-
Radix Astragali prepared from dried roots of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao or Astragalus membranaceus (Fisch.) Bge. is one of the most popular herbs in traditional Chinese medicine (TCM). It is used to strengthen the immune system, protect liver function, fight bacteria and viruses, and treat diabetes, heart failure and seasonal allergies[1, 2]. Isoflavones and triterpenoid glycosides are major small-molecule active compounds present in Radix Astragali[2] (Fig. 1). For example, ononin (5) and astragaloside IV (7) exhibited anti-gastric ulcer effects[3]; and formononetin (4) inhibited the proliferation and metastasis of ovarian cancer cells[4]. Polysaccharides are another major chemical component of Radix Astragali[5], which have demonstrated antioxidant activities[6] and therapeutic potential for glomerulonephritis[7] and cognitive dysfunction[8].
It is well known that chemical consistency can be an issue within individual medicinal plants used in TCM due to genetic and environmental variations[9−11], which has a significant impact on the quality of the TCM material from batch to batch. In recent years, a new form of TCM material, namely ultrafine granular powder (UGP), has been introduced into the market with the application of cell wall-disrupting micronization technology. Technically, crude TCM slices (or pieces) are crushed into ultrafine powder (particle size distribution, D90 < 45 μm) with airflow crushing technology, and then are prepared as granules without excipients[12]. UGP material generally maintains the same chemical profiles as the sliced traditional material (TM), as demonstrated in the UGP material of Salvia miltiorrhiza[12]. Additional studies have shown that the UGP materials of S. miltiorrhiza and Panax quinquefolius possess improved oral bioavailability and pharmacokinetic profiles for selected marker compounds compared to the corresponding TM materials[13, 14].
With the growing use of the UGP material of Radix Astragali in China, an understanding of its chemical profile is critical in assessing its clinical utility. In the present study, the aforementioned two chemotypes of small-molecule compounds, isoflavones and triterpenoid glycosides, were selected as marker compounds to compare the chemical consistency and homogeneity between UGP and TM of Radix Astragali. In addition, polysaccharides, important biologically active constituents of Radix Astragali, were prepared from the two materials for comparison of their chemical profiles and dissolution rates.
-
The bioactive compounds in TCM are the material basis for clinical application. In order to confirm that the UGP material of Radix Astragali retains the same or similar chemical profiles as the corresponding TM material that is commonly prescribed by TCM practitioners, a comparative study between the two materials was conducted using UHPLC/DAD-UV (see the method in the experimental section). The six isoflavones including calycosin (1), calycosin-7-O-β-D-glucose (2), calycosin-7-O-β-D-glucose-6''-O- malonate (3), formononetin (4), ononin (5), and formononetin-7-O-β-D-glucoside-6''- O-malonate (6) and four triterpenoid glycosides including astragaloside IV (7), astragaloside II (8), astragaloside I (9), and acetylastragaloside I (10) (Fig. 1) previously identified in Radix Astragali, were used as marker compounds to facilitate chromatographic comparison (refer to Supplemental Fig. S1 for typical UHPLC-MS-UV chromatograms of the six commercially available standards 1, 2, 4, 5, 7, and 8). The retention time and ESI-MS data (both positive and negative) of all the 10 compounds are summarized in Table 1.
Table 1. Retention time (tR) and characteristic MS data of 10 marker compounds.
Analyte tR (min) MS (m/z) MW DAD 254 nm MS ESI (+) ESI (–) 1 7.187 285 [M + H]+ 283 [M − H]− 284 2 5.219 447 [M + H]+ 491 [M + HCOO]− 446 3 5.810 533 [M + H]+ 475 [M − Malonyl + HCOO]− 532 4 8.791 269 [M + H]+ 267 [M − H]− 268 5 6.411 431 [M + H]+ 475 [M + HCOO]− 430 6 6.928 517 [M + H]+ 267 [M – Malonyl – Glc -H]− 516 7 7.939 807 [M + Na]+ 829 [M + HCOO]− 784 8 8.571 849 [M + Na]+ 871 [M + HCOO]− 826 9 9.737 891 [M + Na]+ 913 [M + HCOO]− 868 10 10.166 933 [M + Na]+ 955 [M + HCOO]− 910 For a direct qualitative analysis by UHPLC/DAD-UV, extraction of TM1 and its corresponding UGP1 was conducted in MeOH under sonication at room temperature for 30 min. It is evident that the UV254 nm chromatograms (Fig. 2a) and the negative and positive ESI-MS chromatograms (Fig. 2b & c) of UGP1 and TM1 are basically identical, indicating the same material basis for the two. A different extraction method involving sonication of the samples in 80% MeOH at room temperature for 30 min was used for UGP2 and TM2, a different batch of Radix Astragali prepared from the same source of the plant material. The results indicated that UGP2 and TM2 produced almost the same chromatographic fingerprints, except for two minor peaks marked as P1 and P2 in the UV- and MS detected chromatograms of UGP2 (Fig. 2d− f). Based on the MS data and UV absorption characteristics (Supplemental Fig. S2), it was speculated that P1 was calycosin 7-O-β-D-(6''-acetyl)-glucoside, while P2 was 6''-acetylononin. Actually, these two compounds were also detected to be present in trace amounts in the extracted ion chromatograms of TM2 (by extracting quasi-molecular ions for both positive and negative ESI-MS modes). They were likely enriched in UGP2 due to different compound dissolution rates in extraction or intermolecular acetyl transfer during the micronization process. Thus, it can be concluded that the UGP and TM materials of Radix Astragali derived from the same source have the same material basis, with slight differences for individual compounds between the two materials.
