-
Polygonati rhizoma is included in the Chinese Pharmacopoeia (ChP) as a traditional Chinese medicinal material[1]. It refers to the dried rhizomes of three different species of the genus Polygonatum Mill, namely Polygonatum sibiricum (called JīTóuHuángJīng in Chinese), Polygonatum cyrtonema (DuōHuāHuángJīng), and Polygonatum kingianum (DiānHuánɡJīnɡ), which have undergone a traditional processing technology 'nine-steaming and nine-drying'. The genus is mainly distributed from eastern Japan to the western Himalayan region, with a few species found in Europe and North America. It originated in the Hengduan Mountain-Himalayan region about 20 million years ago[2]. With the rise of the Tibetan Plateau, global climate cooling, and the strengthening of the East Asian winter monsoon, the genus has gradually diversified, which typically flourishes in humid climates and well-drained moist soils[3].
In recent times, partially due to concerns regarding the adverse effects of chemical medicines, Traditional Chinese Medicine has received substantial global recognition and has become a subject of extensive scientific investigation[4]. Among these traditional remedies, Polygonati rhizoma a traditional Chinese herbal medicine, has emerged as a focus of significant attention due to its abundant nutritional contents and therapeutic constituents[5,6]. It has been widely used in traditional Chinese medicine and has demonstrated remarkable pharmacological effects such as anti-aging, anti-tumor, anti-diabetes, and physiological roles[4,7]. Polygonati rhizoma encompasses a myriad of active components, such as saponins, flavonoids, and alkaloids (Fig. 1), which have been substantiated to possess a wide spectrum of biological activities and pharmacological effects[8] .
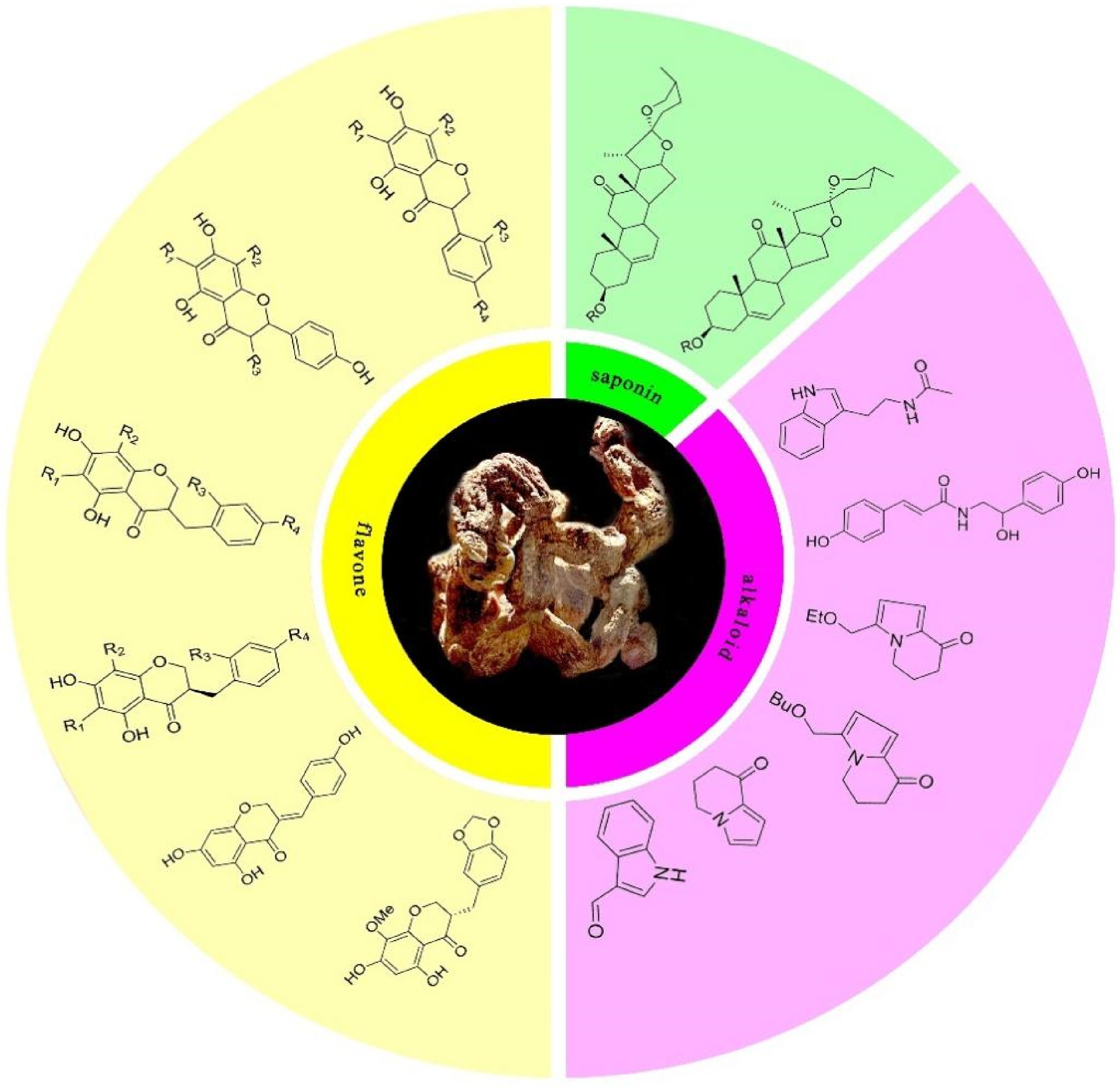
Figure 1.
Bioactive constituents of Polygonati rhizoma. The representative image of Polygonati rhizoma rhizome surrounded by structural formula of saponin, alkaloid, and flavonoid compounds that have been identified within Polygonati rhizoma.
