-
Walnuts (Juglans regia L.), known as one of the 'four big nuts of the world' along with hazelnuts, almonds, and cashews, are rich in various nutrients, such as unsaturated fatty acids and minerals[1]. Currently, the consumption of fresh walnuts has increased due to their fresh flavour, unique taste and crispness[2]. However, their high water content (≥ 17%) increases the physiological metabolism rate and promotes walnut decay. In addition, their delicate green appearance can be easily lost by browning and discolouration (degreening) during storage[3,4]. Maintaining the postharvest quality of fresh walnuts has become a notable research topic of considerable importance.
Researchers have developed several physical and chemical technologies for fresh walnut preservation, such as 60 Co-γ radiation[3], 1-methylcyclopropene (1-MCP)[5], cold plasma[1], improved atmosphere packaging[6], sodium diacetate[7] and ClO2 treatments[8]. Ye et al.[6] reported that treatment with a modified atmosphere (5% O2, 7.5% CO2) promoted the activities of walnut superoxide dismutase (SOD), catalase (CAT), and ascorbic acid peroxidase (APX), inhibited ethylene production, reduced the accumulation of reactive oxygen species (ROS), and delayed browning. Ma et al.[3] found that 60 Co-γ radiation reduced the oxidative degradation of walnuts, thus maintaining better sensory quality and freshness. Nevertheless, it is difficult to apply the abovementioned technologies in practice owing to their extensive technology requirements and limited applicability.
Nitric oxide (NO) is a widely distributed signalling molecule that not only participates in multiple reactions during plant growth, development, maturation, and senescence but also has the ability to induce defence responses and stress tolerance[9,10]. Sodium nitroprusside (SNP) is a common NO donor, and it can have the same effect as fumigation with NO gas[11,12]. Recently, there has been an increase in the application of exogenous SNP (NO) on vegetables and fruits[11,13]. Yang et al.[14] found that NO reduced the decay rate of navel orange fruits and the lesion area on the fruits. Ren et al.[15] reported that SNP treatment not only decreased the rot index but also maintained green colour and reduced weight loss of in the fruit. Additionally, pear browning discolouration was greatly delayed (15 d) by SNP treatment compared with control samples[12].
To date, SNP (NO) is known to function as a fruit and vegetable preservative with the advantages of easy operation, low cost and low safety hazard risk, while there have been few related reports on fresh walnuts. In this study, the ascendancy of SNP (NO) on the preservation of fresh walnuts was assessed. The decay development and colour degradation were recorded and the regulation of reactive oxygen metabolism, lipid oxidation, and sensory qualities was evaluated to achieve some understanding of the application potential of SNP (NO) treatment for fresh walnut preservation.
-
'Liaohe No. 1' fresh walnuts (22−24 g) with green husks at maturity were obtained from the local planting base (Jizhou District, Tianjin, China). Walnuts were stored overnight at 24°C before subsequent sample processing. SNP was purchased from Biyuntian Biotechnology Company in Shanghai, China. The samples were used within three weeks.
The 'Liaohe No. 1' fresh walnuts were devided into four groups randomly and evenly and soaked for 30 min (SNP solutions with concentrations of 0.0 mmol/L (control), 0.1, 0.5, and 1.0 mmol/L). After removal, the surface water was dried in a plastic container, placed in a polyethylene preservation bag, and stored at 24°C.
Appearance observation and decay index
-
Colour parameters were surveyed with a portable precision colorimeter WR-18 (Weifu Optoelectronic Technology Co., Shenzhen, China), The total chromatic difference (ΔE) was calculaed from colorimetric units as ΔE = (a*2 + b*2 + c*2)1/2. After calibration with a standard white reflector plate, six walnuts were taken from each treatment and values were determined at four different places in each walnut.
The browning or decay indices of walnuts were assessed by a visual check. Each walnut was graded on a six-point impact degree: 1, 0% of walnut surface undergoes browning or disease; 2, 1% to 20%; 3, 21% to 40%; 4, 41% to 60%; 5, 61% to 80%; and 6, 81% to 100% of walnut surface undergoes browning or disease[4,8].
Chitinase (CHI) and β-1,3-glucanase (GLU) activities
-
The method of Li et al.[16] was used to determine the CHI activity in fresh walnuts. For enzyme extraction, 10 g of walnut peel was weighed into a precooled mortar, and 10.0 mL of precooled extraction buffer (containing 5 mmol/L β-Mercaptoethanol and 1 mmol/L EDTA) was mixed and ground, centrifuged in a refrigerated centrifuge for 30 min (4 °C, 12000× under g conditions). The supernatant was retained. For enzyme preparation, protein was precipitated using the acetone method. The supernatant was mixed with five times its volume of precooled acetone and then left to stand overnight at −20 °C, and the precipitate was dried with nitrogen gas after centrifugation for 20 min. The precipitate was dissolved in acetic acid sodium acetate buffer (50 mmol/L, pH 5.2) to obtain the enzyme extract. Two test tubes were used, and 0.5 mL of colloidal chitin suspension (10 g/L) and 0.5 mL of acetic acid sodium acetate buffer solution (50 mmol/L, pH 5.2) were added. A total of 0.5 mL of enzyme extract solution was taken to the reaction tube, and 0.5 mL of boiled enzyme extract solution was taken to the control tube. The solution was shaken well and placed in a 37°C water bath for 1 h, 0.1 mL of desalinated snail enzyme (30 g/L) was added, maintained for 1 h, 0.2 mL of potassium tetraborate solution (0.6 mol/L) was added, mixed well and kept for 3 min in a boiling water bath. After cooling the solution, 2.0 mL of p-dimethylaminobenzaldehyde solution was added. After thorough mixing, a colour reaction was carried out at 37 °C for 20 min. Subsequently, the solution absorbance was determined at 585 nm. One CHI activity unit was the amount of enzyme required to break down chitin and produces 1 N-acetylglucosamine molecule per gram of sample per second, equivalent to 10−9 mol.
