-
In angiosperms, double fertilization is a sophisticated and finely regulated process where male-female communication is pivotal in guiding pollen tubes (carrying two sperm cells) to achieve maximum fertility. Upon landing on the stigma, pollen grains initiate male-female communication, involving pollen hydration[1], tube germination, and penetration into the stigma. As the pollen tube advances towards the ovule, it first navigates into the intercellular space of the stigma and style, then progresses through the nutrient-rich extracellular matrix of transmitting tract tissues towards the ovary. Upon sensing attraction signals emitted by the two synergid cells of the ovule, the pollen tube exits from the septum of the transmitting tract[2−5]. It then travels along the placenta's surface, ascending the funiculus towards the micropylar opening of the ovule. Here, it enters the embryo sac that comprises seven cells including the two female gametes—the egg cell and the central cell. Under normal circumstances, only one pollen tube, rather than multiple, emerges from the septum to target the ovule at a time. This phenomenon, known as polytubey block, has its molecular mechanism recently elucidated[6]. Following pollen tube recognition by the synergid cells, during the process called pollen tube reception, one of the two synergid cells (the receptive synergid cell) is triggered to degenerate. The first-emerged pollen tube then resumes growth into the ovule and ruptures, releasing the two sperm cells[7−9]. If successful, double fertilization is completed in approximately 20 min. This results in the development of the embryo and endosperm, ultimately forming a seed. Successful fertilization prompts the rapid degeneration of the remaining synergid cell (the persistent synergid cell)[10,11]. This leads to the fusion of the persistent synergid cell with the fertilized central cell, and a dilution of the pollen tube attractants produced inside the synergid cell[12]. Meanwhile, those pollen tube attractants that have been secreted out of the synergid cell are modified and degraded[13,14]. However, if double fertilization by the first-emerged pollen tube fails, the persistent synergid cell remains viable and continues to produce pollen tube attractants. Since the polytubey block at the septum is lifted shortly after pollen tube rupture[6], secondary pollen tubes can now emerge from the septum to potentially salvage fertilization, known as 'fertilization recovery'[15]. Therefore, both synergid cells are crucial for double fertilization, given their dual roles in pollen tube reception/rupture and fertilization recovery. Our current understanding is that fertilization recovery is synergid cell-dependent, and the number of synergid cells determines the number of potential recoveries.
Recently, a lastest study revealed that the central cell retains an additional avenue for fertilization recovery in scenarios where both synergid cells are inactive. In their recent publication, Meng et al.[16] identified two peptide signals, SAL1 (SALVAGER1) and SAL2, secreted by the central cell. These peptides possess pollen tube attracting properties and can draw additional pollen tubes to the ovule for fertilization when both synergid cells are nonfunctional (Fig. 1). This provides an extra assurance for successful fertilization and optimal fertility in plants. The evidence provided by this study includes: 1) In the hap2 mutant, where both sperm-egg and sperm-central cell fusions are impossible, and synergid cell-mediated fertilization recovery is guaranteed[17,18], the embryo sac that receives two hap2 mutant pollen tubes retains the ability to attract additional pollen tubes (exceeding the number of synergid cells) when both synergid cells are degraded and synergid cell-produced pollen tube attractants are absent; 2) Ovules pollinated with hap2 pollen maintained the capability to attract pollen tubes and complete pollen tube reception; 3) The elimination of synergid cells through synergid-specific over-expression of the toxic protein Diphtheria Toxin A Subunit (DTA) did not completely abolish the ovule's pollen tube attracting activity, resulting in successful fertilization; 4) SAL1 and SAL2 are exclusively expressed within the central cell and are able to attract pollen tubes; 5) SAL1 and SAL2 are secreted from the central cell to attract pollen tubes in cases of failed fertilization or defective synergid attraction function, such as in myb98 mutant. In summary, these two peptides play a crucial role in fertilization recovery when synergid-mediated recovery falls short. While synergid-mediated recovery is paramount in ensuring fertility, this new central cell-mediated mechanism is a significant addition to the regulation of fertilization recovery, particularly for plants lacking synergid cells, such as those in the genera Plumbago, Ceratostigma, Dyerophytum, and Plumbagella[19].