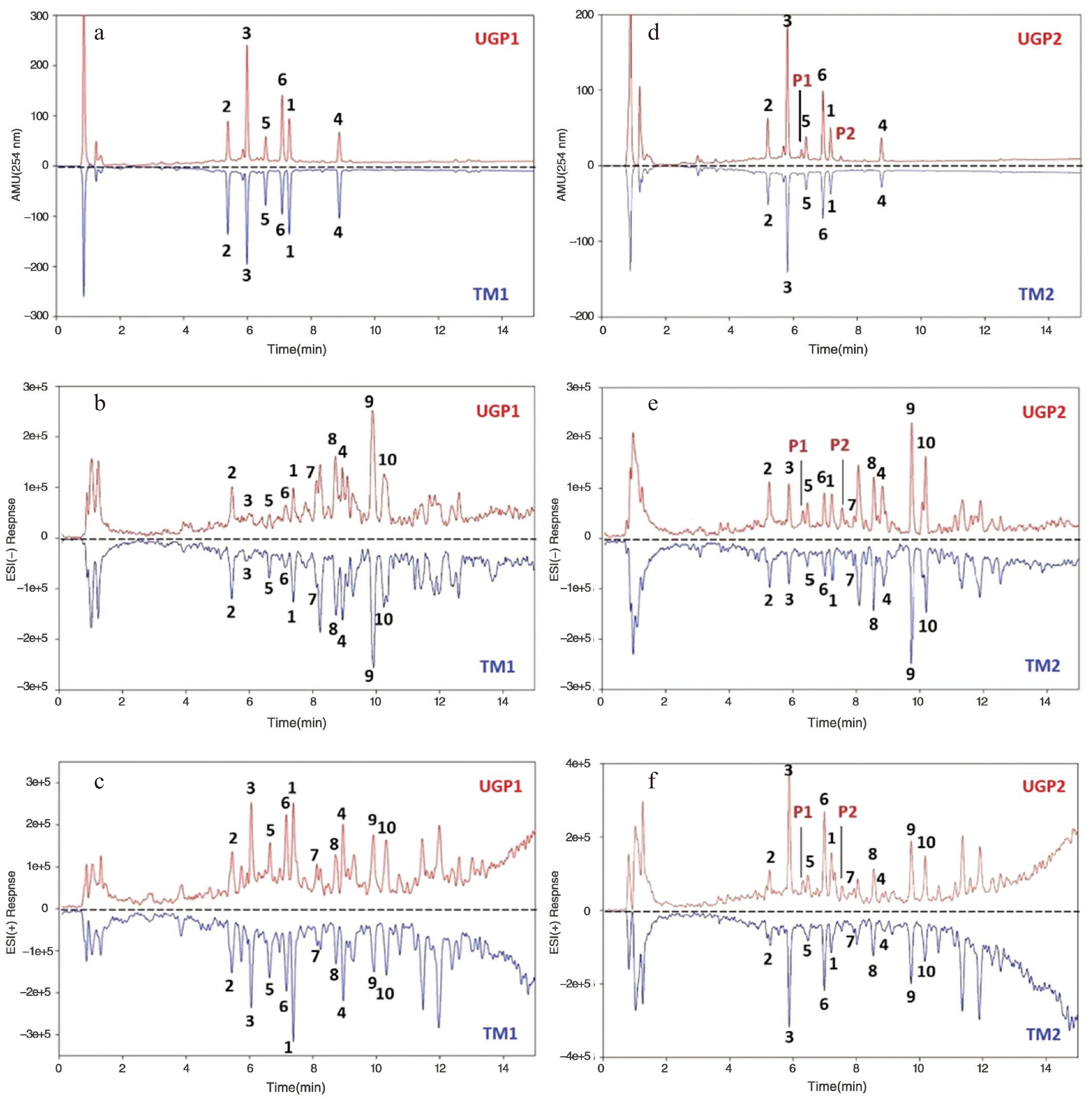
Figure 2.