Although Polygonati rhizoma has a well-established history in traditional Chinese medicine and plays an important role in sustainable agriculture and food systems. The current comprehension of its pharmacological research is still in a nascent stage and confronts certain challenges and gaps[9,10]. In recent years, an increasing number of studies have underscored the broad medicinal significance and potential agricultural importance of Polygonati rhizoma, positioning it as a promising resource for the exploration of novel pharmaceutical drugs and nutritious foods[11]. Therefore, it is of paramount importance to deepen the molecular understanding of Polygonati rhizoma, identify its bioactive compounds, elucidate its mechanisms of action, and probe its potential clinical applications[12]. This review aims to systematically summarize the most recent advancements in Polygonati rhizoma research and provides a comprehensive overview of the distinct mechanisms that underlie Polygonati rhizoma's effects from various perspectives. We prioritize an in-depth exploration of the active constituents of Polygonati rhizoma and deciphering their intricate mechanisms of action. Furthermore, we provide recommendations to rectify the often overlooked facets of Polygonati rhizoma in pharmacological investigations, with the aim of bridging the existing research gaps.
-
Diabetes mellitus is a metabolic disorder distinguished by abnormal metabolism, resulting from either inadequate insulin secretion or insulin resistance[13,14]. It has emerged as a significant health concern and poses a threat to human welfare. A multitude of studies have indicated the potential of Chinese herbal medicine as a natural solution for diabetes and its related complications[15,16]. Polygonati rhizoma has shown promising health benefits, primarily attributed to its polysaccharides known as Polygonati rhizoma polysaccharides (PRP)[17]. Studies have demonstrated the effectiveness of PRP in diminishing fasting blood glucose and hemoglobin A1C levels in diabetic rats. PRP also elicits a dose-dependent elevation in insulin and C-peptide levels. In addition, PRP proficiently mitigates symptoms like polydipsia, polyphagia, polyuria, and weight loss in diabetic rats. In electrophysiological studies, PRP displays a range of advantageous effects on diabetic ocular complications[18]. Moreover, PRP reduces retinal cell apoptosis and promotes cell proliferation and repair, thus mitigating retinal impairment[19]. In summary, PRP showcases promising therapeutic potential against diabetes and its correlated complications.