The extraction and preparation of the GLU enzyme solution were the same as described for the CHI enzyme solution, and the determination method was that of Li et al.[16]. 0.1 mL of Kunbu polysaccharide solution (4 g/L) was added to two test tubes, 0.1 mL of enzyme extraction solution was taken to the reaction tube, and 0.1 mL of boiled enzyme extraction solution was added to the control tube. The solution was mixed well and left for 40 min in a 37°C water bath. Then, 1.8 mL of distilled water and 1.5 mL of DNS reagent were taken into test tubes sequentially. The solution was kept in a boiling water bath for 3 min and dissolved with distilled water to 25 mL after cooling. The mixed solution absorbance was obtained at 540 nm. One GLU activity unit is produced by the enzymatic decomposition of laminarin at a rate of one gram of sample per second, resulting in the release of 10−9 mol of glucose.
Ethylene generation rate and respiratory metabolism rate
-
Five fresh walnuts were randomly placed into the inner cavity, and the internal gas (1 mL) was extracted using a syringe for each treatment. The method of Miranda et al.[17] was referenced to analyze the ethylene (C2H4) concentrations operating a gas chromatograph (Agilent GC7890A, USA). The carrier gas was N2 and the detector was a flame ionization detector (FID). The temperatures of the oven, injector, and detector were 60, 220, and 240 °C, respectively.
Five walnuts were randomly placed in the air chamber of the fruit and vegetable respiration analyser (JFQ-3150H, Beijing, China) according to the method of Du et al.[5]. The detection time was 3 min, and the weight and CO2 changes were recorded.
Content of superoxide anion (O2•−) and hydrogen peroxide (H2O2)
-
Based on actual experiments, previous methods were optimized to determine the rate of O2•− production[18]. Fresh walnut peel (2 g) was added to a precooled mortar, 5 mL of extraction buffer (containing 2% PVP, 0.3% Triton X-100, and 1 mmol/L EDTA) was mixed, and the sample was ground under ice bath surroundings and centrifuged at 4°C and 12,000× g for 20 min. Then, 1 mL of the supernatant, 1 mL of 50 mmol/L pH 7.8 phosphate buffer and 1 mL of 1 mmol/L hydroxylamine hydrochloride solution were sequentially added. The tube was mixed well and kept warm in a 25°C water bath for 1 h. Then, 1 mL of 17 mmol/L p-aminobenzenesulfonic acid solution and 1 mL of 7 mmol/L α-Naphthylamine solution were added, and a 20-min colour reaction was conducted in a 25 °C water bath. Immediately, the mixed solution absorbance was determined at 530 nm (reference control: the same as the above method but without 1 h at 25 °C insulation). The results are expressed as µmol/min·g.
Previous methods were exploited to determine the the H2O2 content[19]. Fresh walnut peel (2 g) was weighed into a mortar, 5 mL of precooled acetone was added at −20 °C to a fume hood. The sample was ground fully and centrifuged at 4 °C and 12,000× for 20 min. Then 1 ml of the sample extraction solution was dissolved to 0.1 mL of a 10% solution of titanium tetrachloride in hydrochloric acid, along with 0.2 mL of concentrated aqueous ammonia. The solution was mixed well and reacted for 5 min. The samples were centrifuged and the supernatants discarded. The precooled acetone at −20 °C was required to repeatedly washed the precipitate to rid the pigment. Eventually, precipitate was completely dissolved in 3 mL of 2 mol/L sulfuric acid solution. A wavelength at 412 nm was used to obtain absorbance, the results are expressed as µmol/g.
Antioxidant enzyme activity
-
The SOD activity was revised and measured by Lotfi et al.[20]. Two grams of fresh walnut peel sample was weighed in a precooled mortar. A total of 5.0 mL of extraction buffer (including 5 mmol/L DTT and 5% PVP) was mixed. The mixture was ground and centrifuged for 30 min at 4 °C and 12,000× g. A total of 1.7 mL of phosphate buffer solution (50 mmol/L, pH 7.8), 0.3 mL of methionine solution (130 mmol/L), 0.3 mL of nitrogen blue tetrazole solution (750 µmol/L), 0.3 mL of LEDTA-Na2 solution (100 µmol/L), and 0.1 mL of enzyme extraction solution were added sequentially to a glass tube. Finally, the reaction was initiated by adding 0.3 mL of riboflavin solution (20 µmol/L). Buffer solution was added instead of enzyme extraction solution to the two control tubes. After mixing, one control tube was layed in a dark location, and the other control tube and measuring tube were layed under a 4,000 lx fluorescent lamp for a 15-min colour reaction. Then, they were placed in a dark place to terminate the reaction. A light source tube was used as a reference for zero adjustment to adjust the absorbance value of other tubes at 560 nm. The results are showed as U/g.
Previously published methods were refered to acquire the CAT activity of fresh walnut peel[21]. The preparation method of the enzyme extract was the same as that detailed in a previous paragraph. 2.9 mL of H2O2 (20 mmol/L) and 0.1 mL of enzyme extract constituted the enzymatic reaction system. The absorbance value was measured at 240 nm. The absorbance at 15 s of reaction was taken as the initial value, and then the measurement was continuously recorded every 30 s to obtain at least six data points. One CAT activity unit was a 0.01 decrease in absorbance change per minute per gram of sample. The results are expressed as U/g.
The ascorbate peroxidase (APX) activity in fresh walnut peels was determined based on the methods of Ye et al. without modification[6]. The APX activity was calculated based on the decrease in absorbance of the reaction system at 290 nm per unit time (ascorbic acid oxidation). The results are showed as U/g.
