-
Mycorrhizae are a type of symbiotic relationship that occurs between fungi and plant roots. There are two broad types of mycorrhiza: ectomycorrhizae and endomycorrhizae. Endomycorrhizae are further classified into five different kinds of mycorrhizal associations. Ectendomycorrhizas are another broad category of mycorrhizal association between plant roots and fungi[1−3]. Ectomycorrhizae form a sheath around the roots of certain plants, such as pines, oaks, and eucalyptus. The fungi involved in ectomycorrhizae forms a thick layer around the roots known as fungal sheath or mantle. They also penetrate between the epidermis and cortex through a structure known as the Hartig net. This allows for the exchange of nutrients and water between the plant and the fungus[4]. Ectomycorrhizae are important for the health and growth of trees in forests, as they help to improve the uptake of nutrients and water from the soil. Endomycorrhizae, on the other hand, form a symbiotic relationship with the plant by actually invading the cells of the root[4,5]. Endomycorrhizae can be categorized into different types, which are summarized in the following paragraph[6].
Monotropoid mycorrhiza are characterized by the fact that the fungus is unable to form a visible sheath around the roots of the host plant, and instead forms a diffuse, intraradical mycelium that is intermingled with the plant's own root tissue. The second type of endomycorrhizae are Ericoid mycorrhiza. There is a symbiotic relationship between certain species of ericaceous plants (such as blueberries, rhododendrons, and heaths) and a group of fungi known as ericoid mycorrhizal fungi (EMF). In this relationship, the EMF colonize the roots of the host plant and form a dense network of fungal structures called hyphae, which increase the plant's ability to absorb water and nutrients, particularly phosphorus. Ericoid mycorrhiza is considered a 'mycoheterotrophic' symbiosis, meaning that the host plant is dependent on the EMF for carbon, which is obtained by the fungus through its association with other plants. The third type are Orchidaceous mycorrhizae which are mutualistic associations between orchid plants and fungi. These relationships are formed in the roots of orchids, where the fungus colonizes the root cells and provide the plant with essential nutrients, such as phosphorus. In return, the orchid provides the fungus with organic carbon through photosynthesis. Orchid mycorrhizae are considered to be obligate, meaning that the orchid cannot survive without the fungus and vice versa. Instead, they rely on