-
Postmortem darkening (melanosis or black spot) is one of the quality problems discovered in crustaceans during processing out[1]. The polyphenol oxidases (PPOs) enzymes accelerate metabolic processes that culminate in melanin buildup (formed from formerly colorless substances) under the crustacean exoskeleton, which causes the undesirable melanosis[2,3]. Color is one of the most crucial characteristics of any food item, according to Shiekh & Benjakul[4]. Although innocuous, customers find the look of melanosis to be unpleasant. Therefore, although the development of melanosis in crustaceans decreases the overall sensory quality, shelf-life, and ultimately the market value of these items, it does not always signify microbial deterioration[1,5].
The cold (in various forms such as chilled water, ice, refrigeration, or freezing) has long been used to avoid melanosis and is the favored technique of preservation of shrimp since the seafood degradation is strongly tied to the storage temperature[1,6,7]. Nevertheless, due to the PPOs' continued activity during refrigeration (with or without ice) and in thawed food, these preservation techniques are insufficient to totally prevent melanogenesis[1,8]. Because of this, many manufacturers utilize chemical additives, which are added during the capturing and/or processing processes in the industry. Since sulfites are thought to be the most effective way to prevent melanosis in crustaceans, sodium metabisulfite (Na2S2O5 – SMS) is frequently used as the main melanosis prevention strategy, either alone or in combination with other compounds like ascorbic, citric, phosphoric, and ethylenediaminetetraacetic (EDTA) acids[1,9]. However, it has long been advised against using sulfites. When used carelessly, SMS's effects – while effective in the treatment of melanosis – are frequently hazardous to human's health. SMS is a known trigger for allergic responses and asthmatic episodes[10,11].
Production facilities, processors, and researchers have been encouraged to look for alternatives to the usage of these compounds because of restrictive regulation and, in particular, customer demand for healthier goods. Technologies like modified atmosphere packaging (MAP), in which the quantities of ambient air gases are changed to protect product quality, are practical and effective choices that are also safe for human health[1,12,13]. Consumers are familiar with and supportive of the use of natural substances derived from plants, seeds, herbs, and fungi, and extensive research has been conducted on a variety of natural substances, including grape seed extract[2], leucine extract[5], edible enokitake mushroom extract[14], an ergothioneine-rich extract from an edible mushroom[15], green and mulberry tea[16], catechin and ferulic acid[17], and chameleon plant extract[18]. These studies, which were thoroughly explored and reported by Gonçalves & Oliveira[1] in a review paper, were designed to examine the potential of such products as melanosis inhibitors.
The chemical composition and concentration of the phytochemicals in the food affect the antioxidant action's efficacy. According to Melo et al.[19], fruit, especially acerola, can be a good source of natural antioxidants since it has a high antioxidant capacity (i.e., a high ability to sequester the radical DPPH, more than 70%). Along with being abundant in minerals like iron, phosphorus, and calcium, as well as vitamins like niacin, thiamine, riboflavin, and carotenoids, acerola also possesses vitamin C (ascorbic acid) concentration up to 30 times higher than that of an orange and the highest antioxidant potential of any tropical fruit[19]. These factors make it conceivable to consider this fruit as a possible melanosis inhibitor. So, the objective of this study was to investigate the combined effect of acerola fruit (AF) or sodium metabisulfite (SMS) with modified atmosphere packaging (MAP) on the quality of white shrimp (Litopenaeus vannamei) stored under refrigeration.
-
A shrimp farm (Mossoró, RN, Brazil) provided 29 kg of live Pacific white shrimp (Litopenaeus vannamei, gramage of 10 g), which were immediately put into a clean insulated box with flaked ice (1:1) and transported to the Laboratório de Tecnologia e Controle de Qualidade do Pescado (LAPESC/UFERSA). No additives were employed, and there was less than an hour between the time of catch and the commencement of the laboratory studies. The shrimp were cleaned (distilled water), weighed, and divided into 96 individual packets weighing 300 ± 0.9 g each before being refrigerated (4 ± 1 °C).
Acerola extract preparation
-
According to Melo et al.[19], the aqueous acerola extract was prepared considering the fruit's 4,962.05 g·mL−1 total phenolic content (or catechin equivalent). An aliquot of 300g of the concentrated acerola pulp (Nordeste Fruit, Brazil) was diluted in distilled water at a ratio of 1:2 (w/v), followed by filtration, and used in the initial testing in order to find the ideal concentration that did not affect the shrimp's taste and flavor (unpublished data). The highest percentage recommended was 100 g·L−1 (10%). Different acerola extract concentrations were not considered in this study until the antioxidant property was confirmed (possible inclusion in subsequent testing).
Experimental design
-
The experimental groups were separated according to the antimelanosic treatments: 32 untreated samples (Control – C); 32 samples immersed in 1.25% food grade sodium metabisulfite (Na2S2O5 – SMS, BASF Brazil) solution for 10 min (according to Otwell & Marshall[10]), and 32 samples immersed in 10% acerola fruit extract solution (AF). After the immersion baths, samples were drained (5 min) and subdivided: 08 samples of each group (C, SMS, and AF) were packed in polyethylene bags, using a Sulpack® Supervac machine (Sulpack® Brazil) coupled to compressor module and gas mixer for three adjustable gases (Gas Mixer KM20-100, WITT Gás Brasil), in the following modified atmosphere packaging: i) AIR [atmospheric air]; ii) MAP1 [70% N2 : 25% CO2 : 5% O2]; iii) MAP 2 [25% N2 : 70% CO2 : 5% O2]; and iv) VAC [vacuum]. The gas and shrimp ratio of 1:1 (v:w) was used in the AIR, MAP1, and MAP2. Each group formed from the combination of the antimelanosic treatments (C, SMS, and AF) and packaging types (AIR, MAP1, MAP2, and VAC) (Table 1) finished with eight identical samples. The packs were kept under refrigeration (4 ± 1 °C) and on day 0, 3, 6, 9, 12, 15, 18 and 21, a sample was withdrawn from each group. The microbiological (total mesophilic and psychrotrophic count, coagulase-positive staphylococci, and Salmonella sp.), physicochemical (TVB-N, TMA, and pH) and sensory (Quality Index Method - QIM) analyses were performed on each of those days.
Table 1. Treatment combinations of samples submitted to antimelanosic and packaging systems.
Package conditions Control Sodium metabisulphite Acerola fruit Air (Atmospheric air) C-AIR SMS-AIR AF-AIR MAP1 (70% N2 : 25% CO2 : 5% O2) C-MAP1 SMS-MAP1 AF-MAP1 MAP2 (25% N2 : 70% CO2 : 5% O2) C-MAP2 SMS-MAP2 AF-MAP2 Vacuum C-VAC SMS-VAC AF-VAC C-AIR: without immersion bath, packed with atmospheric air; SMS-AIR: immersion in 1.25% sodium metabisulphite (SMS), packed with atmospheric air; AF-AIR: immersion in 10% acerola fruit solution, packed with atmospheric air; C-MAP1: without immersion bath, packed with modified atmosphere1; SMS-MAP1: immersion in 1.25% SMS, packed with modified atmosphere 1; AF-MAP1: immersion in 10% acerola fruit solution, packed with modified atmosphere 1; C-MAP2: without immersion bath, packed with modified atmosphere 2; SMS-MAP2: immersion in 1.25% SMS, packed with modified atmosphere 2; AF-MAP2: immersion in 10% acerola fruit solution, packed with modified atmosphere 2; C-VAC: without immersion bath, packed with vacuum pack; SMS-VAC: immersion in 1.25% SMS, packed with vacuum; AF-VAC: immersion in 10% acerola fruit solution, packed with vacuum. All packages were composed of a Combitherm® film (Wolff Walsrode AG, Germany) 0.115 mm thick and grammage 115.0 g·m−2, with the following gas permeability characteristics: water vapor 2.5 g·m−2·day−1; nitrogen <0.1 cm3·m−2·day−1·bar−1; oxygen 0.8 cm3·m−2·day−1·bar−1; carbon dioxide 0.8 cm3·m−2·day−1·bar−1. Sensorial quality through QIM scheme
-
The purpose of developing the Quality Index Method (QIM) scheme for seafood involves the judge's experience, ability to accurately select changes in the analyzed characteristics, and the search for the most suitable one in which the scoring system is well correlated, i.e., a linear increase in the Quality Index. (QI) with ice storage time, estimating the freshness of seafood. So, the QIM was used to estimate the shrimp shelf-life, and the QIM scheme suggested by Oliveira et al.[20] was followed. Six assessors received training[21] in the use of measuring scales, taste and odor detection, and recognition. They were selected based on their prior training in the application of QIM schemes for other seafood species, as well as their achievement of the criteria of research ethics. They described the changes that were occurring during the 21 d of storage of the fresh whole shrimp under refrigeration (4 ± 1 °C) and choose the appropriate scores for each parameter (Table 2). According to the general recommendations for the design of the test room and testing standards[22], all observations of the shrimp were conducted under uniform settings. The sampling for the physicochemical and microbiological studies followed immediately by the sensory analysis (every 72 h) and the assessors were not informed which group they were analyzing at any point. For each storage day, the Quality Index (QI) was calculated. One of the parameters of the QIM was the melanosis index (MI), which was evaluated every 3 d by the trained panel based on the technique presented by Gómez-Guillén et al.[9]. According to Montero et al.[23], the melanosis appeared as black dots (particularly under the shell of the cephalothorax), which were graded on a visual scale: 0 = missing; 2 = modest (up to 20% of 21 shrimp surface affected); 4 = moderate (20%−40% of shrimp surface affected); 6 = significant (40%−60% of shrimp surface affected); 8 = severe (60%−80% of shrimp surface affected); 10 = extremely heavy (80%−100%). Because there were no photos to clearly illustrate the sensory qualities on each evaluation day, the reader struggled to appreciate how freshness was lost during ice storage. As a result, it appears that the quality parameters used to determine freshness, as well as the usage of images to represent the evolution of freshness losses during storage, were not well understood for the QIM schemes and had to be incorporated in the various published research. As a result, the decision was made to publish all photos (images) taken on each day of the sensory assessment (rather than only the beginning and last days of the study), so that readers might correlate them with the sensory aspects that were being evaluated.