The method used by Qiao et al.[22] was slightly modified to determine the peroxidase (POD) activity in fresh walnut peels. Two grams of walnut peel sample was weighed, and 5.0 mL of extraction buffer (containing 1% Triton X-100, 4% PVPP, and 1 mmol/L PEG) was added. The sample was ground under ice bath conditions and centrifuged for 30 min at 4 °C and 12,000× g. The measured reaction system comprise 0.5 mL of enzyme extract and 3.0 mL of guaiacol solution (25 mmol/L), with 0.2 mL of hydrogen peroxide solution (0.5 mol/L) used to initiate the reaction. The absorbance change was surveyed at 470 nm per minute. One POD active unit increased the absorbance variation by 1 per min per gram of sample. The results are shown as U/g.
Sensory evaluation and acid value (AV) and peroxide value (PV)
-
Sensory evaluation of fresh walnut kernels was conducted on day 0 and on the last day of storage, referring to the evaluation method of Habibie et al.[23]. A 15-person evaluation team was formed, and the seed coat colour, kernel colour, odour, taste, and crispness were rated based on a 9-point scoring system (9, like very much; 1, dislike very much). The average score of these five aspects was denoted 'overall consumer acceptance'.
Walnut oil was gained through Soxhlet extraction and based on the method of Wang et al.[24]. Walnut kernel samples (10 g) were put into the extractor. Petroleum ether and extract were added for 24 h, and the heating temperature of the water bath was maintained at 40−50 °C. Then, the mixture was rotary evaporated to remove excess petroleum ether. The AV and PV of the oil sample were determined using the titration methods in GB/T 5009.229-2016 and GB/T 5009.227-2016.
Data analysis
-
SPSS software version 22.0 (IBM SPSS Statistics 22) was used to perform statistical analysis. Data are indicated as the mean ± standard error from triplicate samples. The differences of the different time and treatments on two factors were discussed by two-way analysis of variance (ANOVA) and Duncan's post hoc comparison method was used to compare the obtained data multiple times.
-
Color degradation is a crucial problem occurring during fresh walnut storage[8]. In this study, the appearance of walnuts treated with 0.5 mmol/L SNP solution (20 d) was the most similar to the original appearance (0 d), with a fresh greenness and smooth surface (Fig. 1a). The other groups showed obvious colour degradation (green to faint yellow) and epidermal shrinkage, and the control group and 1.0 mmol/L SNP group also exhibited extensive browning. Figure 1b further reveals that walnuts treated with medium and low SNP concentrations exhibited less browning throughout the whole period. On the 20th day, the browning index decreased by approximately 12.6% and 31.3% in response to 0.1 and 0.5 mmol/L SNP treatment respectively, compared to that in the control samples. Similarly, these two groups maintained lower a* and ΔE values (Fig. 1c & d), and most effectively delayed the process of skin discolouration from green to red and then brown. There was no obvious difference (p ≤ 0.05) in colour parameter contrast with the control group at the high SNP level (except on the 20th day). In addition, water loss is another important factor leading to the shrinkage of the walnut skin[25], and Fig. 1e shows no obvious effect was found among the four groups, indicating that the impact of SNP treatment on the water loss rate of fresh walnuts was relatively small.
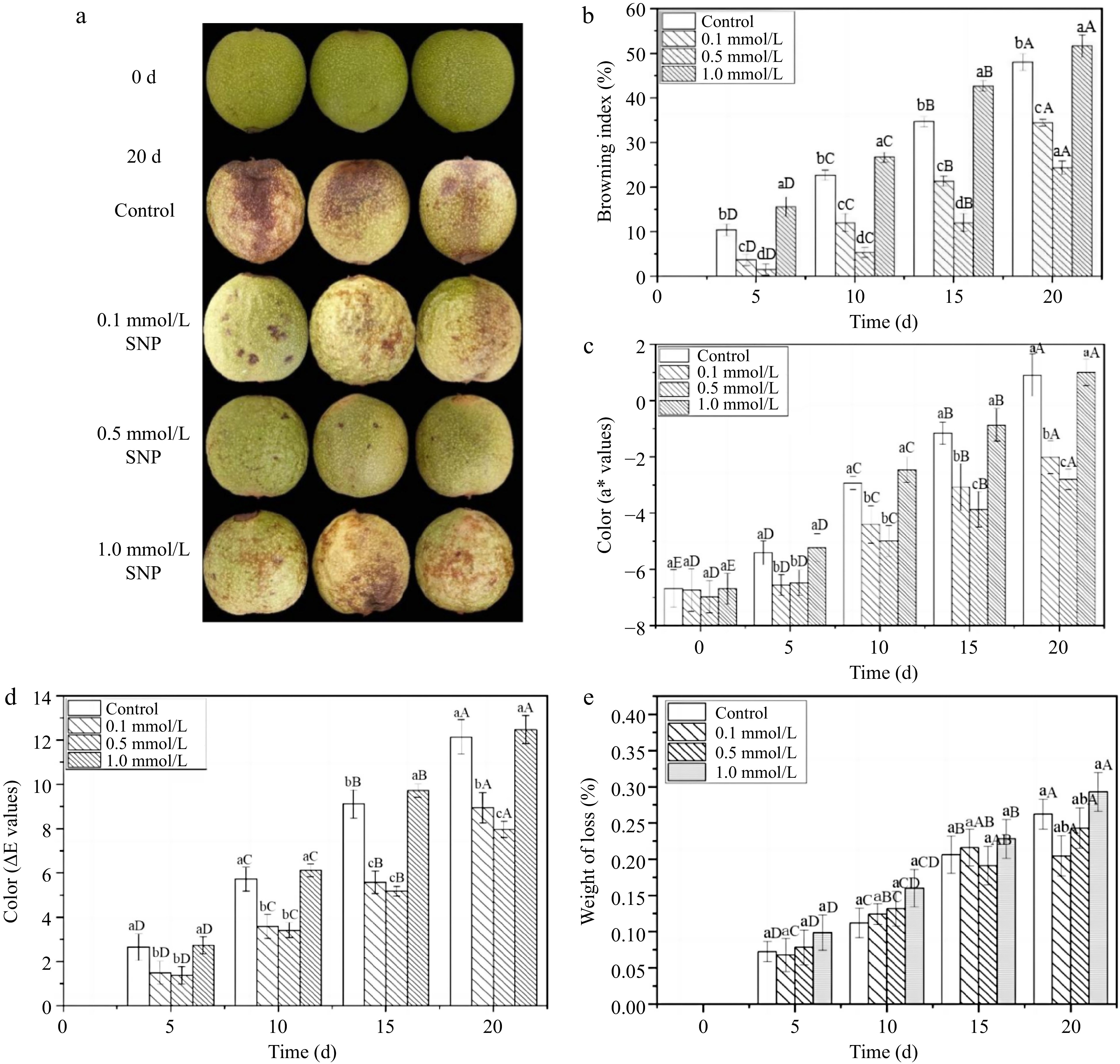
Figure 1.