the fungus to provide them with this essential nutrient. The most common type of endomycorrhizae, Arbuscular mycorrhiza, are a group of soil-borne, obligate symbionts that form mutualistic associations with the majority of land plants. These fungi colonize plant roots, forming unique structures called arbuscules within the root cells. In exchange for sugars produced by the plant through photosynthesis, the fungi provide the plant with phosphorus and other essential nutrients that are otherwise difficult for the plant to acquire from the soil. AM fungi are ancient symbionts that have evolved alongside plants for over 400 million years, and they play important roles in ecosystem functioning and plant productivity[7−10]. Various kinds of mycorrhizal associations are described below in Fig. 1.
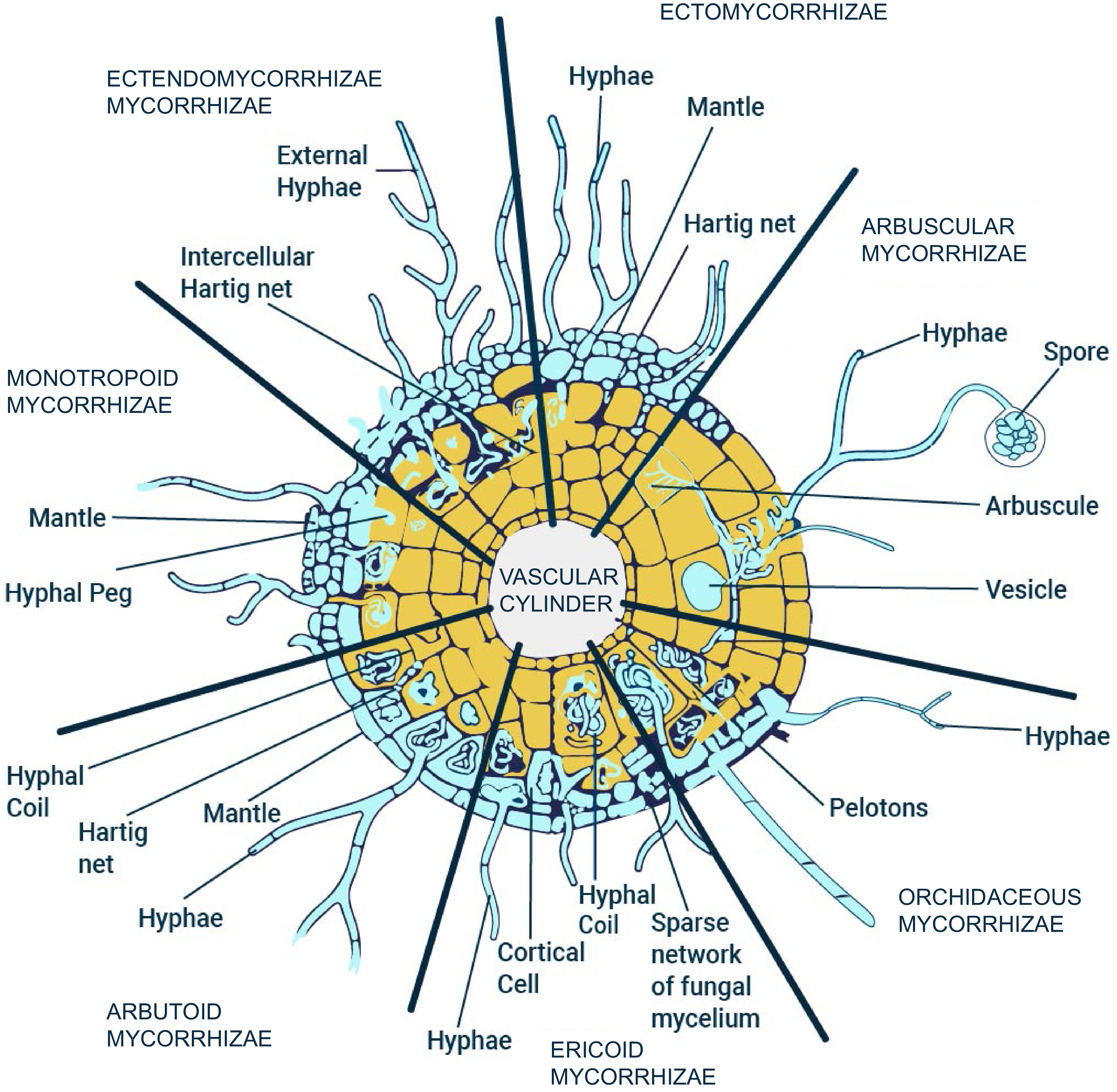
Figure 1.
Different types of mycorrhizal symbiosis in nature: Ectomycorrhizae, Monotropoid mycorrhizae, Arbutoid mycorrhizae, Ericoid mycorrhizae, Orchidaceous mycorrhizae, and Arbuscular mycorrhizae. (Modified image from Selosse & Le Tacon[11])
Soil microorganisms such as AMF reflect a crucial connection between plants and mineral soil nutrients. They are also gaining rising attention as natural fertilisers. AMF are mandatory symbiotics of the phylum Glomeromycota[12], establishing mutualistic symbiosis, with about 80% of the land plants, including many food crops. In return for photosynthetic materials, they provide the host plant with mineral nutrients and water[13]. Some benefits provided by AMF in plant growth and yield are depicted in Fig. 2.
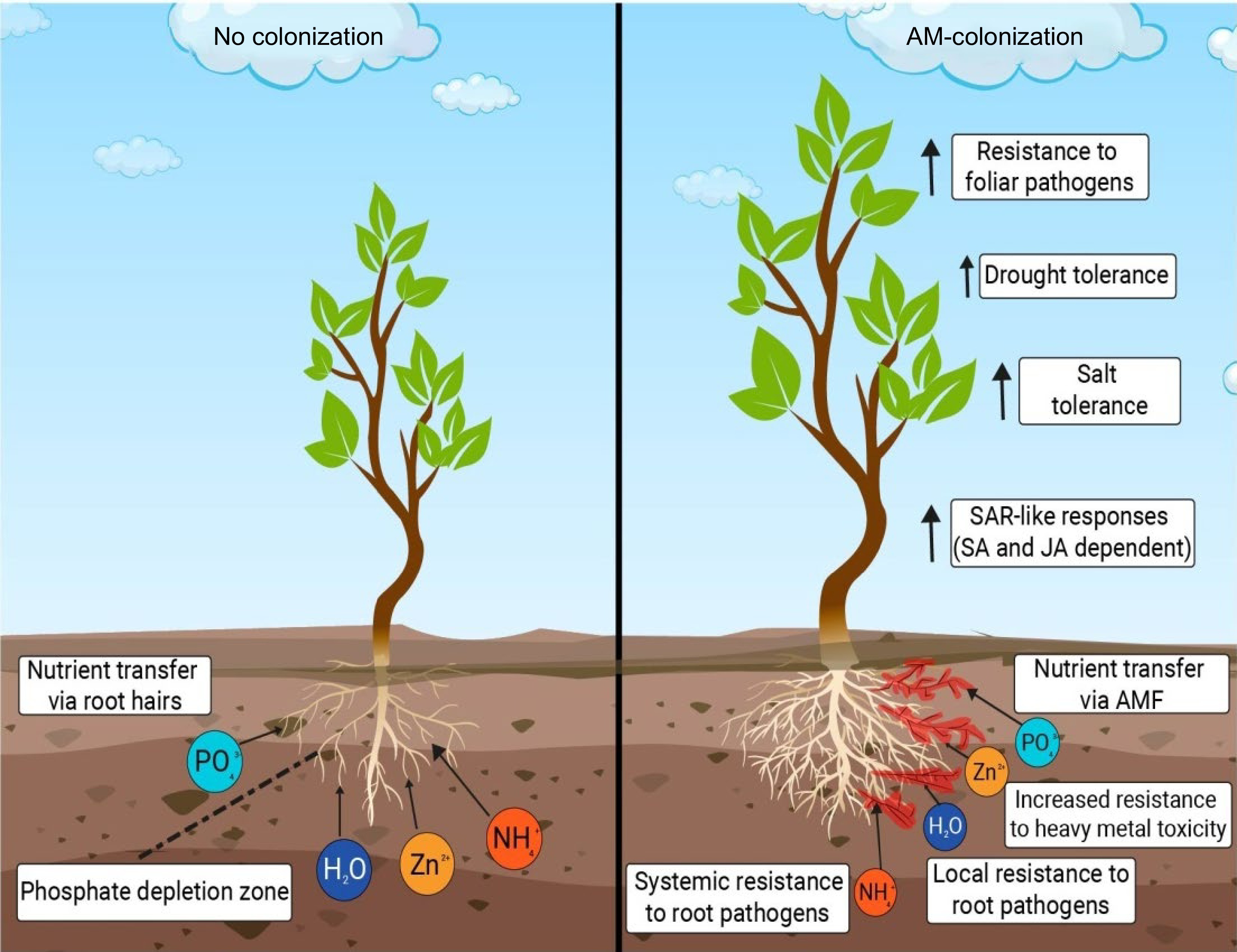
Figure 2.
A pictorial representation of a few of the benefits provided by AMF in plant growth and yield. Increased resistance to foliar pathogens, increased drought tolerance, increased salt tolerance, enhanced defense mechanism, help in uptake of essential nutrients and bioremediation are a few of the many benefits that AMF provide in the growth and development of a plant.
AMF mycelium from the root system is able to extract nutrients from soil volumes that are inaccessible to the roots[14]. In comparison to plant roots, fungal hyphae are far thinner and can reach narrower pores[15]. AM fungal hyphae colonise root cortex predominantly by forming profusely branchy structures inside the cells, i.e. arbuscules, which are known as the functional nutrient exchange sites[16]. AMF therefore eliminates plant growth restrictions imposed by an insufficient supply of nutrients[17,18]. In recent times a non-mycorrhizal state can be regarded as rare