Polygonati rhizoma saponins (PRS) are a type of triterpenoid compound, consisting of a steroidal core with a triterpenoid alcohol and a glycoside group[20]. The glycosidic moiety in saponins can indeed consist of various monosaccharides, such as glucose, galactose, arabinose, and xylose. The specific arrangement and types of sugar molecules in the glycoside portion of the saponin molecule greatly influence its properties, such as water solubility, stability, and biological activity. PRS is an important biological active ingredient found in Polygonati rhizoma[21,22]. PRS has exhibited promising hypoglycemic effects and the capacity to modulate intestinal flora (gut microbiota) in murine models of type 2 diabetes mellitus (T2DM). The hypoglycemic effect of PRS primarily manifests as a substantial inhibition of amylase and glucosidase activities, coupled with the amelioration of insulin resistance in IR-HepG2 cells. Consequently, this cascade leads to heightened glucose consumption and enhanced metabolic activity. Notably, akin to PRP, PRS has yielded favorable outcomes, demonstrating its potential to mitigate clinical manifestations like polydipsia and polyuria in experimental diabetes animal models[18]. An additional mechanism through which PRS operates involves the reduction of blood glucose concentration by augmenting the activity of the insulin signaling pathway and enhancing the liver's glycogen synthesis capacity[23]. These discoveries robustly underscore the noteworthy potential of Polygonati rhizoma in the diabetes treatment paradigm, carrying substantial implications for both diabetes prevention and therapeutic intervention.
In terms of other active ingredients, the phenolic substance, syringaresinol-di-O-beta-D-glucoside in Polygonati rhizoma has demonstrated the capacity to lower blood glucose and glycated hemoglobin levels[24]. This compound also enhances the antioxidant capacity in mice, aligning with the observed inhibition of oxidative stress response attributed to PRS[19]. In comparison to these relatively uncomplicated constituents, extracts or fermentation solutions characterized by more intricate compositions showcase a diverse array of anti-diabetic mechanisms. Notably, the aqueous extract of Polygonati rhizoma has exhibited the capability to enhance blood glucose and lipid metabolism in diabetic mice by modulating the PI3K/AKT signaling pathway[25]. Furthermore, the fermentation fluid of Polygonati rhizoma has demonstrated substantial reductions in blood glucose levels and insulin resistance in diabetic mice (Fig. 2). It effectively reduces total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels[26]. These findings not only offer novel perspectives on the advancement and utilization of Polygonati rhizoma but also lay a groundwork of experimental evidence for enhancing Polygonati rhizoma's efficacy via natural fermentation processing.