The appearance quality and freshness of fresh walnut peels under different treatments. (a) Photographs, (b) browning index, (c) a* value changes, (d) ΔE total chromatic difference, (e) weight of loss in distilled water (Control) and SNP (0.1%, 0.5%, and 1.0%) during storage at 24 °C. Values are presented as the means ± standard errors. Lowercase letters indicate different processing groups under the same time conditions; capital letters indicate different times at the same treatment group level. The different letters indicate significant difference (p ≤ 0.05).
The appearance and colour were consistent, as a lower browning index and colour change were maintained in lower and mid concentration SNP treatments. These results are consistent with those of Adhikary et al., who revealed that SNP (NO) treatment had a positive effect on alleviating early fruit colour transformation and browning[12]. In terms of the dose effect, an optimal dose of SNP was determined ( 0.5 mmol/L). The higher the concentration was, the better the quality. Zhu & Zhou reported NO has a dual effect, and excessive SNP caused an increase in the NO concentration. The interaction between NO and O2•− results in the generation of a large amount of peroxynitrite, which produce a negative impact on the fruit[25,12]. This may be the reason that the quality of the high-dose SNP-treated walnuts remained similar to that of the control walnuts instead of being the best. Thus, it is reasonable to believe that walnuts treated with the appropriate concentrations of SNP can improve or maintain fresh quality and prevent shrinkage and browning.
Decay rate and disease resistance
-
Fresh walnuts are toward to decay and disease infection after harvesting[8]. Ren et al.[15] treated mango fruits with SNP, which greatly reduced the frequency of fruit rot and the probability of disease occurrence. Figure 2a shows the effect of different concentrations of SNP on the decay rate of fresh walnuts. There was no obvious difference between the high SNP (1.0 mmol/L)-treated walnuts and the control samples except on the last day, when a lower degree of decay occurred. Moreover, the medium and low SNP concentrations (0.1 and 0.5 mmol/L) had superior inhibitory effects, at 50% and 24.4% of that of the control treatments after 20 d. Notably, the decay index of SNP (0.5 mmol/L) on day 20 was even lower than that of control on day 15. The fruit integrity was higher, significantly reducing the rot rate of walnuts. This is in line with SNP treatment producing a positive effect on preventing decay in fragrant pear fruits[12].

Figure 2.
The decay rate and disease resistance of fresh walnut peels under different treatments. (a) Decay index, (b) CHI activities, (c) GLU activities in distilled water (Control) and SNP (0.1%, 0.5%, and 1.0%) during storage at 24°C. Values are presented as the means ± standard errors. Lowercase letters indicate different processing groups under the same time conditions; capital letters indicate different times at the same treatment group level. The different letters indicate significant difference (p ≤ 0.05).
Additionally, CHI and GLU are two significant proteins that are commonly found in plants and associated with disease progression (PR), and enhancing their activity can assist plant disease resistance[26]. In Fig. 2b & c, the activity of these two disease-related enzymes significantly increased within 20 d. The CHI activity with 0.1 and 0.5 mmol/L SNP treatment groups remained high and stable for 20 d, increasing on average by 14.5% and 9.0%, respectively, compared to the control group. No difference was observed between the 1.0 mmol/L SNP treatment group and the control group except on day 5. It has also been considered that the combined enzymatic action of CHI and GLU results in the inhibition of fungal growth and disease occurrence[26]. The greater the CHI activity is, the greater the ability of the protein to break down the fungal cell wall[27]. Figure 2c shows that GLU activity was enhanced by SNP treatment, with medium and lower levels increasing these activities and the highest improving activities slightly. Notably, on day 5, the activity increased by approximately 39.7% and 18.1% in the 0.5 and 0.1 mmol/L SNP treatment groups, respectively, compared to the control groups. These results are consistent with those of Zheng et al.[28] and Hu et al.[29] which found that the invasion of pathogens led to a rapid response and enhanced the activity of CHI and GLU during the early stage as a defence, and stabilization at a higher activity during the later stage was beneficial for improving fruit resistance, which prevented further infection of pathogens into fruit tissue. Additionally, Hu et al.[27] suggested that exogenous NO enhanced the CHI and GLU activities in post-harvest fruits as well as improved their resistance to pathogens. Thus, it is reasonable to believe that 0.5 mmol/L SNP treatment significantly improved the activity of the important proteins related to PR (CHI and GLU), enhanced the disease resistance and reduced the decay rate of fresh walnuts.
Ethylene production rate and respiratory metabolic rate
-
The rapid accumulation of ethylene accelerates the process of fruit ripening and ageing, serving an indirect indicator for assessing the storage life of fruits, and is closely related to the respiratory metabolic rate[25,30]. The ethylene production rate of all fresh walnuts continuously increased for 20 d (Fig. 3a). The control group and the high-concentration 1.0 mmol/L SNP treatment showed no significant difference (p > 0.05). An increase in the ethylene content accelerates fruit ripening and ageing, and delaying the ethylene release can delay walnut ageing and increase the storage time[31]. The 0.1 and 0.5 mmol/L SNP treated groups produced lower levels during storage, and the ethylene production rates were reduced by 2.0% and 3.6% compared to the control group on the 20th day. Similarly, an increase in the respiratory rate can easily lead to enhanced fruit metabolism, thereby reducing the quality of fruit storage[30]. SNP treatment of 0.5 mmol/L also resulted in the lowest respiratory rate (Fig. 3b) among the four groups throughout storage. On the 20th day, the respiratory rate of 0.5 mmol/L SNP walnuts was diminished by 14.0% compared with that of the control group. This result is consistent with the findings of Chen et al.[32], who showed that SNP suppressed ethylene production and the respiratory rate, delayed the loss of fruit quality.
