-
Wine color symbolizes both the quality of the grapes and the wine, which has a significant impact on the sensory preferences of consumers. The color of red wine is provided by anthocyanins and their derivatives. With a core structure of 3,5,7-trihydroxyl-2-phenylbenzo-pyran cations, anthocyanins are substances in which anthocyanidins are attached to monosaccharides or oligosaccharides via glycosidic linkages. Anthocyanins exist in a broad range, with more than 700 distinctly identified forms[1], of which five anthocyanins, including delphinidin, cyanidin, petunidin, peonidin, and malvidin, are abundant in wine. During the grape veraison phase, anthocyanins are synthesized via the flavonoid pathway, including the basic flavonoid upstream pathway and the specialized anthocyanin downstream branch. The anthocyanins in grapes are almost entirely located in the skin, except for some teinturier varietals like Yan-73, whose pulp is similarly rich in anthocyanins. The extraction of anthocyanins from berries into must is accompanied by maceration. The maceration time determines the intensity of the anthocyanins and color. The anthocyanin concentration in red wine ranges from 500 to 2,000 mg/L[2].
Wine aging involves redox, esterification, condensation, and polymerization, which reduces astringency and bitterness while enhancing aromatic complexity and color stability. There are two distinct stages of storage: maturation in oak barrels and aging in bottles. Oak barrel aging, a step to improve the condition of most premium wines, enhances wine in two ways. On the one hand, the permeability of oak barrels allows trace oxygen to gradually permeate the vascular bundle of the oak and the cracks between the boards, causing the wine to slowly and continuously oxidize enhancing its flavor and appearance. On the other hand, molecules in the oak, including dissolved guaiacol, oak lactone, and oak eugenol, optimizes the wine bouquet and flavor by interaction[3]. However, the oak barrel aging process presents several drawbacks, such as high cost, a long aging cycle, and susceptibility to harmful microorganisms, like spoilage yeasts and acetic acid bacteria. The use of stainless steel, uncoated concrete, and raw earthenware amphorae for maturation are emerging as reliable options, considering the oenological objectives and market distinction. Every tank material displays unique oxygen permeability and chemical elements that influence the formation and degradation of chemicals during wine aging.
Anthocyanin modification occurs throughout winemaking and aging. The anthocyanins are extracted from the wine via maceration and reach a peak value over 3−4 d[4]. Anthocyanins in fresh wine exist mostly as unstable monomeric substances, while they decline dramatically with aging, from 87% to 39% throughout the two-year storage period. The copigmentation rates also fall from 32%−43% during alcoholic fermentation[5], to 20%−34% after 3 months and 0−5% after 9 months of aging[6]. The decline of monomeric anthocyanins account for three reactions. Firstly, pigment precipitation occurs when anthocyanins combine with the tannins, polysaccharides, and macromolecular proteins in grapes, as well as massive microbial lees. Secondly, environmental forces triggered the deterioration of anthocyanins, such as pH, oxygen, light, heat, and sulfur dioxide. Lastly, the polymerization between the anthocyanins and other chemicals via covalent interaction is also responsible for the decrease in monomeric anthocyanins. Approximately 0–20% of anthocyanins are polymerized in fresh wines, but this percentage rapidly increases during the storage, reaching 30%–45% after 9 months[6]. Physicochemical conversion, such as degradation, precipitation, and polymerization, occurs during wine maturation, promoting the proportion of monomeric anthocyanin shrinkage and stable polymeric anthocyanin formation, altering the wine color from purple to red or even brownish.
The published reviews on food processing recommend modifying the structure and controlling environmental factors to maintain the color and bioactivity of anthocyanins[1,7]. However, some of the solutions are inappropriate for winemaking. For example, encapsulation embeds extractive anthocyanins with solid particles or liquid vesicles, which requires complex process technology and introduces new substances. The entire winemaking process from grapes to wine storage influences wine color stability and comprehensive strategies are warranted to enhance wine anthocyanin stabilization for further exploring the aging potential. Therefore, this review concludes the color variation trend and the reactive mechanism involving pigments in aged wine. Furthermore, from the perspective of grape cultivation to the aging stage, we describe innovative and advanced methods for regulating the compound composition and color degradation with the consideration of chemical reactions and enological practices.
-
The primary type of anthocyanins in grapes are anthocyanidins (or aglycons). As shown in Fig. 1, the core of anthocyanidins is a typical C6-C3-C6 flavonoid skeleton, also known as flavylium nuclei. Anthocyanidins consist of a heterocyclic ring [C] that bonds to an aromatic ring [A] and a third aromatic ring [B] via carbon-carbon bonds. Due to two double bonds in the ring [C], anthocyanidins carry a positive charge, resulting in their susceptibility to environmental factors. The stability of anthocyanidins also depends on their individual structure, including the kind, number, and position of substituents on the flavylium ion. Although electron-donating groups on the ring [B], such as the hydroxyl and methoxy substituents, are basically colorless, they affect anthocyanidin color and stability when they conjugate with the benzene ring[8]. Among them, the methoxy substitution on the ring [B] protects anthocyanidin from oxidizing to ortho-quinones that are instable and accessible to brownish polymeric compounds. It enhances the red hue and improves anthocyanidin stability. In contrast, the hydroxyl substitution intensifies the yellow hue and weakens anthocyanidin stability. Therefore, of the six most usual forms, malvidin is the most stable anthocyanidin, while delphinidin is the last.
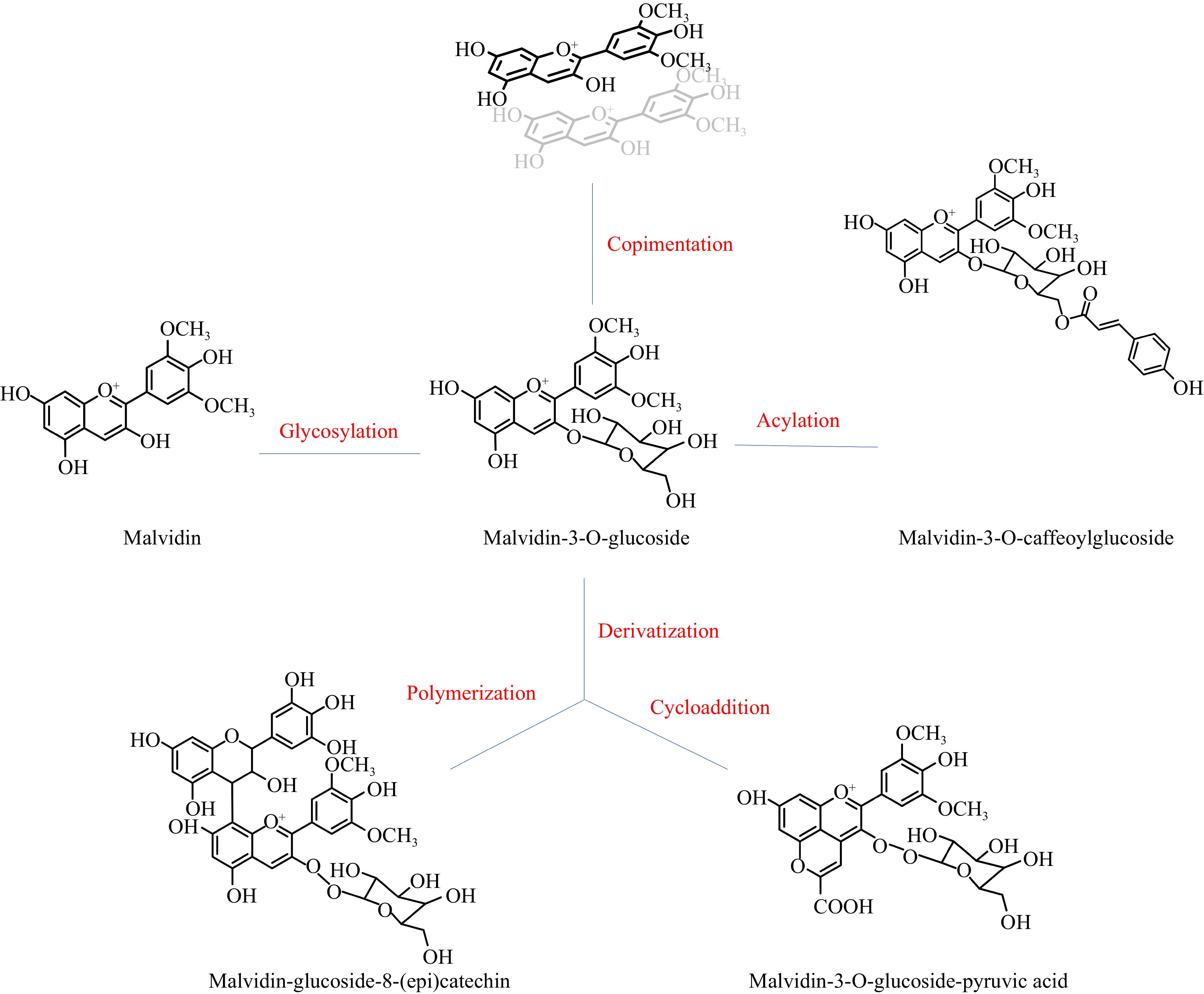
The electronic deficiency of the flavylium nuclei of anthocyanidins results in their high reactivity. During berry growth, wine making and wine aging, anthocyanidins can be involved in many reactions, including glycosylation, acetylation, copigmentation, and derivatization (shown in Fig. 2, malvidin-3-O-glucosides are chosen as an example, since they are abundant in most wine).