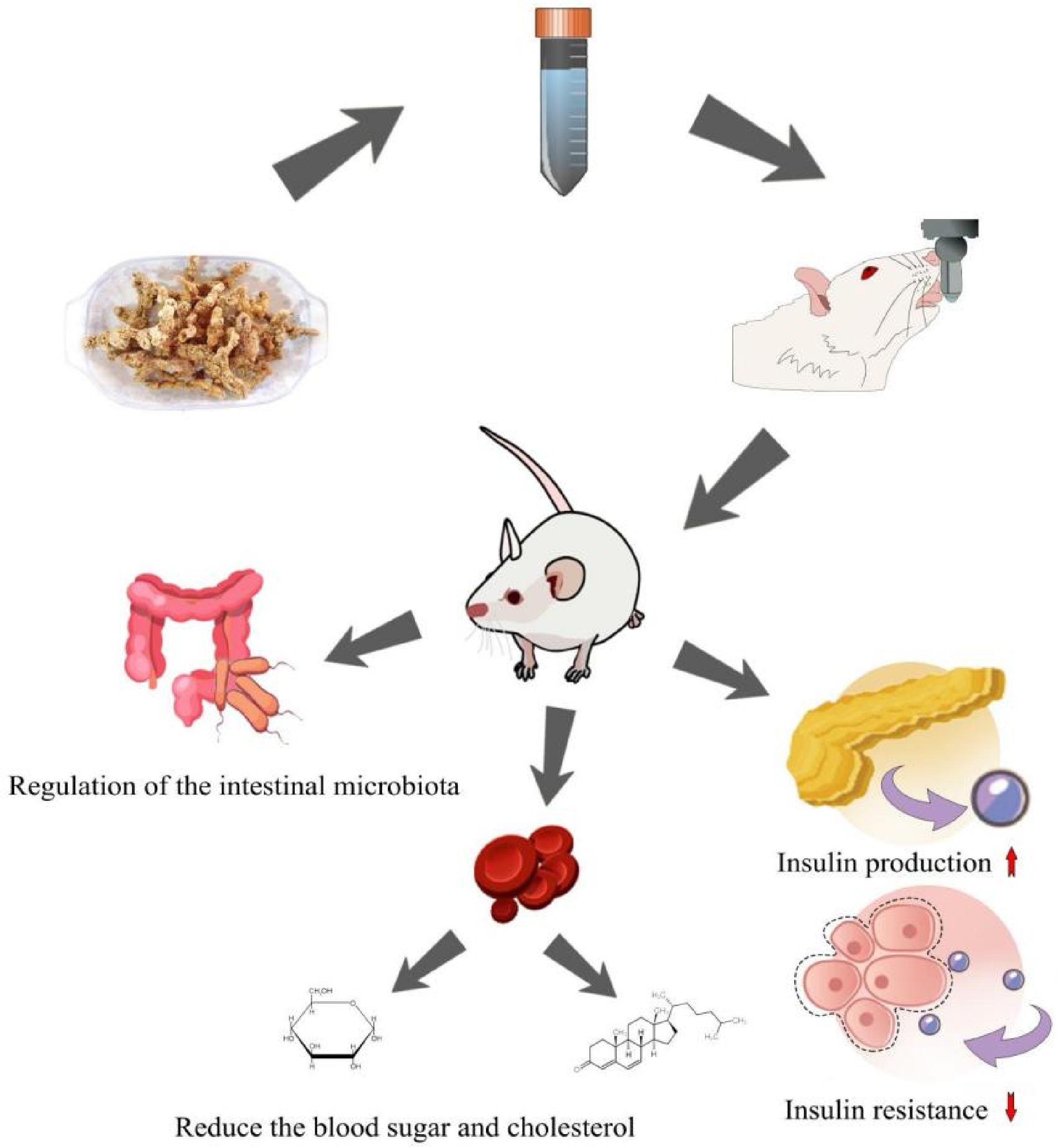
Figure 2.
The influence of Polygonati rhizoma on diabetic model mice physiological regulation. Upon administration of Polygonati rhizoma to diabetic model mice, its regulatory impact is predominantly evident in several aspects, such as modulation of the intestinal microbiota composition, a decline in blood glucose and cholesterol concentrations, enhancement of pancreatic insulin production efficiency, and mitigation of cellular insulin resistance.
In summary, the active ingredients of Polygonati rhizoma, especially PRP and PRS, show obvious hypoglycemic effects and alleviate the multiple complications of diabetes through various regulations. These studies provide an important scientific basis for the application of Polygonati rhizoma in the treatment of diabetes and provide a new perspective and possibility for further development and utilization. Furthermore, the study of complex extracts, such as fermentation liquid, provides a new idea and experimental basis for improving the efficacy of Polygonati rhizoma. Therefore, studying the mechanism of the action of Polygonati rhizoma and its active constituents, and optimizing its efficacy will have an important impact on the prevention and treatment of diabetes. However, there are notable limitations to present studies, which predominantly centered around pathological models, and these outcomes have not yet undergone validation through human studies[27]. Thus, it remains imperative to embark on further research endeavors that can corroborate the safety and effectiveness of Polygonati rhizoma in humans[28]. Moreover, the current multiple studies did not delve into the exploration of suitable dosages and safety profiles for individual constituents or complex formulations of Polygonati rhizoma in human populations. The determination of the optimal dosage and the assurance of safety in diverse modes of administration stand as pivotal considerations necessitating meticulous assessment. To surmount these limitations, additional investigations are warranted to comprehensively explore the safety, efficacy, and dosage optimization of Polygonati rhizoma in humans. Such research endeavors will undoubtedly lay a more robust foundation for the potential clinical applications of Polygonati rhizoma.
