-
According to the Centre for Research on the Epidemiology of Disasters (CRED), the number of reported disasters across the world in the period 2000−2019 increased to 7,348 from 4,212 in 1980−1999[1]. The CRED report also indicates that the reported fatalities increased to 1.23 million in 2000−2019 from 1.19 million in 1980−1999. This implies that the reported fatalities increased by about 3.36% whereas the reported disasters increased by 74.45% from 1980−1999 to 2000−2019. The increase in the number of fatalities is not as significant as the increase in reported disasters. This can be attributed to the rapid advancement in medical science, engineering, and technology. Although the fatalities appear to be under control, the occurrences of disasters have increased rapidly. To continue keeping the fatalities during disasters in check, it is important to learn from past disasters, plan, prepare, and then implement the essential steps accordingly. Disaster risk reduction and planning has always been explored predominantly from a restoration and recovery point of view[2]. To further minimize fatalities and loss, it is also important to consider disaster management from a hazard mitigation perspective. Reducing death and loss from this increasing number of disasters requires better and wider implementation of hazard mitigation techniques and activities. As such, hazard mitigation has gained policy and research across the world. Hazard mitigation is defined as actions, activities, and processes undertaken well in advance of a disaster impact to minimize or eliminate risks to life and property[3−6]. Hazard mitigation provides passive resistance to a hazard which helps minimize the probability of a major adverse impact. Hazard mitigation is relevant for many types of environmental (e.g., flooding), geological (e.g., earthquakes), climate (e.g., sea level rise), technological (e.g., oil spills), and health (e.g., vector-borne diseases) hazards[7].
Community design and implementation of hazard mitigation are guided by mitigation plans. Hazard mitigation plans are developed and adopted by local, state, and national governments and include reviews of past hazard occurrence; past and predicted future disaster damage; identification of vulnerable locations, property, and populations[8] and outline a set of community-determined mitigation strategies to reduce impacts of the hazards identified in the plan[9]. These plans form the basis of community resilience and should connect with other community planning efforts including emergency operations planning for disaster response and recovery as well as community development plans[10, 11].
Hazard mitigation in the United States draws from the Robert T. Stafford Disaster Relief and Emergency Assistance Act (Stafford Act) adopted in 1988[12]. This act requires state, local, tribal, and territorial governments to develop and adopt a hazard mitigation plan in order to be eligible for post-disaster federal assistance. The Federal Emergency Management Agency (FEMA) supports governments in drafting hazard mitigation plans by providing technical support and guidance tools. The Stafford Act also authorized several grant programs for states that developed a FEMA-approved hazard mitigation plan such as: Hazard Mitigation Grant Program (HMGP), Pre-Disaster Mitigation Grant Program (PDM), Public Assistance Grant Program (PA), Building Resilient Infrastructure and Communities (BRIC), and Fire Management Assistance Grant Program (FMAG). After the Stafford Act, some additional laws support hazard mitigation such as the Sandy Recovery Improvement Act (SRIA) of 2013, the National Flood Insurance Act of 1968, and the Water Infrastructure Improvements for the Nation (WIIN) Act of 2016[12]. As of September 30, 2020, all 50 states, the District of Columbia, and five territories (American Samoa, Guam, Northern Mariana Islands, Puerto Rico, and US Virgin Islands) have FEMA-approved state hazard mitigation plans.
The ongoing coronavirus pandemic has attracted the attention of the scientific community and there has been tremendous loss of life and economic activity worldwide[13]. The coronavirus pandemic raises the question of how hazard mitigation planning and plans address this health risk and future similar health hazards. The All-Hazards Approach (AHA) has been the prominent approach taken for disaster risk reduction and planning[14, 15]. This approach is based on the hypothesis that all disasters share some commonalities with regards to their mitigation, impact, and recovery[16]. Although this approach has been implemented globally for disaster planning and hazard mitigation, there has been an increasing focus on exploring hazard specific approach for disaster planning[17]. All-Hazards Approach is often regarded as the most cost-effective approach for disaster planning and hazard mitigation. However, the recent coronavirus pandemic has proven that some disasters, for instance a pandemic, are unique and they need to be considered independently[15]. They need distinct hazard mitigation plans based on their severity and therefore cannot be efficiently tackled using the All-Hazards Approach. Peleg et al. performed a comparative analysis of the ongoing coronavirus pandemic with other natural disasters such as earthquakes[15]. They concluded that the pandemic mitigation and recovery differ in various aspects such as warning indicators, impact, dependence on external assistance, medical response, and politicization. A pandemic can usually give the government a grace period of a few weeks or months to take appropriate action and decelerate the spread of infection. In contrast, other seasonal hazards such as hurricanes or tornados can strike within minutes even with prior warning indicators. The impact of the pandemic on population cannot be determined until the entire population is immune. However, this is not always possible, and impact will vary with time depending on the availability of vaccinations, personal protective equipment (PPE), and willingness of the population to abide by the safety protocols. The impact of other disasters, such as hurricanes or tornados, can be estimated over a relatively shorter period as compared to that of a pandemic. Also, pandemic is a global phenomenon in contrast to other disasters that affect local populations. Therefore, collaboration with other countries to prevent the spread of infection and vaccination plays a vital role. Thus, it can be concluded that pandemics form a different category of disasters and should be scrutinized distinctly.
Merriam has explained the reasons FEMA failed to plan efficiently and even recognize the risk of the coronavirus pandemic[18]. Despite enduring the deadly influenza pandemics in the past, FEMA failed to address and learn from the issues encountered during these pandemics. Merriam reviewed the FEMA documents for pandemic planning and preparedness and concluded that the documents just gave some insight into the statistics of fatalities and economic loss[18]. In one of the documents titled 'Continuity Planning for Pandemic Influenza', the readers were directed to the World Health Organization (WHO) and Center of Disease Control and Prevention (CDC) website instead of addressing the core problem of hazard mitigation. These websites simply highlight the fatalities statistics and describe some general steps that could be taken to avoid infection on a personal level instead of a holistic view or in-depth analysis for drafting mitigation plans[19−21]. There is tremendous research literature available on the impact of influenza pandemics and the measures that could have been taken to control it. The government did plan for the coronavirus pandemic; however, it appears they were unable to build upon the previous research work and implement those plans efficiently. One of the reasons for negligence of pandemics from a hazard mitigation perspective can be the low occurrence frequency of pandemics. Pandemics can be classified as extremely low frequency and high impact events. Because the world had not experienced a pandemic since the deadly influenza pandemic, it might have resulted in complacency regarding considering pandemics as a distinct hazard in the mitigation plans.
Therefore, to draft effective state hazard mitigation plans, it is necessary to consider pandemics as a distinct hazard in the plans instead of an All-Hazard Approach. Also, it is essential to have clear and well-defined steps needed to take appropriate decisions to mitigate the risk of a pandemic. Just referring to some earlier CDC and WHO documents, and websites is not sufficient[18]. In this paper, the research question that we have addressed is whether the state hazard mitigation plans address pandemics, and if yes, are they addressed distinctly or do they simply refer to some other CDC document. We have examined all state hazard mitigation plans to investigate if pandemics were considered in all the plans. Content analysis was performed to investigate the research question mentioned above.