Comparison of UHPLC/DAD-MS UV254 nm, ESI negative, ESI positive chromatograms between ultrafine granular powder (UGP) and sliced traditional material (TM) of Radix Astragali. UV 254 nm chromatograms (a) UGP1 (top) and TM1 (down), (d) UGP2 (top) and TM (down); ESI negative chromatograms (b) UGP1 (top) and TM1 (down), (e) UGP2 (top) and TM2 (down); ESI positive chromatograms (c) UGP1 (top) and TM1 (down), (f) UGP2 (top) and TM2 (down).
Chemical homogeneity between UGP and TM materials of Radix Astragali
-
It is readily assumed that the UGP material would be a chemically homogenized product because it is produced from large amounts of a particular TCM material, whether or not it is from the same or multiple batches of raw plant material. To provide convincing evidence for the homogeneity of the UGP material from Radix Astragali, a quantitative UHPLC/DAD-MS analytical approach was employed to assess the UGP materials in comparison with the TM materials for the contents of the selected marker compounds. The UHPLC/DAD-UV conditions were based on those used for the chemical consistency study.
To achieve good quantitative analysis, the sample extraction method was optimized by using different solvents (0%, 50%, 80%, and 100% methanol in water), and 80% methanol was determined to possess the best extraction efficiency based on relative peak areas of the six isoflavones (Supplemental Fig. S3 and Supplemental Table S1). Therefore, 80% methanol was used to extract the TM and UGP samples. For quantification, four standard isoflavones (1, 2, 4, and 5) and two standard triterpenoid glycosides (7 and 8) were used to construct calibration curves. The standard curves of the isoflavones generated from the UV254 nm chromatograms possessed good correlation coefficients and a wide concentration range, while the triterpenoid glycosides produced good linearity from the negative ESI-MS chromatograms. Quantification of the two commercially unavailable isoflavones 3 and 6 was approximated using the calibration curves of the structurally close compounds 2 and 5, respectively, assuming they produced similar UV responses. Similarly, quantification of the commercially unavailable triterpenoid glycosides 9 and 10 was based on the calibration curve of compound 8. The equations of calibration curves of the ten marker compounds and the limit of quantification (LOQ) of the standard compounds are summarized in Supplemental Table S2. The analytical method was further validated by the accuracy and precision experiments (see experimental section and Supplemental Tables S3 and S4).
Ten accessions of UGP2 and TM2 from the same source were assessed by paired sample t-test for the content of marker compounds 1−6 and 8−10 (Supplemental Fig. S4 and Supplemental Table S5) using statistic software SPSS (Statistical Package for the Social Sciences) (Table 2). Astragaloside IV (7) was shown to have a very low concentration in these samples (under the LOQ), and its quantification was not reliable, thereby not included. Relative standard deviation (RSD) values for the marker compounds were used to judge the homogeneity of the 10 accessions. The RSD values of the nine marker compounds in UGP2 were in the range of 1.70%−8.38%, in comparison with 8.55%−43.80% for these compounds in TM2. For example, compound 1 in UGP2 had a content of 0.059−0.065 mg/g with an RSD value of 3.05%, compared to 0.017−0.071 mg/g with an RSD value of 43.80% in TM2. Comparison of the content of three representative compounds 1, 4, and 8 is illustrated in Fig. 3a. These data indicated that UGP2 possessed greater homogeneity than TM2 in terms of the marker compound content in different accessions. It was also observed that the content of compounds 1 and 4 demonstrated significant differences (p < 0.01) in UGP2 and TM2, with UGP2 having greater concentrations, while the concentrations of their corresponding isoflavone glycosides (compounds 2, 3, 5, and 6) (p < 0.05) and triterpenoid glycosides (8−10) were slightly greater in TM2. This suggests that, to some degree, chemical conversion of glycosides to corresponding aglycones or dissolution rates for some compounds may have occurred in the UGP preparation process.
Table 2. Content of marker compounds 1−6 and 8−10 (mg/g dry material) in UGP2 and TM2 from the same source of Radix Astragali (n = 10).