-
In recent years, traditional Chinese medicine has garnered growing attention in its application to cancer treatment[29,30], particularly evident in the utilization of compounds like polysaccharides and saponins derived from Chinese herbal medicines[7,31]. In general, PRP and PRS exhibit analogous mechanisms to other medicinal plants containing these compounds in their anti-tumor applications. A specific PRS named methylprodipyrimidine (MPD) has demonstrated robust inhibitory activity against cervical cancer[32]. It induces apoptosis primarily by inducing alterations in cancer cell morphology, arresting the cell cycle at the G2/M phase, and escalating the generation of reactive oxygen species (ROS) in cancer cells in a dose-dependent manner. Similar to PRS, PRP fosters apoptosis in tumor cells through a multitude of mechanisms. It triggers the TLR4 signaling pathway, curbs the activation of MAPK and NF-kappa B, amplifies the apoptosis rate of tumor cells and prompts cell cycle arrest. In terms of inhibiting tumor cell growth, PRS diverges from PRP due to its distinct inhibition of the MAPK pathway (Fig. 3). Transcriptomic analysis has highlighted noteworthy differences in an array of genes enriched in the MAPK pathway. Molecular evidence further demonstrates that PRS diminishes the expression levels of MMP2 and MMP9 both in vivo and in vitro. Furthermore, it significantly curtails the phosphorylation levels of MEK, ERK, and JNK[33]. Other Chinese herbs containing saponins exhibit a parallel mechanism of inducing cell cycle arrest[34−36]. For instance, among the saponins found within Siraitia grosvenorii fruit (known as LuóHànGuǒ in Chinese), mogroside induces G0/G1 cell cycle arrest in leukemia tumor cells, culminating in apoptosis[37]. Reactive oxygen species (ROS) encompass chemically reactive oxygen-containing molecules, spanning peroxides, superoxides, hydroxyl radicals, singlet oxygen, and alpha-oxygen[38]. While ROS are natural byproducts of standard oxygen metabolism, they assume pivotal roles in cell signaling and homeostasis[39]. However, under varied adverse conditions, ROS levels can escalate significantly[40]. In the context of cervical cancer cells, MPD prompts the intracellular production of ROS. Conversely, other medicinal plants avert cancer by inhibiting intracellular ROS generation, thereby curbing free radical formation and diminishing cancer susceptibility. Consequently, this study proposes the potential candidacy of MPD as a targeted drug for cervical cancer[32]. In terms of targeted drugs, Polygonati rhizoma total glucoside (TG) has showcased remarkable inhibition of proliferation in three cancer cell lines (A549, HepG2, Caco2). Isolated from TG are seven steroidal glycosides (polygonadine 1-7) and 24 known glycosides. Among these, compound 10 has exhibited the capability to lower the expression of Bcl-2 and pro-caspase3[41]. Molecular docking experiments have unveiled stable interactions between compound 10 and the Bcl-2 protein via hydrogen bonding, consequently impeding tumor cell proliferation. Interestingly, PRP against breast cancer indirectly by protecting hematopoietic cells in the bone marrow[42]. PRP has been identified to propel the differentiation of hematopoietic stem cells into matured red and white blood cells, consequently enhancing hematopoietic capacity. This study also presents a novel therapeutic avenue utilizing PRP to shield bone marrow stem cells from cancer cell influence. Such research holds promise as an adjunct therapy for breast cancer, enhancing patients' quality of life and overall survival.
