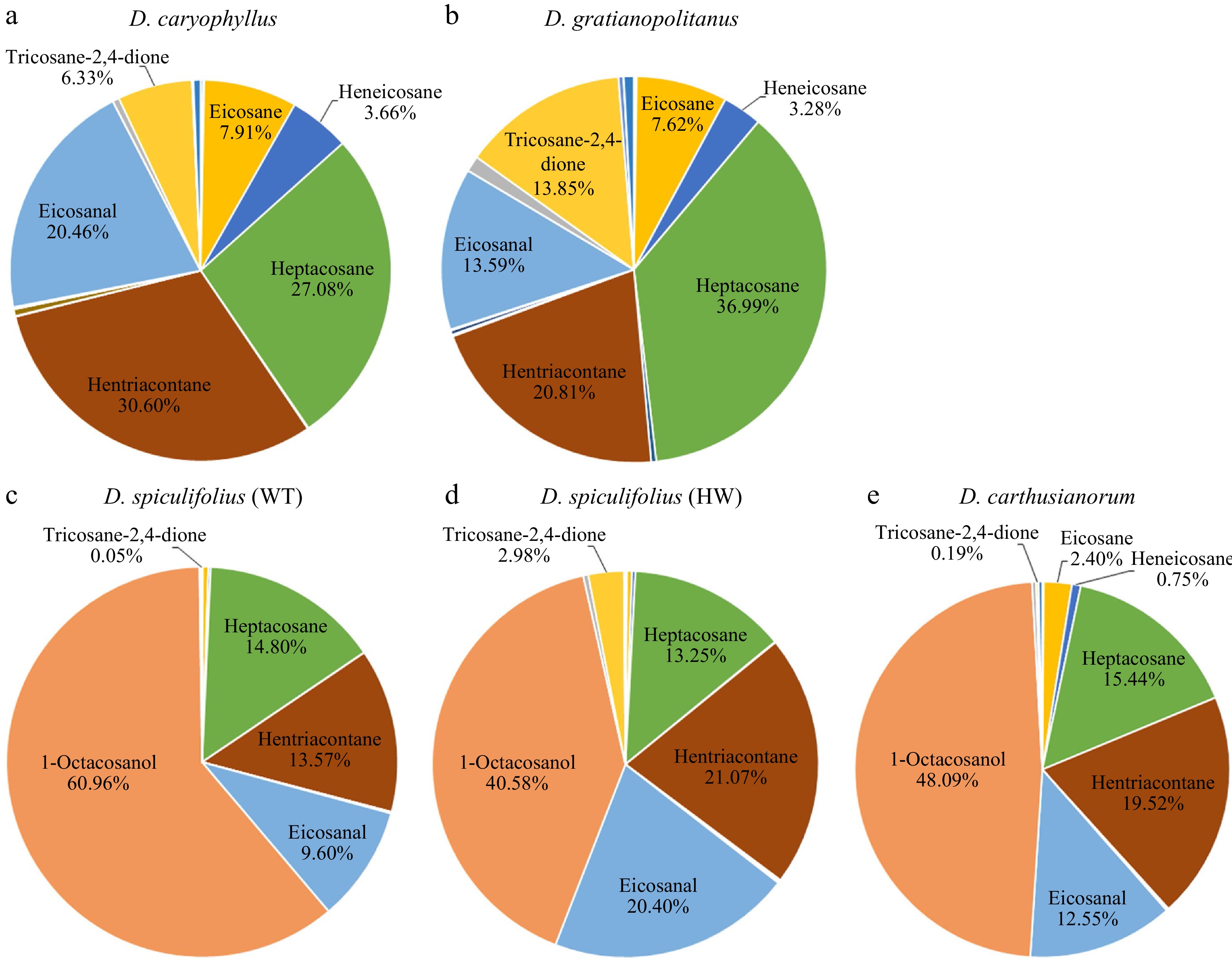
Figure 5.
Main components of cuticular wax on the leaves of five Dianthus plants and their proportion to total wax abundance. (a) D. caryophyllus, (b) D. gratianopolitanus, (c) D. spiculifolius (WT, wild type), (d) D. spiculifolius (HW, high wax content), (e) D. carthusianorum.
The cuticular wax of D. carthusianorum leaves is mainly irregular platelet-shaped wax (Figs 2 & 3), and its non-stomatal water loss rate is the fastest compared to other Dianthus plant leaves (Fig. 4). There are two possibilities: (i) one is that a lower total wax load causes faster non-stomatal water loss, and (ii) the other is that irregular platelet-shaped wax components or arrangement are more likely to cause water loss than tubular wax. As evidence in support of the second possibility is that the total wax load (7.09 ± 1.35 µg/cm2) was higher in D. spiculifolius (WT) leaves than in D. gratianopolitanus leaves (6.85 ± 1.20 µg/cm2) (Table 1), while its non-stomatal water loss rate is faster than that in the D. gratianopolitanus leaves (Fig. 4). The difference between the two is that the cuticular wax of D. spiculifolius (WT) leaves is mainly irregular platelet-shaped wax, while the D. gratianopolitanus leaves are tubular wax (Figs. 2 & 3). Furthermore, D. gratianopolitanus leaves have a higher tubular wax load than D. spiculifolius (WT) leaves (Table 1). This result suggests that a higher tubular wax than the irregular platelet-shaped may better limit non-stomatal water loss on the basis of a similar total wax load. However, D. caryophyllus leaves have higher tubular wax load than D. spiculifolius (WT) leaves, but its non-stomatal water loss is faster, which may be caused by its lower total wax load (Fig. 4; Table 1). These results also suggest that both the composition and total loading of cuticular wax are important for limiting non-stomatal water loss.