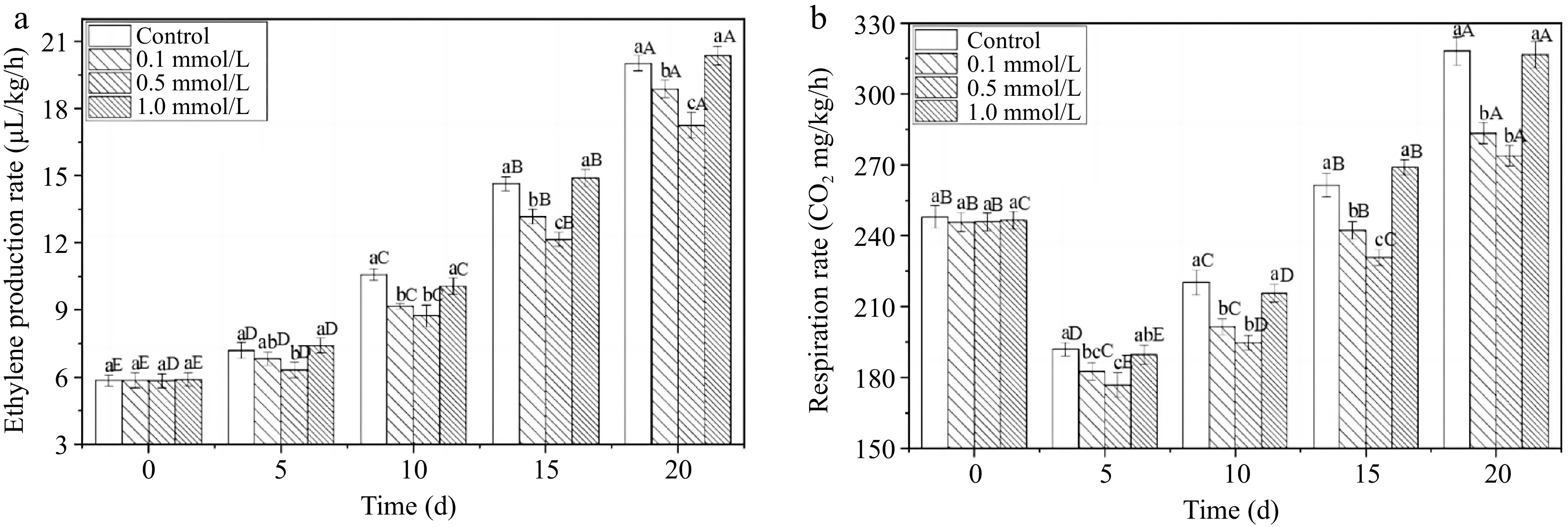
Figure 3.
The ethylene production rate and respiration rate of fresh walnut peels under different treatments. (a) Ethylene production rate, (b) respiration rate in distilled water (Control) and SNP (0.1%, 0.5%, and 1.0%) during storage at 24 °C. Values are presented as the means ± standard errors. Lowercase letters indicate different processing groups under the same time conditions; capital letters indicate different times at the same treatment group level. The different letters indicate significant difference (p ≤ 0.05).
The ethylene production rate and respiratory rate are key physiological parameters that directly determine postharvest quality and storage time. Low- and middle-concentration SNP treatment significantly controlled the rate of increase in these parameters, which may be related to the regulation of key enzymes and gene expression. Zhu & Zhou[25] reported that the most remarked effect was caused by a moderate concentration of SNP, whereas a high dose of SNP harmed the fruits, and a low dose of SNP had little effect on strawberry storage life. These authors suggested that a suitable SNP could effectively inhibited the activity of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and decreased the content of ACC, which contributed to the decrease in ethylene production and the respiratory rate. Cheng et al.[30] proposed that the inhibition of ACC oxidase (ACO) activity and the transcription of the MA-ACO1 gene by NO resulted in decreased ethylene synthesis and a delay in the ripening of banana slice. Consequently, proper SNP treatment may regulate ethylene pathway and delay the respiratory metabolic rates of walnuts and their progression towards decay and ageing.
ROS-redox balance and antioxidant capacity
-
O2•− and H2O2 are two major reactive oxygen species (ROS). The excessive production of ROS leads to oxidative damage and accelerates fruit senescence and deterioration, resulting in a shortened postharvest storage life[33]. Figure 4a & b show that the O2•− production and H2O2 content of all the fresh walnuts increased within 20 d. The increase rates of the 0.1 and 0.5 mmol/L SNP treated groups were obviously lower than those in the other groups, particularly within 10 to 20 d. Controlling the accumulation of ROS could slow the fruit ripening process and reduce the occurrence of spoilage to some extent, similar the findings of Zhang et al.[34]. They showed that SNP treatment decreased H2O2 and O2•− accumulation by 1.2 and 1.4 times compared to that in control rambutans and deferred the deterioration of fruit postharvest quality. Additionally, although SNP treatment with a high-concentration delayed the accumulation of H2O2 and O2•− on the 5th day, it resulted in a rapid increase in the following days, which was not conducive to long-term preservation.