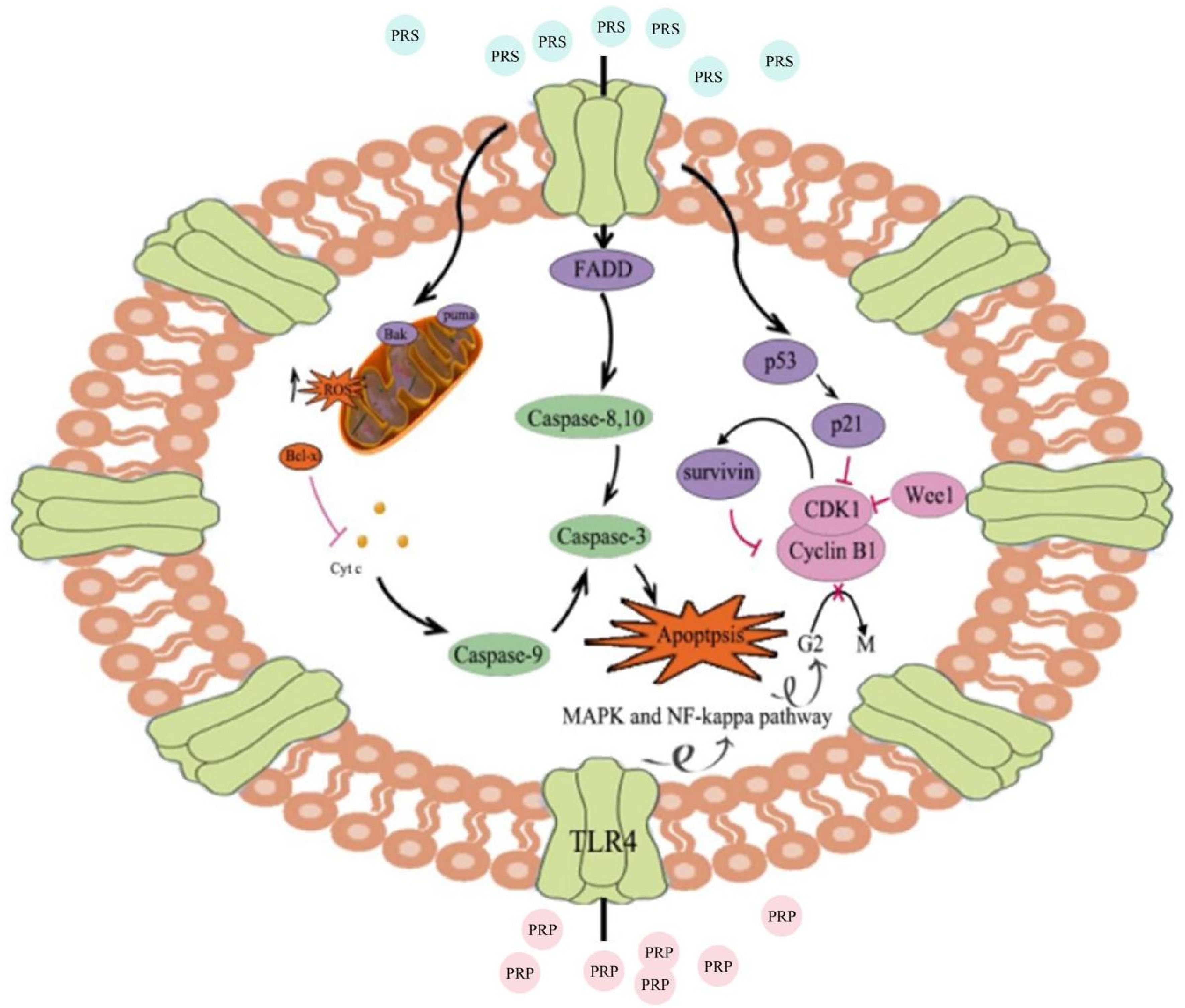
Figure 3.
Diagram of the mechanism model depicting tumor growth inhibition activity of Polygonati rhizoma. PRS effectively achieves its objective of inducing cell cycle arrest and stimulating tumor cell apoptosis by elevating intracellular ROS levels within tumor cells. PRP accomplishes the same objectives through distinct mechanisms, including activation of the TLR4 signaling pathway and suppression of the MAPK and NF-kappa B activation.
