-
The cassava hornworm (Erinnyis ello) Linneaus, 1758 (Lepidoptera: Sphingidae) is an important pest of several plants of the Euphorbiaceae family and is among the main pests of rubber trees and cassava. The larvae can consume large amounts of leaves in a few days. Its occurrence is cyclical and can cause severe damage[1]. The biological cycle of E. ello can vary from 32 to 49 d, depending on the environmental conditions, and the great defoliation potential is concentrated in the fourth and fifth instars of the larval stage[2]. In Brazil, this pest occurs mainly during the periods from September to February, with different attacks according to the regions and usually associated with high temperatures and the beginning of the rainy season, which may not occur in certain agricultural years[3].
The damage caused by the pest directly affects cassava production (Fig. 1). It can occur throughout the year, varying the intensity of the attack. The female can lay up to 1800 eggs during her life cycle, and during the developmental stages the caterpillar consumes, on average, 1,107 cm2 of leaf area, equivalent to 12 well-developed leaves, with 75% of this area being consumed in the 5th instar[4]. As described by Bellotti et al.[5], infestations cause a reduction in root production in the order of 26% to 45% with a single attack, and from 47% to 74% with two attacks, which may vary depending on the variety, age of plants, soil fertility and environmental conditions. A severe occurrence of the pest was observed in rubber plantations in Vale da Ribeira, in the State of São Paulo, Brazil, in 1983, causing defoliation of up to 70% of the plants. At the beginning of the attack, the caterpillars devour the leaves and young branches. In high populations, they destroy the mature leaves and the thinnest branches[1].

Figure 1.
Erinnyis ello larvae infesting cassava crop in Cruzeiro do sul, Acre State, Brazil. (a) Fourth instar larva ingesting cassava leaf. (b) Cassava crop completely defoliated by the E. ello larvae.
The area planted with cassava in Acre reached 43,844 hectares in 2015, earning an income of around US
${\$} $ In Acre, the cassava hornworm caterpillar has been occurring in cassava since the mid-1980s in the region of Cruzeiro do Sul. In 1993 and 1998, new outbreaks occurred with estimated losses of 50%−60% in productivity. Less severe outbreaks also occurred in 2002 and 2007[3]. In 2014, the first cassava hornworm outbreak occurred in rubber trees in the state of Acre, in the regions of Epitaciolândia and Capixaba. There was a high population level of caterpillars and most of the trees were totally defoliated[7].
The methods used to control the cassava hornworm can be cultural, chemical, physical or biological[2]. In the mid-1980s, a virus (Baculovirus erinnyis) pathogenic to cassava hornworm was used as a biopesticide in cassava crops, in a program established by the Empresa de Pesquisa Agropecuária e Extensão Rural de Santa Catarina – EPAGRI[8]. The virus was classified as Erinnyis ello granulovirus (ErelGV), current genus Betabaculovirus of the Baculoviridae family[8]. Granuloviruses produce small, oval-shaped occlusions called granules, about 0.3 to 0.5 μm in length. Occlusion bodies usually have only one virion, rarely two, and virions have only one nucleocapsid per envelope[9].
Erinnyis ello granulovirus (ErelGV) has been shown to be a viable, safe and economical alternative for the control of cassava hornworm[4]. The high virulence of this virus was verified in 1985 in the Brazilian region of Itajaí (SC) under field conditions causing 90% mortality, which was confirmed in laboratory conditions also leading to 90% mortality after nine days of infection[4]. Based on this finding, training actions were carried out for small producers in order to enable the recognition of infected caterpillars, collection and production of the extract for immediate use or frozen storage[10].
The cycle of Baculovirus infection in the insect is widely known. The infection of the caterpillar by the virus begins with the ingestion of the viral particles together with the leaves of the host plant (cassava or rubber tree). Approximately four days after ingestion of the virus, the first symptoms of the disease appear, which are the caterpillar discoloration, loss of movement and the ability to feed. In the final stage of infection, around nine days, the dead caterpillars show negative geotropism behavior, that is, they are found hanging on the petioles of the leaves. After the death of the insect, the granules are released into the environment due to the lysis of the larval cuticle, causing the infection of other insects[8].