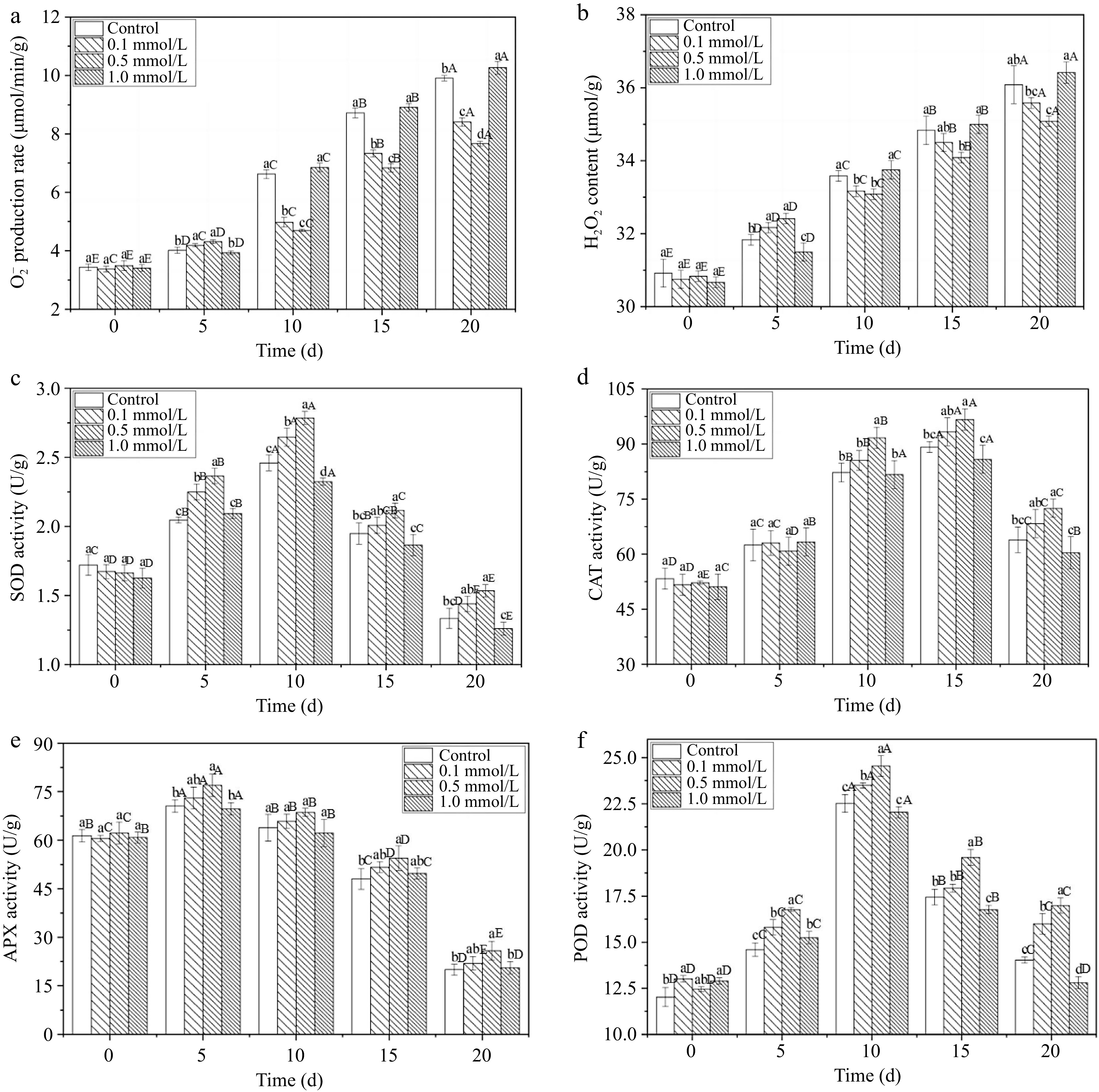
Figure 4.
The ROS-redox balance of fresh walnut peels under different treatments. (a) O2•− production rate, (b) H2O2 content, (c) SOD: superoxide dismutase, (d) CAT: catalase, (e) APX: ascorbic acid peroxidase, (f) POD: peroxidase in distilled water (Control) and SNP (0.1%, 0.5%, and 1.0%) during storage at 24°C. Values are presented as the means ± standard errors. Lowercase letters indicate different processing groups under the same time conditions; capital letters indicate different times at the same treatment group level. The different letters indicate significant difference (p ≤ 0.05).
SOD can remove O2•− and is considered the first line of defence against the powerful toxicity of superoxide[35]. Figure 4c shows that SOD activity peaked on the 10th day. The activity in the 0.5 mmol/L SNP treatment group was approximately 13.2% and 15.0% higher than that in the control group on the 10th and 20th days, respectively (p ≤ 0.05), demonstrating that high SOD activity was induced by the intermediate SNP concentration. CAT is an oxidoreductase that primarily metabolizes H2O2 into H2O and O2, reducing oxidative damage[1]. The CAT activity exhibited a gradually increasing trend within 15 d and then rapidly decreased (Fig. 4d). In the later stage of storage (10−20 d), the 0.5 mmol/L SNP treatment produced higher CAT activity, which was 10.9% higher than that of the control group. Moreover, APX can also interact with CAT to remove H2O2 from fruit tissue, protect the tissue from free radicals, and enhance fruit stress resistance[36]. In this study, there was little difference in APX activity among the various treatments (Fig. 4e), except on days 5 and 20 in the 0.5 mmol/L SNP treatment. POD is an important enzyme not only related to antioxidant defence systems but also contributes to browning[15]. Figure 4f shows that POD activity peaked on the 10th day, and was enhanced by approximately 12.2% at 20 d by 0.5 mmol/L SNP treatment than that of the control group.