in natural habitats for most organisms[19], even though the AM fungal populations below the ground are significantly different depending on the species composition, soil and seasonal form or variation of these factors[19]. AM association provide plants with additional advantages, in addition to an increased food source, such as enhanced drought and salinity resistance[20,21]. While several studies have been performed on the impact of AM symbiosis on plant reaction to abiotic stress such as drought, salinity and flooding in the last few years , the processes that have contributed to an improved plant stress resistance still remains somewhat elusive[20,22−25]. Metals such as Iron (Fe), Copper (Cu) and Zinc (Zn) perform important functions in a variety of sub-cellular compartments, but they constitute a highly reactive community of elements that are toxic at large concentrations[26].
AMF have been documented to minimise the toxicity of heavy metals in host plant and to withstand high metal concentrations in soil[26−30]. Metal transporters play a vital function in homeostasis of heavy metals. A Zn transporter was identified in Glomus intraradices (GintZnT1)[31] and multiple putative genes coding Cu, Fe and Zn transporters were identified in a genome-wide study of the recently published Rhizophagus irregularis (formally, Glomus intraradices) genome[32]. The next steps would be characterisation of these carriers and discerning their role in the symbiosis. AMF may also have a significant influence on the environment, as they promote soil structure and aggregation[33−36] and control the development and production of plant populations[37].
The impact of AMF symbiosis has also recently been studied on greenhouse gas (GHG) emission[38,39]. Evidence presented by Bender et al. suggests that AMF may have a role to play in climate change mitigation due to their ability to significantly reduce emissions of N2O, a key greenhouse gas. By enhancing plant nitrogen (N) absorption and assimilation, AMF may be able to reduce N2O emissions by decreasing soluble N in soil and, in turn, denitrification[38]. Correlations between AMF abundance and genes involved in primary N2O production (nirK) and consumption (nosZ) suggest that AMF promote shifts in soil microbial biomass and community composition that lead to reduced N2O emissions. According to Lazcano et al., AM symbiosis aids in N2O emission management at high soil moisture levels, and it was suggested that AM plant N2O emission control may be mediated by higher soil water use rather than increased N absorption[39].