Glycosylation
-
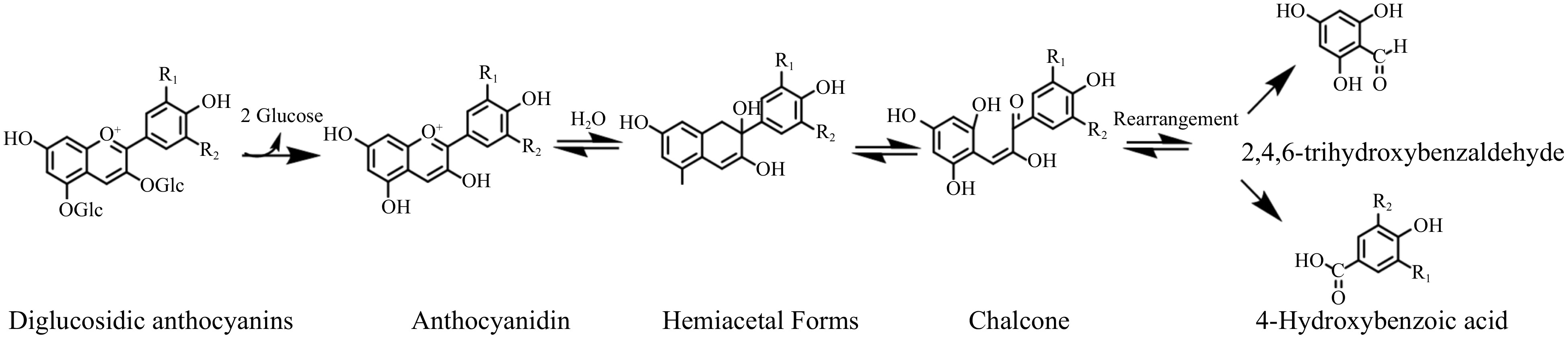
Anthocyanidins are exceedingly unstable. In the form of glycosides, anthocyanins are more widespread in nature. The C-3, C-5, and even C-7, C-3', and C-5' of anthocyanidins are usually bonded to glucose, galactose, rutinose, arabinose, and rhamnose via glycosidic linkages. Substitutes are beneficial for improving the stability of anthocyanidins by inhibiting the chalcone ring-opening cleavage into aldehydes and phenolic acids.
The anthocyanins in wine are typically glycosidic substitutes, of which monoglucosides and diglucosides are the most common forms. Brauch et al. reported that at pH 3.6, the half-lives of 3-O-glycosides were substantially shorter than those of the respective 3,5-O-diglycosides[9]. Figure 3 demonstrates the degradation process of anthocyanins diglucosides. They are usually more stable than their monoglucoside counterparts in aqueous solution with two glucosides hindering the ring from opening. However, the anthocyanins in wine exhibit the opposite phenomenon. Since glycosides are substituted at position 5 in the ring [A], anthocyanin diglucosides are not only unable to form stable pyran rings during aging but also less effective at polymerizing with flavonols, making it challenging to form stable polymeric anthocyanins. Furthermore, anthocyanin diglucosides is less resistant to oxidative and thermal conditions, triggering wine browning and discoloration over a short period[10]. However, the exact structure of the browning material remains unclear.
Acetylation
-
The sugar moiety (glycosyl) in anthocyanins can be esterified by different aromatic acids (such as p-coumaric, caffeic, ferulic, and sinapic acid) or aliphatic acids (such as acetic, malic, malonic, oxalic, and succinic acid) under the catalysis of acyltransferase, which refers to acetylation. Acylation reactions are effective in altering molecular mass, polarity, and steric hindrance.
Acylated anthocyanins can be classified as monoacylated and polyacylated anthocyanins according to the number of acyl groups. A higher number of acyl groups on the anthocyanin molecule often increases its stability[11]. Flower pigments contain large amounts of polyacylated anthocyanins, while the acylated anthocyanins that naturally exist in fruit and vegetables, such as grapes, are mostly monoacylated anthocyanins. The proportion of acylated anthocyanins in red wine, made from Vitis Vinifera, ranges from 0 to 53.5%[12,13]. The acylated proportion is different depending on the grape variety, cultivation terroir, as well as the viticultural and winemaking management systems.
Acetylation groups change the spatial conformations through non-covalent interactions (hydrogen bonds and Van der Waals forces) and therefore influence copigmentation of anthocyanin complex. Via intramolecular copigmentation and self-association reactions, the acylation shields the flavylium chromophore from nucleophilic water attack and reduces the anthocyanin sensitivity to pH changes, promoting their stability to heat and light in aqueous solutions. However, a study revealed that the steric hindrance caused by anthocyanin glycosyl acylation impedes intermolecular copigmentation in model wine solutions[14]. The intermolecular copigmentation of acetylated anthocyanins is not as stable as intramolecular copigmentation. At high temperature (> 60 °C), intermolecular aggregates are disrupted but intramolecular interactions remain[15]. Overall, acetylation is helpful for improving the stability of anthocyanins.
Copigmentation
-
Copigmentation is a phenomenon in which pigments and copigments form complex associations via non-covalent interaction. The flavylium ion forms of anthocyanins are deficient in p-electrons and the copigments are usually abundant in electrons. Therefore, the electron distribution imbalance between anthocyanins and copigments links them together.
Four copigmentation mechanisms have been identified: intramolecular copigmentation, intermolecular copigmentation, self-association, and metal complexation. Intramolecular copigmentation occurs between an anthocyanin chromophore and its aromatic residue, forming polarizable planar nuclei via hydrophobic interaction (π-π stacking). Intermolecular copigmentation occurs between the anthocyanin ring [B] and planar ring [B] of other flavonoids, whose copigmentation effect is distinctive depending on their structure. The pattern of the substituents on anthocyanin ring [B] dramatically impacts their ability to bind to copigments. The tendency to form the complexes is also inconsistent due to different copigments. The polyphenols with larger planar structures are more accessible for hydrophobic interaction with the anthocyanins, consequently presenting a better copigmentation effec. Since hydroxybenzoic acid has a smaller planar structure and flavanols have non-planar structures, the copigmentations of hydroxybenzoic acid and flavanols are weaker than hydroxycinnamic acid and flavonols. When the aromatic nuclei of anthocyanins are stacked parallel to each other, self-association occurs, in which anthocyanins are viewed as copigments in this particular instance of intermolecular copigmentation. Metal complexation occurs between metals (Mg2+, Fe3+, and Al3+) and anthocyanins with o-di-hydroxyl groups in the B ring (Cy, Dp, and Pt).
Copigments are generally colorless, but when mixed with anthocyanins, they can form copigment-pigment complexes via Van der Waals interactions, hydrophobic effects, and hydrogen bonding. In young red wine, copigmented complexes support 30%–50% color. The association slows down the conversion of anthocyanins from colorful flavylium ions to colorless hemiacetals and exerts a hyperchromic effect (intensity absorbance in the visible spectrum) and bathochromic shift (the maximum absorbance changes to a longer wavelength). The copigmentation also plays a crucial role in the red hue remain and yellow hue restrain by enhancing the stability of anthocyanins against external environment. It provides protection against nucleophilic water attack in the 2 positions of the flavylium ion[16] and against other species, such as peroxides and sulfur dioxide, in the 4 position.
Derivatization
-
Monomeric anthocyanins interact with grape polyphenols and yeast metabolites during wine fermentation and aging. The interaction produces anthocyanin derivatives, predominantly polymeric anthocyanins, and pyranoanthocyanins. Polymeric anthocyanins in wine provide a purplish-red color. Pyranoanthocyanins, with the exception of portisin and pyranoanthocyanin dimers, present a yellow color[17]. Polymeric anthocyanins and pyranoanthocyanins increasingly replace the monomeric anthocyanins during wine aging, which substantially improves the color stability and accelerates the wine transition from a vivid purplish red to brick red or even brownish red.