Compound Detector UGP2 (mean ± SD mg/g) RSD (%) TM2 (mean ± SD mg/g) RSD (%) 1 UV 254 0.062 ± 0.002** 3.05 0.038 ± 0.016** 43.80 2 UV 254 0.190 ± 0.010* 5.09 0.239 ± 0.040* 17.94 3 UV 254 0.768 ± 0.015* 1.94 0.909 ± 0.185* 20.31 4 UV 254 0.078 ± 0.002** 1.91 0.064 ± 0.006** 8.55 5 UV 254 0.123 ± 0.004* 3.13 0.148 ± 0.020* 13.65 6 UV 254 0.388 ± 0.007 1.70 0.403 ± 0.082 18.96 Total isoflavonoids 1.609 ± 0.029 1.83 1.827 ± 0.329 18.00 8 ESI- 0.091 ± 0.007 7.68 0.096 ± 0.017 18.30 9 ESI- 0.283 ± 0.021 7.58 0.292 ± 0.028 9.58 10 ESI- 0.113 ± 0.009 8.38 0.115 ± 0.015 12.82 Total astragalosides 0.487 ± 0.019 3.82 0.503 ± 0.046 9.11 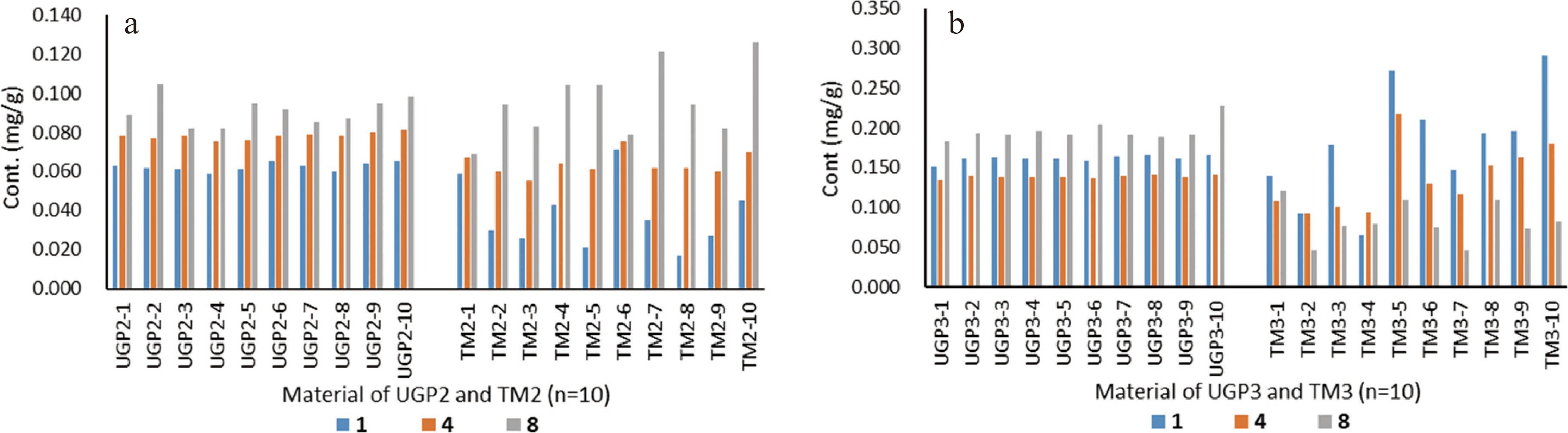
Figure 3.
Comparison of content (mg/g dry material) for marker compounds 1, 4, and 8 between ultrafine granular powder (UGP) and sliced traditional material (TM) of Radix Astragali. (a) UGP2 and TM2 accessions (n = 10); (b) UGP3 and TM3 accessions (n = 10).
To expand the comparative scope for chemical homogeneity of Radix Astragali, a sliced traditional material (TM3) and a UGP material (UGP3) were purchased from a local TCM drug store. It is unknown whether TM3 and UGP3 were derived from the same or different batches of raw plant materials. Analysis of UGP3 and TM3 (each with 10 accessions, Supplemental Fig. S5 and Supplemental Table S6) indicated that the two materials had significant differences in terms of the contents of marker compounds 1−6 and 8−10 (Table 3). The RSD values for the nine marker compounds were in the range of 1.25%−10.90% in UGP3 versus 13.63%−37.74% in TM3 (Table 3), indicating UGP3 was much more chemically homogenized than TM3. Significant variances for the concentrations of compounds 1, 4, and 8 in the 10 accessions of TM3 are also evident in Fig. 3b.
Table 3. Content of marker compounds 1−6 and 8−10 (mg/g dry material) in UGP3 and TM3 from unknown batch sources of Radix Astragali (n = 10).