Table 2. QIM scheme developed especially for this research.
Quality
parameterDescription Score Odor Fresh, smooth as seaweed 0 Faint, reminiscent of sea salt 2 Light ammonia sent 4 Heavy ammonia sent, putrid 6 Melanosis Absent 0 Slight, small isolated black spots, occurring in up to 50% of the sample's shrimp 2 Moderate, small isolated black spots, occurring in over 50% of the sample's shrimp 4 Moderate, larger black stains, occurring in up to 50% of the sample's shrimp 6 Heavy, larger black stains, occurring in over 50% of the sample's shrimp 8 Heavy, blackening of the shrimp, occurring in 100% of the sample's shrimp 10 Texture Normal 0 Softened 2 Shell Strongly adhered 0 Mildly adhered 2 Weakly adhered 4 Head Strongly adhered 0 Mildly adhered 2 Weakly adhered 4 Appearance Excellent 0 Great 2 Good 4 Bad 6 Awful 8 Unacceptable 10 Total Quality Index (TQI) 0−36 Adapted from Oliveira et al.[20] . Microbiological and physicochemical analyses
-
Total mesophilic count, total psychrotrophic count, coagulase-positive staphylococci inquiry, and Salmonella sp.[24,25] were all done in accordance with Brazilian standards[26]. Hydrogen ion concentration (pH) was measured using a digital pH meter (Hayonik® Model FTP905, Brazil), the total volatile bases nitrogen (TVB-N) and trimethylamine (TMA) were performed according to Brazil[26]. According to Tarladgis et al.[27], the TBARS assay (for thiobarbituric acid-reactive compounds) was carried out. Each analysis was carried out three times.
Statistical analysis
-
The data distribution was checked for normality using the Shapiro-Wilk normality test before being subjected to One-way ANOVA and the Tukey test to compare means and detect significant differences with p < 0.05. The data was tabulated in MS Excel, statistical tests were run, and graphs were created in SigmaPlot. To develop the QIM method and anticipate the shelf-life of seafood held on ice, the calibration curve must be produced using a simple linear regression (done in SigmaPlot), where the intercept at the y-axis is critical and should be close to zero.
-
The total mesophilic bacterial analysis (Fig 1a) revealed an initial decrease at the beginning of the experiment (0 to 3rd day) due to the difference of the initial shrimp temperature (~10 °C) and the refrigeration temperature (4 ± 1 °C), but from the 3rd day an increase was observed with higher counts on the 6th day of storage (C-AIR) that exceeded the maximum threshold (5.4 log CFU g−1) established by Brazil[26]. Although this value decreased on the 9th day, it continued to increase until the end of storage (Fig 1a). SMS-AIR and AF-AIR reached the maximum value (5.4 log CFU g−1) on the 15th and 18th day, respectively. Significant differences (p < 0.05) among groups were observed along the experiment (until the 12th day) between C-AIR and SMS-AIR or AF-AIR. Fig. 1b presented the psychrotrophic bacteria growth along with the experiment. No counts were observed in any groups at the beginning of the experiment (day 0) demonstrating no initial contamination. However, the counts increased significantly (p < 0.05) along with the experiment possibly due to the exponential (log) phase of bacterial growth[28]. C-AIR, SMS-AIR, and AF-AIR exceeded the maximum threshold of 5.4 log CFU g−1 on the 12th day. Independently, there was no difference (p < 0.05) between the total psychrotrophic count in any of the treatments during the period of analysis (Fig 1b) except on 6th and 9th day. The coagulase-positive staphylococci count, and the Salmonella sp. was negative in all samples throughout all the days of the experiment. These microorganisms are associated with poor cleanliness in facilities and manipulators[29]. In the current investigation, the freshly obtained shrimp were washed before packing and stayed in the refrigerator (5 ± 1 °C) with minimal modification for the next 21 d, which could explain the absence of these bacteria in the tested samples. TVB-N levels were high (~10 mg·N·100g−1) at the start of the experiment and remained stable over the course of the 21-d storage period (Fig. 1c), remaining below the permissible maximum threshold (30 mg·N·100g−1) for fish freshness[30]. Although the SMS-AIR had lower TVB-N values than the other groups, no significant difference (p > 0.05) was found. Sensory rejection occurred at a TVB-N value of 20−21 mg·N·100g−1 determined using the steam-distillation method for farmed shrimp (L. vannamei) maintained under refrigeration[31]. TMA data followed the same pattern, with starting values decreasing during the trial, and 3.95 mg·N·100g−1 being the highest observed value (C-AIR, 18th day) among the samples (Fig. 1c). The pH analysis revealed that all groups began the experiment with values of 6.2, and a significant increase (p < 0.05) was detected on the 3rd day (~7.6) and on the 6th day (~7.9), after which it stabilized (from 7.6 to 8.1) until the end of the experiment (Fig. 1d). After the 6th day, the majority of the samples would be regarded to be outside the limits (7.85) stipulated by Brazilian regulation[32].
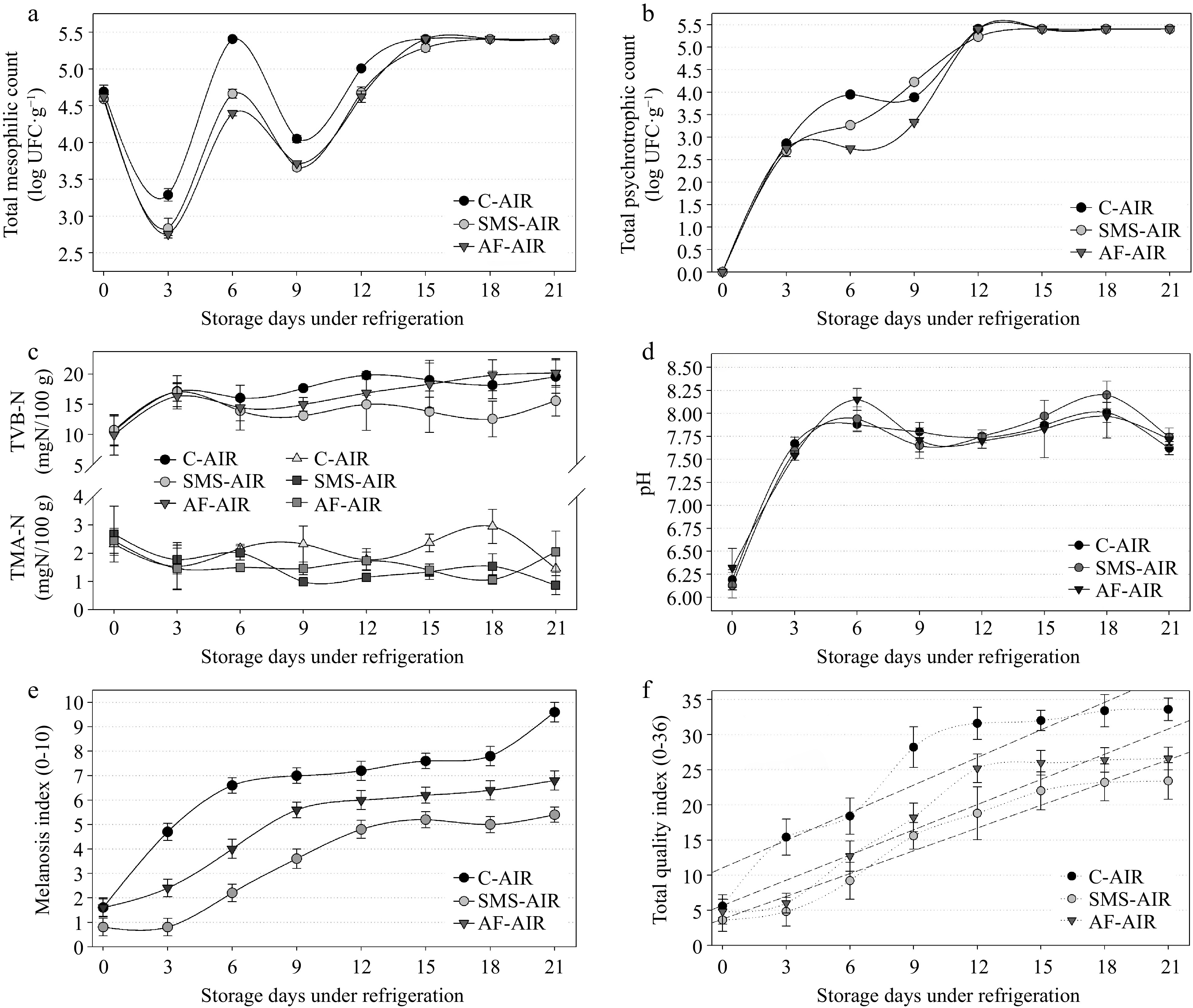
Figure 1.