The antioxidant enzymes APX, CAT, SOD and POD constitute a powerful protective system in fruit that can effectively eliminate ROS and free radicals. SNP treatment not only enhanced the antioxidant activities of CAT, SOD and POD but also reduced the H2O2 and superoxide anion radical levels compared with those in the control group. The increase in these enzyme activities indicated that the antioxidant capacity of the fruit improved, which may help maintain its freshness and alleviate browning and ageing[11,37]. The above results are consistent with those of Ren et al.[15], who reported that SNP sensibly enhanced fruit antioxidant enzyme activity, suppressed the respiratory rate, and decreased the peel colour index, and the rot index in mango fruit. Jing et al.[35] also showed that treatment with an appropriate concentration of NO delayed the decrease in the mitochondrial permeability transition and reduced the content of ROS in mitochondria. Therefore, it is concluded that proper SNP treatment can enhance antioxidant levels, reduce ROS accumulation and help maintain postharvest quality (Fig. 5).
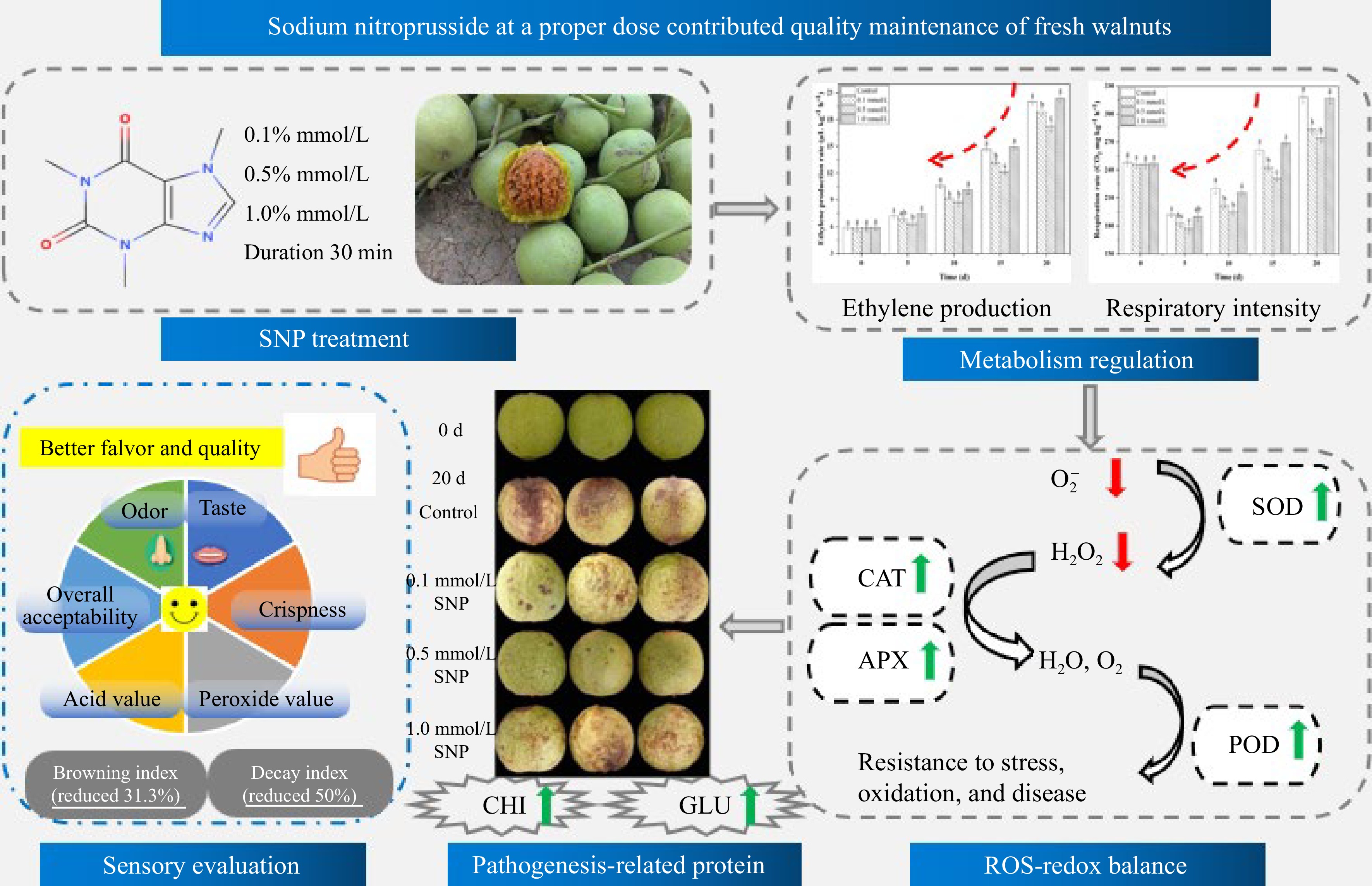
Figure 5.
Possible mechanism whereby treatments of SNP treatment maintain the postharvest quality of fresh walnuts.
Sensory quality of walnut kernels
-
The sensory quality of walnut kernels to some extent reflects the storage quality and acceptability for consumers[3]. The higher the sensory evaluation score, the greater the quality of the fresh walnut kernels. Table 1 shows that the sensory quality of walnut kernels decreased on day 20, particularly as the seeds browned. In terms of concentration, 0.5 mmol/L SNP treatment achieved the highest score, and the overall consumer acceptance was 30% higher compared to the control group. Not only the browning degree of walnut seeds and kernels was the lowest, but also the odour, taste, and crispness were significantly improved (p ≤ 0.05). These results were similar to those of Dai et al.[38], in which potatoes soaked in SNP minimized the damage from surface colour and chewing features (34.3%), resulting in optimal storage quality and acceptance.
Table 1. Sensory evaluation, acid value, and peroxide value of fresh walnut kernels in distilled water (Control) and SNP (0.1%, 0.5%, and 1.0%) during storage at 24°C.