Prior to the use of a certain baculovirus isolate as a biopesticide it is necessary to characterize it morphologically and genetically. The presence of an intact occlusion body is essential for the environmental persistence of baculovirus biopesticides[9]. The genetic characterization allows monitoring the genetic stability of the isolate, in order to prevent the appearance of isolates with losses of virulence such as defective isolates[11]. In addition, the virus DNA information is necessary for molecular tools to verify biopesticide quality control, avoiding contamination problems that could cause differences in control efficiency in the field[12]. The restriction profile is a molecular analysis with advantages for routine use such as speed and lower costs compared to DNA sequencing. In this work, we present further genetic characterization, morphological characterization and field efficiency parameters of a Baculovirus isolate occurring in populations of Erinnyis ello in the municipality of Cruzeiro do Sul, Acre aiming its use as a biopesticide.
-
Cassava hornworm larvae (Erinnyis ello) that presented the characteristic symptom of Baculovirus infection (Fig. 2) were collected in a private area (family farming) in the municipality of Cruzeiro do Sul, Acre (Latitude −7.55086; Longitude −72.72570) in the year 1999.
Plastic containers with a capacity of 200 mL, equipped with a screw cap, were used to collect the larvae. The material was initially taken to an extraction room, where the caterpillars were placed in groups of two to five individuals, in a mortar with a capacity of 55 mL, adding approximately 5.0 mL of distilled water. Then, maceration was carried out with a pestle until a homogeneous mass was obtained. The liquid thus obtained was strained through gauze, obtaining a viscous extract ready to be used or stored under freezing (−20 °C).
Viral particles purification
-
Granules were purified according to Maruniak[13]. Infected caterpillars were ground in homogenization buffer (1% ascorbic acid; 2% SDS; 0.01 M Tris pH 7.8; 0.001 M EDTA pH 8.0). After filtering through gauze layers with glass wool and centrifuging at 10,000 rpm for 15 min at 4 °C (Sorvall SS 34 rotor), the macerate was washed by centrifugation in TE buffer (Tris-HCl 10 mM pH 8.0 and 1 mM EDTA pH 8.0). Then, the sample was applied on a sucrose gradient from 1.17 to 1.26 g/mL and submitted to ultracentrifugation at 24,000 rpm for 40 min at 4 °C (Sorvall AH 627 rotor). The band of granules formed in the lower third of the tube was collected and diluted with TE buffer. After centrifugation at 12,000 rpm for 15 min at 4 °C (Sorvall SS 34 rotor), the pellet containing the granules was suspended in water and stored at −20 °C.
Viral DNA extraction
-
The DNA purification process was performed according to Sambrook et al.[14]. Initially, a total of 250 μL of 3X alkaline solution, pH 10.9 (0.3 M Na2CO3, 0.51 M NaCl and 0.03 M EDTA in 50 mL of distilled water) was added to 500 μL of the suspension of purified granules, the samples being kept at 37°C for dissolution of the granulin. After 30 min, 25 μL of 20% SDS and 12.5 μL of Proteinase K enzyme (20 mg/mL) were added, followed by incubation at 37 °C overnight. After the samples were centrifuged, the supernatant was subjected to cycles of an equal volume of phenol saturated with 5 M sodium chloride (1:1), phenol : chloroform : isoamyl alcohol (25:24:1) and chloroform : isoamyl alcohol (24:1). Finally, the precipitation of the aqueous phase containing the DNA was carried out at −20°C, after addition of two volumes of 95% ethanol and 10% of the final volume of 3 M sodium acetate, pH 5.2.
Cleavage of viral DNA with restriction enzymes
-
Restriction profiles of viral DNA samples were obtained by cleavage with Bam HI, Eco RI, Hind III and Pst I enzymes, according to the manufacturer's instructions (Invitrogen). The digestion system for each enzyme was performed in a reaction volume of 40 μL containing 1.5 mg of DNA template (ErelGV), 1U of the enzyme and 4 μL of the buffer (10 X). Digestion reactions took place at 37 °C in a water bath.