-
Pandemics have been common throughout the history of humanity, and today still pose a high risk for large loss of life if not mitigated or controlled once they begin[19, 20]. Influenza-type pandemics are common and thus contribute to a large loss of life[20]. The timeline of past influenza pandemics is shown below in Fig. 1. Millions of people all over the world have lost their lives during these pandemics[20].
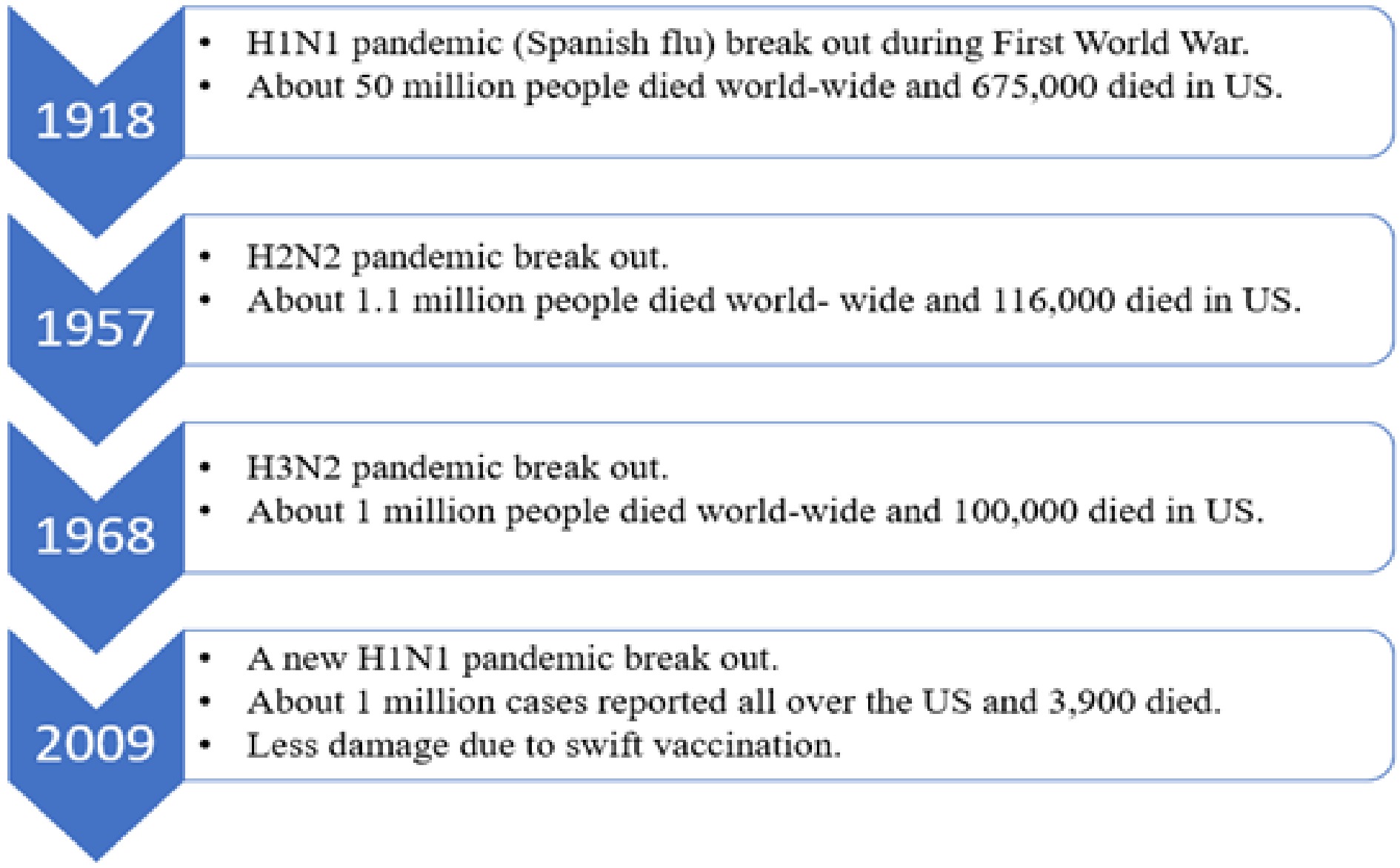
Figure 1.
Timeline of influenza pandemics and estimated number of deaths[[20]].
As we can see from Fig. 1, influenza pandemics occurred repeatedly due to mutations in the influenza viruses. The current coronavirus pandemic, at the time of writing in February 2021, is close behind the 1918 Spanish Flu in loss of life in the US and has surpassed death tolls for the more recent flu pandemics listed. Both pandemics and epidemics are a serious health hazard to the world. The major distinction between a pandemic and an epidemic is that the epidemic affects population in a particular area at a community level whereas a pandemic is an enhanced epidemic which affects populations across different countries[19].
Besides influenza, the US also experienced an outbreak of the West Nile virus in 1999. The first West Nile virus epidemic was reported in Israel in 1951 where 123 cases of infection were identified[22]. Later in 1999, 62 residents of Queens in New York City, US were found to be infected with the West Nile virus. The rapid outbreak led to spread of virus across the west coast of the US. The West Nile virus causes an arboviral disease which means that it needs an arthropod such as a mosquito to transmit it to different hosts. In 2018, a total of 2,813 cases of arboviral diseases were reported of which 2,647 cases were caused by the West Nile virus[23]. Therefore, CDC recommended mitigation strategies such as aerial spraying of adulticides or larvicides for mosquito control at household and community level[24]. Mitigation strategies like these could help in curbing the spread of such viruses.
Another example of mitigation is the CDC response to the Ebola epidemic of West Africa in 2014. Cases of infection were also reported in other countries such as the UK, the US, Italy, and Nigeria[21]. According to CDC, the spread of this epidemic could have been a result of increased travelling across the countries and crowding in urban areas. Therefore, CDC recommended mitigation strategies such as exit screening of travelers leaving West Africa and enhanced entry screening of travelers arriving from affected countries. This ensured that the probability of spread of the epidemic was minimized. This is an example of mitigation strategies being implemented on an international level.
Mitigation strategies have dramatically reduced the death rates for many health hazards, especially in high income countries. For example, advances in basic sanitation and water distribution have reduced the impact of many bacteria-based health hazards[25, 26]. Viruses, with their capacity to mutate, make mitigation more difficult than other types of health hazards. It might take several years for the scientific community to manufacture a vaccine, with the coronavirus vaccine development in 2020 being an amazing quick breakthrough. Hence, to prevent such tremendous loss of life, it is necessary to identify some mitigation strategies that will help in reducing the human and economic losses. Scholars are calling for research on mitigative opportunities of the built environment, such as housing size, density, access to outdoor spaces, ventilation systems, etc., that could reduce the potential of pandemics or spread of them[26−30]. Some of the suggestions for research involve building code changes, which are a common strategy to reduce damage from natural hazards like hurricanes or floods. There have been attempts to model the characteristics of built environment and the clusters of reported COVID-19 cases mathematically[29]. Researchers have found significant direct and indirect influences of built environment characteristics such as transportation infrastructure and places of public gathering on occurrences of infectious clusters[29, 30]. Similar research studies could be instrumental in improving the preparedness for the next pandemic and taking better mitigative steps. However, after a pandemic begins, it might take a long time to analyze all its characteristics and manufacture a vaccine. There is a possibility that the initial preparedness and response measures may fail. In such situations, the learnings obtained from the consequence analysis of previous pandemics can be used to design better and safer mitigation strategies[28]. Thus, while preparedness and response are the most crucial once a pandemic has begun, considering ways to reduce spread are mitigative actions appropriate for consideration in hazard mitigation planning.