Polymerization
-
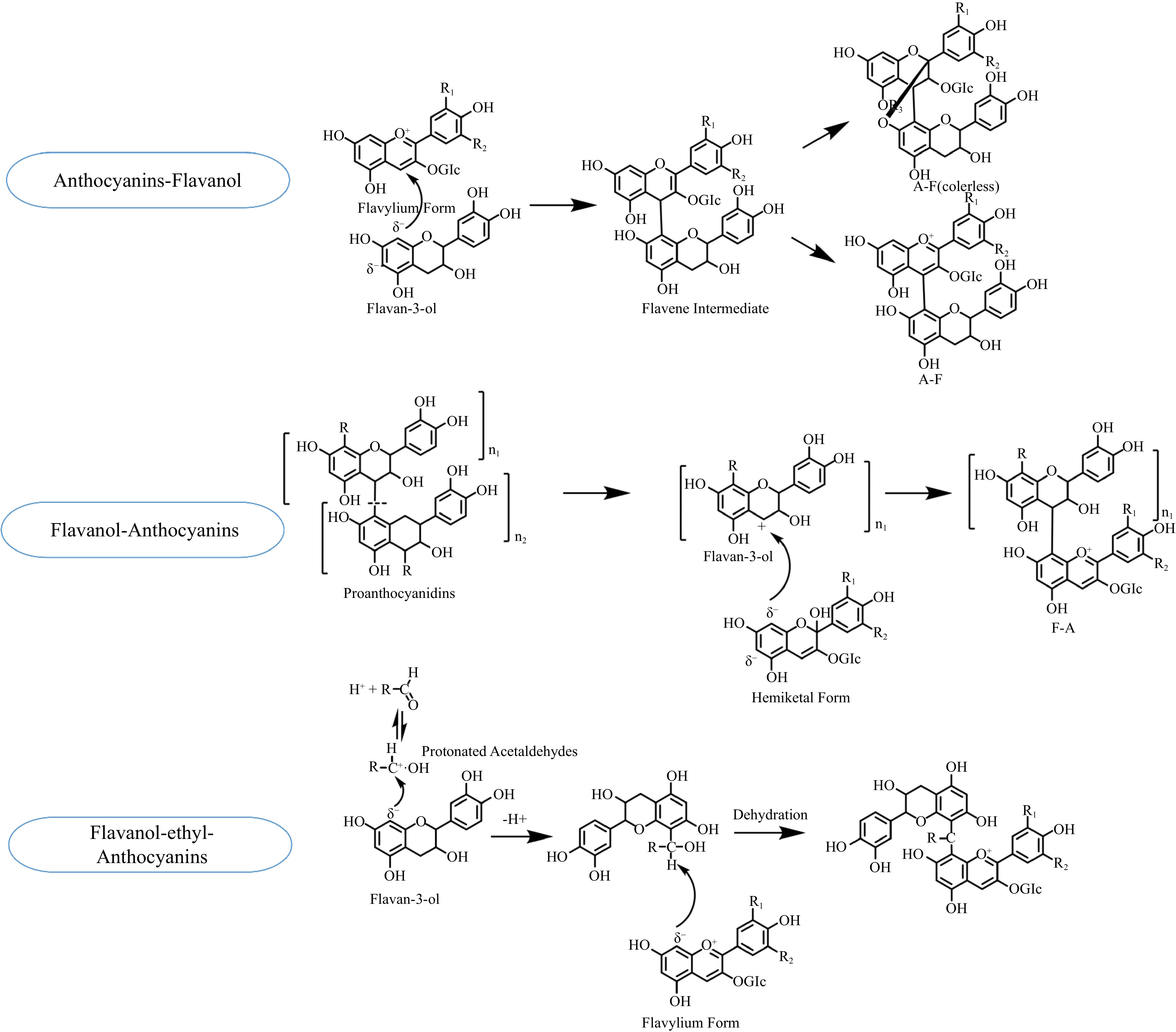
In the process of polymerization, monomeric anthocyanins or pyranoanthocyanins, either directly or mediated by aldehydes, condense with the monomers or oligomers of flavan-3-ols, forming polymeric anthocyanins. It is difficult for polymeric tannins with large molecular weights to react with anthocyanins because of their tendency to precipitate. The direct polymerization of flavanol-anthocyanins is divided into Flavanol-Anthocyanins (F-A) and Anthocyains-Flavanol (A-F) (Fig. 4). First of all, when the nucleophilic C8 or C6 position in the ring [A] of the anthocyanin molecule attacks the electrophilic C4 position in the ring [C] of a flavan-3-ol or a terminal unit of oligomeric proanthocyanidins, the anthocyanins in hemiketal form can generate the F-A adducts with tannins. The hemiketal forms of original anthocyanins are colorless. After dehydration reaction, they are modified to the corresponding colored flavylium forms of polymeric anthocyanins, enhancing color expression. F-A types of adducts can induce a red shift in the absorption of the anthocyanins and give the wine a blue-red hue. Secondly, A-F type anthocyanins are formed when the electrophilic C4 position in the ring [C] of anthocyanins in the flavylium form is attacked by the nucleophilic C8 or C6 position in the ring [A] of a flavan-3-ol[18]. The structure of A-F type anthocyanins is disrupted by ether bonds, resulting in their colorlessness[18]. Lastly, the process of acetaldehyde-mediated anthocyanin–flavanol formation involves the nucleophilic addition of flavanol to protonated acetaldehydes. Then, the nucleophilic addition of the carbon-positive ion intermediate to anthocyanins ultimately yields a flavanol-ethyl-anthocyanins[19].
Once anthocyanins are extracted into wine, polymerization begins and continues throughout the process from winemaking to aging. As acetylation increases the stability of anthocyanins, it takes a longer time for acylated anthocyanins to polymerize. Furthermore, the polymerization reaction is much more difficult for acetyl and caffeoyl anthocyanins than coumaroyl anthocyanins[22]. The synthesis and degradation of polymeric anthocyanins occur simultaneously in wine. Acetaldehyde-bridged flavanol-anthocyanins are generated earlier, during wine fermentation and short-term aging. However, they are not as stable as directly polymerized anthocyanins, with their content declining rapidly after synthesis. In specific circumstances, acetaldehyde-bridged flavanol-anthocyanins are transformed into anthocyanin-ethyl-flavanol dimers and vinyl-flavanol pyranoanthocyanins. The direct polymeric F-A anthocyanins is relatively stable. Ellagitannins supplied by oak products, such as barrel and oak chips, release procyanidins, which are reaction substrates for F-A polymeric anthocyanins synthesis. Thus, the content of F-A polymeric anthocyanins fluctuation increases when the wine ages with oak products.
The accumulation of polymerized anthocyanins usually reaches a peak at 6−8 d of alcoholic fermentation[23]. Almost all polymeric anthocyanins decrease during bottle aging since they are less stable than pyranoanthocyanins. However, with their descending rate considerably slower than monomeric anthocyanins, the status of polymeric anthocyanins still strongly improves wine stability as wine aging over a longer term[5].
Cycloaddition
-
Pyranoanthocyanins result from the cycloaddition of anthocyanins monomers and other organics. The nucleophilic substitution in the C-4 position on the anthocyanin moiety leads to the cyclization and subsequent formation of an additional ring between the OH group at C5 and the C4 of the anthocyanin pyranic ring. Not detected in grapes or unfermented must yet, pryanoanthocyanins evolve from monomeric anthocyanins during fermentation and aging. They are mostly responsible for a gradual color succession from red-purple to a stable orange hue. Furthermore, pryanoanthocyanins also contribute to wine color stability. When exposed to a specific environment, like sulfur dioxide, pH, heat, and oxygen, pyranoanthocyanins are more resistant to nucleophilic attack and display increased stability compared to their precursors.
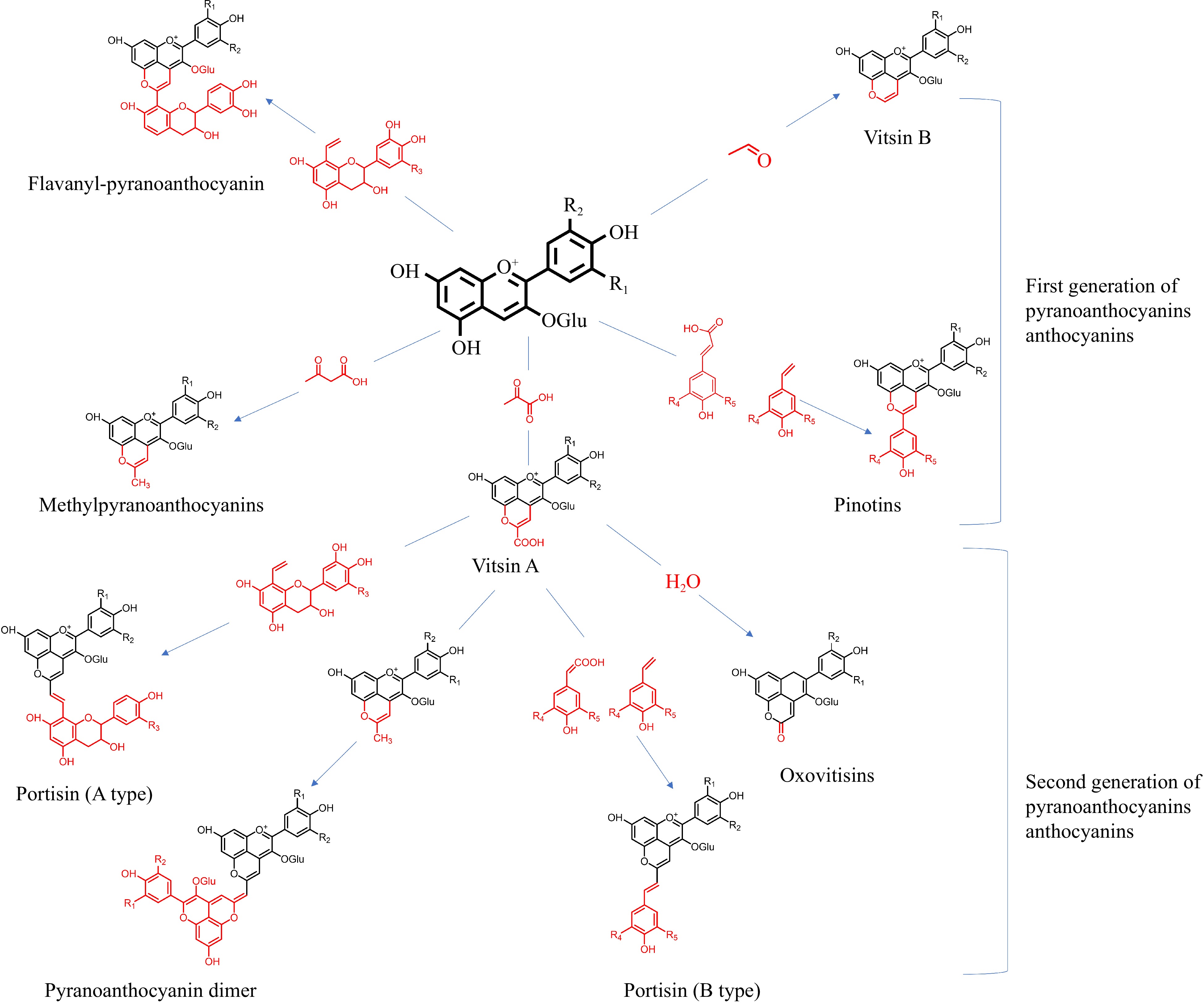
Aged wine includes pyranoanthocyanins in a variety of forms. Recent research extracted pyranoanthocyanins polymerized between monomeric anthocyanins and acetaldehyde, pyruvic acid, 4-vinyl phenol, hydroxycinnamic acid, and vinyl flavanol. As shown in Fig. 5, the following paragraphs discuss first- and second-generation pyranoanthocyanins.