Therefore, AMF are primary biotic soil elements, which, when absent or degraded, for instance, by anthropic input, will contribute to a less effective functioning of the ecosystem. The process of re-establishing AMF may be a promising solution to industrial fertilisation methods in order to achieve organic cultivation, a significant goal for farmers in the midst of a global recession and an environmentally friendly consumer[40,41] . The key technique for achieving this aim is to directly reintroduce AMF (inoculum) propagules in the target soil. However, in the application of these fungi, awareness of how AMF adapts and reacts to the objective of soil management and ecosystem management and the events which result in a functional symbiosis, including the mechanisms involved in the transfer of nutrients is important[42]. This paper gives a brief discussion of the latest studies on the nutritional aspects of AM symbiosis and a short description of the challenges of development of AMF inoculum, descriptions of the application of AM fungi are mentioned and addressed, both under regulated and open field conditions, with specific emphasis on identifying factors contributing to success of the biofertilizer.
-
The symbiosis of AMF with plants was originally discovered 400 million years ago[43]. Such links are a series of biological processes that have beneficial impacts on the ecosystems of both wild and cultivated biota[44]. An example of a reciprocal interaction that may regulate the growth and advancement of a plant is the symbiotic relationship between AMF and its partners. The plant's roots may absorb nutrients that would not otherwise be available thanks to the mycelial fungal network that has grown throughout them[45]. The fungal mycelium colonises the roots of several plants, even if they are from different species, and creates a shared mycorrhizal network. According to Pringle et al.[46], this mycorrhizal network is regarded as the main element of the terrestrial ecosystem for many plant populations, including invasive species, and it facilitates the transfer of phosphorus (P) and nitrogen (N) to plants via fungi[13,47]. They may improve soil qualities and encourage plant development in both natural and challenging environments[48−50]. Plant resilience to harsh environments is increased by AMF colonisation, which results in several improvements in morpho-physiological traits[49−52]. Researchers encourage the use of AMF as influential bio-fertilizers in sustainable agricultural production, having been employed as bioinoculants[53] . In comparison to untreated soils, AMF-inoculated soil often forms more consistent masses and much more extraradical hyphal mycelium[54] . Glomalin-related soil protein is thought to keep soils with complex abiotic stressors moist[55], which then controls water levels between soil and plants and naturally promotes plant development. Glomalin contains 30%–40% carbon (C) and related chemicals to protect soil from desiccation by enhancing its ability to retain soil-water[56]. Growth-related processes that affect AMF inoculation include stomatal conductance, leaf water capacity, relative water content (RWC), PSII quality, and CO2 assimilation[57,58]. By altering the biochemistry of the organ and tissues above ground, AMF also contributes to improving water stress tolerance[24]. Additionally, AMF inoculation promotes the accumulation of dry matter and improves moisture uptake, boosting plant tolerance to stressors like salt and drought. AMF extraction for plant growth in various biological conditions will significantly boost organic farming's ability to promote growth and raise production[59].
Here are the key characteristics of AM fungus symbiosis:
a) Establishment: The AM fungus forms a symbiotic relationship with plant roots through the process of colonization, in which the fungus penetrates the root cells and forms structures known as arbuscules[60].
b) Nutrient exchange: The AM fungus enhances the plant's nutrient uptake by extending the absorptive surface area of the root system and solubilizing soil nutrients. In return, the plant provides the AM fungus with carbon[61,62].
c) Soil structure improvement: AM fungi can contribute to soil structure improvement through the production of extraradical mycelium, which helps bind soil particles and improve soil stability[40].
d) Enhanced plant growth: The symbiotic relationship between AM fungi and plants results in improved plant growth and health, with higher root and shoot biomass, enhanced root system architecture, and increased stress tolerance[45].
e) Wider host range: AM fungi have a wide host range, forming symbiotic relationships with many different plant species, including some of the most important crops such as maize, wheat, and soybean[4, 63−66] .
f) Environmental importance: AM fungi play a crucial role in the ecosystem, by improving soil fertility and plant productivity. They also help to maintain soil health and reduce soil degradation by contributing to soil aggregation and water retention. Overall, the AM fungus symbiosis is a vital component of the terrestrial ecosystem, providing many benefits to plants and the environment[67,68] .