Besides being a unique class of hazards, pandemics can impact the functioning of societies across the world from the individual to the governmental scale. Therefore, hazards such as pandemics demand a high level of flexibility to adapt to the dynamic nature of the hazard. Handmer & Dovers proposed a typology of resilience for institutions against hazardous environmental challenges[31]. They classified the response to external hazards into three categories: resistance and maintenance, change at the margins, openness and adaptability. Resistance and maintenance are the inability of the system to change its operation and denial of the possible threat. Change at the margins refers to minor changes in the system's operation, which slowly mitigates the risk of the hazard over time. These two types of responses are favorable for hazards which affect a small proportion of the population at a local scale. They can be handled by a small team of hazard mitigation professionals[31]. However, for hazards such as pandemics that affect the population on a global scale, openness and adaptability to changing hazard conditions are necessary to minimize the severity of the impact. The state hazard mitigation plans fall under this category of response because they prescribe the strategies that need to be implemented to tackle such hazards based on the learnings from past incidents. These strategies can involve drastic measures such as prohibition of public gatherings, transport services, etc. which impact the population on a global scale. Although the plans are based on past incidents, they are not a standard set of instructions that should be followed exactly in the event of a pandemic because it is very difficult to predict the severity of a hazard that is ever changing. However, the plans still serve as a guide for the steps that could be taken on a governmental as well as individual level. The aim of this research is to analyze the hazard mitigation plans of all the states in the US for their ability to address pandemics. The content analysis of plans will help in identifying the strengths and weaknesses in the pandemic mitigation planning. This will be instrumental in promoting adaptability among people at an individual and governmental level by updating the state hazard mitigation plans. The mitigation strategies promoted by CDC for influenza pandemics are used as a standard to gauge the ability of the plans.
In 2017, the CDC recommended some community mitigation strategies to prevent influenza pandemics[32]. The strategies recommended by the CDC are non-pharmaceutical interventions which help in restricting the spread of disease during an influenza pandemic. This implies that these strategies can be implemented on an administrative as well as personal level with the help of emergency management and public health officials, local leaders, organizations, and stakeholders. Therefore, these strategies can be extended to any pandemic in general and would be appropriate to be addressed in all plans. We briefly review these strategies below.
Use of personal protective equipment (PPE)
-
PPE type will vary based on the properties of the disease but include items such as face masks (disposable surgical, medical, or dental procedure masks), which have been widely used by health care workers to prevent respiratory infections. Masks can also be worn by infected people during pandemics to prevent spread of infection to household members and others in the community. Face shields can also be used to avoid infections due to droplets dispersed in air. Gloves are another example of PPEs which can help in preventing the spread of infection through contact. The beginning of the COVID-19 pandemic saw a lack of PPE available or stock-piled.
Self-isolation/quarantine
-
Self-isolation is an important strategy for containing the spread of infection during a pandemic. If a person feels sick or is showing the symptoms of the disease, then he/she should isolate or quarantine themselves to prevent the spread of infection. This will help in containing the disease to a minimum and result in eventual eradication of the disease. The hazard mitigation plans should address strategies for setting up quarantine centers for infected people. Quarantine centers are essential to ensure the infected person does not spread the disease to their family member at home. The plans should mention strategies for allocating places for quarantine centers and what factors should be considered while choosing such places.
Hygiene
-
The plans should address strategies for educating the public and promoting hygiene habits such as avoiding handshakes to prevent spread of disease through contact, cleaning surfaces which are regularly accessed by people, and sanitizing or washing hands before consuming food. Such habits are easy to follow and can be effective in preventing the spread of disease. Social distancing strategies should also be addressed to prevent the spread.
Restricted workplaces, schools, travel, and mass gatherings
-
Restricting access to areas of mass gatherings such as workplaces and schools is an important strategy that must be addressed in hazard mitigation plans. The influenza pandemic of 1918 proved to be so lethal because of over-crowding and travelling of soldiers during the First World War. Travel across states and countries could be restricted as well to prevent the spread of the disease globally. Some other areas of mass gathering such as parks, movie theaters, etc. could also be restricted.
Vaccination
-
Vaccination helps reduce the probability of getting infected by a disease in future. It is one of the important strategies for preventing pandemics. State hazard mitigation plans must address the vaccinations available for various diseases that are usually encountered in that state. Plans must also address strategies for vaccinating all the people in a community in a systematic way. Whenever a vaccine is discovered for a disease, it should be updated in the plan. Some diseases are seasonal in nature. Vaccination drives for such diseases should be organized for before the beginning of season for all diseases.
Vulnerability assessment
-
Vulnerability assessment is instrumental in identifying the most vulnerable populations to a particular disease. Most of the time they are people above the age of 65 and young children between 0 to 5 years of age but vary depending on how the disease spreads and affects the body. Identifying the vulnerable population helps in prioritizing the vaccination plan. Vulnerability is not only age specific, but it can also depend on population density and climatic factors. People living in a densely populated region would be more vulnerable to a disease like influenza or any other airborne disease. Similarly, people living in a region where the climate is conducive to the growth of disease vectors such as mosquitoes will be more vulnerable. Vector-based diseases are diseases which need carriers like mosquito for transmission. All these factors must be addressed in hazard mitigation plans. Vulnerability assessments are commonplace in mitigation plans for risk and hazards[8, 33]. These assessments for health are especially important to address disparities in risk of infection and severity of impacts[34]. Early research on the ongoing COVID-19 pandemic show that some aspects of social vulnerability used to understand those that are most at risk during natural hazards[16, 35, 36] also correlate with COVID-19 infection[37, 38].
For instance, Kim & Botswick developed a social vulnerability index using sociodemographic factors such as percentage of poverty, median household income, employment ratio, percentage of less than high school education, and the percentage of female-headed households with children[38]. The values of these indicators were retrieved from US census. Similarly, a health risk score was created using factors such as stroke deaths, heart related deaths, diabetes, obesity, etc. Finally, the correlation between the social vulnerability index, heath risk score, and the reported COVID deaths were calculated for people belonging to a particular section of the population. This analysis can be carried out to determine the vulnerability of people of a particular ethnicity, socioeconomic status, or health conditions. The correlation between the indices will be proportional to the vulnerability of that section of the population.
Economic consequences
-
Economic analysis helps in estimating the economic loss that could be endured during a pandemic. Shutting down places of mass gatherings like movie theaters, restaurants, etc. can have a significant impact on the economy of a state. An increase in homelessness and joblessness has been particularly observed during the ongoing coronavirus pandemic. These factors can adversely affect the mental stability of people leading to suicides or other mental illnesses. Therefore, estimating the economic loss based on the data from past pandemics is immensely important. It can be instrumental in preparing the economy to be resilient to such economic damage. This strategy must be addressed in all hazard mitigation plans.