First-generation pyranoanthocyanins
-
Vitisin-type anthocyanins are usually the simplest and the most abundant class of pyranoanthocyanins. Vitisin A (at a maximum absorption wavelength of 508–518 nm) and vitisin B (at a maximum absorption wavelength of 490–498 nm) are separately formed between malvidin-3-O-glucoside and pyruvate or acetaldehyde. Methyl-pyranoanthocyanin (at a maximum absorption wavelength of 478 nm) is synthesized by acetoacetic acid or acetone reacting with monomeric anthocyanins via a similar mechanism. The synthetic substrates for vitisins are metabolized by yeast and lactic acid bacteria in wine. The active microbial metabolism during fermentation results in an increase in the vitisin concentration[24]. However, the key time for vitisin A and B accumulation is distinctive. For example, as an essential substrate for vitisin A, pyruvate is metabolized by yeasts. Therefore, the concentration of vitisin A rises during the peak period of alcoholic fermentation at 3–4 d. The specific accumulation time is related to the free pyruvic acid in wine. During the first 6 months of aging, Liu et al. discovered that wine fermented with high-producing pyruvic acid yeast gradually produced vitisin A, with levels rising by 26%–54%. Between 6 and 12 months of aging, vitisin A tended to decline by 2.2%–10.2%[25]. Since acetaldehyde can be both converted from acetyl CoA during fermentation and oxidized from ethanol during aging, vitisin B formation during the late stages of alcoholic fermentation and constantly accumulates until several months after the end of fermentation. The time for the total vitisin reaches peak varies between 3 to 6 months depending on the wine style and aging circumstances[26]. This process is also affected by the amount of acetaldehyde and pyruvate in the wine and the presence of compounds like ellagitannins in the oak barrels.
Pinotins represent another kind of pyranoanthocyanins, exhibiting a brick-red color. Pinotins are formed when anthocyanins are polymerized with 4-vinyl phenol or hydroxycinnamic acid. The pyranoanthocyanins formed by caffeic acid and anthocyanins is known as pinotin A (at a maximum absorption wavelength of 511–514 nm) and are widely applied in wine. Pinotins are hardly found in wines aged 1–2 years. Even if the reactive substrates for pinotins, are always present in fermented wines, the synthesis of pinotins do not occur until the wine goes through a long aging process. During 2.5–4 years of aging, the ratio of hydroxycinnamic acid to free anthocyanins gradually rises with the rapid oxidation of monomeric anthocyanins, which accelerates pinotin A to be a significant anthocyanin component, conveying a tawny hue to the aged wine[27]. Blanco-Vega et al. examined the composition of anthocyanin derivatives in 283 different wines of Cabernet Sauvignon, Grenache, Merlot, and Petit Verdot wines, revealing that the pinotin A proportion in wines stored for 6–7 years still showed an increasing trend[28]. However, Schwarz et al. discovered that the pinotin A degradation rate exceeded the rate of synthesis and showed a decreasing trend when Pinotage wines were aged for more than five years[27]. The declining speed of the pinotin A was much slower than other forms of anthocyanin during long-term aging, so its content declines but proportion rose, which might account for the disparity between the findings of the two papers.
Flavanyl-pyranoanthocyanins are synthesized via the cycloaddition reaction between anthocyanins and vinyl-flavanols, exhibiting better stability than the polymer. Vinyl-flavanols do not naturally exist in grapes and they are derived from the dehydration of flavanol-ethanol adducts and the decomposition of the methylmethine-linked flavanol adducts, both of which can result from the reaction between flavanols and acetaldehyde[29]. The formation of flavanyl-pyranoanthocyanins is related to the oxygen content and acidity of the wine. According to Catania et al., increasing the oxygen permeability of the wine before and after malolactic fermentation are helpful to increase the content of flavanyl-pyranoanthocyanins and stabilize wine color[30].
Second-generation pyranoanthocyanins
-
During prolonged wine aging, first-generation pyranoanthocyanins can also be reactive substrates for anthocyanin derivation, forming second-generation pyranoanthocyanins, mainly including oxovitisins, portisins, and pyranoanthocyanin dimers. Second-generation pyranoanthocyanins have more complicated structures and are typically characterized by distinctive spectrum properties[29]. They are primarily found in port wines with their slow development. Additional evidence is required to confirm the presence of pyranoanthocyanins in older red wines.
He et al. first discovered that the nucleophilic addition of water molecules to the C10 position of vitisin A could form a hemiacetal form of pyranoanthocyanins, known as oxovitisins, which are yellow in color with a maximum absorption wavelength of 373 nm[31]. However, no research is available regarding oxovitisins in aged wine.
Portisin-type anthocyanins are formed when vitisin A polymerizes with either vinyl-flavanol (type A; at a maximum absorption wavelength of 575 nm) or hydroxycinnamic acid/vinyl phenol (type B; at a maximum absorption wavelength of 550 nm). Pyranoanthocyanins and flavanol molecules can stack vertically to facilitate intramolecular copigmentation in portisins, presenting a better electron conjugation effect than vitisin A. Furthermore, it further improves anthocyanin stability and is thought as the origin of the intense blue color. Portisins have mostly been found in port wines and have received less attention in dry red wines. However, Zhang et al. recently discovered portisins in dry red wines, proving that its levels rose considerably as the dry red wine aged[32].
Oliveira et al. firstly discovered the pyranoanthocyanin dimer in port wine aged for nine years[33]. It represented a polymerized adduct of vitisin A and methyl-pyranoanthocyanins, exhibiting a blue-green tint and a maximum absorption wavelength of 680–730 nm[33]. However, no reports are currently available demonstrating that dry red wine contains pyranoanthocyanins dimers.
-
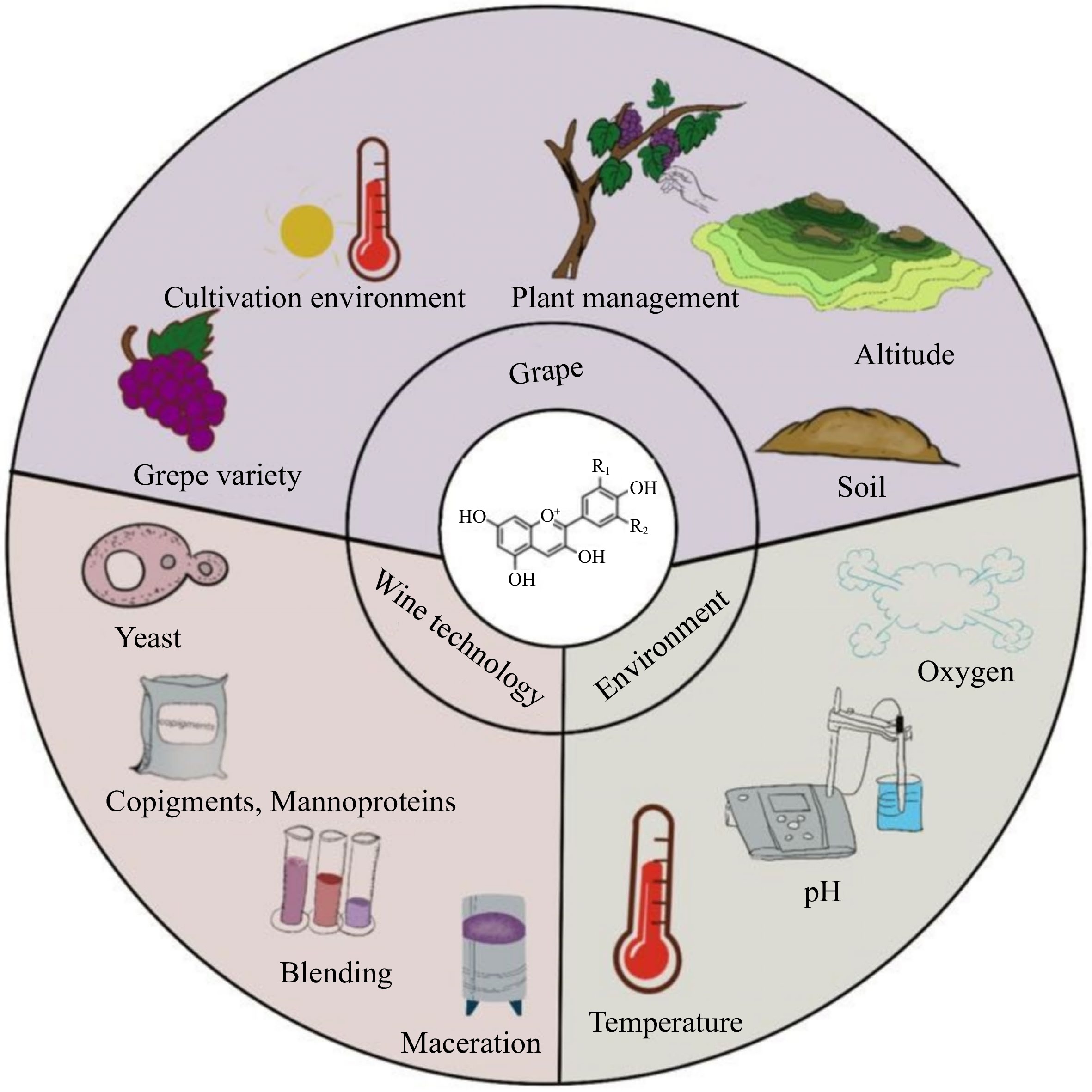
Based on their bioactivity, anthocyanins are inherently unstable. Figure 6 lists the factors that affect anthocyanin concentration and stability. They are susceptible to a wide range of environmental parameters, including extreme humidity, oxygen, pH, heat, light, and sulfites. In particular, the complex wine making process and long aging time of wines can further accelerate the degradation of these pigments. The following paragraph provides an overview of the factors that affect the stability of anthocyanins, from grapes to aging circumstances.