-
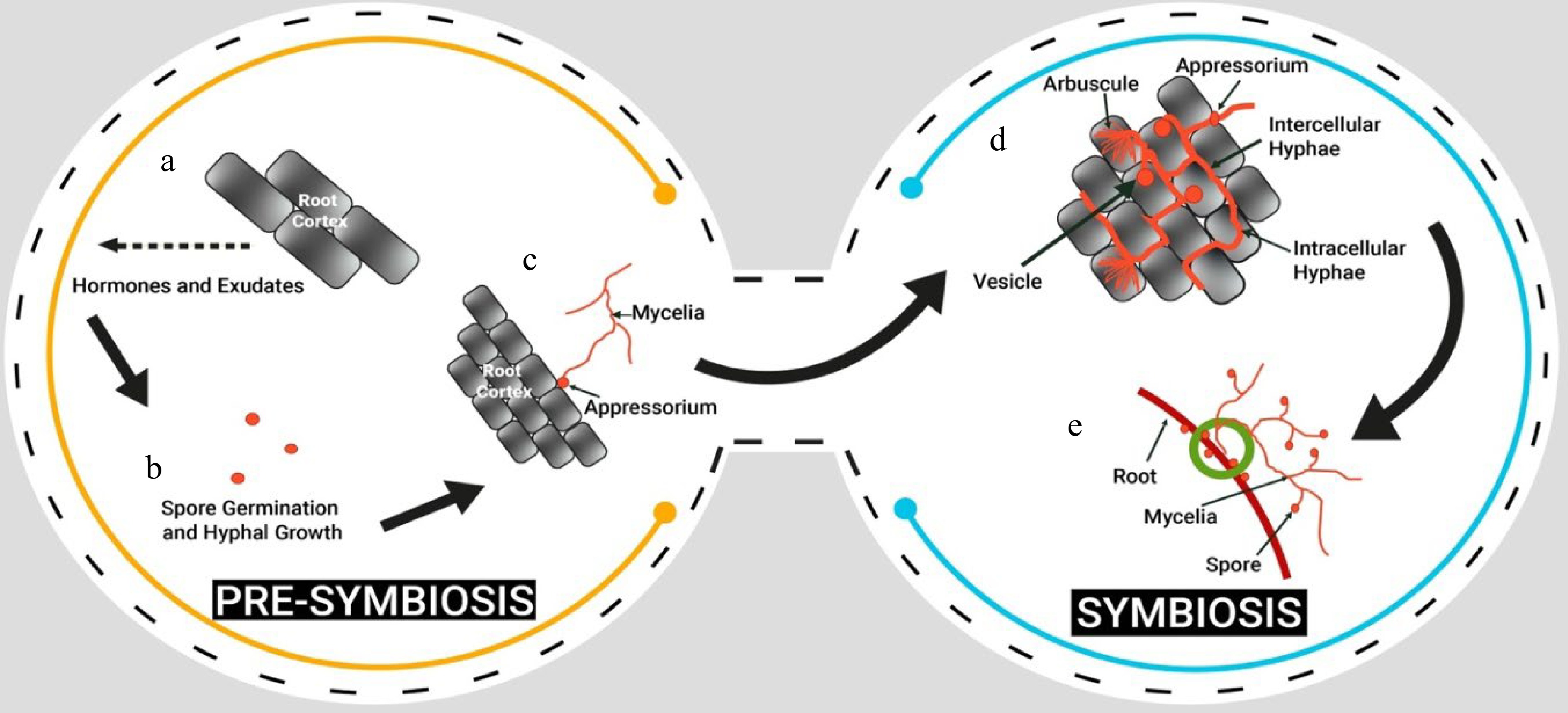
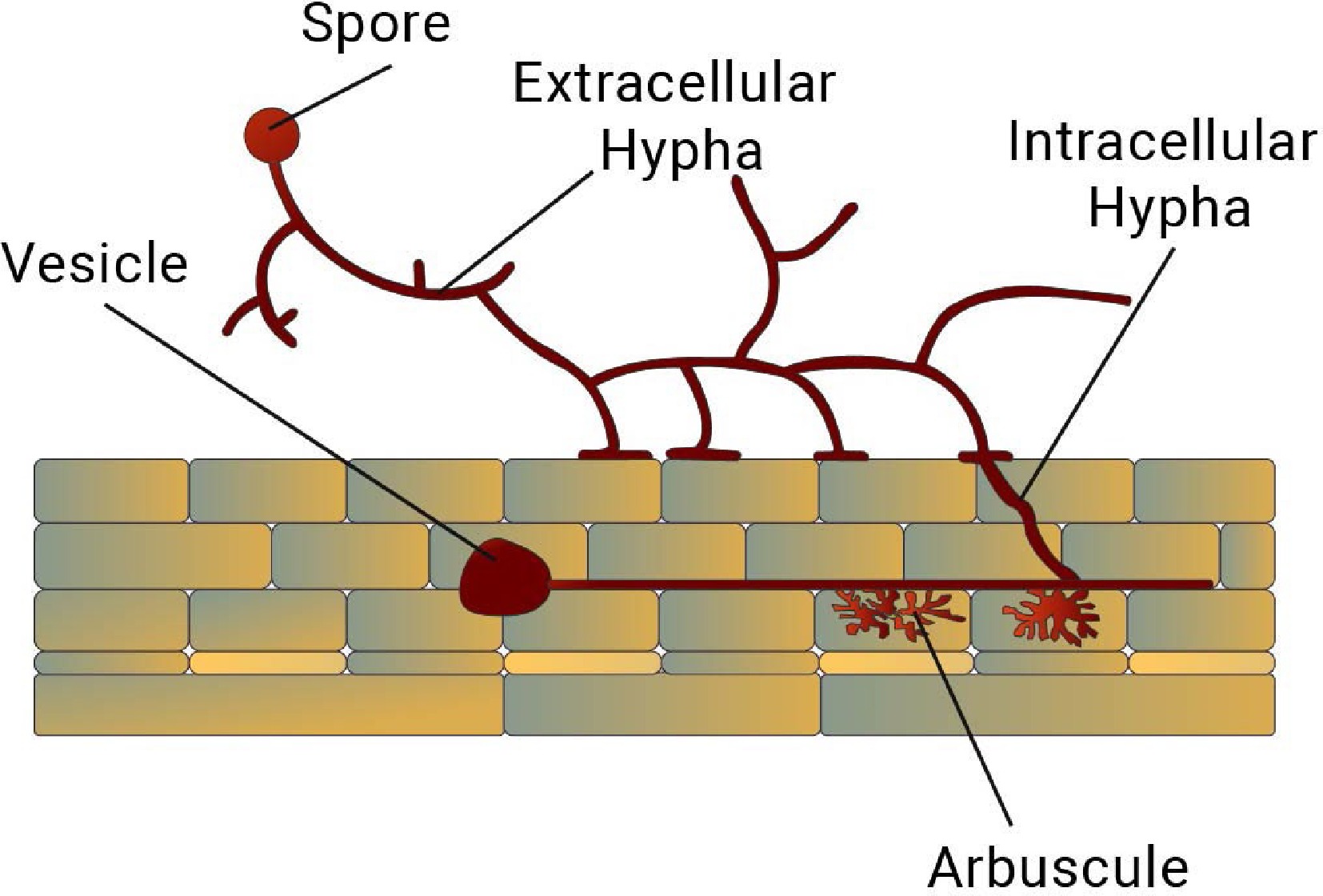
The life-cycle of AMF is basically divided in two stages; pre-symbiosis and symbiosis (Fig. 3). Pre-symbiosis is characterized by the following steps: Plant root exudating certain chemicals, spore germination and hyphal growth, and attachment of appressorium to the root. This step is followed by the symbiosis stage: the hyphae of the AMF colonize the root system of a host plant by penetrating the root cells and form arbuscules, and vesicles[69,70] . The hyphae of the AMF extend into the soil and increase the absorptive surface area of the root system (Fig. 4). The relationship between the AMF and the host plant becomes established, and the fungus continues to grow and spread throughout the root system. Extraradical mycelium also gives rise to formation of chlamydospores on soils at maturity. When the host plant dies, the AMF begins to decompose the plant's organic matter and return nutrients to the soil. The AMF spores, in time will make association with other plants and continue its life cycle.
-
Reviewing the work from the past few decades, the most successful form of AMF propagation was the use of trap cultures using certain plants as a host species, and it accounted for almost 75% of AMF propagation and interestingly, only marginalised usage of other approaches was observed. Approaches such as aeroponics and hydroponics, are also contributing to the development of pure clean spores and the maximisation of the host plant growth conditions[71]. The monoxenic root organ culture is another approach that permits the effective large-scale propagation of AMF that can be directly used as inoculum. The system consists of culture of inoculated excised roots (the so-called hairy roots), which have acquired the capacity to proliferate without developing any epigeous portion, after processing with the soil-borne plasmid Ri (root-inducing) Agrobacterium rhizogenes[72]. Within a few months, a significant number of spores, mycelium and colonised