Results of (a) total mesophilic count, (b) total psychrotrophic count, (c) nitrogen of total volatile bases (TVB-N) and trimethylamine (TMA), (d) pH, (e) melanosis index, (f) total quality index obtained in samples of white shrimp (L. vannamei), packed in atmospheric air, submitted to different antimelanosic treatments, stored for 21 d (4 ± 1 °C). C-AIR: without immersion bath, packed with atmospheric air; SMS-AIR: immersion in 1.25% SMS, packed with atmospheric air; AF-AIR: immersion in 10% acerola fruit solution, packed with atmospheric air; Error bars represent the standard error of three replicates.
All groups had rising melanosis scores over the storage period, with the C-AIR group having the highest melanosis index (MI) (Figs 1e & 2). Although a few samples had melanosis on day zero, most likely due to incorrect processing, and considering that the dark spots were few and small, melanoses began to form in the C-AIR on the 3rd day; in the AF-AIR, melanosis began to increase significantly on the 6th day, and in the SMS-AIR on the 9th day. The other criteria examined showed growing ratings over time as well. These were combined, along with the melanosis score, to produce the Quality Index (or QI), a metric used to assess the shelf-life of sample groups. The initial QI for all groups was similar, and the C-AIR had a considerably greater QI after the 3rd day (p < 0.05). Although the SMS-AIR had lower QI averages during the storage period, there was no difference between this group and the AF-AIR (p < 0.05). However, both groups differed considerably from the C-AIR (higher QI) (Figs 1f & 2). Each sample group's shelf life was predicted using linear regressions. Considering that the greatest index value for shrimp that is still regarded sensorially acceptable is 65% of the maximum QI (i.e., 36 points, Table 2). As a result, scores higher than 23.4 are no longer regarded acceptable. The shelf lives of the C-AIR, SMS-AIR, and AF-AIR stored at 4 ± 1 °C were determined using linear regression models, and the maximum acceptable QI to be 10.7, 14.7, and 13.7 d, respectively (Table 3, Figs 1f & 2).
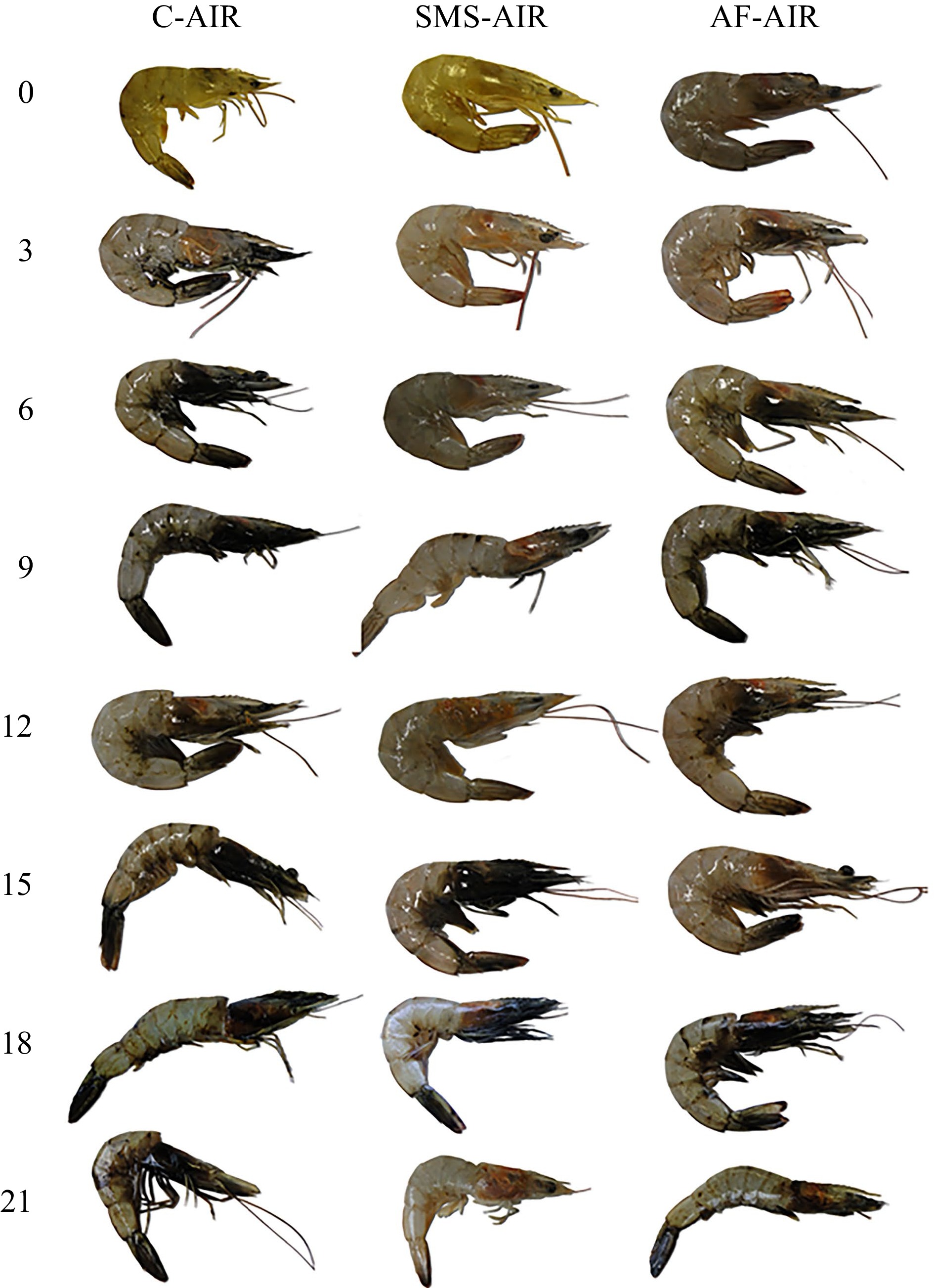
Figure 2.
Photographs of white shrimp (L. vannamei) packed in atmospheric air, submitted to different antimelanosic agents, and stored for 21 d (4 ± 1 °C). C-AIR: without immersion bath, packed with atmospheric air; SMS-AIR: immersion in 1.25% SMS, packed with atmospheric air; AF-AIR: immersion in 10% acerola fruit solution, packed with atmospheric air (photographs were taken after the shrimp were removed from their packaging for sensory evaluation).
Table 3. Results from the linear regression and estimated shelf life after 21 d of storage (4 ± 1°C).