Grapes
-
The anthocyanins in wine are derived from grapes. The quantity and composition of them are influenced by grape variety, growing environment, and cultivation practices. Furthermore, these factors can also affect the progression of anthocyanin degradation during wine aging, resulting in variations in the rate and extent of color changes over time.
The variety of grapes primarily determines the composition and content of anthocyanins in wine, with various degrees of methylation, acylation, and glycoside substitution observed among different grape varietals. Although malvidin is typically the most abundant anthocyanin in grapes[34], the ratios of monomeric anthocyanins vary. For example, Cabernet Sauvignon tends to have higher levels of malvidin, while Merlot is known for producing more hydroxyl-substituted anthocyanins such as delphinidin[32].
The glycoside substitution pattern in grape species exhibits variation. The synthesis of 3,5-O-diglucoside anthocyanins is regulated by the 5-O-glucosyltransferase enzyme. In Vitis vinifera grapes, the gene encoding this enzyme is inactive, resulting in the dominance of 3-O-glucoside anthocyanins. Contrarily, non-vinifera grapes such as Vitis riparis, Vitis rotundifolia, Vitis davidii, Vitis amurensis, exhibit high levels of gene expression, leading to the production of large amounts of 3,5-O-diglucosides. In fact, some researchers have even considered 3,5-O-diglucoside and acylated 3,5-O-diglucoside anthocyanins to be marker compounds for non-vinifera species or hybrid red grapes. As mentioned above, diglucosides anthocyanins are not as stable as monoglocosides anthocyanins, leading a short shelf life of most non- vinifera wine.
The proportion of acylated anthocyanins in wine is distinct due to differences in the ability to synthesize acyltransferases. Non-vinifera grapes have lower levels of acylated anthocyanins due to the absence of genes regulating acyltransferases. However, the absence of acylated anthocyanins is a rare genetic trait in Vitis vinifera, with the exception of the Pinot family of cultivars[35]. Anthocyanin acylation can enhance intramolecular copigmentation and stability, resulting in improved color stability during aging. Therefore, Pinot Noir and non-vinifera, which have few acylated anthocyanins, tends to change color quickly. The Brazilian hybrid grape variety, Violeta, which contains high amounts of coumaroyl anthocyanins (3,5-diglucosides 1,146.45 mg/L and p-coumaroylated-3,5-diglucosides 408.87 mg/L), showed better color stability when aged at 25 and 35 °C for 120 d than other wines with only a large proportion of diglucosidic anthocyanins[36].
The grape cultivation environment affects the concentration and composition of anthocyanins by altering light and heat parameters. Sunlight plays a crucial role in the biosynthesis and regulation of anthocyanins, influencing their varieties and profiles. For instance, excluding sunlight decreases the relative proportion of 3′,4′,5′-hydroxylated anthocyanins and increases that of 3′,4′-hydroxylated anthocyanins in berry skin[37,38]. Furthermore, high light intensities during veraison are generally considered to promote anthocyanin biosynthesis in skin due to the increased transcript abundance of VvUFGT, VvMybA1, VvMybA2, and VvMyc1[37,39]. However, it should be noted that the optimal sunlight intensity for anthocyanin synthesis varies among different grape varieties. For example, Merlot shows maximum color accumulation at low light intensities of less than 10% ambient photosynthetic photon flux density (PPFD), while Pinot noir is at 18% PPFD and Cabernet Sauvignon is at 20% PPFD[40]. When light conditions within a canopy receive sufficient light, then light may not necessarily be a limiting factor for anthocyanin synthesis. However, these effects may be temperature-related.
Temperature plays a critical role in regulating the efficiency of anthocyanin accumulation. Heat is necessary for anthocyanin synthesis, and generally, anthocyanin concentration increases with an improvement in the average temperature. However, when berries are exposed to excessively high ambient temperatures (> 30 ~ 35 °C) for a significant period during the day, the expression of genes related to anthocyanin synthesis (VlMYBA2, UFGT) is reduced, leading to the inhibition of anthocyanin synthesis[41]. Additionally, high temperatures can shift the anthocyanin profiles, resulting in a higher proportion of malvidin anthocyanins and acylated derivatives due to the overexpression of the acyltransferase gene Vv3AT[38,42]. In regions with high levels of sunlight and low temperatures, such as Xinjiang and Gansu in western China, grapes contain a high concentration of anthocyanins, but non-acylated anthocyanins are more prevalent[43]. Since non-acylated anthocyanins are known to be unstable, wines made primarily from them tend to experience color fading.
Wine making technology
-
Wine making involves the fermentation of grapes by yeast, which extracts anthocyanins from the grapes into the wine. The wine making technology not only affects the anthocyanin profile but also the precursors of anthocyanin derivatives, ultimately determining the final color development.
Maceration is an essential step in red wine making, during which the must is in contact with the raw materials (grape seeds, pulp, and skins) to extract phenolics, polysaccharides, nitrogen compounds, minerals, and volatile compounds into the wine. It represents a key period for determining the content and profile of anthocyanins. Extended skin contact time is theoretically related to a higher anthocyanin concentration in wine. Nevertheless, some studies have found no direct and even a negative relationship[44] between the length of maceration and the final anthocyanin content, because the anthocyanins might be partially fixed in the solids or reduced to a colorless form. More controlled and accurate research is needed to illustrate the relation between maceration and anthocyanins.
The stability of wine color can be enhanced through copigmentation during short-term wine aging. Natural compounds found in wine with π-conjugation systems, such as alkaloids, amino acids, polysaccharides, and polyphenols, can act as copigments. They can interact with the chromophores of anthocyanins through π-π stacking.
Yeast plays a dominant role in the alcoholic fermentation process by converting glucose into ethanol. Recent studies have indicated that preferential yeasts not only reduce the adsorption of anthocyanins by cell walls, but also promotes the formation of pyranoanthocyanins and polymeric anthocyanins in wine. On the one hand, the numerous mannoproteins present in yeast cell walls can adsorb pigmented compounds, leading to the loss of anthocyanins. Mannoproteins are biopolymers composed of proteins covalently linked to mannose (80%–95%). These molecules can be naturally extracted from grape skin cell walls and yeast cell walls. The release of mannoproteins varies depending on the metabolic phase of the yeast, with a higher release occurring when yeast mortality is high. Mannoproteins have been shown to inhibit the crystallization, adsorb ochratoxin A, enhance malolactic bacteria growth and improve mouth feeling. Mannoproteins also play a significant role in stabilizing wine color by the 'structure-stability' relationship. The non-covalent interactions involved in this relationship represent a key intermolecular force that increases the stability of anthocyanins. Recent research has also revealed the involvement of covalent linkages in the connection between mannoproteins and anthocyanins, as not all polyphenolic compounds can be released from their combined compounds[45]. However, further research is needed to clarify this mechanism. The extent of anthocyanin adsorption varies among yeast species and strains, with some strains exhibiting adsorption rates ranging from 2% to 9%. Therefore, preferential yeast fermentation can mitigate anthocyanin loss resulting from yeast sedimentation . On the other hand, yeasts accelerate the synthesis of anthocyanin derivatives through two pathways. The first pathway involves the metabolism of pyruvate and acetaldehyde, which combine with anthocyanins to generate pyranoanthocyanins such as vitisin A and vitisin B, and promote the synthesis of acetyl-bridged polymeric anthocyanins[46]. The second involved the metabolism of hydroxycinnamic acid decarboxylase that transforms the hydroxycinnamic acid in the must into vinyl phenols, and yield flavanyl-pyranoanthocyanins through polymerization[47].
Environmental factors
-
The environment during wine fermentation and aging is a critical factor affecting anthocyanin stability. Factors such as oxygen exposure, pH, and temperature can affect the rate of polymeric anthocyanin synthesis and monomeric anthocyanin degradation.
Oxidation reactions during winemaking include active enzymatic oxidation and non-enzymatic oxidation (also known as chemical oxidation). Peroxidase, polyphenol oxidase, and β-glucosidases are the main enzymes that catalyze enzymatic oxidation. These enzymes become less active with an increase in the alcoholic strength of the wine. Therefore, enzymatic oxidation reactions principally occur in grapes and must. Non-enzymatic oxidation reactions are primarily responsible for the degradation of anthocyanins during aging. Anthocyanins and other polyphenols, containing an ortho-dihydroxybenzene moiety (a catechol ring) or a 1,2,3-trihydroxybenzene moiety (a galloyl group), cannot be directly oxidized by oxygen in wine[48]. The redox cycle of Fe3+/Fe2+ and Cu2+/Cu+ mediates the successive oxidation of these substrates to semiquinone radicals and benzoquinones while oxygen is reduced to hydrogen peroxide[49]. The quinones formed during the oxidation of polyphenols are unstable and may spontaneously combine with nucleophilic compounds (including some phenols, thiols, and amines) due to their high electrophilic characteristics. These dimers or polymers in coupled oxidation reactions display higher redox potential than their initial phenols and are more readily oxidized, accelerating the oxidation process. The Fenton reaction, involving hydrogen peroxide in association with ferrous ions, produce hydroxyl radicals that can damage practically every organic molecule found in wine, resulting in anthocyanin degradation and color browning[50].