Compound Detector UGP3 (mean ± SD mg/g) RSD (%) TM3 (mean ± SD mg/g) RSD (%) 1 UV 254 0.162 ± 0.004 2.21 0.179 ± 0.067 37.74 2 UV 254 0.212 ± 0.008 3.82 0.188 ± 0.044 23.23 3 UV 254 0.656 ± 0.010** 1.47 0.392 ± 0.126** 32.14 4 UV 254 0.139 ± 0.002 1.40 0.136 ± 0.040 29.31 5 UV 254 0.124 ± 0.003** 2.36 0.088 ± 0.012** 13.63 6 UV 254 0.312 ± 0.004** 1.25 0.151 ± 0.029** 18.88 Total isoflavonoids 1.603 ± 0.027 1.67 1.133 ± 0.241 21.29 8 ESI- 0.196 ± 0.012** 5.89 0.082 ± 0.024** 29.20 9 ESI- 0.498 ± 0.026** 5.18 0.228 ± 0.047** 20.71 10 ESI- 0.182 ± 0.020** 10.90 0.072 ± 0.023** 31.66 Total astragalosides 0.876 ± 0.026 2.96 0.382 ± 0.088 22.90 Chemical profiles and content of polysaccharides in UGP and TM of Radix Astragali
-
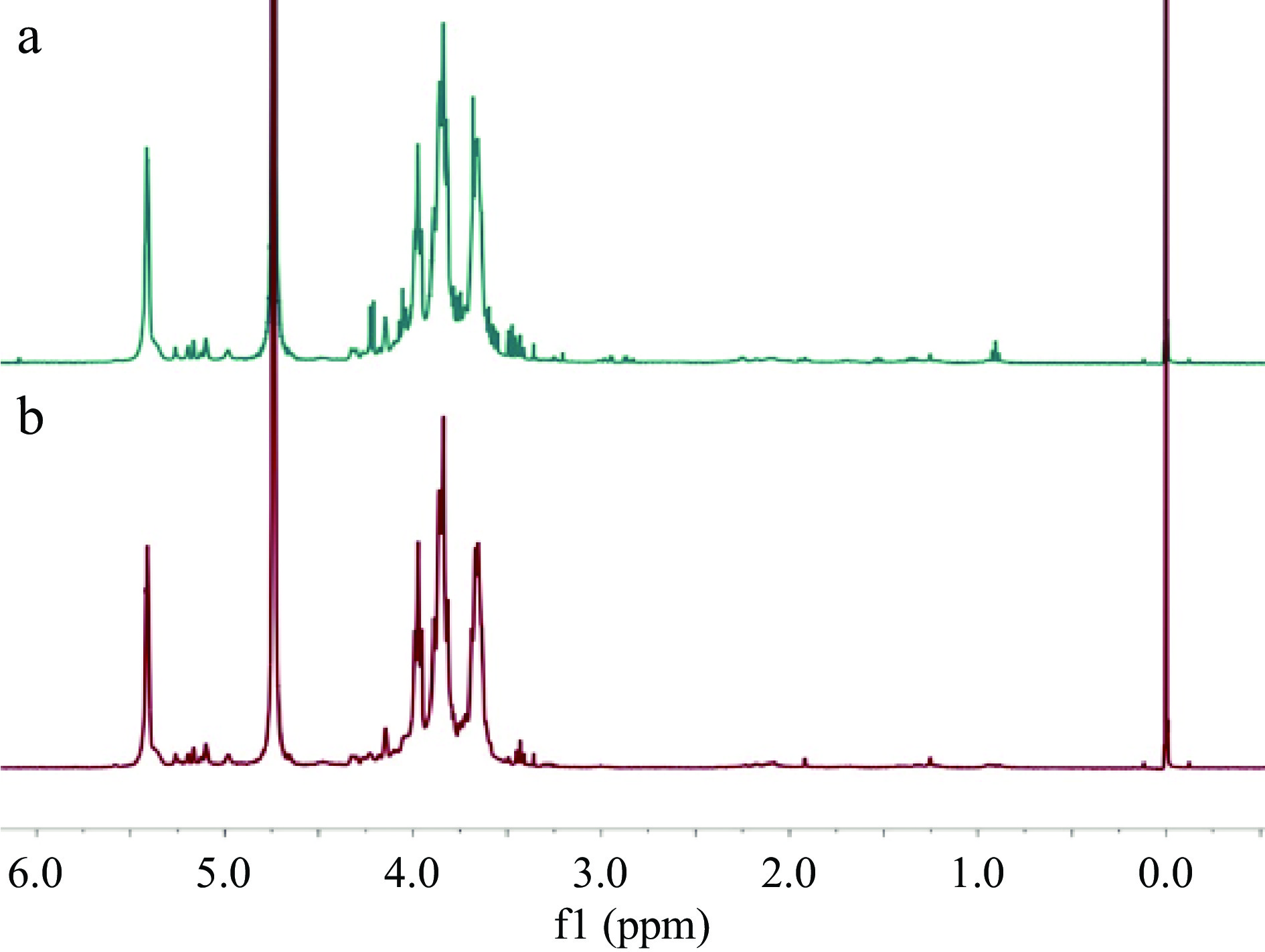
The polysaccharides present in Radix Astragali are considered to play an important role in the efficacy of this TCM. The preparation of Radix Astragali polysaccharides (RAPS) was achieved by extraction of TM4 and its corresponding UGP4 (prepared from TM4) with hot water, and then treated with the Sevag reagent to remove proteins, followed by ultrafiltration to remove small molecules and oligosaccharides. The resultant RAPS were subjected to 1H NMR analyses, which demonstrated that RAPS from both TM4 and UGP4 were very similar (Fig. 4), indicating chemical consistency of the two materials. It was also confirmed that these 1H NMR profiles were similar to those of APS-I and APS-II, two anti-tumor polysaccharides purified from Astragalus mongholicus[15]. The yields of RAPS resulted from TM4 and UGP4 were 3.39 ± 0.20% and 7.22 ± 0.35%, respectively. This indicated that the UGP materials had excellent dissolution rates for RAPS, and appear to be more efficient than TM in terms of releasing RAPS during the course of usage.