Groups Linear regression model r2 Shelf life (d) C-AIR y = 10.67 + 1.1890 x 0.8795 10.7 (See Fig. 1e) SMS-AIR y = 3.30 + 1.3670 x 0.8497 14.7 AF-AIR y = 5.70 + 1.2860 x 0.9456 13.7 C-AIR y = 10.67 + 1.1890 x 0.8795 10.7 (See Fig. 3e) C-MAP1 y = 9.50 + 1.1330 x 0.9184 12.3 C-MAP2 y = 11.63 + 0.9110 x 0.8403 12.9 C-VAC y = 9.53 + 1.0250 x 0.8796 13.5 SMS-AIR y = 3.30 + 1.3670 x 0.8497 14.7 (See Fig. 5e) SMS-MAP1 y = 6.30 + 1.1095 x 0.7624 15.4 SMS-MAP2 y = 2.83 + 1.2310 x 0.8200 16.7 SMS-VAC y = 1.23 + 1.1970 x 0.9500 18.5 AF-AIR y = 5.70 + 1.2860 x 0.9456 13.7 (See Fig. 7e) AF-MAP1 y = 7.17 + 1.1510 x 0.9317 14.1 AF-MAP2 y = 6.84 + 1.1476 x 0.9035 14.4 AF-VAC y = 3.67 + 1.3420 x 0.8879 14.7 y = maximum acceptable TQI (65% of total, i.e., 23.4); x = refrigerated days; r2 = coefficient of determination. Influence of the packaging method (without antimelanosic agents) on the quality of white shrimp
-
Throughout the trial, we observed microbial growth independent of packing style, and bacterial growth rates in the C-AIR were significantly higher than and distinct from the other groups. However, the mesophilic bacteria count for C-MAP1, C-MAP2, and C-VAC remained steady and < 5.4 log CFU g−1[26] throughout storage. When compared to the other groups, C-MAP2 grew at a slower rate, which was expected given that it had the highest concentration of CO2 (70%), a gas with documented bacteriostatic action. Throughout the storage, the C-MAP1 and C-VAC groups had identical findings, i.e., there was no significant difference (p < 0.05) between the means of bacterial counts performed during the experiment (Fig. 3a). These findings imply that when compared to control (C-AIR) groups, an acceptable proportion of gases in the MAP and an adequate application of VAC limit the growth and proliferation of mesophilic bacteria. Fig. 3b shows that psychrotrophic bacteria did not grow on day 0, most likely due to the adaptation period (lag phase) that these microorganisms experience when exposed to low temperatures; however, there was significant growth (p < 0.05) from the 3rd to the 15th day; however, there was no significant difference between C-MAP1 and C-VAC (p > 0.05) on the 3rd, 12th and after the 15th day. Throughout the investigation, all samples tested negative for coagulase-positive staphylococci and Salmonella spp. As previously stated, these results were most likely owing to the freshness of the samples, the fact that they had been washed before packaging, and the fact that they had received little manipulation during processing. The TVB-N readings increased (p < 0.05) on the 3rd day but remained within the freshness limits for total volatile bases (Fig. 3c), which are 30 mg·N·100g−1[30]. The rise might be attributed to a significant increase (p < 0.05) in psychrotrophic bacteria during the same time period. In the current study, the levels of TVB-N decreased and remained constant until the end of the storage period (Fig. 3c), whereas the counts of mesophilic and psychrotrophic bacteria increased (Fig. 3a & b), indicating that the formation of bases volatiles was probably more related to the action of endogenous enzymes. Fig. 3c shows that, regardless of the type of packaging used, TVB-N averages did not differ significantly (p > 0.05) over the 21-day experiment, though they were lower in packs with high CO2 concentrations and showed differences in the microbiological means (Fig. 3a & b). Despite the high values obtained in the mesophilic, psychrotrophic, and TVB-N analyses (Fig 3a−c), there were no significant variations (p < 0.05) in TMA values across groups or between days of analysis. Fig. 3d depicts a rapid rise in pH over the first three days of storage. This parameter remained high and did not differ significantly (p > 0.05) between groups until the end of the experiment, most likely due to the formation of alkaline compounds, such as ammonia and other volatile nitrogenous bases, caused by the deterioration of shrimp tissues by the action of endogenous or bacterial enzymes[11]. The melanosis index (MI) rose during the trial (Figs 3e & 4); and because melanogenesis is an oxidative process, the C-AIR group had the highest final mean value, followed by C-MAP1 and C-MAP2 (they did not differ substantially from each other). It is worth mentioning that these are the groups with the highest oxygen content, and oxygen is present in both its simple (O2) and mixed forms (CO2). The lowest mean was discovered in the C-VAC group, which also had the lowest quantity of O2. The sensory analysis revealed that the overall quality index (QI) increased during storage (p < 0.05) (Figs 3f & 4). Although there was no difference (p > 0.05) between the C-MAP1 and C-MAP2 groups, as expected, the C-VAC samples had lower values (significantly different) until the 9th day, after which it did not differ significantly from the other groups (C-MAP1 and C-MAP2). The C-AIR was significantly different from the other groups and had the highest final average, with a QI of 23.2 at the end of the experiment. Using the linear regression equation (Table 3, Figs 3f & 4) it was possible to anticipate that the shelf-life of group C-AIR was 10,7 days. The C-MAP1 and C-MAP2 groups obtained nearly identical results of 12.3 and 12.9, respectively, indicating that the difference in the measured concentrations of CO2 and N2 gases was inadequate or insufficient to improve shelf-life. The C-VAC group had the longest shelf-life (13.5), most likely due to bacterial growth suppression or undesired oxidative reactions caused by vacuum packaging, which corroborated the results of microbiological and physicochemical investigations. The QIM results support the microbiological and physicochemical findings, primarily from the 12th or 15th day of storage (Fig. 4).
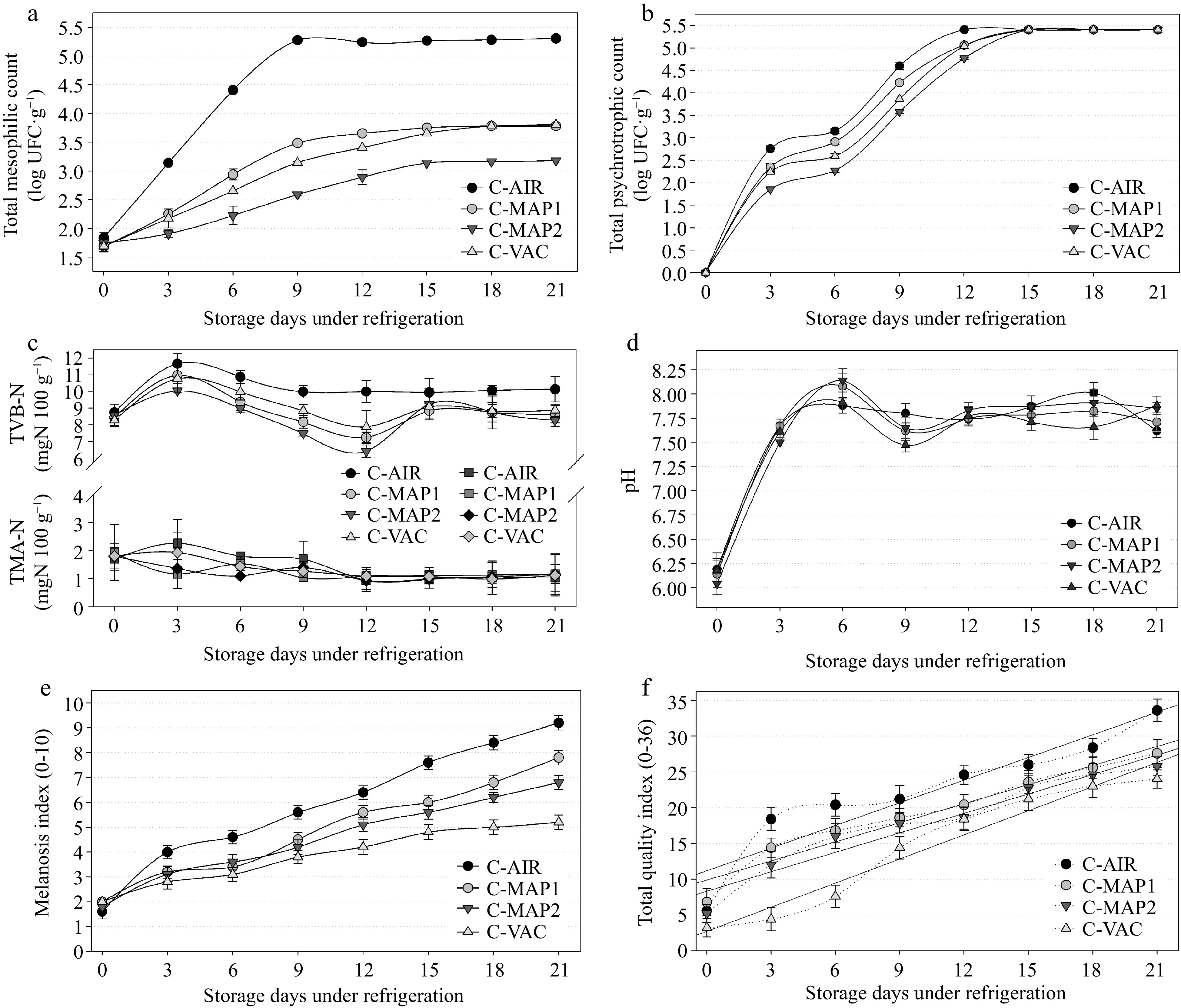
Figure 3.
Results of (a) total mesophilic count, (b) total psychrotrophic count, (c) nitrogen of total volatile bases (TVB-N) and trimethylamine (TMA), (d) pH, (e) melanosis index, (f) total quality index obtained in samples of white shrimp (L. vannamei), without different antimelanosic treatments, stored for 21 d (4 ± 1 °C). C-AIR: without immersion bath, packed with atmospheric air; C-MAP1: without immersion bath, packed with modified atmosphere1; C-MAP2: without immersion bath, packed with modified atmosphere 2; C-VAC: without immersion bath, packed with vacuum pack; Error bars represent the standard error of three replicates.

Figure 4.