Although excessive oxygen exposure can have a negative impact on wine quality, an appropriate amount of oxygen during the aging process can have several positive effects. It can help to soften tannins, reduce green flavors, enhance aroma, and promote the production of anthocyanins. When wine is exposed to weak, controlled, and continuous oxygen, a small amount of ethanol can be converted into acetaldehyde. Acetaldehyde can then either contribute to the formation of anthocyanin dimers and vitisin B or react with the C6 and C8 positions of flavanols, generating acetyl-flavanols that can adduct with anthocyanins to create acetal-bridged polymeric anthocyanins[51]. Although these processes may decrease the concentration of monomeric anthocyanins, they lead to the formation of complex anthocyanin derivatives that contribute to a more stable color in aged wines.
The state of anthocyanins is pH-dependent, as shown in Fig. 7. At pH 2, anthocyanins exist predominantly as red flavylium cations. As the pH increases, the flavylium cations undergo dehydration to form colorless hemiacetal forms, which can be protonated to form yellow chalcones. Alternatively, the flavylium cations can undergo direct deprotonation to form blue-purple quinonoidal bases. The electrical properties of anthocyanin forms at different pH levels in wine can affect their reactivity. Flavylium and hemiacetal forms are the primary anthocyanin species in grapes and wine, and are susceptible to oxidation and degradation.
Acidity during maceration is a crucial factor for organic compound extraction. Lower acidity levels promote flavanol extraction and acetaldehyde production, which can enhance the potential for anthocyanin polymerization during aging. Thus, compared to wine fermentation, in which the pH is maintained at 3.2 throughout, a higher pH (3.5 or 3.9) during maceration and fermentation significantly increases the production of polymeric anthocyanins during a 1–2-year aging period, even when the wine is acidified to 3.2 after fermentation[52]. Additionally, anthocyanins tend to copigment in a less acidic environment[53], but more anthocyanins are extracted into wine under high acidic conditions. During wine aging, an acidic environment promotes the formation of pyranoanthocyanins and acetyl-bridged polymerized anthocyanins, especially during micro-oxygenation.
Therefore, acidity generally accelerates anthocyanins extraction and copigmentation during maceration and encourages the conversion from monomeric anthocyanins to polymeric anthocyanins during aging. However, lower wine acidity during the maceration stage accelerates the extraction of tannin and flavanol that polymerize with anthocyanins, hindering the fade of red tones and emergence of yellow tones during wine aging.
Temperature can also affect the pace of chemical polymerization and degradation during the process of maceration and aging. High temperatures promote the polymerization reaction of polyphenols, while wine stored at low temperatures is less likely to spoilage but takes longer to mature.
-
Grapes are a major source of anthocyanins, and their ability to synthesize these compounds is determined by their genetics and cultivation environment.
Determined by gene, some grapes, such as non-vinifera, are naturally full of unacetylated anthocyanins and diglycosidic anthocyanins, resulting in the instability of color. To improve color stability, interspecific varieties are created by the hybridization of Vitis vinifera and non-vinifera, which can reduce the 5-O-glucosyltransferase expression and the proportion of anthocyanins digluccosides in non-vinifera. Furthermore, anthocyanin structure modification is another strategy. Previous studies have demonstrated that the acylation proportion of anthocyanins can be increased by combining anthocyanins with lauric acid for 48 h[54] or by directly adding acyltransferase and acylation substrate[55]. Commercial application of physical and enzymatic methods is constrained by operational complexity, high expense, and sensitive environmental requirements, and no reports on how to increase the acylation proportion of anthocyanins in the brewing sector are available to date. The method of hybridization is faced with a long breeding circle. The more practical way to improve the anthocyanin stability is optimizing the grape cultivation environment.
As over-high temperatures decrease anthocyanin synthesis, in climates where temperatures regularly exceed 35 °C, moderately open canopies by pruning and leaf removal appear to create the best conditions for color development. Additionally, in some wine appellations, the growing season did not simultaneously provide sufficient light and temperature for optimal grape development. These plant management techniques such as spike thinning, pruning, and leaf removal during veraison can also improve the light-exposed area and temperature of the microenvironment. Therefore, it promotes anthocyanin concentration and enhances their polymerization, potentially improving the stability of grape color in cooler regions[56]. High altitude[57] and sunny slope planting[58] can be implemented to improve light transmission in the grape microenvironment. For example, high-altitude planting in the Hengduan Mountains of Yunnan, China, increases the light intensity and duration for Cabernet Sauvignon and Merlot during the grape growth phase, resulting in wines with higher flavonoid and anthocyanin content compared to those made from grapes grown at lower elevations[57].
Soil is another important factor affecting grape coloration. Grapes planted in soils with a low nitrogen content were more conducive to anthocyanin production[59]. Meanwhile, deficiency irrigation raised the expression profiles of genes involved in pigment manufacturing and increased anthocyanin content[60]. Grown in soils with less water and organic matter, grapes also tend to present a more stable anthocyanin profile, producing higher levels of 3′5′-substituded, O-methylated and acylated anthocyanins[61]. Therefore, grapes are adaptive to adverse cultivation conditions, and their anthocyanin content and stability can increase when grown in environments with low soil nutrients and water holding capacity.
Different grape varieties produce wines with distinctive pigment profiles. Therefore, to ensure wine color stability, grapes with a high proportion of unstable and vulnerable anthocyanins should be cultivated in optimal surroundings with moderate sunlight and temperatures to effectively adjust their pigment profile. In cooler regions, precise viticultural practices and early-maturing grape varieties should be considered to increase heat and anthocyanin accumulation, thereby prolonging the color stability of wines.
Delay time and addition of exogenous substances during maceration
-
Even though the direct impact of extended maceration is still unclear, it is a consensus that extended maceration can increase flavan-3-ol, proanthocyanidins and flavonols, providing bitterness and astringency to the wine. In addition, the phenolics are crucial for stabilizing the anthocyanins during copigmentation and derivatization. Hence, extended maceration can protect monomeric anthocyanins from degradation.
Furthermore, other winemaking techniques employed during maceration can also improve color stability. For instance, partial stem maceration may lead to reduced anthocyanin content in the resulting wine, as a significant proportion of these compounds are absorbed by grape stems during the fermentation process. However, the addition of procyanidins derived from grape stems can enhance the polymerization of colored pigments in wine, resulting in greater color stability compared to conventionally macerated wines even after 12 months of storage[62]. Exogenous enzymes, including pectinase and cellulase, are commonly employed in the production of red wine to degrade the pecto-cellulosic cell walls present in the berry skins through the hydrolysis of structural polysaccharides. The addition of maceration enzymes has been shown to increase the release of anthocyanins from the skins by 8% to 15% and reduce anthocyanin degradation at different maceration times[63]. However, despite the emphasis on the influence of exogenous enzyme addition on anthocyanin content, its impact on anthocyanin stability has been overlooked.
Adding copigmented substances
-
The copigmentation plays an important role in stabilizing young wine. However, the copigments naturally present in wine may be insufficient, and their concentration may decrease rapidly, which limit the formation of anthocyanin copigmentation. As a result, researchers have attempted to enhance copigmentation by artificially adding flavonoids, hydrolyzed tannins, and phenolic acids during the winemaking process. The effect of copigments on copigmentation differs significantly, the added flavonoid includes rutin > quercetin > baicalein > daidzein[64], while phenolic acid copigmentation includes ferulic acid > caffeic acid > coumaric acid > protocatechuic acid > gallic acid > salicylic acid[64,65].
The direct addition of chemically synthesized copigments on enological applications faces with regulation restrictions and high expense. Consequently, mixing grape pomace[66], oak products[67], and phenolic extracts from natural foods[68,69] to increase copigmentation and stabilize wine color has attracted considerable research attention in recent years. Rivero et al. added overripe grape seeds high in phenolic compounds like catechins, epicatechin, and proanthocyanidins and macerated with wine for 30 or 60 d during malolactic wine fermentation. The wines displayed better color stability after 3 months of aging by increasing the grape seed additions[70]. By adding rosemary and cinnamic acid substances extracted from aromatic plants, Bimpilas et al. increased the copigmentation of anthocyanins in wines, reducing the color loss in Merlot wines during 3 months of aging. However, this did not affect polymeric anthocyanin formation, while the copigmentation effect produced by the addition of extracts vanished after 6 months of aging[68]. Zhao et al. added epicatechin and gallic acid to model wines containing five monomeric anthocyanins, after 231 d of aging the wine with added copigments, the wine unexpectedly exhibited more yellow and less red hues[71]. Overall, the addition of copigments can only offer short-term stabilization of wine color, as the copigmentation effect may decline rapidly or disappear after 6 to 9 months of aging.
Mannoproteins can also be involved in the non-covalent interactions with anthocyanins. However, most mannoproteins are released slowly during the aging process on lees, which can be both time-consuming and expensive, and may sometimes result in microbiological spoilage. As a result, mannoproteins are often artificially added during winemaking to enhance wine color stability. In a study, the addition of three types of commercial mannoproteins after fermentation promoted the formation of pigmented polymers from the 6th month in Aglianico and Sangiovese wines[72]. The timing of mannoprotein addition is also important. Addition during post-fermentation and storage can lead to unpredictable results depending on the wine type and the amount added. Introduction before fermentation has been shown to have a stronger protective effect on color stability and antioxidant capacity.
Correspondingly, although barrel aging can hinder the decline of non-covalent connection, it is still regarded as the first step in wine color evolution over a long period. To maintain their color, long-aged wines must undergo derivation with durable covalent connections.