-
Small-molecule compounds in A. membranaceus var. mongholicus and A. membranaceus were reported to have significant organ-, age,- and variety-specificity based on LC-MS and NMR analyses[9−11]. In cultivated Radix Astragali, isoflavonoids and astragalosides vary in concentrations in different organs of the plants harvested from different regions[16]. The Radix Astragali polysaccharides also demonstrate notable differences of carbohydrate composition in the plant materials from different geographic origins[17]. This implies the traditional slices of Radix Astragali differ from batch-to-batch and even within the same batch, as demonstrated in this study (refer to Fig. 3) in terms of the content of bioactive compounds, which significantly affects the material quality and potential clinical efficacy. Production of the Radix Astragali UGP material generally involves a large amount (kg to ton scale) of sliced material. Even though the sliced material is from different batches of the plant, the UGP production process will physically improve chemical homogeneity, with minor chemical conversions in the UGP production process which would not negatively impact the overall metabolite profile.
It should be pointed out that UGP can be used like tea leaves in hot water for direct administration while TM is generally decocted in a mixture form in routine TCM practice. For any UGP or TM material, the content of bioactive compounds in the extracts depend on the extraction process. For example, decoction of a specific TCM prescription involves multiple TCM materials in boiling water, which is a complex extraction process leading to specific content and ratios of bioactive compounds required for pharmacological effects. The current study was performed under the same extraction conditions to compare their chemical consistency, homogeneity, and dissolution. It appears that our results are consistent with previous studies demonstrating a higher dissolution rate of ginsenosides from UGP of Panacis Quinquefolii Radix[18] and an improved bioavailability of flavonoid compounds present in UGP of Radix Astragali compared to respective TM material[19].
-
This study confirmed that the UGP material of Radix Astragali was not only identical or similar to the traditional slices in terms of small-molecule metabolite profiles, but also chemically homogeneous over traditional slices, laying a scientific foundation to support the use of UGP in TCM. In addition, the UGP material demonstrated chemical profiles of the bioactive polysaccharides similar to those of TM, however, it possessed a greater dissolution rate compared to the traditional slices. The UGP material is an advancement in overcoming the batch-to-batch inconsistency of traditional slices, and has great potential to be used in TCM prescriptions.
-
Three batches of sliced traditional material TM1 (code 20170802), TM2 (code 181101), and TM4 (code 20170601) of Radix Astragali prepared from Astragalus membranaceus var. mongholicus and their corresponding ultrafine granular powder UGP1 (code 20170908), UGP2 (code DP20190130), and UGP4 (code 20180706), as well as two batches of sliced traditional material TM3 (code 181005) and ultrafine granular powder UGP3 (code 20190105) purchased from a TCM drug store were supplied by Zhongzhi Pharmaceutical Co. Ltd. Each accession of TM and UGP were randomly selected and powdered with a grinder to a particle size of < 0.25 mm.
Chemicals and solvents
-
Reference standards of calycosin (> 99%), calycosin-7-O-β-D-glucose (> 98%), formononetin (> 98%), ononin (> 98%), astragaloside II (> 99%), astragaloside IV (> 99%) were purchased from AvaChem Scientific (San Antonio, TX, USA). HPLC grade methanol and acetonitrile were purchased from Merck. Formic acid (HPLC grade) was from Sigma-Aldrich. Deuterated trimethylsilylpropanoic acid (TMSP, 2,2,3,3-D4, 98%) in D2O (D, 99.9%) (0.02% (W/V)) was purchased from Cambridge Isotope Laboratories. Inc. (50 Frontage Road, Andover, MA 01810, USA). Chloroform and n-butanol (ACS grade) were obtained from Merck. 10 kDa molecule weight cut-off (MWCO) Spin–X UF concentrators was manufactured in Corning, NY, USA.
UHPLC/DAD-MS analysis
UHPLC/DAD–MS conditions
-
Agilent UHPLC 1290 (Agilent Technologies, Santa Clara, CA, USA) consists of a dualistic solvent delivery system, an autosampler, and a column temperature controller. An Agilent Eclipe Plus C18 1.8 µm, 2.1 × 100 mm column was used for the separation. The mobile phase consisted of 0.05% formic acid (v/v) in water (A) and acetonitrile (B) with a flow rate of 0.25 mL/min. The gradient program was as follows: 0–1 min, 5% B; 1–15 min, 5%–95% B; 15–16 min, 95%–100% B. The DAD wavelength was set at 254 nm. The column temperature was set to 30 °C and the injection volume was 2 µL. The mass spectrometer was an Agilent 6120 quadrupole. The ESI source was set in both positive and negative modes for isoflavones and astragalosides. The parameters were as follows: capillary voltage 4.0 kV; gas temperature: 325 °C; gas flow 12 L/min; nebulizer 35 psi; vaporizer temperature: 225 °C; mass range (m/z): 100−1000.