Governments have used all the above strategies during the current COVID-19 pandemic, with much critique for slow implementation of mitigation strategies, confusing public communication, and unsystematic analysis of vulnerability and economic consequences[39]. Hazard mitigation plans provide an opportunity for a jurisdiction to identify risks and the actions, resources, and coordination needed to mitigate those risks[9, 40]. Mitigation actions should reduce the costs of response and recovery, and actions identified in plans already offer pre-identified strategies that also should speed response such as pre-identified vaccination locations, pre-developed messaging content and channels, and contracts for sourcing supplies. Thus, this recent crisis prompts the question of how CDC recommended mitigation actions were or were not included in hazard mitigation plans. A lack of inclusion could help explain the slow response to the COVID-19 crises and point toward future hazard mitigation planning changes. Thus, we asked how did states in the US address pandemics in their hazard mitigation plans?
-
Content analysis is an analytical method used to evaluate various forms of printed, visual, or audio materials[41, 42]. It has been used to assess emergency management and hazard mitigation plans for their inclusion (or lack thereof) particular topics, concerns, or population groups. Bennett, for example, performed a content analysis of state emergency management plans to assess their inclusion of populations known to be at risk of impact during a disaster, such as older persons, persons with disabilities or chronic disease, children, and those with low-incomes or limited transportation options, among other populations[43]. She concluded that vulnerable populations aged above 65 years of age and population aged above 5 years of age with disabilities have not been sufficiently addressed in emergency management plans and therefore they tend to suffer more during disasters. Purdum & Meyer, similarly, analyzed state emergency operations plans for the inclusion of prison inmates in disaster mitigation, preparedness, response, and recovery[44]. They developed a typology of different hazard-related tasks that may be assigned to inmates. Several content analysis studies assess the quality of hazard mitigation plans at various jurisdictional levels[45, 46].
Performing a content analysis of state hazard mitigation plans for their ability to address pandemics can reveal valuable insights into the steps proposed by states to counter pandemics as well as the effectiveness of these steps. With this goal, we have performed a content analysis of all the state hazard mitigation plans to identify the mitigation strategies for pandemics which are addressed in the plans. We have also identified the strategies which are not sufficiently addressed in the plans. This is the first study, to the best of our knowledge, which analyzes mitigation plans from a pandemic perspective. This study will be instrumental in providing some guidance while drafting updated hazard mitigation plans so that communities can be better prepared for another pandemic like the recent coronavirus pandemic.
We analyzed the hazard mitigation plans for 49 states, the District of Columbia, and four territories (American Samoa, Guam, Northern Mariana Islands, and Puerto Rico). The Oklahoma hazard mitigation plan was updated in 2019, however the plan was not available on the official website. Hence, we excluded Oklahoma from our analysis. The New York hazard mitigation plan document was not available, however there is a website dedicated entirely to the hazard mitigation strategies for New York state which we analyzed. Similarly, we analyzed the hazard mitigation website for US Virgin Islands because the document was not available.
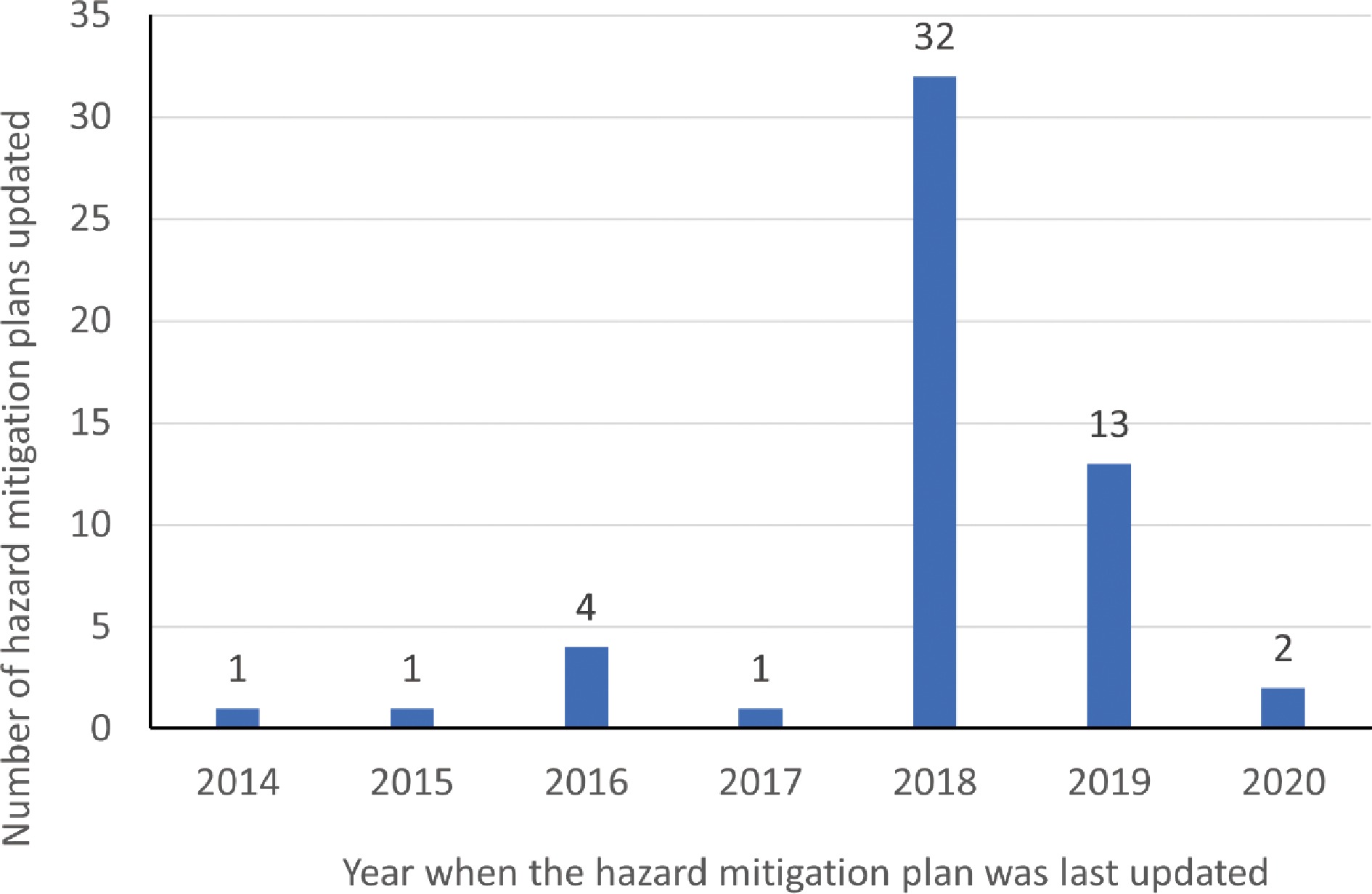
Figure 2 shows the number of hazard mitigation plans that had been updated every year since 2014. It can be observed from Fig. 2, that most of the plans (32 out of 54) were updated in 2018, which is a year before the advent of the ongoing coronavirus pandemic. The hazard mitigation plans which were updated in 2018 and after 2018 hardly addressed mitigation strategies for pandemics. However, during the ongoing coronavirus pandemic all the state governments had been working with federal government and the CDC to control the spread of the pandemic. The government authorities and the scientific community has been studying the nature of the coronavirus pandemic extensively. Therefore, we can hope for more mitigation strategies for pandemics in the next updated versions of the plans. Also, the District of Columbia and the territory of American Samoa had their mitigation plans last updated in 2014 and 2015. Therefore, those plans need to be updated soon to account for the impacts of a pandemic.