roots were produced[73]. Since, AMF may use a variety of propagules in order to expand and colonise new roots with different levels of efficiency[74], the selection of the source of the inoculum is a factor of primary importance for a successful colonisation. Spores, mycelium fragments fragmented from the lower hyphal network and other complexes inside both living and dead root fragments are all components of the extraradical and intraradical systems of AMF. The main cause of regrowth for some AM fungal organisms was in specific intraradical vesicles[75]. Different AM fungal taxonomic ranks vary in their capacity to disperse from a given propagule. The propagation of mycelial fragmentation appears to be of greater importance for organisms of the Glomeraceae family, whereas for representatives of other groups such as Gigasporaceae, Acaulosporaceae and Scutellosporaceae spore germination would be preferential methods of propagation[76]. The most effective and user-friendly method to apply a multi species inoculum, as propagation through trap culture is the most widely utilised strategy, is to sieve the substrate and finely cut the root of the trap culture plant so that all the various kinds of fungal propagules (crude inoculum) can be retrieved.
-
In most of the reviews studied, the latest general tendency is to pursue one or more types of AM fungi for individual inoculation (monospecies inoculum). Gosling et al.[77] have examined the impact on plant growth after inoculating diverse AM fungal populations with functionally distinct characteristics, fewer fungal species that are able to mitigate stress are likely to be of utmost benefit to the hosts when a host plant is subjected to one cause, such as greenhouse experiments. In the case of shoot biomass, single species inoculation experiments are more effective than inoculation experiments with more than one species concurrently used. Another greenhouse research has shown that species composition instead of variety may be more critical in deciding how the species works[78].