Sample extraction
-
To 500.0 mg of each TM or UGP material, 10 mL of solvent was added. The suspension was sonicated at room temperature for 30 min. After removal of the supernatant, the residual material was extracted twice in the same manner. The combined supernatants were filtered by a 0.22 µm micro membrane prior to UHPLC-MS-UV analysis. First, the TM1 sample and its corresponding UGP1 sample were extracted with MeOH for chemical consistency study. Next, the extraction method was evaluated using different solvent systems (0%, 50%, 80%, and 100% methanol), leading to the identification of 80% methanol possessing the greatest extraction efficiency based on relative peak areas of six isoflavonoids (Supplemental Fig. S3), which is consistent with previous reports[20]. Thus, 80% methanol was used to extract TM2, UGP2, TM3, and UGP3.
Preparation of calibration solutions
-
The stock solutions of calycosin (1) (3,200 µg/mL), calycosin-7-O-β-D-glucose (2) (3,000 µg/mL), formononetin (4) (1,500 µg/mL), ononin (5) (1,200 µg/mL), astragaloside IV (7) (3,400 µg/mL), and astragaloside II (8) (2,900 µg/mL) were individually prepared with methanol. All stock solutions were stored at 4 ℃. The stock solutions of the standards were further diluted with methanol to produce combined standard working solutions at a series of concentration of 0.11–533.33 µg/mL for 1; 0.10–500.00 µg/mL for 2; 0.05–250.00 µg/mL for 4; 0.04–200.00 µg/mL 5; 0.11–566.67 µg/mL for 7; 0.09–483.33 µg/mL for 8.
Method validation
-
The intra-day and inter-day precision were determined by analyzing UGP2 sample during a day and on three different days, respectively. Accuracy of this method was verified by a recovery test. The standard solution was spiked to the UGP2 material to make two different final concentrations of 10.67 (5.33), 10.00 (5.00), 5.00 (2.50), 4.00 (2.00), 11.33 (5.67), 9.67 (4.83) µg/mL for calycosin (1), calycosin-7-O- β -D-glucose (2), formononetin (4), ononin (5), astragaloside IV (7), astragaloside II (8), respectively. The detailed data are shown in Supplemental Table S3 & S4.
Statistical analysis
-
The statistical analysis was performed using the software SPSS (Statistical Package for the Social Sciences, IBM, NY, USA)
Analysis of RAPS in UGP and TM materials
RAPS preparation
-
Five hundred mg of TM4 or UGP4 material (n = 3) was extracted twice with 10 ml of water at 100 ℃ for 15 min. The combined filtrates were fixed to a final volume of 20 ml, which was treated with Sevag reagent (CHCl3: n-BuOH = 4:1)[7] (20 mL × 3) to remove proteins by centrifugation. The resulting water layer was subjected to ultrafiltration[21] using a 10 kDa Spin–X UF concentrator (20 mL Corning). Briefly, the water layer was added to the sample reservoir of the concentrator, and centrifuged at 3000 rpm. After the water layer was reduced to approximately 1.5 mL, the concentrator was refilled with 20 mL of deionized water and centrifugation was continued. This procedure was repeated twice. Finally, the residual solution in the sample reservoir was collected and lyophilized to afford the desired polysaccharides with yields of 3.39 ± 0.20% and 7.22 ± 0.35% from TM4 and from UGP4, respectively.
1H NMR analysis
-
1H NMR profiles were used to characterize the RAPS obtained from UGP and TM materials. The 1H NMR measurements were conducted on an Agilent DD2-500 NMR spectrometer equipped with a One NMR probe operating at 499.86 MHz for 1H, operating at a temperature of 300 K (27 °C). A scan number of 128 for each sample was used for data collection, with a relaxation delay of 5.0 s and a pulse width of 7.8 ms (90 degree). A 0.3 Hz line broadening was used for apodization and the data sets were zero-filled to 256 k points prior to processing. Chemical shifts (δ) expressed in ppm and referenced to TMSP proton signals at δH = 0.0 ppm.
This research was supported by the USDA Agricultural Research Service Specific Cooperative Agreement No. 58‐6060‐6‐001 and Zhongshan Zhongzhi Pharmaceutical Co. Ltd. We thank Mr. Mohammad A. Daghestani for his sustenance of drying samples during the work.
-
The authors declare that they have no conflict of interest.