We manually analyzed the plans to identify keywords. It was found that keywords such as 'pandemic', 'epidemic', 'influenza', 'virus', and 'disease' have been frequently used throughout the plans while documenting the mitigation strategies. This finding was expected because the mitigation strategies in the plans have been based on the learnings from the past influenza pandemics. Some plans have used the words 'pandemic' and 'epidemic' interchangeably. This is understandable because pandemics are epidemics which occur throughout the world in different countries. The word 'disease' is used frequently because many plans address specific human and animal diseases that are encountered frequently in a particular state. Along with influenza virus, many other viruses, and diseases such as malaria, hantavirus, nipha virus, smallpox, measles, zika virus, cholera, mumps, Ebola, chikungunya, HIV, etc. were mentioned in the plans. Figures 3 & 4 show that influenza, West Nile virus, and plague are the most frequently discussed public health crises. About 31% (29 out of 54) of the plans address influenza, which is understandable because of the devastating impact of influenza pandemics in the past.
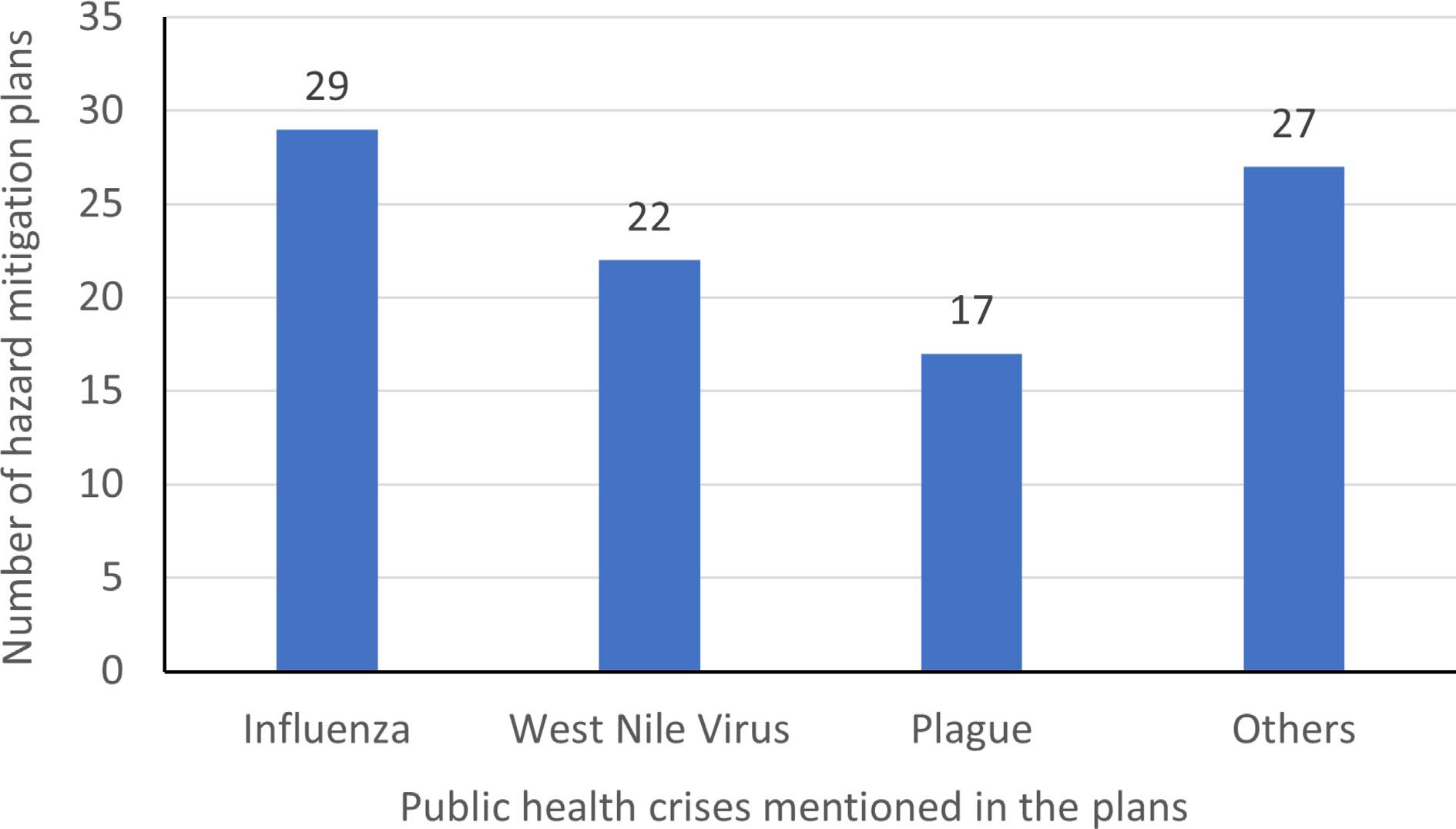
Figure 3.
Graph depicting the different public health crises mentioned in the hazard mitigation plans.