In the present study, we investigated and compared the micromorphology of cuticular wax crystals on the leaves of five Dianthus plants and their contribution to leaf non-stomatal water loss. Our results suggest that the deposition of tubular epicuticular wax contributes to the limitation of non-stomatal water loss in leaves. Further analysis of the chemical components revealed that tubular epicuticular wax was mainly formed of tricosane-2,4-dione. We believe that this is an important trait that will aid the improvement of many important crops for drought tolerance.
-
Clones of four Dianthus species and one mutant, D. caryophyllus, D. gratianopolitanus, D. spiculifolius (wild type, WT, and a mutant with high wax content, HW), and D. carthusianorum, were grown in greenhouses and in an open field at the Northeast Agricultural University (Harbin, China; 128.4° E, 45.0° N). Healthy, mature leaves of three-month-old plants were selected for the experiments.
Scanning electron microscopy (SEM and cryo-SEM)
-
For SEM analysis, leaf samples from the five Dianthus plants were prepared using three methods: the standard sample fixation method wherein leaf samples were fixed with 2.5% glutaraldehyde, then gradually dehydrated using alcohol, and dried to the critical point in liquid CO2; leaf samples were dried at 40 °C; or leaf samples were air-dried at room temperature. Leaf samples prepared by the three methods were then sputter-coated with an electrically conductive gold layer before imaging using a scanning electron microscope (Hitachi SU-8010, Tokyo, Japan) at 5 kV.
For cryo-SEM analysis, leaf samples of the five Dianthus plants were sprinkled onto a perforated aluminum stub and plunged into a liquid nitrogen slush (−196 °C). The frozen samples were transferred to a cryosystem (PP3010T; Quorum Technologies, Lewes, UK), sputter-coated with platinum, transferred to the scanning electron microscope cold stage, and examined at −140 °C at a beam voltage of 5 kV and probe current of 10 mA.
Water loss and chlorophyll leaching assays
-
For non-stomatal water loss assays, three-month-old plants grown under normal conditions were dark acclimated for 12 h to ensure stomatal closure. The stems with leaves were excised and placed at 38 °C. Leaves were weighed at the indicated time points (0−240 min). For Chl leaching assay, the leaves of dark-acclimated plants were soaked in 80% ethanol for the indicated time periods (0−24 h). The absorbances of Chl a (645 nm) and Chl b (663 nm) were determined using a UV/Vis spectrophotometer (Specord 205, Analytik Jena, Germany), with three biological replicates and three technical replicates. The total Chl content was calculated as the sum of Chl a and Chl b. The measurements were conducted in darkness to avoid degradation of photosynthetic pigments.
Wax extraction and gas chromatography mass spectrometry (GC-MS) analysis
-
Leaf samples were placed into 50 mL tubes, and 30 mL chloroform (CH3Cl) was added to each tube. The samples were then vortexed for 30 s at room temperature. The leaves were removed from the tubes and 20 μL of internal standard (tetracosane, 0.5 mg mL−1 stock) was added to each tube. GC-MS analysis of the extracted leaf waxes was performed using an Agilent 7890 GC-MS as previously described[22].
-
This research was supported by the National Natural Science Foundation of China (31902052 and 32172619), Natural Science Foundation of Heilongjiang Province of China (YQ2020C006).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Zhiyan Wan, Haizhen Zhang
- Supplemental Fig. S1 Scanning electron microscopy (SEM) images of cuticular wax crystals on the abaxial leaf surface of five Dianthus plants obtained by three leaf sample fixation methods. (a) Cuticular wax morphology of leaf samples fixed with glutaraldehyde (2.5%) and then dehydrated with alcohol. (b) Cuticular wax morphology of leaf samples dried at 40 °C. (c) Cuticular wax morphology of leaf samples air-dried at room temperature. Red arrows indicate rodlet-shaped wax crystals, and red arrowheads indicate irregular platelet-shaped wax crystals. Bar = 1 μm. WT, wild type; HW, high wax content.
- Supplemental Fig. S2 Total ion chromatograms (TIC) of cuticular wax components extracted from the leaf surface of five Dianthus plants.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wan Z, Zhang H, Ren L, Zhang H, Feng S, et al. 2022. Tubular epicuticular wax is an important trait for limiting non-stomatal water loss from leaves in several Dianthus species. Ornamental Plant Research 2:15 doi: 10.48130/OPR-2022-0015
Tubular epicuticular wax is an important trait for limiting non-stomatal water loss from leaves in several Dianthus species
- Received: 22 April 2022
- Accepted: 05 September 2022
- Published online: 22 September 2022
Abstract: Cuticular wax plays an important role in plant drought tolerance by limiting non-stomatal water loss and has a diverse micromorphology and composition. However, the contribution of different wax components to limiting non-stomatal water loss remains unclear. We investigated and compared the micromorphology of cuticular wax on the leaves of five Dianthus plants and its role in limiting non-stomatal water loss. Furthermore, we further analyzed the chemical components of the cuticular waxes. Our results showed that cuticular wax crystals on the leaves of five Dianthus plants were mainly composed of irregular platelets or tubular epicuticular wax. The deposition of tubular wax may be related to better limiting non-stomatal water loss than platelets wax in the leaves of five Dianthus plants. Chemical component analysis revealed that the tubular wax was mainly composed of tricosane-2,4-dione. Our study suggests that tubular wax composition is an important trait for limiting non-stomatal water loss in several Dianthus species and contributes to the improvement of drought tolerance in other Dianthus plants.
-
Key words:
- Dianthus /
- cuticular wax /
- tubular wax /
- non-stomatal water loss /
- chemical component