- Supplemental Fig. S1 Typical UHPLC-MS-UV chromatograms of six standard compounds. Standard compounds: calycosin (1), calycosin-7-O-β -D-glucose (2), formononetin (4), ononin (5), astragaloside IV (7),and astragaloside II (8).
- Supplemental Fig. S2 ESIMS spectra of two minor compounds calycosin 7-O-β -D-(6''-acetyl)-glucoside (P1) and 6''-acetylononin (P2) in UGP1 and TM1. A: ESI (+) and B: ESI (-) of P1; C: ESI (+) and D: ESI (-) of P2.
- Supplemental Fig. S3 Peak areas (UHPLC/DAD-MS UV254 nm) of isoflavonoids (1-6) extracted by solvent systems of 0%, 50%, 80%, and 100% methanol in water. Marker compounds: calycosin (1), calycosin-7-O-β-D-glucose (2), calycosin-7-O-β-D-glucose-6''-O-malonate (3), formononetin (4), ononin (5) and formononetin-7-O-β-D-glucoside-6''-O-malonate (6).
- Supplemental Fig. S4 Contents (mg/g) of marker compounds (1-6 and 8-10) in UGP2 and TM2 from the same source of Astragali Radix (n=10). Marker compounds: calycosin (1), calycosin-7-O-β-D-glucose (2), calycosin-7-O-β-D-glucose-6''-O-malonate (3), formononetin (4), ononin (5), formononetin-7-O-β-D-glucoside-6''-O-malonate (6), astragaloside II (8), astragaloside I (9), and acetylastragaloside Ⅰ (10).
- Supplemental Fig. S5 Contents (mg/g dry material) of marker compounds (1-6, 8-10) in UGP3 and TM3 materials (n=10). Marker compounds: calycosin (1), calycosin-7-O-β-D-glucose (2), calycosin-7-O-β-D-glucose-6''-O-malonate (3), formononetin (4), ononin (5), formononetin-7-O-β-D-glucoside-6''-O-malonate (6), astragaloside II (8), astragaloside I (9), and acetylastragaloside Ⅰ (10).
- Supplemental Table S1 Peak areas (UHPLC/DAD-MS UV 254 nm) of isoflavonoids (1-6) a extracted by different solvent systems of methanol/water (0%, 50%, 80%, and 100% of methanol in water).
- Supplemental Table S2 Regression data for the marker compounds in UHPLC/DAD-MS.
- Supplemental Table S3 Intra-day and inter-day precisions by analyzing UGP2.
- Supplemental Table S4 Recovery test for determination of six standard compounds in Radix Astragali (by using sample of UGP2, n = 3).
- Supplemental Table S5 Contents (mg/g dry material) of marker compounds (1-6 and 8-10) a in UGP2 and TM2 from the same source of Radix Astragali (n=10).
- Supplemental Table S6 Contents (mg/g dried material) of marker compounds (1-6 and 8-10) a in UGP3 and TM3 materials (n=10).
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Cao L, Wang M, Zhao J, Peng LH, Cheng JL, et al. 2022. Comparative analysis of chemical profiles of Radix Astragali between ultrafine granular powder and sliced traditional material. Medicinal Plant Biology 1:4 doi: 10.48130/MPB-2022-0004
Comparative analysis of chemical profiles of Radix Astragali between ultrafine granular powder and sliced traditional material
- Received: 05 May 2022
- Accepted: 05 September 2022
- Published online: 30 September 2022
Abstract: Radix Astragali, one of the most popular herbs in traditional Chinese medicine (TCM), is used to strengthen the immune system, protect liver function, fight bacteria and viruses, and treat diabetes, heart failure and seasonal allergies. In recent years, a new form of Radix Astragali material processed by cell wall disrupting technology, namely ultrafine granular powder (UGP) has been introduced into the market. In order to determine chemical consistency and homogeneity of the UGP material prepared from sliced traditional materials (TM) of Radix Astragali, multiple batches of the UGP and TM samples derived from Astragalus membranaceus var. mongholicus were analyzed by UHPLC/DAD-MS using isoflavones and triterpenoid glycosides as marker compounds. The results demonstrated that the chemical profiles of UGP was identical or similar to that of TM, but UGP was highly homogeneous in terms of marker compound contents as assessed, e.g., by the relative standard deviation values of the nine marker compounds in the range of 8.55%−43.80% for TM2 compared against 1.70%−8.38% for UGP2. Macromolecular component preparation and 1H NMR analyses indicated that TM4 and its corresponding UGP4 produced similar polysaccharides, but the later had approximately two-fold dissolution rate of the polysaccharides when compared to the former (yield 7.22 ± 0.35% vs 3.39 ± 0.20%). This study confirms that UGP of Radix Astragali is chemically consistent and homogenous, supporting its use as a promising material in TCM prescriptions.
-
Key words:
- Radix Astragali /
- Ultrafine Granular Powder /
- Isoflavones /
- Triterpenoid glycosides /
- Polysaccharides