Inoculating excellent yeasts
-
Appropriate yeast inoculation is effective in anthocyanin derivative production. However, one of the biggest challenges of improving pyranoanthocyanins by Saccharomyces cerevisiae inoculation is low yield of pyruvate and acetaldehyde[71]. Non-Saccharomyces cerevisiae is not only more successful in wine smell and flavor enhancement than Saccharomyces cerevisiae, but also significantly promotes anthocyanin derivative synthesis by metabolizing more pyruvate and acetaldehyde[47]. Fermentation inoculating Metschnikowia pulcherrima, Zygosaccharomyces bailii, Candida zeylanoides, and Torulaspora delbrueckii can increase pyranoanthocyanin production[73]. Escott et al. demonstrated that mixed inoculation with Schizosaccharomyces pombe or other non-Saccharomyces cerevisiae strains resulted in higher acetaldehyde levels than single inoculation with Saccharomyces cerevisiae, which promoted the formation of proanthocyanidins and polymeric anthocyanins, consequently increasing color intensity significantly[46]. This finding suggests that mixed inoculation may be a beneficial approach to enhance wine color quality. Regarding sensory analysis, mixed inoculation fermentation with non-Saccharomyces did not impair wine flavor even though it resulted in more acetaldehydes. This process enhanced the quantity of volatile compounds such as esters, thereby increasing the richness of the aroma[46].
When it comes to the production capacity of hydroxycinnamic acid decarboxylase of yeasts, there are variations among yeast species and strains, with metabolic rates ranging from 0−91.1%[74]. Of these, Pichia guilliermondii, S. pombe, and Wickerhamomyces anomalus yeasts have been found to have the highest potential for its production. Bozic et al. revealed that fermentation with the yeast Pichia guilliermondii ZIM624 showed a 50-fold higher production of malvidin-3-O-glucoside-4-vinylphenol in model wine containing Pinot Noir anthocyanin skin extract[75]. However, non-Saccharomyces yeasts are typically less efficient in ethanol conversion and tolerance during wine fermentation. Therefore, mixed inoculation with Saccharomyces cerevisiae is commonly used to ensure successful completion of the fermentation process. Except for yeast species, the way of inoculation is also a crucial factor in pyranoanthocyanins synthesis. Studies have shown that fermentation with sequential inoculation can nearly double the content of pyranoanthocyanins in comparison to co-inoculation[74].
In conclusion, the inoculation of yeasts with high production of acetaldehyde, pyruvate, and hydroxycinnamic acid decarboxylase, and low adsorption of anthocyanins can effectively increase the production of anthocyanin derivatives and reduce wine color loss. This approach may be a promising strategy for enhancing wine color and quality.
Blending
-
Blending is a commonly used technique in the wine industry to enhance wine quality by balancing the chemical composition of different wines and addressing flaws that may be present. Additionally, blending can also be used to manage the color of wine by combining grapes or wines with different anthocyanin profiles.
Wines with low inherent pigment content benefit from the addition of teinturier grape varieties to increase color intensity. Wines, in some cases, may have a deeper yellowish color and low stability of anthocyanins. In such instances, blending in wines with higher levels of flavonols and flavanols, such as Marselan and Petit Verdot, can enhance copigmentation and polymerization, improving the color stability of the wine during the oxygen exposure process[76]. A study conducted from 2008 to 2011 involving 160 single-varietal and blended commercial wines further proved that blending increased the copigmentation and chroma values of young wines[77]. However, a study reported that the color difference values after 100 d of aging was higher in all blended wines than single wines. It is the blending behavior, rather than the level of chemical proportion that is responsible for color instability, because different blending ratios ranging from 0−100% were relatively consistent[78]. The definitive impact of blending behavior and ratio on the color stability of aged wines remains to be confirmed by further research.
The blending percentage and wine characteristics affect the outcomes. Therefore, small-scale trials must be conducted before large-scale blending operations. Moreover, the timing of blending should not be overlooked. Blending grapes prior to fermentation allows for more prolonged interaction and better color stabilization during aging compared to blending after fermentation. However, this approach poses significant challenges in oenological practice, such as the need for simultaneous grape harvesting and stricter contamination control.
Supplying micro-oxygen environments for wine
Aging in barrels
-
Barrel aging is a traditional method used to create a superior micro-oxygen environment for wine. The vascular bundles in the barrel wall introduce oxygen into the wine during the aging process, maintaining a constant oxygen concentration of 0.1−0.5 mL/L/month.
Depending on the oak species and the growth environment, the density and thickness of the barrel walls alter the amount of oxygen that can pass through the walls. The oxygen transfer rate (OTR) of oak barrels is also significantly influenced by workmanship, like the toasting temperature and degree of usage[3]. Typically, OTR is higher in American barrels than French barrels[79] and higher in new barrels than old barrels. Oak barrels with a higher oxygen permeability are better for improving color intensity during barrel aging for 3 to 12 months, but may results in a visible increase in yellow hue during later bottle aging. In more recent research, Martínez-Gil et al. found that wine receiving lower oxygenation formed more polymeric pigments and accelerated the release of low molecular weight wood compounds than wine aged in higher oxygenated barrels[80]. Therefore, oak barrels with lower oxygen permeability are preferred for ensuring the stability of long-aged wine.
In addition to providing a micro-oxygen environment, oak wood in contact with wine also releases ellagitannins and other polyphenols. The oxidative nature of ellagitannins contributes to the formation of ethylidene-bridged anthocyanin-tannin adducts through the formation of hydroperoxyl radicals, which reduces the loss of monomeric anthocyanins. Furthermore, ellagitannins have also been shown to bind covalently to a wide variety of grape-derived compounds, including flavanols and thiols, altering wine flavor and aroma. Last but not least, due to their chemical structure, ellagitannins are naturally the first barrier against phenolic compound oxidation, but they can be degraded during barrel processing, especially during toasting. Higher toasting temperatures and longer toasting durations lead to greater degradation of ellagitannins. Therefore, to improve color stability through barrel aging, low to medium toasted barrels should be taken into consideration.
Aging in other tanks
-
Aging in other tanks represents an emerging way for a micro-oxygenated environment by artificially providing the wine in stainless steel tanks with continuous oxygen. The oxygen supply by micro-oxygenation is generally higher than oak barrel aging, and is between 10 and 30 mL/L/month[81]. Oak surfaces are suitable for dangerous yeast strains like Brettanomyces, while aging in stainless steel tanks reduces the risk of wine contamination and saves the cost of oak barrel purchase.
Both tank aging and oak aging can reduce the content of monomeric anthocyanins, and increase polymeric anthocyanins over a short-term period. After 6 months of bottle aging, wine aged in oak barrels forms more anthocyanin derivatives[82] since oak barrels enrich wine with ellagitannins, phenolic acids, and xylenol. Alcalde-Eon et al. used model wine to demonstrate how ellagitannins facilitate the cyclization of pyruvate and anthocyanin in pyranoanthocyanins formation[26], further indicating that apart from creating micro-oxygenated surroundings, the abundant phenolics in oak barrels also accelerate anthocyanin stabilization[83]. Therefore, micro-oxygenation is combined with the addition of wood chips, wood blocks, and oenological tannins to better replicate the effect of oak barrel aging on wine sensory quality. Lisanti et al. discovered that wine micro-oxygenated for 15 d by adding wood chips and blocks caused a 4%−14% decrease in monomeric anthocyanins and a 7%−21% increase in anthocyanin polymerization, while it took wine aged in oak barrels 6 months to achieve the same effect. However, the micro-oxygenation effect did not enhance wine vanilla, smoke, and toast flavors as much as oak barrel aging[84]. Micro-oxygenation presents a larger interaction area and higher oxygen transmission rate between oak products and wine. Although it has less effect on wine flavor, micro-oxygenation is more efficient for the polymerization of anthocyanins and proposes an instant method to stabilize wine color.
Furthermore, except for stainless steel, other tank materials also provide specific conditions for oxygen permeability. The physical and chemical parameters of tanks determine their permeability, including thickness, size, and materials. Concrete samples exhibit OTR values between1.08 and 1.19 × 10−9 cm3/m2 d. Additionally, earthenware samples vary from 0.12 to 14.65 × 10−4 cm3/m2 d, while claystone samples range between 0.11 and 0.22 × 10−4 cm3/m2 d, and granite samples are between 1.03 × 10−6 and 1.86 × 10−6 cm3/m2 d[85]. Consequently, when wine is aged in tanks, anthocyanin succession is influenced by distinctive oxygen accessibility. Maioli et al. discovered that after 6 and 12 months of aging in tanks and 6 months in glass bottles, Sangiovese wines aged in earthenware raw amphorae and uncoated concrete registered a higher content of polymeric pigments than wine aged in new oak barrels, better promoting wine color stabilization[86].
Due to variations in the levels of antioxidant compounds such as phenolics, sulfur dioxide, and ascorbic acid, different wines have distinct capacities for micro-oxygenation. Wines with lower total phenol indexes tend to exhibit greater evolution and more substantial variability, and even trace amounts of oxygen may increase the risk of over-oxidation[87]. Therefore, the availability and method of oxygen exposure during the aging process must be determined by the specific characteristics of the wine.
Regulating maceration and aging temperature
-
Maceration temperature affects color intensity and stability. Cold maceration extracts more anthocyanins and less other phenolic compounds. Therefore, less macromolecular pigments form via polymerizing with tannins in a cooler environment, providing better protection for monomeric anthocyanins. Regarding the formation of derivative anthocyanins after cold maceration, contradictory results have been reported. Several studies indicated that cold maceration increased polymeric pigment formation while others found no effect[88]. Cejudo-Bastante et al. reported a detrimental effect of cold maceration on the color stability of Shiraz wine[89]. The different research results may be related to the initial ratio of anthocyanins to flavanols in the must. It is assumed that cold maceration may benefit grapes with less anthocyanins by enhancing the extraction rate and promoting the formation of derivative anthocyanins, but have minimal impact on grapes with abundant anthocyanins and less reaction substrate for derivatives.