According to Opik et al.[79], for single species inoculation, three AMF species viz. Rhizophagus intraradices, Rhizophagus irregularis and Funneliformis mossae are subjected to most scientific studies because these are extremely versatile symbionts that can colonise a wide range of host plants, maintain long-term storage, disperse widely across the world and quickly and massively reproduce. These species have been ideal for premium inoculum components due to the above described characteristics. Several studies have shown that different isolates from the same species can have a greater impact on plant response compared to the variations among different species[80−82]. This suggests that the exclusive use of individual AMF organisms such as R. intraradices, R. irregularis and F. mossae should not be considered a drawback in inoculation experiments as these species may have significant functional diversity. In this context, in the presence of R. irregularis reference genome[32,83] the partial genome re-sequencing of several isolates from various geographical backgrounds can open the door to exploring the roles of genetic variation in AMF communities so that it is possible to develop and choose more successful and productive AMF for crop plants. Another factor that needs to be addressed is that the receptivity of plant organisms, including seeds, to AMF inoculation differs greatly[84−86]. The plants' reaction to AM fungi can be used as a selection function in modern farming, resulting in varieties or cultivars with different genetic differences.
-
Bio-fertilizers are a combination of naturally occuring microbes used to increase soil fertility. These fertilisers are very useful for soil health and plant growth[87]. Various scientific experiments on AMF in the last two decades have demonstrated their innumerable benefits in terms of soil quality and crop productivity. Therefore, it is commonly assumed that AMF may, in the foreseeable future, be seen as a substitute for inorganic fertilisers, since mycorrhizal applications will effectively reduce the quantitative use of the chemical fertiliser input, especially phosphorus[88]. Owing to the adverse influence on food safety, crop health, and air and water systems by inorganic fertilisers, herbicides and fungicides, the continued usage of these have triggered numerous land, plant and human health concerns[89]. AMF may be able to minimise chemical fertiliser usage up to 50% for optimal agricultural output, although this calculation depends on the variety of plant species and the prevalence of stressful environments.In order for sustainable agriculture to be accomplished, AMF as a biofertilizer becomes more significant because the proper treatment of these symbiotic fungi might significantly minimise the usage of agrochemicals. Inoculation of AMF propagules (inoculum) into a target soil is the key technique embraced for this aim. Sadly, AMF are obligate symbionts and cannot be produced without the host plants in pure cultures. The large-scale development of AMF inocula is very difficult and complex because of this constraining feature.
There are three major forms of AMF inocula. First, AMF soil may be used as an inoculum from the root zone of a plant since it usually includes colonised root parts, AMF spores, and hyphae. However, without adequate knowledge regarding propagule quantity, variety and infectivity, soil inocula cannot be effective and may be at risk of transmitting weed seeds and pathogenic agents. Spores removed from soil can be used instead as starters for the development of crude inoculum. Crude inoculum can be collected from the inert medium adapted for AMF propagation after the known AMF isolate and the host trap plant (i.e. plant which can be colonised with a lot of AMF species) have been cultivated together[90−92]. This is the most common form of inoculum used for large-scale inoculation, since it normally includes a more condensed collection of propagules of the same sort present in the soil inoculum. Finally, colonised root fragments alone from an established AMF host that are isolated from a trap crop may even be used as an inoculum source. AMF's large-scale development of crude inoculum remains very demanding even while new mass processing methods[71], and the technology of seed coating have been developed in recent years[44]. The biggest challenge to an AMF inoculum is the unavoidable symbiotic conduct of the AMF, i.e., its requirement for a host facility to expand and complete its life cycles. Which implies that the propagation stage must involve a time and space-demanding process of cultivation with the host plant. As a result, the establishment of AMF reference collections involves methodologies which are very different and more binding than those for other microbial collections[93−96]. In addition, the lack of a prompt way to determine when and how often the host plant is colonised by AMF often contributes to threatening AMF 's agricultural usability. The management of the high inoculum needed for large-scale usage is also a challenging operation. However, AMF inoculation for plant manufacturing systems with a transplant stage is simpler since smaller quantities of inoculum are required.