Photographs of white shrimp (L. vannamei) without different antimelanosic treatments, stored for 21 d (4 ± 1 °C). C-AIR: without immersion bath, packed with atmospheric air; C-MAP1: without immersion bath, packed with modified atmosphere1; C-MAP2: without immersion bath, packed with modified atmosphere 2; C-VAC: without immersion bath, packed with vacuum pack (photographs were taken after the shrimp were removed from their packaging for sensory evaluation).
The combined effect of antimelanosic treatment (SMS or AF) with different packaging methods (MAP and VAC) on the quality of white shrimp
-
The combined effect of immersion in SMS or acerola extract linked with MAP was examined by comparing the SMS-AIR, SMS-MAP1, SMS-MAP2, and SMS-VAC groups (Figs 5 & 6), as well as the AF-AIR, AF-MAP1, AF-MAP2, and SMS-VAC groups (Figs 7 & 8). The total counts of mesophilic bacteria revealed that only the AF-AIR group attained the limit of 5.4 log CFU g−1 by day 12 (Fig. 7a), while the SMS-AIR group did so on day 18 (Fig. 5a). The other groups maintained consistent bacterial growth throughout the storage period, which was more obvious in the SMS-treated groups (Fig. 5a). There was no significant difference (p > 0.05) between the samples treated with SMS and packaged in different systems (Fig. 5a), but their findings were higher than those observed for the groups treated with acerola, AF-VAC, and AF-MAP2 (Fig. 7a). Psychrotrophic bacteria growth was not observed in any of the sample groups at the start of the experiment (day zero), but there was a significant increase (p < 0.05) throughout the storage time and between treatments (SMS and AF) until day 15, when bacterial growth stabilized and there was no further significant difference between treatments (p > 0.05). The means in the two treatments (SMS and AF) were fairly comparable, and in the SMS treatment, MAP2 had lower psychrotrophic numbers, followed by MAP1 and VAC (Fig. 5b). For the AF treatment, the MAP2 group showed lower counts than the VAC and MAP1 groups (Fig. 7b). When compared to the SMS groups, the samples with AF treatment performed better (reduced microbial growth). Throughout the trial, the search for Salmonella sp. and coagulase-positive staphylococci yielded negative findings in all samples. These outcomes have already been mentioned in prior items and most likely occurred because samples were fresh and handled hygienically. TVB-N results (Fig. 5c) showed mean initial values of 9.4 mg·N·100g−1 (day zero) for all groups, and began to significantly increase by the 3rd day, coinciding with the increase of psychrotrophic bacteria (Fig. 5b) in all treated groups, followed by a decrease in growth rate and stabilization beginning on day 6. SMS-AIR had higher TVB-N values, followed by SMS-VAC, but SMS-MAP1 and SMS-MAP2 remained significantly equal (p < 0.05) until the completion of the experiment (values ranging from 7.64 to 9.96 mg·N·100g−1). Despite the proliferation of mesophilic and psychrotrophic bacteria, these findings indicate freshness stability because they stayed below the maximum threshold of 30 mg·N·100g−1 advised in the literature[30]. The estimated shelf-life (Table 3) of 14 to 20 days (SMS) and 12 to 14 d (AF) verifies this assumption because the shelf-life of the groups did not reach as long even with low TVB-N levels up to 21 d. During the 21-d experiment, the TMA levels for both treatments (SMS and AF) showed the similar trends. Samples with SMS treatment (Fig. 5c) had values ranging from 3.56 (initial) to 2.05 (21st day) mg·N·100g−1, while samples with AF treatment (Fig. 7c) had values ranging from 3.18 (initial) to 2.44 (21st day) mg·N·100g−1. The AF-AIR group had higher means (3.73 and 3.30 mg·N·100g−1) while the other treatments had no significant difference and stayed close to 2.15 mg·N·100g−1. TMA values for the AF samples were generally greater than those for the SMS groups, albeit the difference was modest and non-significant (p < 0.05). On the first day of analysis (day zero), the pH of all groups was 6.19 for SMS groups (Fig. 5d) and 6.22 for AF groups (Fig. 7d). These values are more acidic than usual, most likely due to the post-mortem production of acidic chemicals in shrimp muscle[30]. The pH of the SMS-treated groups increased significantly beginning on the 3rd day (average of 7.53 on the 3rd day, and 8.02 on the 6th day), but did not differ significantly across groups and remained in that pH range until the 21st day. From the 3rd day, the pH in the AF-treated groups increased dramatically, with averages of 3.36 (AF-MAP2), 7.61 (AF-MAP1 and AF-VAC), and 7.95 (AF-AIR), maintaining in this range with more variability between groups until the 21st day. Overall, the mean pH of all groups (SMS and AF) on the 3rd day was 7.63 ± 0.09, demonstrating that the shrimp retained fresh qualities. Interestingly, when the two treatments (SMS and AF) were compared, the first influenced little the pH in combination with the different packaging atmospheres - there was no significant difference between the packages for each day (Fig. 5d), but when the latter (AF) was observed in combination with the different packaging, different results were observed - higher pH variability between packages for each day (Fig. 7d). The melanosis index reported in groups subjected to SMS solution treatment (Figs 5e & 6) was lower than the index identified in samples subjected to AF solution treatment (Fig. 7e). Melanosis was present in certain shrimp on the first day (MI~1.0), stayed steady until the 6th day, then grew until the 21st day of storage (MI~6.5 - SMS-AIR; MI~4.5 - other groups). The control group (SMS-AIR) was significantly different from the 6th day, with a quicker and nearly linear increase from the 9th day (Fig. 5e). The other groups did not differ significantly from one another. There was, however, an increase in melanosis, where was evident in the AF-treated groups (Fig. 7e) on the 1st day (MI~1.8) and increased up to the 21st day of storage (MI~8.0 - AF-AIR; MI~4.9 - other groups). These findings support SMS's antimelanosic activity. However, it should be noted that the acerola fruit solution was prepared by simple dilution, there was no type of purification, concentration, or separation of the fruit's different molecular compounds, and the final mean results were very similar to the SMS groups (MI~4.5 - SMS, MI~4.9 - AF). In the current study, the vacuum-packed groups achieved the lower MI (average) value, whereas the higher values (excluding the control) were found on most days in the samples packed in MAP1 [70% N2 : 25% CO2 : 5% O2], most likely due to O2 concentration in the atmosphere. In terms of QI, samples treated with SMS had lower mean scores during the storage period (Figs 5f & 6) than samples treated with AF (Figs 7e & 8). This was expected given the extremely comparable results for the MI, most likely due to a high link between melanosis and sensory quality of the shrimp, since it effects the overall appearance of the product[9]. Simultaneously, the MI and QI results reflected the estimated shelf-life of the groups (SMS and AF), and samples immersed in acerola solution (AF) (Table 3) retained sensory quality for less time than SMS (Figs 6 & 8). Again, the vacuum packaging groups had the longest shelf-life, with SMS-VAC being suitable for up to 18.5 d and AF-VAC remaining acceptable for up to 14.7 d (Table 3).
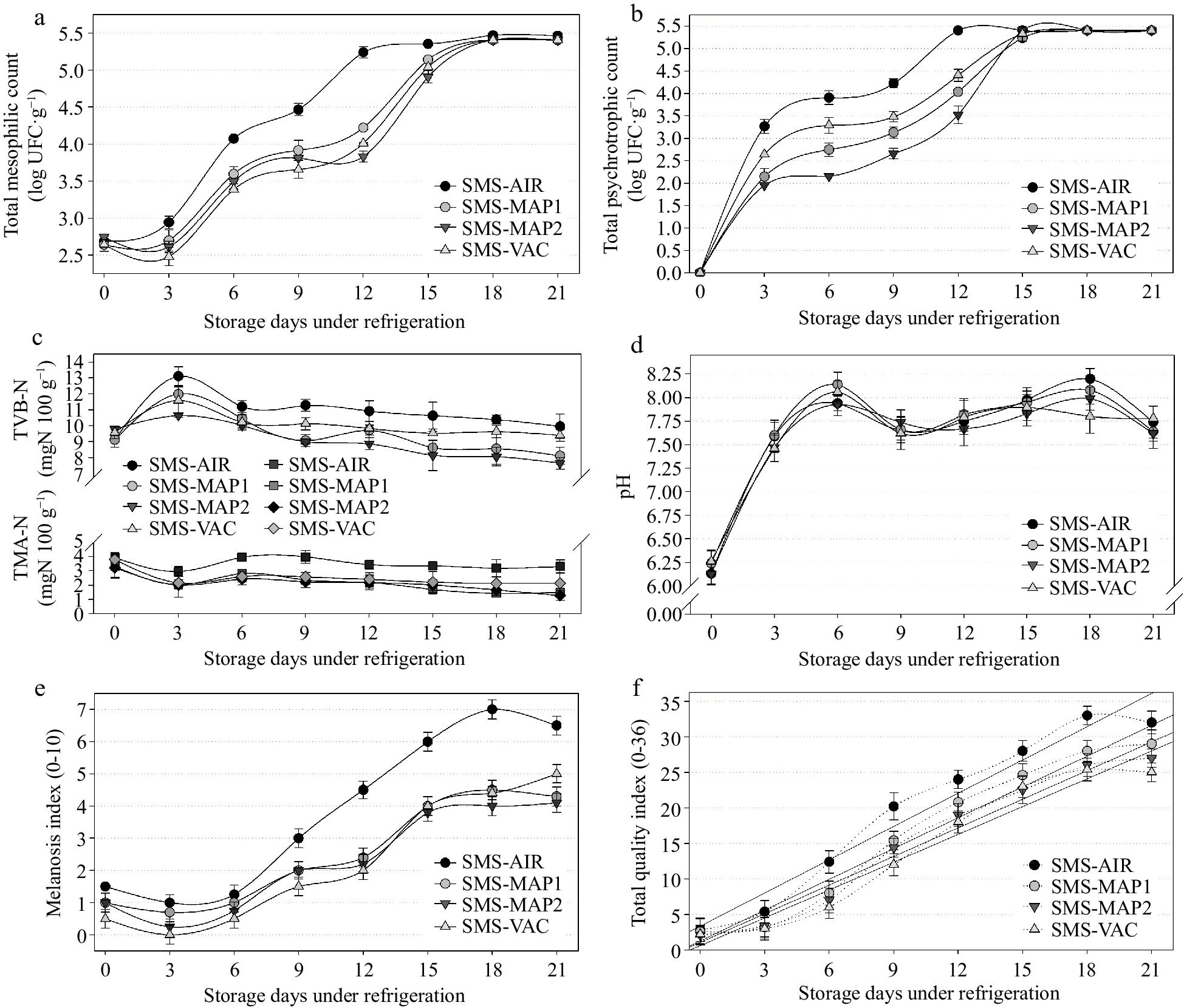
Figure 5.