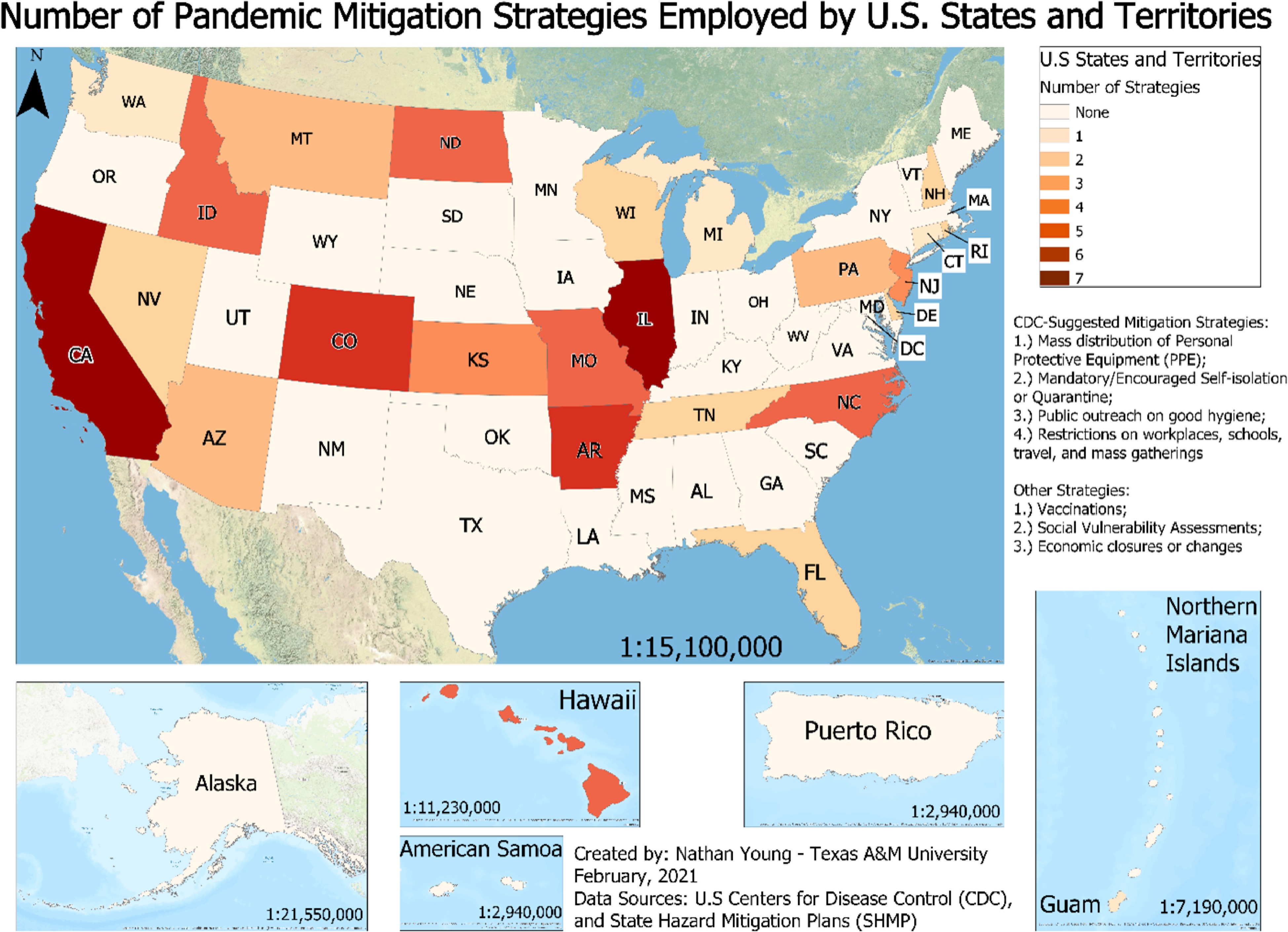
Figure 4.
State wise distribution of number of pandemic mitigation strategies employed by US states and territories.
Some plans addressed mitigation strategies for pandemics in the context of bioterrorism. We counted those strategies in our analysis because those strategies were also applicable in pandemics. For example, social distancing and self-isolation strategies are valid during a bioterrorism incident as well as a pandemic. However, bioterrorism is different to a pandemic. Bioterrorism is a manmade hazard which involves intentional outbreak of a disease on a large scale. Pandemics are not intentional.
Apart from influenza, West Nile virus, and plague, all the other viruses and diseases are addressed in varying proportions across all the plans. This might be because of the demographic and climatic factors of the state to which the plan belongs. For instance, a state with climatic conditions conducive to the growth of mosquitoes will focus on diseases like malaria in their hazard mitigation plan. Whereas a state with impure water supply problem will focus more on waterborne diseases in their hazard mitigation plan. The 54 state hazard mitigation plans were analyzed manually to identify the public health crises addressed by them. A summary of the different public health crises addressed in hazard mitigation plans for 49 states, the District of Columbia, and four territories (American Samoa, Guam, Northern Mariana Islands, and Puerto Rico) is shown in Table 1. The results of the analysis were arranged as shown in Table 1 with an 'x' denoting the public health crises addressed in the corresponding state hazard mitigation plan. We have classified the results into four categories.
Table 1. Summary of different public health crises addressed in hazard mitigation plans for 49 states, the District of Columbia, and four territories (American Samoa, Guam, Northern Mariana Islands, and Puerto Rico).
Hazard mitigation plans Influenza West Nile virus Plague Others Alabama Alaska American Samoa x Arizona x x x x Arkansas x x x California x x x x Colorado x x x x Connecticut x x Delaware x x District of Columbia Florida x x x Georgia Guam x x x Hawaii x x x Idaho x x x x Illinois x Indiana x x x Iowa Kansas x x Kentucky Louisiana x Maine Maryland Massachusetts x Michigan x Minnesota x x x Mississippi Missouri x x x x Montana x x x x Nebraska Nevada x x x New Hampshire x x x New Jersey x x x New Mexico New York North Carolina x x x x North Dakota x x x x Northern Mariana Islands Ohio x x x x Oregon Pennsylvania x x x x Puerto Rico Rhode Island x x South Carolina x x South Dakota Tennessee x x x Texas Utah Vermont x x x x Virginia x x x x Washington West Virginia Wisconsin x x Wyoming Finally, the plans were analyzed for the mitigation strategies proposed by the CDC. After manually analyzing the 54 state hazard mitigation plans, the coding scheme shown in Table 2 was proposed. Keywords 'pandemic' and 'influenza' were used to search through the documents to locate the relevant information on pandemic hazard mitigation. All the information related to any type of pandemics were thoroughly analyzed manually to identify the strategies that were being proposed in the 54 state hazard mitigation plans. Based on the results of the analysis, the strategies proposed in the plans were arranged as shown in Table 2 with a 'x' corresponding to every strategy that was mentioned in the plans. Keywords 'pandemic' and 'influenza' were used together because the influenza pandemics were found to be addressed the most frequently in the plans. We considered the strategies proposed for influenza pandemic specifically in our results because those strategies could be extended for other similar types of pandemics in the future.
Table 2. Summary of different mitigation strategies for pandemics addressed in hazard mitigation plans for 49 states, the District of Columbia, and four territories (American Samoa, Guam, Northern Mariana Islands, and Puerto Rico).
Hazard
mitigation
plansMitigation strategies as suggested by CDC Vaccination Vulnerability assessment Economic consequences Only acknowledges the need for addressing pandemics Refers
to CDC
guidelinesRefers to
some other
Influenza
pandemic planUse of Personal Protective Equipment (PPEs) Self-isolation/ quarantine hygiene Restricted workplaces, schools, travel, and mass gatherings Alabama x Alaska American Samoa Arizona x x x x Arkansas x x x x x x x x California x x x x x x x x x Colorado x x x x x x x Connecticut x x Delaware x x x District of Columbia x Florida x x x x Georgia Guam x x Hawaii x x x x x x Idaho x x x x x x Illinois x x x x x x x x x Indiana x Iowa x x Kansas x x x x x Kentucky Louisiana x x Maine x Maryland Massachusetts x x Michigan x Minnesota x x x Mississippi x Missouri x x x x x x x Montana x x x x x Nebraska Nevada x x x New Hampshire x x x x New Jersey x x x x x New Mexico New York North Carolina x x x x x x North Dakota x x x x x x Northern Mariana Islands Ohio x Oregon x x Pennsylvania x x x x Puerto Rico Rhode Island x x x South Carolina x South Dakota x Tennessee x x x Texas Utah x Vermont x x Virginia x Washington x x West Virginia Wisconsin x x x Wyoming -
We analyzed the hazard mitigation plans for the nine strategies identified above. Figure 4 shows the number of hazard mitigation plans addressing different mitigation strategies for pandemics. According to Fig. 5 and Table 2, about 57% (31 out of 54) of the hazard mitigation plans refer to the CDC guidelines.
