-
Anthocyanins are important secondary metabolites in plants, responsible for the pigmentation of fruits. Anthocyanin biosynthesis begins with phenylalanine and is regulated by various hormone signals[1]. The phytohormone gibberellin (GA) is widely involved in regulating plant growth and development, including anthocyanin biosynthesis[2]. However, investigations on the regulation of anthocyanin biosynthesis by GA mainly focus on the model plant Arabidopsis[3,4], and its regulation mechanism is still not fully understood. In this study, we explored the mechanism by which GA signal regulates anthocyanin biosynthesis through the MdRGL2a-MdTCP46-MdMYB1 module in apple (Malus × domestica).
Teosinte branched1/cycloidea/proliferating (TCP) is a class of plant-specific transcription factors (TFs) that exist widely in plants. It is typically characterized by the TCP domain containing atypical basic helix-loop-helix (bHLH) motifs. To date, 23 and 22 TCP TFs were identified in Arabidopsis and rice, respectively[5]. Extensive studies have shown that TCP TFs are involved in multiple plant growth and development processes, as well as biotic and abiotic stress responses. TCP TFs also act as interfaces between plant growth and various hormone signals[6]. Here, we investigated the response patterns of MdTCP46 to several hormone signals. Abscisic acid (ABA), methyl jasmonate (MeJA), and 1-aminocyclopropane carboxylic acid (ACC) all slightly induced MdTCP46 expression at the transcriptional level (Fig. 1a). On the contrary, GA3 (GA) treatment dramatically inhibited the expression of MdTCP46, while MdTCP46 had no significant response to naphthylacetic acid (NAA) treatment (Fig. 1a). Further qRT-PCR analysis showed that GA continuously inhibited the expression of MdTCP46, and reached the highest inhibitory effect 2 h after GA treatment (Fig. 1b). The MdTCP46 promoter fragment was cloned to pCAMBIA1300-GUS vector and the recombinant vector was genetically transformed into apple callus. Transgenic apple callus with overexpression of the MdTCP46 promoter fragment was treated with GA and GA biosynthesis inhibitor paclobutrazol (PAC), and the response of the MdTCP46 promoter to GA and PAC was evaluated by GUS staining and activity detection. As expected, GA treatment reduced the activity of the MdTCP46 promoter, while PAC had activation effect on the MdTCP46 promoter (Fig. 1c, d). Above results suggest that MdTCP46 is a negative regulator of GA signaling response.
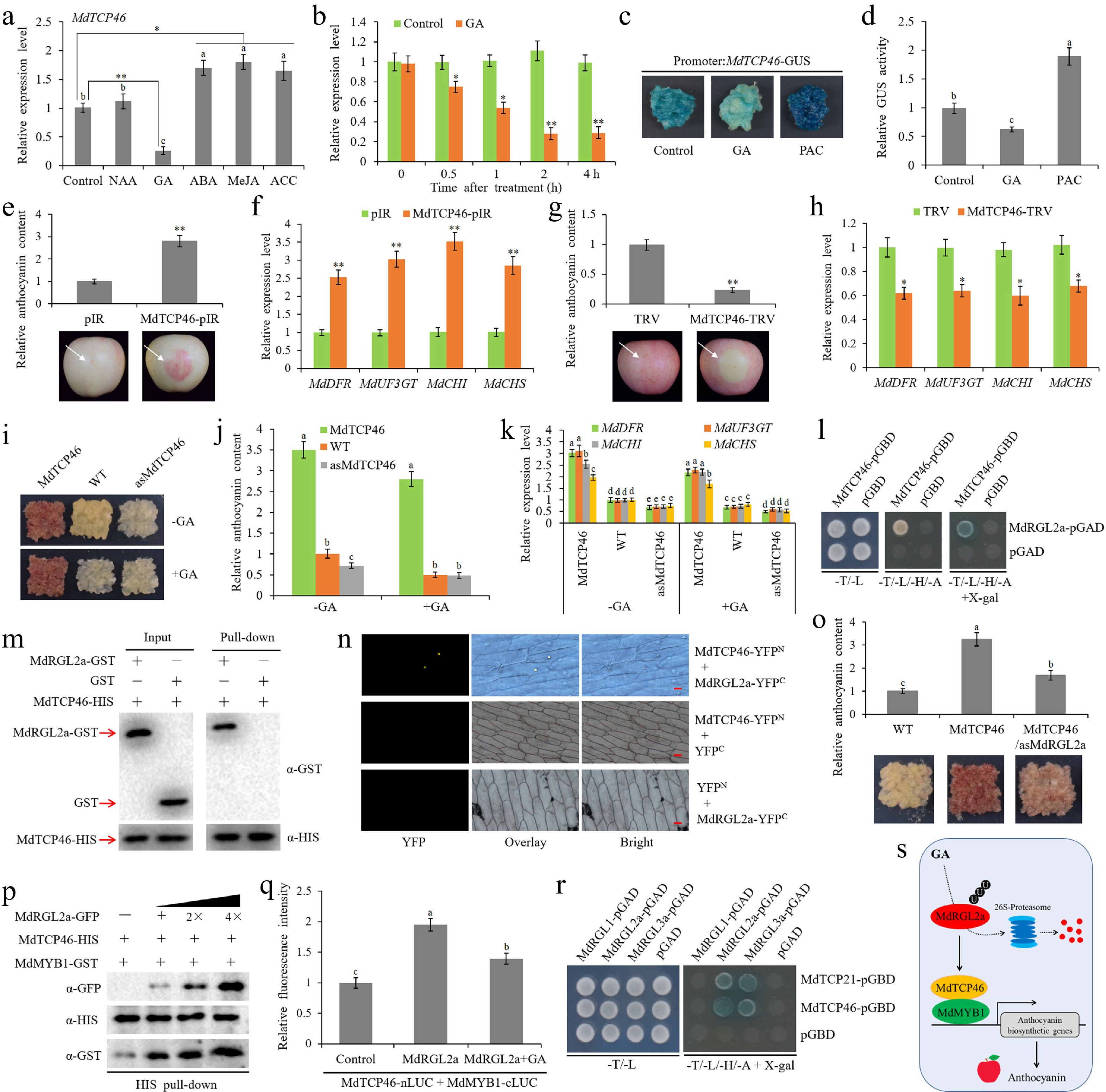
Figure 1.