0 d 20 d CK 0.1 mmol/L SNP 0.5 mmol/L SNP 1.0 mmol/L SNP CK 0.1 mmol/L SNP 0.5 mmol/L SNP 1.0 mmol/L SNP Color of seeds 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 3.40 ± 0.51bB 3.87 ± 0.64abB 4.33 ± 0.72aB 3.53 ± 0.52bB Color of walnut kernel 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 6.67 ± 0.72bB 6.93 ± 0.80abB 7.33 ± 0.49aB 6.60 ± 0.51bB Odor 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 4.20 ± 0.77bB 5.53 ± 0.64aB 6.07 ± 0.80aB 4.13 ± 0.52bB Taste 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 4.33 ± 0.62bB 5.33 ± 0.90aB 5.67 ± 0.98aB 4.26 ± 0.59bB Crispness 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 4.87 ± 0.64bcB 5.47 ± 0.64bB 6.67 ± 0.90aB 4.53 ± 0.74cB Overll consumer
acceptance9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 9.00 ± 0.00aA 4.69 ± 0.26cB 5.43 ± 0.45bB 6.01 ± 0.29aB 4.61 ± 0.31cB AV (mg/g) 0.39 ± 0.01aB 0.37 ± 0.04aB 0.36 ± 0.04aB 0.37 ± 0.04aB 0.99 ± 0.05aA 0.87 ± 0.07aA 0.71 ± 0.06bA 0.95 ± 0.09aA POV (mg/100g) 0.11 ± 0.01aB 0.10 ± 0.01aB 0.11 ± 0.01aB 0.10 ± 0.01aB 0.32 ± 0.03aA 0.28 ± 0.01abA 0.25 ± 0.01bA 0.31 ± 0.02aA Lowercase letters indicate different processing groups under the same time conditions; uppercase letters indicate different times at the same treatment group level. The different letters indicate significant difference (p ≤ 0.05). Additionally, walnuts are rich in unsaturated fatty acids, and their AV and PV are important indicators for measuring fat oxidation and rancidity, reflecting the edible value and safety of walnut kernels[7]. The AV and PV continuously increased with the increasing storage time in this study and remained within the safe range (AV ≤ 3 mg/g, PV ≤ 80 mg/100 g, based on fat). The 0.5 and 0.1 mmol/L SNP treatment groups exhibited controlled increases (P ≤ 0.05). Notabely, the AV was reduced by 8.4% and 25.3%, and the PV was significantly reduced by 9.7% and 19.4%, respectively, compared with the control walnuts. Dai et al.[39] also stated that SNP treatment increased the expression levels of key genes associated with fatty acid synthesis, maintained membrane structural integrity, and slowed the occurance of internal oxidation and rancidity. Therefore, it was inferred that 0.5 mmol/L SNP can control rancidity and oxidation, maintain good sensory characteristics, prolong the fruit quality and improve sales quality of fresh walnut kernels.
-
In this study, low- and moderate-concentration SNP treatment effectively preserved the postharvest quality of fresh walnuts, and 0.5 mmol/L caused the least decay and colour change. SNP activated two crucial disease-related proteins (CHI and GLU), and retarded, to some extent, respiratory metabolism and ethylene production in walnuts. Furthermore, the metabolism of reactive oxygen species (O2•− and H2O2) was regulated and the antioxidant enzymes activities such as SOD, CAT, APX, and POD increased. Sensory evaluation revealed a greater overall consumer acceptance, and lower levels of AV and PV were achieved by SNP treatment. It was concluded that optimal SNP treatment may mediate the physiological metabolic rate, activate disease-related enzymes, regulate the ROS-redox balance, and therefore maintain postharvest quality (Fig. 5). This study offers an innovative solution for promoting environmentally friendly and minimizing resource waste.
This project was supported by the National Natural Science Foundation of China (No. 32001765. No. 32272395); Postdoctoral Research Foundation of China (No. 2022M712375); and Open Project Program of State Key Laboratory of Food Nutrition and Safety (No. SKLFNS-KF-202316).
-
The authors confirm contribution to the paper as follows: conceptualization: Qiao L, Deng X, Jiao Y; methodology: Yu X, Jiao Y; investigation: Yu X, Jiao Y; data curation: Deng X, Feng M; formal analysis and visualization: Qiao L, Jiao Y; writing-original draft: Qiao L, Deng X; writing-review & editing: Qiao L, Deng X, Feng M; funding acquisition: Qiao L, Lu L, Wang Y; supervision: Lu L, Liu X. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Qiao L, Deng X, Yu X, Feng M, Jiao Y, et al. 2024. Appropriate sodium nitroprusside dose contributes to the quality maintenance of fresh walnuts. Food Innovation and Advances 3(1): 42−51 doi: 10.48130/fia-0024-0006
Appropriate sodium nitroprusside dose contributes to the quality maintenance of fresh walnuts
- Received: 01 February 2024
- Revised: 11 March 2024
- Accepted: 14 March 2024
- Published online: 28 March 2024
Abstract: Fresh walnuts (Juglans regia L.) are challenging to store due to their high water content and delicate green appearance. It has been reported that sodium nitroprusside (SNP, a nitric oxide donor) can promote stress tolerance. However, whether SNP affects the postharvest quality of fresh walnuts remains unknown. This research showed that appropriate SNP treatment contributed to walnut preservation; in particular, 0.5 mmol/L SNP treatment resulted in a better appearance and less decay (59.7%). Compared with the control, this treatment not only increased the levels of proteases related to fresh walnut disease (chitinase and β-1,3-glucanase) but also increased the overall antioxidant level and reduced oxidant damage. Moreover, respiratory metabolism and ethylene release were greatly suppressed (9.5%), and the overall sensory evaluation did not reveal any adverse effects associated with a lower acid or peroxide content. Thus, it was inferred that the optimal SNP dose activated disease-related enzymes, mediated the physiological metabolism rate, regulated the ROS-redox balance and therefore reduced decay and maintained the walnut quality. This is the first report of SNP (NO) application for the preservation of fresh walnuts and may provide information to facilitate practical application of this potential innovation.
-
Key words:
- Fresh walnuts /
- Sodium nitroprusside /
- Disease resistance /
- Decay and quality /
- ROS-redox balance /
- Sensory evaluation.