Agarose gel electrophoresis
-
The gel was prepared at a concentration of 0.8% agarose in 1 X TAE buffer (0.04 M Tris-acetate, 0.001 M EDTA, pH 8.0) according to Sambrook et al.[14]. After solidification, the gel was submerged in the tank containing 1 X TAE and the samples containing the DNA fragments, suspended in a buffer containing bromophenol blue and glycerol, were introduced into the wells. Molecular markers λ/Hind III and λ/Pst I were also used. After an electrophoretic run, with a constant voltage of 80 V, the gel was immersed in a 1 X TAE solution containing 0.5 mg/mL of ethidium bromide. Finally, for band size and molar analysis, the gel was visualized and photographed using an ultraviolet transilluminator and a photodocumentation system (LAB Trade).
Transmission electronic microscopy
-
The treatment of viral particles (ErelGV granules) for transmission electron microscopy was carried out according to Bozzola & Russell[15]. The purified granules were initially fixed in 0.1 M sodium cacodylate and 2.5% glutaraldehyde buffer. After treatment with post-fixation buffer containing 1% tetroxide, the material was subjected to a series of dehydrations in 30%, 70%, 90% and 100% ethanol, and conditioned in spurr resin. Then, the block was cut into thin sections with an ultramicrotome (diamond knife) and placed on copper screens. Finally, the screens with the samples were stained with 2% uranyl acetate and visualized under a Jeol 1011 transmission electron microscope.
Field management parameters
-
The potential of this isolate of ErelGV for cassava pest control was evaluated in the Juruá River Valley (Acre State) where observation units were installed. Five areas around 1.5 ha were chosen at the 'Badejo do Meio' community located in Cruzeiro do Sul region according to parameters previously tested by Fazolin et al[3].
-
Erinnyis ello larvae, collected in the municipality of Cruzeiro do Sul (Acre), showed typical symptoms of baculovirus infection (Fig. 2), such as flaccid body, color change, loss of movement and negative geotropism[9,11]. After the death of the insect, there was intense lysis of the larval cuticle and dispersion of the viral particles.
The ultrastructural analysis of the purified viruses revealed the presence of oval-shaped bodies, containing only one virion per occlusion body and ranging in size from 0.3 to 0.5 μm. The virus was identified as belonging to the genus Betabaculovirus (extinct genus Granulovirus) of the family Baculoviridae (Fig. 3).
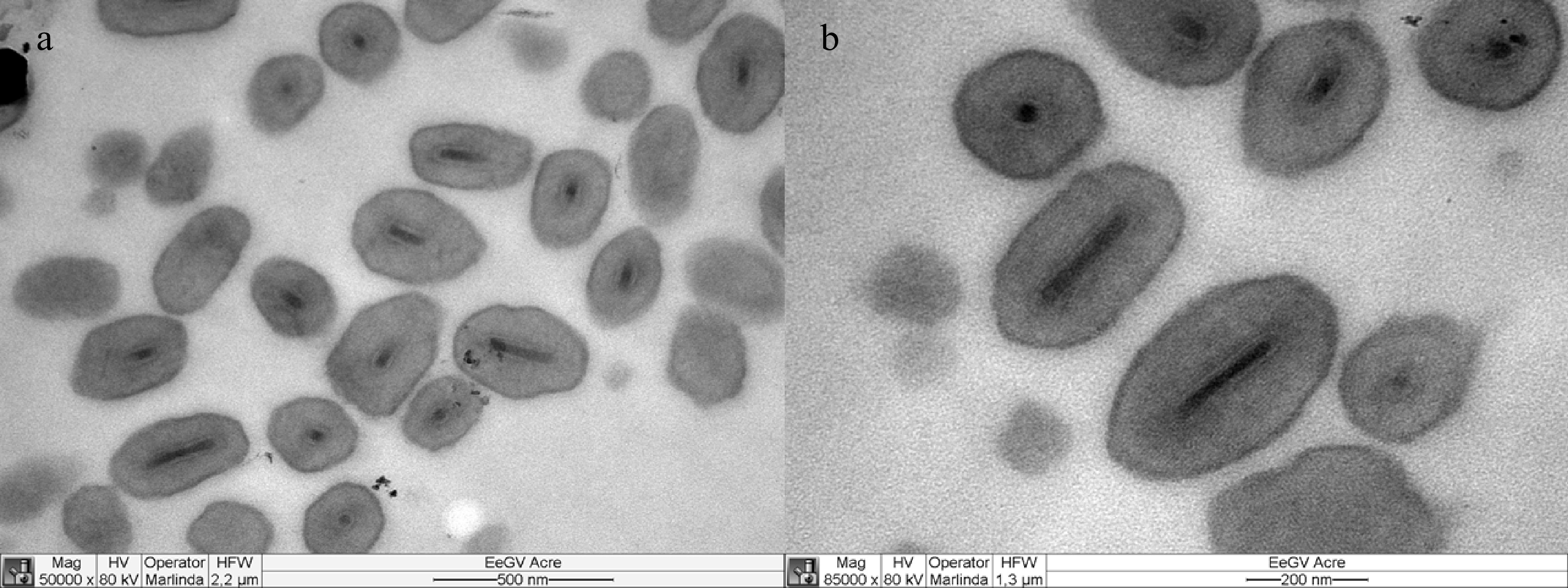
Figure 3.