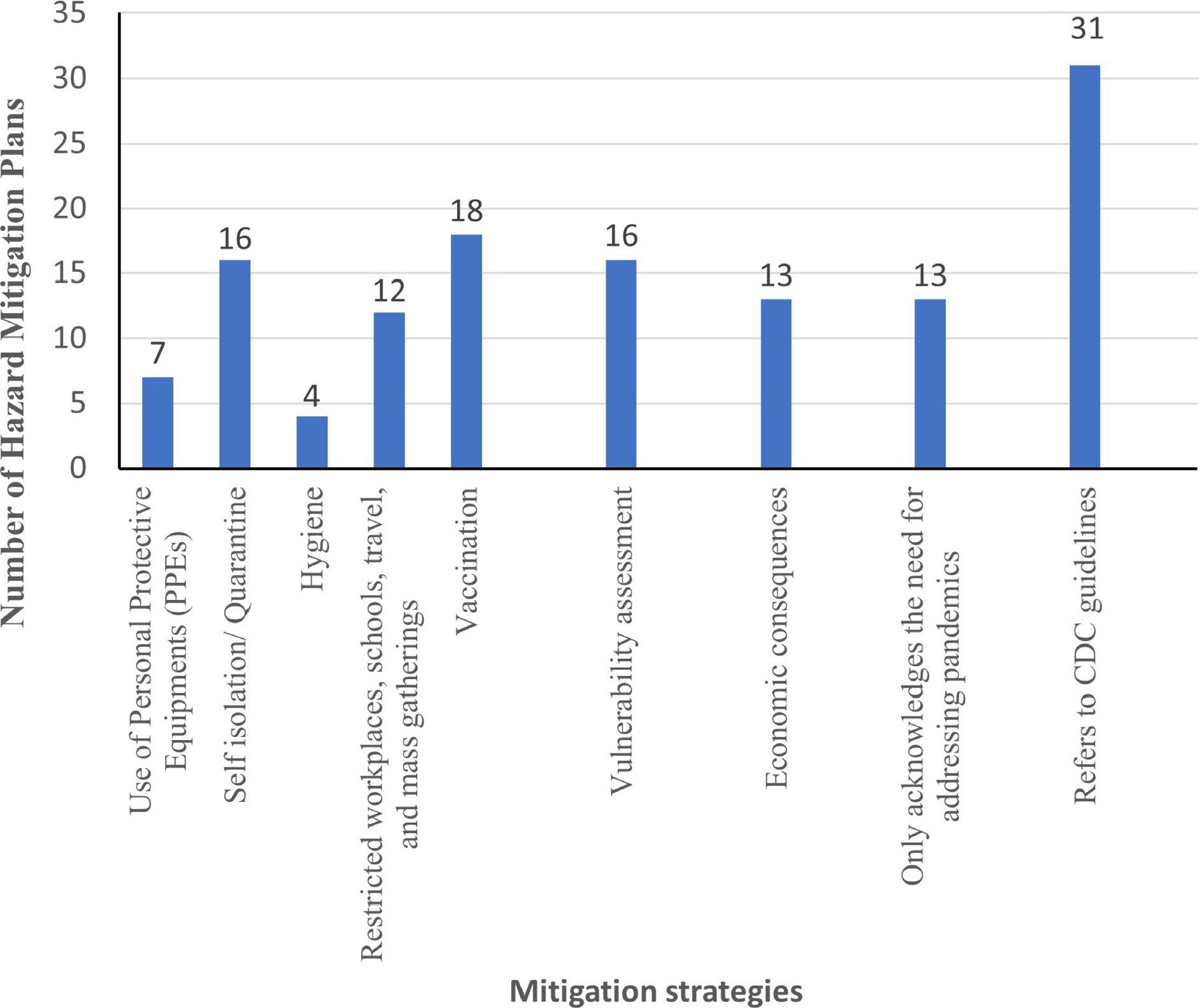
Figure 5.
Graph depicting number of hazard mitigation plans addressing different mitigation strategies for pandemics.
About 24% (13 out of 54) of the plans only acknowledge the need for addressing pandemics. These states include Alabama, Iowa, Louisiana, Massachusetts, Minnesota, Mississippi, Oregon, South Carolina, South Dakota, Utah, Vermont, Virginia, and the District of Columbia. This is an alarming situation considering the impact of the ongoing coronavirus pandemic. Therefore, these states need to update their hazard mitigation plans to include the mitigation strategies for pandemics.
Table 2 provides a summary of different mitigation strategies for pandemics which are addressed in the hazard mitigation plans. From Table 2, we found that about 24% (13 out of 54) of the plans do not even acknowledge the need for addressing pandemics. These states include Alaska, Georgia, Kentucky, Maryland, Nebraska, New Mexico, New York, Texas, West Virginia, Wyoming, and territories of American Samoa, Northern Mariana Islands, and Puerto Rico. These states are in dire need of a mitigation plan update. If a pandemic were to strike again later, these states could be worst affected. Therefore, these states also need to update their hazard mitigation plans based on the CDC guidelines, local characteristics, and population. About 18% (10 out of 54) of the plans refer to some other earlier proposed emergency plans for influenza pandemics. The hazard mitigation plans for Arkansas, California, Florida, Illinois, Iowa, Louisiana, Minnesota, Mississippi, Montana, and New Hampshire refer to other mitigation plans which are designed specifically for influenza pandemics. Therefore, guidelines should be made in accordance with the nature of a pandemic such as airborne, waterborne, respiratory disease, etc.
As shown in Fig. 4, only a few states address all the mitigation strategies described in Table 2. Figure 4 shows the number of pandemic mitigation strategies employed by US states and territories. Use of PPEs is only addressed in about 13% (7 out of 54) of the plans. These states include Arkansas, California, Colorado, Florida, Illinois, North Carolina, and Wisconsin. Promoting the use of PPEs appeared to be a simple task. However, this misconception was highlighted during the coronavirus pandemic in 2020. Some people had been avoiding the use of PPEs due to their set of beliefs and ideologies. Also, political agendas played an important role in further exploiting this issue. Therefore, more states need to address this issue of promoting the use of PPEs effectively in their hazard mitigation plans. Hygiene was also hardly addressed in mitigation plans. Only 7% (4 out of 54) of the plans addressed the importance of proper hygiene to restrict the spread of pandemic. These states include Arkansas, California, Connecticut, and Illinois. Hygiene practices are ignored because they are usually considered too simple and obvious. However, the mitigation plans need to include these because they are critical to restrict the spread of pandemics.
California and Illinois are the only two states that address all the nine mitigation strategies identified above. They have separate chapters in their hazard mitigation plans which are solely dedicated to infectious diseases outbreak and pandemics. Also, Arkansas and Colorado address eight out of the nine mitigation strategies identified above. Other states can draw some parallels by comparing their plans with California, Illinois, Arkansas, and Colorado.
Finally, Fig. 5 suggests that all the mitigation strategies identified above are scarcely addressed in the hazard mitigation plans. This might be because pandemics are low probability disasters, but they have a high impact. The next occurrence of a pandemic cannot be predicted. The uncertain nature of pandemics makes them more worthy of consideration in hazard mitigation plans. Therefore, all states, the District of Columbia, and the territories need to address mitigation strategies for pandemics thoroughly while updating their hazard mitigation plans.