The MdRGL2a-MdTCP46-MdMYB1 module mediates anthocyanin biosynthesis in response to GA. (a) Expression of MdTCP46 after treatment with 10 μM of 1-naphthaleneacetic acid (NAA), gibberellin (GA), abscisic acid (ABA), methyl jasmonate (MeJA), l-aminocyclopropane-l-carboxylic acid (ACC) for 4 h using qRT-PCR. The value of control without any treatment was set to 1. (b) Expression of MdTCP46 in response to 10 μM of GA3 (GA) for 4 h. The value of control before treatment was set to 1. (c) Phenotypes of transgenic apple callus carrying the MdTCP46 promoter sequence fusion with the GUS tag after GUS staining for 1 h. GA, apple callus treated with 10 μM of GA; PAC, apple callus treated with 2 μM of paclobutrazol (PAC); apple callus without any treatment was used as control. (d) Determination of the GUS activity of apple callus shown in (c). The value of control was set to 1. (e), (f) Functional identification of MdTCP46 using overexpression assays in apple fruits. The anthocyanin content and anthocyanin biosynthetic gene expression around the infiltration sites were measured 3 d after infiltration. Each treatment was performed in triplicate, and each replicate comprised 8–10 apple fruits. Representative pictures are shown. The anthocyanin content and gene expression level in the sites infiltrated with empty vector were set to 1. pIR, IL60-1 + IL60-2; MdTCP46-pIR, IL60-1 + MdTCP46-IL60-2. (g), (h) Functional identification of MdTCP46 using suppression assays in apple fruits. The anthocyanin content and anthocyanin biosynthetic gene expression around the infiltration sites were measured 6 d after infiltration. Each treatment was performed in triplicate, and each replicate comprised 8–10 apple fruits. Representative pictures are shown. The anthocyanin content and gene expression level in the sites infiltrated with empty vector were set to 1. TRV, TRV1 + TRV2; MdTCP46-TRV, TRV1 + MdTCP46-TRV2. (I-K) Functional identification of MdTCP46 via stable transformation in apple callus. Fifteen-day-old apple callus were transferred to -GA or +GA (10 μM) medium and incubated under constant light at 24 °C for 5 d. Each treatment was performed in triplicate and each replicate contained 3–5 callus masses. WT, wild-type apple callus; MdTCP46, MdTCP46-overexpressing apple callus; asMdTCP46, apple callus with suppression of MdTCP46. The anthocyanin content and anthocyanin biosynthetic gene expression in WT grown on -GA medium were set to 1. (l) Y2H assays showing the interaction between MdTCP46 and MdRGL2a. (m) Pull-down assays. (n) BiFC assays. (o) Validation of synergistic effects of MdRGL2a on MdTCP46-mediated anthocyanin accumulation in apple callus. Fifteen-day-old apple callus were incubated under constant light at 24 °C for 5 d. Each treatment was performed in triplicate and each replicate contained 3–5 callus masses. WT, wild-type apple callus; MdTCP46, MdTCP46-overexpressing apple callus; MdTCP46/asMdRGL2a, overexpression of MdTCP46 and silencing of MdRGL2a. Anthocyanin content in WT was set to 1. (p) Competitive binding assay. The mixture of MdRGL2a-GFP and MdMYB1-GFP was added to immobilized MdTCP46-HIS. The gradient indicates the increasing amount of MdRGL2a-GFP. Western blot analysis was performed with anti-GFP, anti-HIS, and anti-GST