Alcoholic fermentation is also a continuous extraction process and usually holds a higher temperature. In contrast with pre-fermentative cold maceration, alcoholic fermentation mainly extracts the tannins that can be found in seeds and skins. In addition, fermentation temperature has an impact on yeast metabolism. At higher temperatures (above 25 °C), yeasts produce more related metabolite, such as acetaldehyde, pyruvate, and hydroxycinnamic acid decarboxylase, which plays as a synthetic precursor for derived anthocyanins.
During wine storage, low temperatures inhibits anthocyanin deterioration. Hellstrom et al. found that anthocyanins were retained in fruit juices at various temperatures, with a half-life of 3 weeks at 21 °C, 9.8 weeks at 9 °C, and 20.3 weeks at 4 °C[90]. However, a relatively high temperature can stabilize wine color. Gomez-Plaza et al. discovered that the wine synthesized more polymeric anthocyanins and displayed stronger color intensity after a year of bottle aging at 20 °C even if the total anthocyanin content was lower than wine aged at 15 °C[91]. Arapitsas et al. further proved that Sangiovese wines stored at higher temperatures (20–27 °C) than those kept at relatively low temperatures (15–17 °C) encouraged more flavanol hydrolysis and pinotin formation[92]. A recent study indicated that model wine formed more vitisin A (21.3%) when artificially aged at 30 °C, but it disregarded wine quality[75]. Nevertheless, the quality of the wine can be adversely affected by excessively high aging temperatures. Over-high temperatures also causes ring opening of anthocyanins, forming carboxylic acids and trihydroxybenzaldehyde by proton transfer and rearrangements[93]. For example, Malbec wines stored at 25 °C displayed a perceptible color difference (E*ab > 3) to average observers at 6 months, while those stored at 15 °C did so until 15 months[94]. Additionally, wines aged at low temperatures link to adorable sensory perceptions, whereas aging wine at above 25 °C resulted in an unacceptable dry vegetable aroma[94].
Therefore, low temperature aging benefits wine aroma preservation and monomeric anthocyanins stability, but it restrains polymerization reactions. However, excessively high temperatures undoubtedly accelerate wine oxidation, dissipate wine aromas, and rapidly cause a brown hue. The ideal temperature for wine color maturation is between 15 and 20 °C, which can support anthocyanin derivation and give wines a stable orange-red hue.
-
Wine color degradation is typically of concern during long aging periods. However, color changes during storage are determined by various factors, including grape cultivation, maceration, fermentation processes, and more. Firstly, grapes with different genomes have unique capacities for the synthesis of specific anthocyanins. In the same variety, the growth environment, such as light, temperature, and irrigation, affects anthocyanin formation. Secondly, during fermentation, the non-covalent connections formed between anthocyanins and copigments enhance molecular interaction and protect anthocyanins from nucleophilic attacks. Yeasts release pyruvate, acetaldehyde, and hydroxycinnamic acid decarboxylase, which accelerates the transition from free anthocyanins to derivative forms. Last but not least, aging conditions, especially oxygen, pH and temperature, also play a significant role in wine color stability. Consequently, future studies are needed to further explore practical and effective technologies for color stability in the field of enology.
Currently, there has been some progress in improving the color of wines, and Table 1 summarizes the methods available for enhancing the color of wines. However, the colors of most non-grape wines are quite fragile and may degrade significantly during the aging process. Although researchers have found that diglycosidic anthocyanins are mainly responsible for this issue, effective and targeted approaches to solve this defect have not been developed. Direct hybridization of non-vinifera and Vitis vinifera retains only about half of the diglycosidic anthocyanins, and the color stability remains low. Furthermore, hybridization also changes the unique flavor and resistant ability of non-vinifera. With the advancement of gene editing technology, methods need to be developed to knock down the gene controlling the synthesis of 5-O-glucosyltransferase or inhibit the expression of the transferase to improve the aging potential of non-vinifera.
Table 1. Examples used in improvement of anthocyanin stability.
Species Methods Outcomes Reference Cultivation environment Grapes Cabernet Sauvignon Spike thinning Total and acylated anthocyanins increased. [95] Frontenac, Marquette, and Petite Pearl Pruning The percentage of polymeric color increased by rising berry temperature and photosynthetically active radiation. [56] Nebbiolo Leaf removal The 3′-hydroxylated anthocyanin decreased and the 3′,5′-hydroxylated anthocyanin increased. [96] Cabernet Sauvignon and Merlot High altitude Total tannins increased. [57] Cabernet Sauvignon Deficiency irrigation Gene expression levels in the anthocyanin biosynthetic upregulated and the proportion of Malvidin-3-O-glucosides increased. [60] Maceration Grapes Primitivo Stems addition The rate of polymeric anthocyanins increased, resulting in greater color stability. [62] Cabernet Sauvignon and Nebbiolo Exogenous enzymes addition Extract more anthocyanins from skins and reduce the anthocyanins degradation. [63] Copigmentation Grapes Syrah Overripe grape seeds addition Increased chromatic stability, improved chroma values and bluish hues [70] Syrah American barrel wastes Wine color chromatically more stable [67] Merlot Phenolic extracts addition Color loss reduction in 3 months [68] Mannoproteins additionn The color stability and antioxidant capacity improved [97] Yeast Yeast Metschnikowia pulcherrima, Zygosaccharomyces bailii, Candida zeylanoides, and Torulaspora delbrueckii Mixed inoculation with S. cerevisiae Pyruvate and acetaldehyde content increase, promoting polymeric anthocyanins formation. [46,73] Pichia guilliermondii, S. pombe, and Wickerhamomyces anomalus Mixed inoculation with S. cerevisiae Hydroxycinnamic acid decarboxylase increased, resulting in more pyranoanthocyanins synthesis. [75] Wine Blending wine Outcomes Blending Cabernet Franc, Cabernet Sauvignon Marselan and Petit Verdot a* and red hue increased; b* and yellow hue decreased. [76] Aging time Methods Outcomes Micro-oxygen 3~6 months Barrel aging and tank aging with micro-oxygen Monomeric anthocyanins decreased and vitisin-related anthocyanins increased. Oak matured wine showed a more stable color after six months botting aging, [82] 12 months Barrel aging Wine aging in lower oxygen permeability barrel receives more polymeric anthocyanins. [80] 15 d Tank aging with adding wood chips and blocks Monomeric anthocyanins decreased 4%−14%, polymeric anthocyanins increased 7%−21%. [84] Temperature Method Outcomes Temperature 20 °C Bottle aging at 20 °C Synthesize more polymeric anthocyanins than wine aging at 15 °C. [91] 20−27 °C Bottle aging at 20−27 °C Synthesize more pinotin than wine aging at 15−17 °C. [92] Considering that acylated anthocyanins are resistant to color fading and play important roles throughout the aging period, further research needs to apply chemical and enzymatic methods to promote acetylation in the winemaking sector. Although research exists on improving acylation ratios in industrial pigments, there are no reports on wine anthocyanins. The effects of organic acid addition and enzyme catalysis on anthocyanin acylation need to be assessed in wine. Furthermore, Vv3AT has been shown to encode a BAHD acyltransferase protein, and the lack of acyltransferase in Pinot Noir is attributed to a nonsense mutation in Vv3AT[98]. Adopting gene engineering to transfer foreign genes in vitro into grapes with a large ratio of non-acylated anthocyanins may provide another approach to changing anthocyanin profiles in grapes.
Copigmentation between anthocyanins and polyphenols decreases with wine aging, and the concentrations of polymeric anthocyanins increase. Copigmentation is generally considered as the first step for covalent bond linkage in the formation of polymers, but the necessity and holding time of non-covalent bond formation before covalent bonds are unclear. The factors that interfere with the anthocyanin transition from copigmentation to their polymerized forms and methods to improve the transition efficiency also need to be clarified. Further research is necessary to establish a solid foundation for the relationship between copigmentation and polymerization.
-
The authors confirm contribution to the paper as follows: study conception and design: Zhan J, Huang W, You Y, Cheng S; data collection: Cheng S, Wu T, Gao H, Han X; analysis and interpretation of results: Cheng S; draft manuscript preparation: Zhan H, Cheng S. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This work was supported by the National Natural Science Foundation of China (U21A201207-1). The mechanism of microbial role in the formation of flavor characteristics of wines from the eastern Helan Mountains.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Cheng S, Wu T, Gao J, Han X, Huang W, et al. 2023. Color myth: anthocyanins reactions and enological approaches achieving their stabilization in the aging process of red wine. Food Innovation and Advances 2(4):255−271 doi: 10.48130/FIA-2023-0027
Color myth: anthocyanins reactions and enological approaches achieving their stabilization in the aging process of red wine
- Received: 19 June 2023
- Accepted: 08 September 2023
- Published online: 06 November 2023
Abstract: Color is a crucial sensory indicator of wine quality. However, changes in anthocyanin concentration and profile occur during wine aging, resulting in noticeable reductions in chroma and shifts in hue from purple to brick red. This is because monomeric anthocyanins degrade and derivative anthocyanins form. The rate of color changes can vary depending on complex factors, such as the anthocyanin content of the must, oenological technology, and environmental conditions, which makes the management of red wine color evolution challenging. To address this issue, appropriate winemaking techniques are required to achieve an elegant wine color. This review summarizes the mechanisms related to anthocyanin stability, including glycosylation, acetylation, and derivatization. The review also discusses factors influencing red wine color fading for specific grape varieties and wine appellations, offering time- and cost-efficient techniques to accelerate anthocyanin derivatization and color stabilization.
-
Key words:
- Wine color /
- Color stability /
- Wine aging /
- Anthocyanins