Results of (a) total mesophilic count, (b) total psychrotrophic count, (c) nitrogen of total volatile bases (TVB-N) and trimethylamine (TMA), (d) pH, (e) melanosis index, (f) total quality index obtained in samples of white shrimp (L. vannamei) submitted to 1.25% sodium metabisulfite (SMS) treatment, packed in different atmospheric air, and stored for 21 d (4 ± 1 °C). SMS-AIR: packed with atmospheric air; SMS-MAP1: packed with modified atmosphere (70% N2 : 25% CO2 : 5% O2); SMS-MAP2: packed with modified atmosphere (25% N2 : 70% CO2 : 5% O2); SMS-VAC: vacuum packed. Error bars represent the standard error of three replicates.

Figure 6.
Photographs of white shrimp (L. vannamei) submitted to 1.25% sodium metabisulfite (SMS) treatment, packed in different atmospheric air, and stored for 21 d (4 ± 1 °C). SMS-AIR: packed with atmospheric air; SMS-MAP1: packed with modified atmosphere (70% N2 : 25% CO2 : 5% O2); SMS-MAP2: packed with modified atmosphere (25% N2 : 70% CO2 : 5% O2); SMS-VAC: vacuum packed. Error bars represent the standard error of three replicates (photographs were taken after the shrimp were removed from their packaging for sensory evaluation).

Figure 7.
Results of (a) total mesophilic count, (b) total psychrotrophic count, (c) nitrogen of total volatile bases (TVB-N) and trimethylamine (TMA), (d) pH, (e) melanosis index, (f) total quality index obtained in samples of white shrimp (L. vannamei) submitted to 10% acerola fruit (AF) treatment, packed in different atmospheric air, and stored for 21 d (4 ± 1 °C). AF-AIR: packed with atmospheric air; AF-MAP1: packed with modified atmosphere (70% N2 : 25% CO2 : 5% O2); AF-MAP2: packed with modified atmosphere (25% N2 : 70% CO2 : 5% O2); AF-VAC: vacuum packed. Error bars represent the standard error of three replicates.

Figure 8.
Photographs of white shrimp (L. vannamei) submitted to 10% acerola fruit (AF) treatment, packed in different atmospheric air, and stored for 21 d (4 ± 1 °C). AF-AIR: packed with atmospheric air; AF-MAP1: packed with modified atmosphere (70% N2 : 25% CO2 : 5% O2); AF-MAP2: packed with modified atmosphere (25% N2 : 70% CO2 : 5% O2); AF-VAC: vacuum packed (photographs were taken after the shrimp were removed from their packaging for sensory evaluation).
-
In the same way as observed in Fig. 1a, samples of pink shrimp (P. longirostris) maintained at low temperature (2 °C, 14 d) without antimelanosic treatments showed total mesophilic bacterial counts exceeding 5.4 log CFU g-1 on the 11th day of the experiment performed by López-Caballero et al.[33]. Aubourg et al.[6] discovered no difference in total mesophilic counts in Norway lobster (N. norvegicus) between the control and 0.5% SMS groups after 9 d of experimentation, with both showing levels slightly greater than 4 log CFU g−1. Nirmal & Benjakul[34] discovered similar results in a study utilizing feluric acid as an organic alternative to sulfites in the inhibition of melanosis. On the 10th day of storage, they discovered levels of 4.5 and 4.2 log CFU g−1 in samples of whole shrimp (L. vannamei) treated for 15 min at 4 °C with a 1 and 2% solution of feluric acid, respectively. Other researchers, on the other hand, did not find identical results to those discovered in this study, despite the fact that their scores were higher and/or reached such values earlier. Martinez-Álvarez et al.[35] discovered that Norwegian lobsters (N. norvegicus) samples treated with 6% (v/v) commercial sulfite-based products and also containing sodium ascorbate, citric acid, and EDTA obtained counts of 5.27 log CFU g−1 on the 7th day of storage at 4 °C. Lu[36] discovered that total aerobic counts in both whole and headless Chinese shrimp (F. chinensis) samples packed in atmospheric air and without antimelanosic treatment exceeded 5.5 log CFU g−1 as early as the 5th day of storage at 21 °C. Mastromatteo et al.[37] used thymol essential oil as an alternative to traditional sulfite-based antimelanosic treatments, but by the 7th day of the experiment, peeled shrimp (P. serratus) immersed in aqueous solutions containing varying concentrations of thymol (500 to 1500 ppm) had counts greater than 7 log CFU g−1. Other researchers have used green tea extract to inhibit melanose in white shrimp (L. vannamei) on two occasions: during ice storage[16], where all samples showed counts greater than 5 log CFU g−1 on the 12th day of experiment; and during refrigerated storage at 4 °C associated with MAP[38], where control group samples and the groups treated with green tea extract and MAP obtained counts greater than 5.97 and 3.98 log CFU g−1, respectively, from the 10th day. This same research group used catechin extracted from teas to treat white shrimp (L. vannamei) and found total counts of psychrotrophic bacteria of 5.17, 5.13, 4.81, and 4.61 log CFU g−1 on the 10th day of storage in the control, SMS, 0.05%, and 0.1% catechin solution groups, respectively[38]. Dong et al.[29] investigated the usage of active packaging impregnated with rosemary and cinnamon essential oils and discovered that the psychrotrophic bacteria count was approximately 6.2 log CFU g−1 after 10 d of storage at 4 °C. The high TVB-N values (Fig. 1c) corresponded with the higher initial mesophilic bacterial counts and were likely maintained at higher levels due to psychrotrophic bacteria growth (log phase) throughout the first 3 d of storage, as seen in Fig. 1b. Although Nirmal & Benjakul[17] associate TVB-N levels with microorganisms, particularly degrading bacteria, Martínez-Álvarez et al.[39] confirm that these values are more easily related to ammonia synthesis by tissue enzymes than to bacterial action. Martínez-Álvarez et al.[39] employed a volatile base measurement method similar to the one used in this investigation to assess TVB-N in pink shrimp (P. longirostris) subjected to various cooking methods and antimelanosic treatments. On the 23rd day of the trial, their results exceeded the permitted limit, with the groups treated with sulfite-based formulae having the highest averages. Similarly, when treated with metabisulfite, Norway lobsters (N. norvegicus) held in crushed ice and examined by steam distillation exhibited a considerable increase in the synthesis of TVB-N[6]. Chinese shrimps (F. chinensis) treated with ozonated water and bactericidal chemicals required 13 and 17 d, respectively, to surpass the limit of 30 mg·N·100g−1[36]. López-Caballero et al.[40] packed the pink shrimp (P. longirostris) in different atmospheres and used the modified Conway micro diffusion method to estimate TVB-N values; by the 7th day of storage, all samples had already surpassed the limit. Bono et al.[31] treated and packaged the pink shrimp (P. longirostris) on board and stored it at −18 °C for 12 months, but the results show that all samples received TVB-N levels more than 30 mg·N·100g−1 within three days of capture. According to Don et al.[41], the TMA acceptable limit is 3 mg·N·100g−1. Our findings are consistent with those found in Norway lobsters (N. norvegicus) held on slurry ice with or without SMS[6] and thawed pink shrimp (P. longirostris) exposed to 4-hydroxylresorcinol or sulfites solutions[33]. Mejlholm et al.[42] found no TMA formation in any of the shrimp (Pandalus borealis) samples tested using MAP, even when they were considered sensorially injured. TMA was similarly not detected in samples of white shrimp (L. vannamei) treated with feluric acid by Nirmal & Benjakul[34]. However, López-Caballero et al.[40] discovered that TMA exceeded 10 mg·N·100g−1 before the 7th day of storage in pink shrimp (P. longirostris) packed in MAP. The increase in pH observed on the 3rd day was most likely due to the formation of basic compounds by bacterial activity or endogenous enzymes, which was aided by the fact that crustaceans naturally have high levels of non-protein nitrogen[40], which corroborated the increase in TVB-N observed in the first three days of the experiment (from 10 to 17 mg·N·100g−1). Similar results were observed in pink shrimp (P. longirostris) packed in different atmospheres[43]; in Norwegian lobsters (N. norvegicus) stored in slurry ice and treated with SMS[6]; in shrimp (P. serratus) treated with thymol essential oil[37]; in white shrimp (L. vannamei) treated with catechin[35]; and in white shrimp (L. vannamei) treated with chameleon plant (H. cordata) extract[18]. Similar melanosis outcomes were seen in white shrimp (L. vannamei) treated with tea extracts[16,38], frozen pink shrimp (P. longirostris) treated with non-organic antimelanosic agents[33], and Norway lobsters (N. norvegicus) treated with sulfite powder[35]. Nirmal & Benjakul[44] discovered that melanosis scores in samples of white shrimp (L. vannamei) and samples treated with sodium metabisulfite, as well as control samples, increased significantly after the 4th day. According to Gonçalves et al.[43], after 4 d of ice storage, samples of pink shrimp (P. longirostris) from the control group showed evidence of melanosis in the cephalothorax. Oliveira et al.[20] determined that the maximum permissible QI in whole white shrimp (L. vannamei) maintained on ice was 60%, and their samples achieved that level on the 12th day of the experiment. White shrimp (L. vannamei) packaged in modified environment had shelf lives of 15 and 10 d, respectively[38]. Furthermore, even when treated with sodium metabisulfite, Norway lobsters (N. norvegicus) maintained on crushed ice were only sensorially acceptable until the 5th day of analysis[6], and pink shrimp (P. longirostris) held on ice became undesirable after 7 days[43]. Gonçalves & Santos[45] discovered that the control group (white shrimp (L. vannamei) packed in air) had a predicted shelf-life of 7.57 d, the shrimp group treated with chlorine and packed in MAP (100% CO2) had a predicted shelf-life of 11.45 d, and the shrimp group treated with ozone and packed in MAP (100% CO2) had a predicted shelf-life of 24.18 d.