antibodies. (q) Luciferase complementation imaging assay in N. benthamiana leaves. Control, co-expression of MdTCP46-nLUC/MdMYB1-cLUC and pXSN-GFP empty vector; MdRGL2a, co-expression of MdTCP46-nLUC/MdMYB1-cLUC and MdRGL2a-pXSN-GFP; MdRGL2a + GA, co-expression of MdTCP46-nLUC/MdMYB1-cLUC and MdRGL2a-pXSN-GFP along with the treatment of 10 μM GA. The fluorescence intensity of the control was set to 1. (r) Y2H assays. (s) A working model of MdTCP46 functioning in GA-mediated anthocyanin biosynthesis. Error bars denote standard deviations. Different lowercase letters indicate significant difference at p < 0.05 based on one-way ANOVA test. Asterisks indicate statistical significance based on t-test. *, p < 0.05; **, p < 0.01.
In previous[7] and current studies (Fig. 1e−k; Supplemental Fig. S1a−S1c), MdTCP46 was verified to positively regulate anthocyanin biosynthesis through both apple fruit transient expression and apple callus stable transformation systems. To investigate the effect of MdTCP46 on GA-inhibited anthocyanin biosynthesis, wild-type and MdTCP46 transgenic apple callus were transferred to the medium containing GA for anthocyanin accumulation assays. GA treatment significantly inhibited anthocyanin accumulation in wild type and transgenic apple callus with MdTCP46 suppressed expression, while GA inhibition effect on anthocyanin biosynthesis was not obvious in MdTCP46 overexpressed callus (Fig. 1i, j), indicating that MdTCP46 negatively regulates the anthocyanin biosynthesis inhibited by GA.
DELLA proteins are core regulators of GA signaling response[8]. Although DELLA proteins do not possess DNA-binding domains, they extend their functions by recruiting interaction partners[9]. In Arabidopsis, DELLAs have been reported to be involved in regulating anthocyanin biosynthesis[4,10]. Apple DELLA protein MdRGL2a positively regulates anthocyanin biosynthesis[11]. Considering the similar roles of MdTCP46 and MdRGL2a in regulating anthocyanin biosynthesis, we hypothesize that there may be a regulatory relationship between them. Then, yeast two-hybrid (Y2H) assay was performed to explore the interaction between MdTCP46 and MdRGL2a. Only the yeast cells expressing both MdTCP46-pGBD and MdRGL2a-pGAD could grow normally on selective medium (Fig. 1l), indicating that MdTCP46 directly interacts with MdRGL2a in yeast cells. Meanwhile, the interaction between MdTCP46 and MdRGL2a was further verified by pull-down and bimolecular fluorescence complementation (BiFC) assays (Fig. 1m, n). To determine the dependence of MdTCP46 on MdRGL2a in regulating anthocyanin biosynthesis, we obtained co-transgenic apple callus with MdTCP46 overexpression and MdRGL2a suppressed expression (Supplemental Fig. S1d). Anthocyanin accumulation assays showed that inhibition of MdRGL2a expression significantly weakened the promoting effect of MdTCP46 on anthocyanin biosynthesis (Fig. 1o).