Electron micrographs of the occlusion bodies. Oval-shaped granules containing only a single virion. (a) 50,000× magnification. (b) 85,000× magnification.
The analysis of the restriction profiles of the Erinnyis ello GV isolate (Fig. 4) collected in Acre, obtained with the enzymes Bam HI and Hind III, revealed a total of seven and three fragments with similar molar ratios, respectively. Upon cleavage with Pst I, eight full molar and three submolar fragments were identified. In the digestion with Eco RI, 21 DNA fragments were observed, with some submolar bands present.
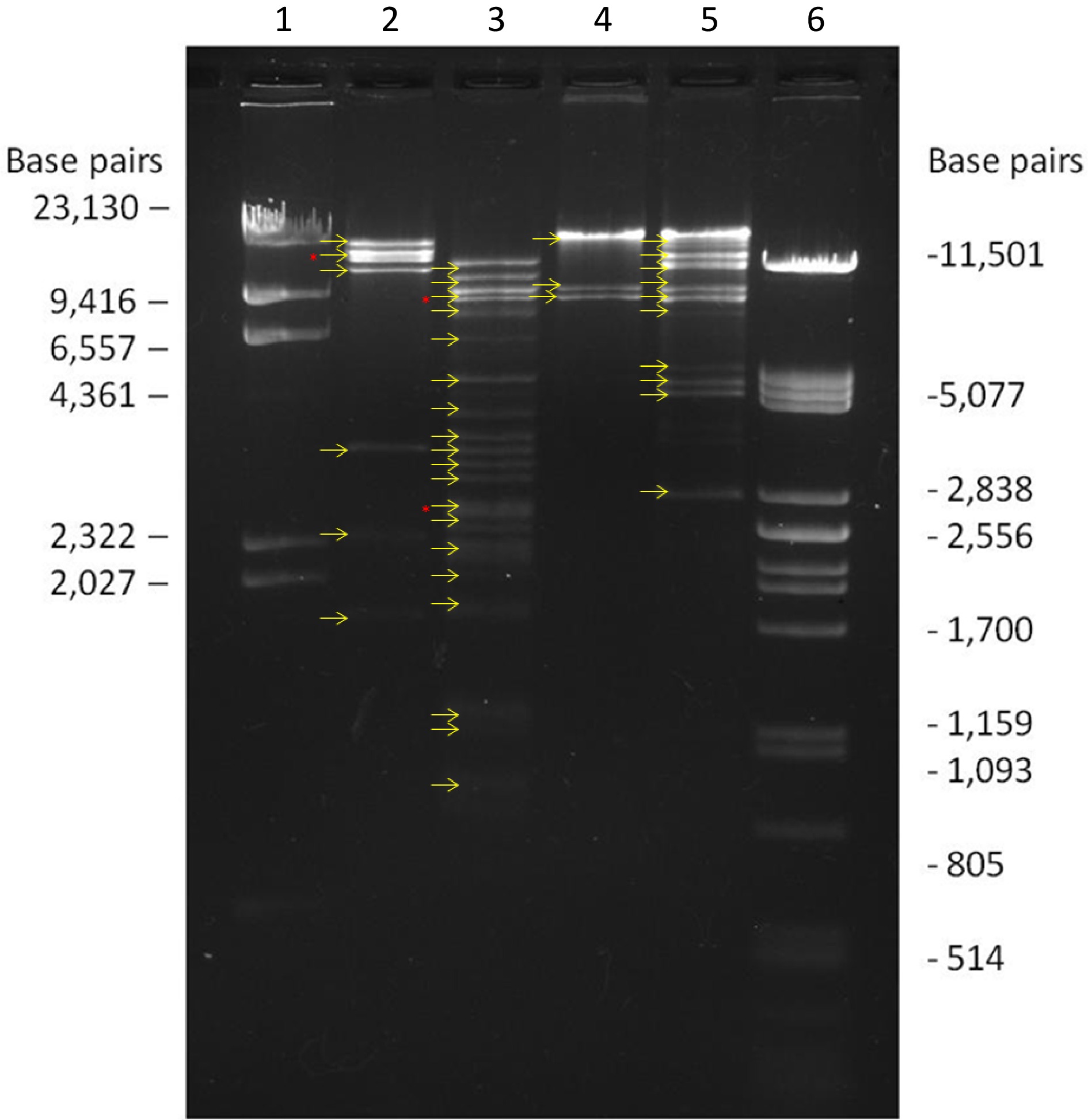
Figure 4.