Influence of the packaging method (without antimelanosic agents) on the quality of white shrimp
-
When López-Caballero et al.[40] studied pink shrimp (P. longirostris) packed with [3 N2 : 4 CO2 : 3 O2] or [5 N2 : 4,5 CO2 : 0,5 O2], they found that the total bacterial count was lower in samples treated with MAP compared to those in the control group, but by the 9th day of storage, all groups had exceeded the 5 log CFU g−1 threshold. After the 9th day of storage, samples of Chinese shrimp (F. chinensis) treated with bactericidal chemicals for 10 min and stored in packages of [3 N2 : 4 CO2 : 3 O2] or [100% CO2] modified atmosphere[36] showed counts more than 5.5 log UFC g−1. However, the fact that the C-VAC had the highest average bacterial count when compared to C-MAP2 and similar to C-MAP1 was unexpected, because authors report that the use of this type of packaging extends the shelf-life of the products and inhibits the growth of aerobic bacteria[11], so the high counts were most likely caused by anaerobic characteristics in the microorganisms present in the samples, their initial bacterial load, or problems with the vacuum packaging (Fig. 3a). Boziaris et al.[28] discovered a 24-h lag phase in samples of Norway lobsters (N. norvegicus) kept at 5 °C. Nirmal & Benjakul[38] discovered that samples stored in MAP [4.5% N2 : 5% CO2 : 0.5% O2] had lower counts of psychrotrophic bacteria than control samples, and only the control group exceeded the value of 5 log CFU g−1 during the study period, which they attributed to the CO2 concentrations in the packages. Psychrotrophic counts were fewer than 4 log CFU g−1 during a 12-d experiment[13] utilizing whole and headless L. vannamei that had been subjected to antimelanosic treatment (4-HR and pyrophosphate), packaged in MAP [10% N2 : 80% CO2 : 10% O2] and stored at 4 °C. Gonçalves & Santos[45] discovered higher counts of mesophilic bacteria in the first days of cold storage (4 °C) of white shrimp (L. vannamei) in [100% CO2] MAP (day 0 was 9.3 log CFU g−1 and day 3 was 9.2 log CFU g−1) but the counts decreased significantly toward the end of the experiment, with the total mesophilic count standing at 1.37 log CFU g−1 on day 12. When these findings are compared to the current study, it is feasible to conclude that the average counts were lower, most likely due to the higher CO2 content of the packages. Three days following capture, samples of Norway lobster (N. norvegicus) packed in a vacuum or in the MAP with [100% N2] or [5% N2 : 5% CO2] revealed high TVB-N values ranging from 33.5 to 36.5 mg·N·100g−1, according to Bono et al.[31]. The results obtained by Lu[36] for Chinese shrimps (F. chinensis) using the steam distillation method (similar to the one used in the current experiment) demonstrated that the group of headless shrimp samples packed in air exceeded the limit of TVB-N on the 13th day, but all other sample groups reached this value either on the 17th or on the last day of the experiment, and the group that had the heads removed and was submitted to the MAP with [3% N2 : 4% CO2 : 3% O2] was the one with the lowest average on this day. Mejlholm et al.[42] employed the modified Conway micro diffusion method to assess TVB-N and TMA in cooked, shelled, and packaged shrimp (P. borealis) under MAP [3% N2 : 5% CO2 : 2% O2] and found no TMA production even when the shrimp became sensory unsatisfactory. The current results were higher than those obtained by Thepnuan et al.[13] for shrimp subjected to MAP [10% N2 : 80% CO2 : 10% O2] or [20% N2 : 80% CO2], which found values ranging from 0 to 1 mg·N·100g−1. According to these experts, low levels of TMA may be linked to CO2's bacteriostatic effects. Our results, however, were similar to those obtained by Gonçalves and Santos[45] in white shrimp (L. vannamei) packed in [100% CO2], who reported averages of 1.16 mg·N·100g−1 on the first day of the trial and 3.14 mg·N·100g−1 on the 12th (final) day. Mejlholm et al.[42] packed shrimp (P. borealis) in [3% N2: 5% CO2: 2% O2] and stored it at 5°C, finding identical pH (7.8) values. Pink shrimp (P. longirostris) samples[43] packed in [3% N2 : 4% CO2 : 3% O2] or [5% N2 : 4,5% CO2 : 0,5% O2] showed an increase in pH values up to the 4th day of storage and then stabilized until the end of the analyses, with pH varying between 7.08 and 7.6; in the same study, atmospheric air samples obtained slightly higher pH values, ranging between 7.08 and 7.83. Gonçalves et al.[43] also found lower melanosis scores in groups of pink shrimps (P. longirostris) exposed to changed atmospheres [30% N2 : 40% CO2 : 30% O2] or [50% N2 : 45% CO2 : 5% O2], and as expected, melanosis was more visible in groups with higher oxygen concentrations. Similarly, Thepnuan et al.[13] found that L. vannamei packed in MAP with [20% N2 : 80% CO2] had lower melanosis scores than L. vannamei packed in MAP with [80% CO2 : 20% O2]. Nirmal & Benjakul[34] discovered comparable results in the sensory examination of L. vannamei, namely that on the 10th day of storage at 4 °C, both control samples and those packed in MAP [45% N2 : 50% CO2 : 5% O2] obtained unacceptable scores. Lu[36] concluded that Chinese shrimp (F. chinensis) subjected to [30% N2 : 40% CO2 : 30% O2] or [100% CO2] had higher acceptability scores than those of the group packaged in atmospheric air that had exceeded bacterial growth limits on the 17th d of analysis, whereas those subjected to MAP reached such counts on the 21st d, which is to be expected given that samples had been treated with ozone or bactericides. Rutherford et al.[46] demonstrated that samples of ready-to-eat shrimp stored in vacuum packaging at 3 and 7 °C were not considered spoiled even after 15 d, most likely due to the processing that such samples were subjected to (i.e., the prawns were cooked, peeled, gutted, and passed through rapid freezing). Mastromatteo et al.[37] discovered that peeled shrimp (P. serratus) treated with thymol essential oil (1,000 ppm) and MAP [95% CO2 : 5% O2] had a shelf-life of 14.37 d, while samples without the thymol essential oil treatment but packed in MAP had a shelf-life of 9.87 d and samples with only the thymol treatment had a shelf-life of 5.09 d. In a study using pomegranate peel extract on white shrimp (L. vannamei) stored on ice for 10 d, Fang et al.[47] discovered that, while the effect of treatment at 15 g·L-1 on melanosis inhibition and overall shrimp quality improvement was good, it still underperformed when compared to treatment with 12.5 g·L−1 of SMS.