-
Merriam observed that the FEMA documents for pandemic planning and preparedness usually just redirect the readers to CDC or WHO websites and barely analyzes the issue of hazard mitigation[18]. This was also observed from the results of the content analysis. This can be one of the potential reasons for the slow implementation of mitigation measures during the recent coronavirus pandemic. Although the CDC prescribed some steps that could potentially hinder the spread of infection, the state hazard mitigation plans should have analyzed, modified, and then implemented those measures according to the geographic, social, and economic characteristics of the state. Also, the compliance of hazard mitigation plans with the requirements proposed by the Disaster Mitigation Act of 2000 and FEMA is generally low[47]. This further implies that the plans would not be able to tackle pandemics efficiently which was observed during the recent COVID pandemic. However, a drastic change in planning could be expected after the recent pandemic from the local as well as federal government officials in terms of pandemic mitigation when the state hazard mitigation plans are revised in future[48].
It was also concluded that although most mitigation plans consider pandemics and epidemics to some extent, they still addressed them with an All-Hazards Approach described earlier. Most mitigation plans described the same general mitigation steps that could be taken on a community level for one public health crisis such as influenza while merely illustrating the steps that need to be taken for other different types of crises. This was also observed in Merriam's analysis of the FEMA documents where the same general links to CDC websites were cited[18]. A thorough analysis is further required to recommend mitigation steps based on the nature of the health crises. For instance, water borne diseases will need different mitigation strategies than air borne diseases. None of the mitigation plans analyze the health crises based on their nature or characteristics. Also, local characteristics of the population such as vulnerability, economics, geography, etc. should be considered to propose mitigation plans tailored according to the local characteristics. This is one of the important drawbacks observed in all the mitigation plans that needs to be addressed in future. A Top Hazards Approach (THA) is being considered as a suitable alternative to AHA[15]. THA is based on the hypothesis that every disaster risk is unique and requires a distinct mitigation plan. It will be interesting to see if the planners consider this approach in the updated plans. Although it is reasonable to refer to such plans for guidance, it would be preferrable to include proper guidelines in the respective state hazard mitigation plans. The plans currently tend to be influenza pandemic specific; this discrepancy should be addressed appropriately in the updated versions of the state hazard mitigation plans.
-
This analysis indicates there is much room for improvement to connect pandemic mitigation into hazard mitigation planning. Furthermore, the current coronavirus pandemic and the strategies undertaken across the world bring many more mitigation strategies that future planners could learn from and apply in mitigation plan revisions. Additional mitigation strategies for pandemics should be explored and could coincide with more traditional natural hazard mitigation actions. For instance, long-term mitigation strategies related to the built environment, for both reducing risk and speed of spread as well as pre-identifying needed strategic locations for testing, vaccination, and quarantine.
Also, the THA approach for mitigation planning needs to be implemented while drafting the new state hazard mitigation plans. This approach will be instrumental in developing more effective plans which are suited to the state population. Along with the THA approach, FEMA should mandate the inclusion of pandemics as a distinct class of hazards in multi-hazard mitigation planning like other hazards. Most state hazard mitigation plans do not consider pandemics as a distinct class of hazards and instead focus on a particular pandemic hazard such as influenza. After learning through the outcomes of the coronavirus pandemic, the hazard mitigation planners can add much detailed analysis of the mitigation steps necessary to curb the pandemic to avoid such disaster in future.
This pandemic with its economic consequences and social distancing requirements also highlights mental health impacts as a risk to be estimated and mitigated if possible. Strategies for addressing the mental stability of health care workers, which face long working hours are needed. Currently, Arkansas hazard mitigation plan addresses this issue. Therefore, more work needs to be done to address mental stability of health care workers. As the pandemic continues across the US, these mental health consequences are increasing for other populations as well, and preplanning for surge in these areas is needed.
Contact tracing is also not addressed adequately in the plans. A proper methodology needs to be proposed for carrying out contact tracing efficiently. Contact tracing has been implemented heavily in the ongoing coronavirus pandemic. A lot can be learned about contact tracing from the outcomes of the coronavirus pandemic. This will help in addressing contact tracing in future mitigation plans.
The impact of unavailability of public services on people and the economy needs to be addressed. This is an important issue which was not addressed in any plans we analyzed. Unavailability of public services such as transport, restaurants, essential groceries, etc. during pandemics can have a tremendous impact on people. Plans need to address this issue of shortage of public services. Also, shortage in services can lead to economic losses of many locally owned businesses. This can lead to an increase in homelessness and joblessness. These conditions can adversely impact the mental wellbeing of people and lead to mental illnesses. Mitigation plans also need to address this issue.
Moving forward, hazard mitigation planners can use lessons learned during the COVID-19 pandemic to identify needed mitigation features for future pandemics, thinking creatively about health in our built and social environments. Future researchers could evaluate how COVID-19 has changed hazard mitigation plans in the future and how well mitigation plans integrate with public health planning efforts.
A limitation of this research could be that the pandemic hazard mitigation strategies used for the content analysis of the 54 plans are adopted from CDC based on their recommendations for the recent coronavirus pandemic. More strategies could be identified and adopted along with the nine strategies mentioned in this paper for the content analysis based on the review of CDC recommendations for past pandemics.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Tech University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Pawar B, Meyer M, Wang Q. 2022. Content analysis of state hazard mitigation plans and their ability to address pandemics. Emergency Management Science and Technology 2:12 doi: 10.48130/EMST-2022-0012
Content analysis of state hazard mitigation plans and their ability to address pandemics
- Received: 12 August 2022
- Accepted: 10 November 2022
- Published online: 29 November 2022
Abstract: Hazard mitigation plans address hazards such as earthquakes, hurricanes, landslides, tornados, flooding more explicitly than pandemics or other public health crises. This paper analyzes and identifies pandemic-related mitigation strategies in state-level hazard mitigation plans across the United States. This study identifies common and uncommon strategies that could be included in plan updates such as: use of personal protective equipment (PPE), self-isolation/quarantine, hygiene, restricted gatherings, vaccination, vulnerability assessment, economic consequences analysis, acknowledging the need to address pandemics, and referring Centers for Disease Control and Prevention (CDC) guidelines. The 54 state-level plans are compared based on their inclusion of the nine strategies. Findings show that vaccination, self-isolation/ quarantine, and vulnerability assessment are the strategies most listed in plans. Additional concerns made evident by the 2020 coronavirus pandemic, such as contact tracing, environmental impact, and public services, were limited across the plans. It was concluded that long-term mitigation strategies for reducing the risk and speed of spread such as identifying strategic locations for testing, vaccination, and quarantine should be devised and implemented. Pandemics should be included as a distinct class of hazards while formulating state hazard mitigation plans. Strategies for addressing the impact of long working hours on the mental health of healthcare workers should be explored. Economic and psychological impact of unavailability of essential public services, such as transport and groceries, should be investigated and strategies should be formulated in the mitigation plans accordingly.
-
Key words:
- Pandemics /
- Hazard mitigation plans /
- Public health /
- Content analysis /
- Mitigation strategies