Restriction profiles of the ErelGV-Acre isolate. 1 DNA λ/Hind III marker; 2 ErelGV DNA cleaved with Bam HI; 3 ErelGV DNA cleaved with Eco RI; 4 ErelGV DNA cleaved with Hind III; 5 ErelGV DNA cleaved with Pst I; 6 DNA λ/Pst I marker. The yellow arrows indicate the presence of DNA fragments and red arrows indicate double bands.
Since baculoviruses are specific biopesticides agents, it was possible to combine their use with other natural enemies such as parasitoids and predators, which are important agents of Integrated Pest Management (IPM). Therefore, the first step for the management was to monitor the incidence of E. ello eggs in the cassava leaves. The adoption of control measures was taken when less than 50% of eggs present signs of natural parasitism (deformed eggs or black color). The larvae population in the areas consisted in six to eight individuals/plant and 75% of the larvae were in the second and third instars. The areas were sprayed with 40 mL of baculovirus concentrated extract/ ha using volume application rate of 200 L/ha in a knapsack sprayer. After 6 d of spraying in the areas the average mortality of larvae was 96%. It was observed the occurrence of natural predator, wasps of genera Polybia (Hymenoptera: Vespidae) in both infected and healthy larvae of E. ello. The presence of this wasp species can contribute for spread of baculovirus around the areas. The control with baculovirus was adopted in the interval of 3 to 5 d after inspection and varies according to the number of larvae per plant and the larval instar, but in average the control threshold of six larvae (size up to 3 cm) per plant was considered.
-
The isolate was named ErelGV-Acre and deposited in the Embrapa Invertebrate Virus Collection (CVI), in Brasilia, Brazil (CVI code = BRM060712). DNA restriction profile analysis of different seasonal isolates collected in the region of Itajai / Jaguaruna (SC), including the isolate ErelGV 1986, has been previously described[16]. Our comparison of the restriction profile of the ErelGV isolate collected in Acre with that described for isolates from Santa Catarina revealed a high similarity between them, based on digestion with the enzymes Bam HI, Eco RI and Hind III. However, it was not possible to compare the restriction profile by cleavage with Pst I enzyme, since the DNA digestion of the Santa Catarina seasonal isolates with this enzyme was incomplete.
The complete genome of the Erinnyis ello granulovirus isolate collected in Santa Catarina State in 1986, called Br-S86, was sequenced[17]. This isolate has a size of 102.759 bp, being phylogenetically closer to the viruses Choristoneura occidentalis granulovirus (ChocGV) and Pieris rapae granulovirus (PiraGV). Moreover, genomic analyses of Brazilian ErelGV field isolates were recently carried out. Their genomes were sequenced and their inter- and intrapopulational sequence diversity were analyzed[18]. The ErelGV-Acre isolate has shown to be the most diverse population, with a unique pattern of polymorphisms. Also, in terms of phylogenetics, Santa Catarina isolates have shown to be distantly related to the Acre isolate.