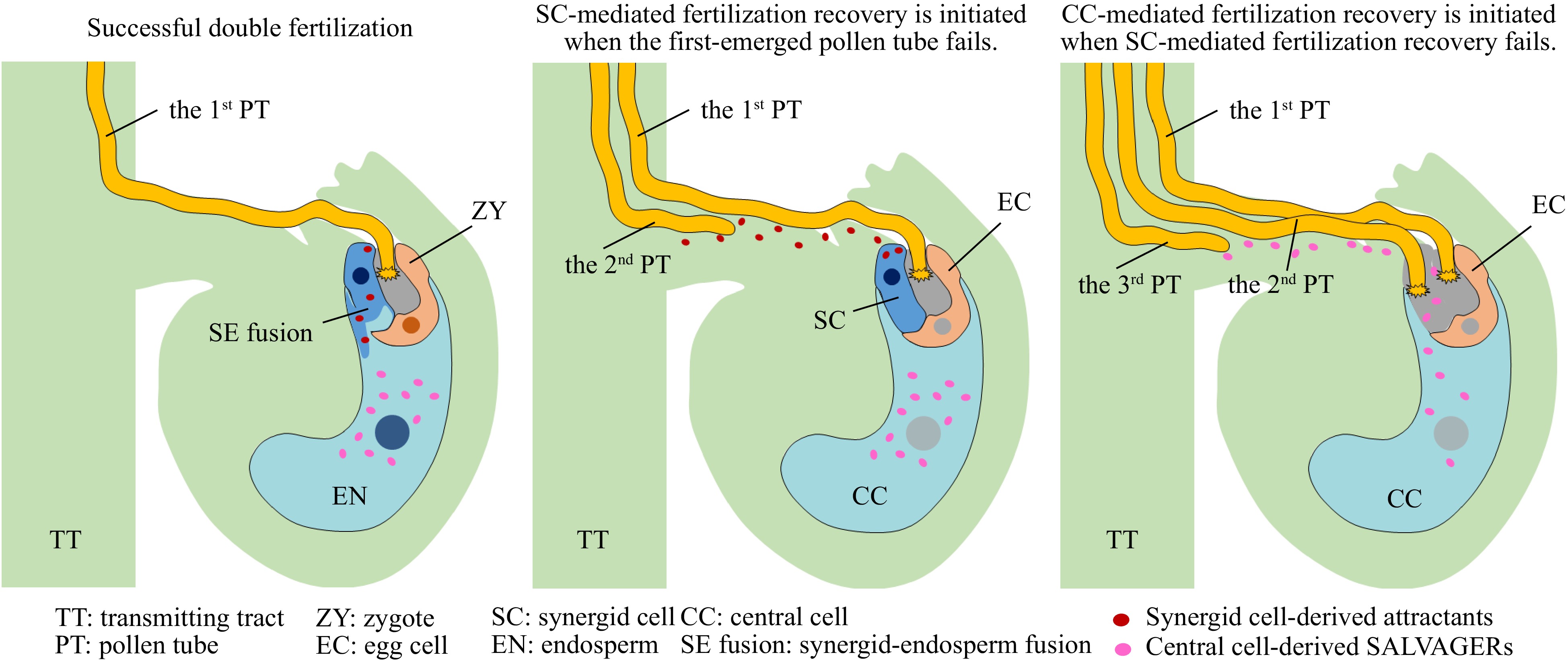
Figure 1.
Diagram of the double fertilization and fertilization recoveries. The successful fertilization by the first-emerged pollen tube results in the fusion of the persistent synergid cell with the fertilized central cell, leading to a dilution of the pollen tube attractants so that no more pollen tubes are attracted. If the double fertilization by the first-emerged pollen tube fails, however, the persistent synergid cell remains viable and continues to produce pollen tube attractants to attract a second pollen tube to salvage the fertilization, i.e., the synergid cell-mediated fertilization recovery that cause the degeneration of the persistent synergid cell. When the two pollen tubes fail to fertilize and both the synergid cells are nonfunctional, the two central cell-secreted peptide signals, SAL1 (SALVAGER1) and SAL2, are in place to draw additional pollen tubes to the ovule for fertilization, i.e., the central cell-mediated fertilization recovery.
This intriguing study also raises some important questions for future exploration. Firstly, the current model proposes that the pollen tube only ruptures after being recognized by the synergid cell[7,8]. Therefore, it would be interesting to investigate how the pollen tube, attracted by the central cell, is recognized and subsequently ruptures to release sperm cells, especially when both synergid cells have already degenerated. Secondly, previous reports have indicated that cells within the embryo sac can undergo cell fate alterations under certain circumstances[20]. It would be fascinating to further explore whether and how the egg cell or the central cell undergoes full or partial alterations in cell fate, acquiring some features or functions of synergid cells for pollen tube reception/rupture while still retaining their gamete potential for fertilization. Thirdly, from the data provided in this study we observed an interesting phenotype in wild-type pistils that were first pollinated with hap2 pollen and then with wild-type pollen 32 h later. The resulting seeds were primarily located in the upper part of the pistil, which differs from conventional pollination where pollen tubes often emerge first in the middle part of the pistil. This phenomenon suggests that central cell-mediated pollen tube guidance might differ from synergid cell-mediated pollen tube guidance during the emergence of pollen tubes from the septum. This aspect deserves further exploration in future studies. Fourthly, it will be a challenging endeavor to decipher the working mechanism of SAL proteins. This includes tasks such as identifying their receptors in the pollen tubes, elucidating the mechanism by which SAL proteins are inhibited from secretion prior to synergid elimination, and understanding how SAL-mediated signaling is attenuated after fertilization. Fifthly, considering that single fertilization of the central cell also triggers fertilization recovery[21], further investigation is warranted to determine whether the egg cell also produces similar peptide signals to attract pollen tubes.
With the functional identification of an increasing number of peptide ligands in male-female interactions in plants, we are gradually gaining a clearer understanding of how plants utilize peptide ligands to facilitate fertilization.
HTML
-
The authors confirm contribution to the paper as follows: draft manuscript preparation: Lan Z, Song Z, Zhong S, and Qu LJ. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
This work was supported by the National Natural Science Foundation of China (Grant No. 31991202 and 31830004 to Qu LJ, and 32122014 and 32070854 to Zhong S), and the Young Elite Scientists Sponsorship Program by China Association of Science & Technology (2019QNRC001 to Zhong S). Qu LJ was supported by the New Cornerstone Science Foundation.
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Zijun Lan, Zihan Song
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Hainan Yazhou Bay Seed Laboratory. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Lan Z, Song Z, Zhong S, Qu LJ. 2023. The central cell: another opportunity for fertilization recovery in plants. Seed Biology 2:22 doi: 10.48130/SeedBio-2023-0022 |