MdTCP46 promotes anthocyanin accumulation by forming a complex with MdMYB1, a core regulator of anthocyanin biosynthesis[7]. To assess the effect of MdRGL2a on MdTCP46-MdMYB1 interaction, competitive binding and luciferase complementation imaging assays were conducted. For the competitive binding assay, with the addition of the MdRGL2a-GFP protein precipitated from the transgenic apple callus, the interaction between MdTCP46 and MdMYB1 was gradually enhanced (Fig. 1p). Similar results were also found in the luciferase complementation imaging assay, where the addition of MdRGL2a improved the fluorescence activity stimulated by MdTCP46 and MdMYB1 (Fig. 1q). These results indicated that MdRGL2a plays a key role in MdTCP46-induced anthocyanin biosynthesis by improving the interaction between MdTCP46 and MdMYB1. Meanwhile, we also found that GA treatment interfered with the activation of MdRGL2a on MdTCP46-MdMYB1 interaction (Fig. 1q), revealing that GA-RGL2a-TCP46-MYB1 pathway is an important module of anthocyanin biosynthesis regulated by GA.
In addition to MdRGL2a, we found that MdTCP46 also interacted with MdRGL3a (Fig. 1r). In addition, the randomly cloned MdTCP21 was also determined to interact with MdRGL2a and MdRGL3a in yeast cells (Fig. 1r), suggesting that TCP and DELLA proteins may have extensive interactions. Based on previous studies of TCP and DELLA interactions in other species[9,12], we hypothesize that the DELLA-TCP module may be an interface for TCP TFs to integrate GA signaling response with plant growth and development. In previous studies, MdTCP46 was found to respond to different light intensities at transcriptional and post-translational levels[7]. Combined with the current research on TCP in GA signaling response, we believe that TCP46 may be an important component in the coordination of light and GA signals in plants, which needs further exploration.
A model diagram illustrates the role of the RGL2a-TCP46-MYB1 module in GA-mediated anthocyanin biosynthesis (Fig. 1s). DELLA protein RGL2a positively regulates anthocyanin biosynthesis by enhancing the interaction between TCP46 and MYB1. Exposed to GA, RGL2a is ubiquitinated and degraded through the proteasome pathway[11], resulting in the weakened interaction between MdTCP46 and MdMYB1, which leads to the inhibitory regulation of anthocyanin accumulation by GA. Combined with the previous report on RGL2a mediated anthocyanin biosynthesis through RGL2a-WRKY75-MYB1 and RGL2a-MYB308-bHLH3/33 pathways[11], we believe that the RGL2a-TCP46-MYB1 module may be the third elucidated parallel regulatory pathway mediated anthocyanin biosynthesis in apple. In conclusion, the discovery of the RGL2a-TCP46-MYB1 regulatory module not only enriches the transcriptional regulatory network of anthocyanin biosynthesis, but also provides reference for in-depth exploration of GA signaling pathway and the synergistic regulation between GA and light signals.
HTML
This work was financially supported by grants from the Development Plan of the Youth Innovation Team of the Higher Education Institutions in Shandong Province (2022KJ326), Natural Science Foundation of Shandong Province (ZR2022YQ24), and Wuhan Botanical Garden Scientific Research Support Project (E3559901).
-
The authors declare that they have no conflict of interest.
- Supplemental Fig. S1 Identification of transgenic materials.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| You C, Han Y, An J. 2023. The RGL2a-TCP46-MYB1 module regulates GA-mediated anthocyanin biosynthesis in apple. Fruit Research 3:21 doi: 10.48130/FruRes-2023-0021 |