Once AMF biodiversity has been preserved and well developed, and an AMF-friendly management such as fall cover[97] and conservation tillage[98] is introduced, the AMF population will prosper and if no damage is done before and after cultivation, it is understood that in the future, the network of mycorrhizal hyperbiotics of biodiversity will remain unaltered and contagious. Few of the experiments carried out to study the effectiveness of AMF as a biofertilizer either alone or in combination of other soil microorganisms are as follows: The maximum grain production of Triticum aestivum at phosphorus levels was recorded on being inoculated with Pseudomonas striata, followed by Glomus fasciculatum[99]. Rajendran & Jayasree[100] have examined the impact of biofertilizers such as Rhizobium, AMF etc. for Acacia nilotica and also demonstrated a substantial improvement in the length of seedlings and biomass compared with control. Microbial bioagents have been explored in chickpea for the prevention of collar rot disease[101]. When they co-inoculated Rhizobium with AMF, optimum decrease in mortality was observed as compared to control (100% seedling mortality). Different yield parameters have been effectively improved in each of the adjustments by disease control and seedling mortality reduction. The impact of single and dual inoculum of AMF species such as that of Gigaspora rosea, Glomus intraradices + Gigaspora rosea, and Glomus etunicatum + Glomus intraradices on dry shoot and root weight of Medicago sativa plants was studied by Khan et al.[102] and they reported a significant growth in both the parameters compared to control. Increase in nutrient absorption in inoculated plants was also reported. The inoculation of AMF is promising because it is inexpensive, simple to manage and promotes the growth of plants and the seed quality. Growth and return reaction to inoculation of Cicer arietinum with Rhizobium sp. and AMF has been studied by Giri[103]. These findings showed an increase of 10.83% of the total weight and 9.0% of the germination over control in pot experiments.
-
According to some estimates, the global population will reach 9 billion by the year 2050[104]. In order to preserve both human and environmental health, the world's agricultural sector is now faced with the problem of almost tripling the amount of food that is produced while simultaneously lowering farmers' dependency on agricultural chemicals. The increase in yield that is anticipated is more than the current capacity for the production of food throughout the world[104], which highlights the need for the development or revitalization of environmentally friendly technologies such as AMF-based biofertilization. Despite the enormous potential it has, agriculturalists have not yet entirely accepted the use of AMF. According to the findings of this study, overall AMF inoculation has beneficial effects on plant growth in both controlled and open-field conditions. This is mostly due to the many nutritional advantages that this class of soil fungus symbionts provides to the host plant. In point of fact, it has been shown that AMF inoculation in the field is just as effective as inoculation in the greenhouse, where, in contrast to open field conditions, non-inoculated controls are often free of AMF. Because of this, the next significant step for the consistent use of AMF in agriculture is to conduct large-scale field experiments and a cost-benefit study, such as the one suggested by Ceballos et al.[105], to enable future end-users to be better aware of the benefits of AMF inoculant. Farmers are urged to create their own AMF inoculant from their local soils since research has shown that the indigenous AMF is as potent as or stronger than commercial or cultural isolates. Even farmers in developing nations like India, who are in desperate need of a technique of crop production that is sustainable, would have an easier time gaining access to biofertilization technology as a result of this.
-
The authors confirm their contribution to the paper as follows: study conception and design: Jha SS, Songachan LS. Data collection: Jha SS; analysis and interpretation of results: Jha SS, Songachan LS. All authors reviewed the results and approved the final version of the manuscript
-
The data supporting the findings of the study are available within this published article.
-
The authors are thankful to the Head of the Department of Botany, Banaras Hindu University, India for providing laboratory facilities.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Jha SS, Songachan LS. 2023. Exploring the potential of Arbuscular Mycorrhizal Fungi as a biofertilizer. Studies in Fungi 8:17 doi: 10.48130/SIF-2023-0017
Exploring the potential of Arbuscular Mycorrhizal Fungi as a biofertilizer
- Received: 15 July 2023
- Accepted: 22 September 2023
- Published online: 24 November 2023
Abstract: Arbuscular Mycorrhizal Fungi (AMF) are a group of soil-borne fungi that form symbiotic relationships with the roots of most plants, including crops. In this relationship, the fungus provides the plant with nutrients, such as phosphorus, in exchange for carbohydrates produced by the plant through photosynthesis. The use of AMF as a biofertilizer involves the application of these fungi to soil to enhance plant growth and improve nutrient uptake. Studies have shown that AMF can increase plant growth, drought tolerance, and nutrient uptake, leading to improved crop yields. The fungi form a network of hyphae in the soil, which helps to increase the soil's water-holding capacity, as well as its ability to retain nutrients. This can lead to improved plant growth and health, even in nutrient-poor soils. In addition, the use of AMF as a biofertilizer can help to reduce the dependence on synthetic fertilizers, which can have negative environmental impacts. AMF can help to improve soil fertility, increase plant nutrient uptake, and reduce soil erosion, leading to more sustainable agriculture practices. However, it is important to note that the effectiveness of AMF as a biofertilizer can vary depending on several factors, including the species of AMF used, the type of crop being grown, and the conditions of the soil. Additionally, the proper application and management of AMF is important to ensure its effectiveness. In conclusion, the use of AMF as a biofertilizer has the potential to enhance plant growth, improve nutrient uptake, and promote sustainable agriculture practices.
-
Key words:
- Arbuscular mycorrhizal fungi /
- Biofertilizers /
- Inoculum /
- Microbiome /
- Sustainable agriculture