The biological control of cassava hornworm with ErelGV has been done with success since the 1980s. It started with observations performed by CIAT in Colombia, having nowadays culminated in a biopesticide developed, in this country, by AGROSAVIA[19]. In Brazil, a program established by the Agricultural Research and Rural Extension Company of Santa Catarina - EPAGRI in mid-1980 was implemented in cassava crops of Itajaí region (SC). It was observed 90% of larvae mortality 9 d post baculovirus spraying and a high dispersion capacity through agents like wind, cultivation practices, parasitoids and predators[8]. The ErelGV virus was later applied in Paraná State and Northeast region of Brazil[20]. In Brazil, so far, the ErelGV has been produced only 'on farm'. In addition to natural agents in the IPM, other methods for cassava hornworm control can be adopted, such as manual removing of larvae (recommended for areas with size up to 2 ha) and light traps equipped with ultraviolet lamps for monitoring and control of adult individuals[3].
In conclusion, the ErelGV Brazilian isolate from Cruzeiro do Sul, Acre State, has a good potential for cassava hornworm control. However, there is a need to carry out more research on the rearing of E. ello as well as large-scale production of the ErelGV virus to support a commercial biopesticide with suitable cost-benefit for growers. The DNA restriction profile analysis will be also an important tool to monitor the distribution and behavior of the virus applied as biopesticide in the field.
-
The authors confirm contribution to the paper as follows: conceptualization: de Souza ML, Fazolin M; methodology: Sihler W, de Souza ML; validation: Sanches MM, Fazolin M, de Souza ML; investigation: Sihler W, Sanches MM, Fazolin M, Estrela JLV, de Souza ML; resources: de Souza ML, Sanches MM, Fazolin M; data curation: de Souza ML, Sanches MM, Fazolin M; writing—original draft preparation: Sihler W, de Souza ML, Sanches MM, Fazolin M; supervision: de Souza ML; project administration: de Souza ML; funding acquisition: de Souza ML. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
We thank Rosana Falcão (Embrapa Agroenergia) for the Transmission Electronic Microscope support. This research was funded by Brazilian Agricultural Research Corporation - EMBRAPA, grant number 10.20.02.009.00.02.003.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Sihler W, Sanches MM, Fazolin M, Estrela JLV, de Souza ML. 2024. Erinnyis ello granulovirus for cassava hornworm control in the Acre State of Brazil. Technology in Agronomy 4: e001 doi: 10.48130/tia-0023-0019
Erinnyis ello granulovirus for cassava hornworm control in the Acre State of Brazil
- Received: 19 October 2023
- Revised: 25 November 2023
- Accepted: 28 November 2023
- Published online: 02 January 2024
Abstract: The hornworm (Erinnyis ello) is an important pest of cassava and rubber tree with high capacity of migration and can be controlled with biological control agents such as baculoviruses. Baculoviruses are rod-shaped enveloped viruses with circular double-stranded DNA genomes infecting insects of the order Lepidoptera. Prior to large-scale use of certain baculovirus, it is essential to assess its genetic and morphological information. This work aimed to characterize the baculovirus isolate occurring in Erinnyis ello populations at small farms in Cruzeiro do Sul, Acre State, Brazil and to develop parameters for field management using this isolate. Viral particles were purified from infected larvae through ultracentrifugation in sucrose gradient. After treatment and negative staining with uranyl acetate 2%, viral particles were visualized using transmission electron microscope. The virus with ovicylindrical occlusion body presenting only one virion per envelope and size less than 0.5 μm was identified as a member of the genus Betabaculovirus. Viral DNA was extracted through phenol/chloroform cycles, digested with different restriction enzymes and separated with agarose gel electrophoresis. The restriction enzyme patterns obtained with the enzymes Bam HI and Hind III revealed seven and three DNA fragments, respectively; cleavage with Pst I, generated eight molar fragments and three submolar fragments and Eco RI digestion resulted in 21 fragments, with some submolar bands. Comparison of the DNA restriction pattern from ErelGV isolated in Acre with that described for ErelGV isolated from SC State showed high similarity. This isolate named as ErelGV-Acre presented potential for control of E. ello populations.
-
Key words:
- Baculovirus /
- Biological Control /
- Pest management /
- Manihot esculenta