The combined effect of antimelanosic treatment (SMS or AF) with different packaging methods (MAP and VAC) on the quality of white shrimp
-
On the 10th day of storage at 4 °C, researchers[48] using the same shrimp species (L. vannamei) immersed in cinnamaldehyde solution (1 or 5 g·kg−1) obtained mesophilic bacteria counts higher than 6.21 and 4.54 log CFU g−1, respectively, demonstrating that there may have been an antibacterial effect, even if small, in the groups treated with SMS and acerola, since even the samples packaged in air reached lower count values after a longer period of time. The same can be said for Noordin et al.[7], who studied the effects of essential oils (cinnamon, garlic, and lime) and organic acids (lactic acid, tartaric acid, and sodium diacetate) in tiger shrimp (Penaeus monodon) at 4 °C for 10 d and discovered that the combination of cinnamon oil and tartaric acid showed the highest antibacterial activity against aerobic organisms, presenting counts of 6.65 log CFU g−1, which is still higher than the counts found in our study. Mastromatteo et al.[37] used peeled shrimp (P. serratus) treated with thymol oil packed in MAP [95% CO2 : 5% O2] and kept at 4 °C and found that the mesophilic bacteria count reached 5 log CFU g−1 on the 16th day of the experiment, which they attribute to the high carbon dioxide concentration in their packages. The L. vannamei stored in ice and treated with 0.1% green tea extract or 0.1% green tea extract + ascorbic acid had total psychrotrophic counts of 4.26 and 4.13 log CFU g−1 on the 12th day of storage, respectively; both were lower than the counts of samples treated with SMS (5.89 log CFU g−1) in the same experiment[49]. Another study involving L. vannamei treated with ethanolic extract of green tea (with chlorophyll removed a priori) revealed that samples exposed to 5 and 10 g·L−1 of the extract had total psychrotrophic counts below 5 log CFU g−1 on the 10th day of ice storage, whereas samples exposed to SMS had counts above this value[16]. On the 4th day of storage at 4 °C, the same researchers[17] recorded total psychrotrophic counts of 4.56 and 3.93 log CFU g−1, respectively, when studying the influence of catechin (0.2%) and feluric acid (3%) on melanosis and overall quality of shrimp (L. vannamei). Some investigations comparing white shrimp (L. vannamei) treated with SMS to those treated with feluric acid[34], catechin[44], or green tea extract[49] found that while TVB-N values were higher in the SMS groups, the efficiency in controlling freshness was present. TVB-N levels were lower in the current investigation when samples were treated with AF (Fig. 7c) versus SMS (Fig. 5c). These findings support the findings of Sivertsvik et al.[10], who found that TVB-N levels in fish packed in CO2-modified atmospheres were lower than the maximum limits even after sensory analysis indicated deterioration. In contrast to the results shown in Fig. 5c, Nirmal & Benjakul[34] found that SMS-treated shrimp (L. vannamei) had 0.14 mg·N·100g−1 after 6 days of ice storage and that no TMA production occurred in samples treated with 1% or 2% feluric acid until the completion of the trial (10th day). Mejlholm et al.[42] found no TMA production in cooked, peeled P. borealis packed in [30% N2 : 50% CO2 : 20% O2] before it was deemed sensorially undesirable. Mastromatteo et al.[37] discovered different pH values in peeled shrimp (P. serratus), with the pH of the samples packed in atmospheric air being greater than that of the MAP samples [95% CO2 : 5% O2]. When working with quality changes in L. vannamei treated with chameleon plant extract (E-DC) and kept in cold storage at 2 °C for seven days, Phan et al.[18] discovered that at the end of the experiment, samples treated with water had pH values of 7.96 ± 0.10, samples treated with 1.25% SMS had pH values of 7.49 ± 0.10, and samples treated with 0.025% E-DC had pH values of 7.43 ± 0.20. Such findings are similar to the levels measured for the AF groups (excluding AF-MAP2), but lower than the pH values discovered in this study for the SMS groups. Encarnacion et al.[50] discovered decreased melanosis production in SMS-treated crabs (Chionoecetes japonicus), while there was no significant difference between those, and the samples treated with 1% edible mushroom extract (Flammulina velutipes). Qian et al.[51] studied the effect of varied levels of oxygen in a modified atmosphere on shrimp (L. vannamei) held at 4 ± 1 °C. There was delayed melanosis in the packages with low O2 concentrations [55% N2 : 40% CO2 : 5% O2], whereas at high O2 concentrations [5% N2 : 40% CO2 : 55% O2], melanosis production was expedited, and the sensory quality of the shrimp declined rapidly. Shiekh & Benjakul[4] used pulsed electric field (PEF) treatments on white shrimp (L. vannamei) to assess quality alterations, particularly melanosis development, after 10 d of refrigerated storage (4 °C). They employed the 10-point scale developed by Montero et al.[23] and discovered that at day 10, the control group had a melanosis score of about 10 points, while their highest performing group PEF-T3 (15 kV·cm−1, 600 pulses) had a score of about ~7 points.
-
The results are encouraging and point to an antimelanosic potential in acerola fruit. Regarding the type of packaging that has kept the sensory quality of the white shrimp (L. vannamei) longer, it was possible to observe that vacuum packaging stood out, extending shelf-life more than any other, even though this product has a physical characteristic (the rostrum) that hinders the integrity of the vacuum packaging. The antimelanosic treatments (sodium metabisulphite and acerola fruit) improved quality and extend the shelf-life of white shrimp (L. vannamei) when associated with MAP. Nevertheless, vacuum packaging in combination with SMS and AF showed better results. In most analyses, no significant difference was found between treatments (SMS and AF), indicating that acerola fruit can inhibit melanosis as efficiently as SMS. The estimated shelf-life for shrimp treated with SMS and AF was similar and improved in combination with a MAP. The shelf-life time calculated with the linear regression model obtained through the QI results coincided with the days when the microbiological and physicochemical values exceeded their respective maximum limits. In the absence of a modified atmosphere packaging system, the vacuum packaging associated with the acerola treatment should be considered a good option. Overall, results indicate that the efficacy of SMS prevailed in the control of melanosis, in addition, the AF was also effective and can potentially be used as a natural alternative for melanosis control. However, the antioxidant activity of acerola is recognized due to its high levels of vitamin C and phenolic compounds therefore, it is advisable that other studies be carried out, preferably looking to isolate specific parts of the fruit (skin, pulp, seeds) to identify where the highest content of phenolic compounds is, or even to purify acerola extracts, so that the true antimelanosic potential of this fruit is fully understood.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001. The authors thank CAPES for the research grant awarded to the second author under Master project (Animal Science Post-Graduation Program).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Gonçalves AA, de Oliveira ARM. 2023. Combinate effect of antimelanosic agents (acerola fruit extract and sodium metabisulphite) with the modified atmosphere packaging on the quality of white shrimp (Litopenaeus vannamei) stored under refrigeration. Food Innovation and Advances 2(3):233−246 doi: 10.48130/FIA-2023-0025
Combinate effect of antimelanosic agents (acerola fruit extract and sodium metabisulphite) with the modified atmosphere packaging on the quality of white shrimp (Litopenaeus vannamei) stored under refrigeration
- Received: 07 July 2023
- Accepted: 31 August 2023
- Published online: 18 September 2023
Abstract: The current study set out to find out how shrimp quality in cold storage (4 °C) for 21 d was impacted by antimelanosic treatments (10% acerola fruit extract (AF) and 1.25% sodium metabisulphite (SMS) solutions for 10 min) in combination with modified atmosphere packaging (MAP, including vacuum): Atmospheric air (AIR), MAP1 [70% N2 : 25% CO2 : 5% O2], MAP2 [25% N2 : 70% CO2 : 5% O2], and vacuum (VAC). Untreated samples were considered as Control (C). Every three days, microbiological, physicochemical, and sensory investigations were conducted. Overall, the findings show that MAP improve the shelf-life of shrimp stored under 4 °C. In the battle to control melanosis, SMS's effectiveness – either alone or in combination with MAP – was overwhelming. Notwithstanding, the AF was also efficient and can be an effective and a natural substitute in the control of melanosis. When considering the results of the physico-chemical and microbiological results, the SMS often did not differ from the treatment with AF, demonstrating the excellent viability of the AF on the quality of the shrimp stored under refrigeration. However, given that acerola's high levels of vitamin C and phenolic compounds are known to have antioxidant activity, it is advised that additional studies be conducted. Preferably, these studies should aim to isolate specific fruit parts (peel, pulp, seeds) to determine where the highest concentration of phenolic compounds is found, or even to purify acerola extracts to comprehend the fruit's true antimelanosic potential.
-
Key words:
- Shrimp /
- Melanosis /
- Shelf-life /
- Acerola fruit