In summary, PRP and PRS are the main active components of Polygonati rhizoma for inhibiting tumor growth. They are similar in their modes of action, especially in controlling the cell cycle. However, they differ in the inhibitory mechanism of the MAPK pathway. There's no denying that the studies discussed provide valuable insights into the potential of Polygonati rhizoma as a source of anti-proliferative agents in tumor cells, highlighting its potential as an adjuvant therapy. The results unequivocally underscore the significance of traditional medicinal plants as promising founts for novel drug development, following an extensive historical tradition of employment across diverse cultures for malady alleviation[43]. Laden with an array of bioactive compounds, traditional medicinal plants have demonstrated therapeutic attributes that encompass intricate mechanisms of action[44]. Studies on Polygonati rhizoma and its constituents, including PRP, PRS, and TG, unequivocally substantiate their capacity to stifle the proliferation of tumor cells, provoke apoptosis, govern cell cycle advancement, and orchestrate signaling pathway modulation. This corpus of findings casts a spotlight on the imperative role of probing the vast biodiversity inherent to traditional medicinal plants and their constituent compounds as latent repositories for pioneering therapeutic agents[45]. Traditional medicine stands as a potent repository for vital cues in drug discovery, propelling the advancement of innovative treatments for an array of afflictions, including cancer[46]. Nonetheless, a continued pursuit of further research and inquiry remain essential to comprehensively unravel the mechanisms of action, refine optimal dosage regimens, and validate the efficacy and safety of these compounds through human clinical trials. By capitalizing on the latent potential harbored by traditional medicinal plants, we can unravel novel drugs and therapeutic paradigms that hold the potential to ameliorate patient outcomes and address unmet medical exigencies.
-
Triterpenoids constitute a major class of active compounds found in Polygonati rhizoma. Accumulating evidence has demonstrated the anti-inflammatory potential of triterpenoids, operating through diverse pathways. In-depth investigation into the rhizome constituents of Polygonati rhizoma has identified 27 chemical components. Two specific compounds within these components exhibited significant inhibitory effects on the production of inflammatory factors in RAW264.7 macrophages, encompassing NO, TNF-α, IL-6, and IL-1β[47]. Notably, these compounds effectively suppressed the protein expression of COX-2 and iNOS, alongside the NF-κB pathway. COX-2 and iNOS play pivotal roles in the inflammatory process[48−50], with their transcriptional expression being regulated by the NF-κB pathway[51]. Inhibition of NF-κB activation consequently diminishes the expression of these genes, leading to the mitigation of the inflammatory and immune response[52]. Interestingly, PRP operates via a distinct anti-inflammatory mechanism. By activating the AMPK pathway, PRP curtails the inflammatory response triggered by a high-fat diet, echoing the mechanism exhibited by the aforementioned compounds[53]. Activation of AMPK can inhibit the inflammatory response by preventing activation of the NF-κB pathway[54]. When NF-κB remains in an inactive state, it is restrained by the IκB protein family. However, upon stimulation by signals, IκB undergoes degradation, thereby enabling the activation of NF-κB. In a broader context, the AMPK and NF-κB signaling pathways exhibit intricate interplay and a regulatory relationship, capable of mutually influencing one another and overseeing essential physiological processes, including cell metabolism and immune responses. Consequently, it is reasonable to posit that the constituents within Polygonati rhizoma engage in mutual interactions pertaining to their anti-inflammatory actions.
Delving into the therapeutic potential of PRP in lipopolysaccharide (LPS)-induced acute lung injury (ALI), PRP emerges as a regulator of macrophage polarization. It ameliorates ALI via the Toll-like receptor 4 (TLR4)-MAPK/NF-κB signaling pathway[55]. PRP mitigates LPS-induced ALI by reducing levels of inflammatory cytokines and lung injury markers. Moreover, PRP exerts control over macrophage polarization, diminishing pro-inflammatory M1 macrophages and amplifying anti-inflammatory M2 macrophages. The therapeutic promise of PRP extends to sepsis, a high-fatality systemic inflammatory response syndrome, wherein it restrains hepatic pyroptosis through the attenuation of NLRP3 and GSDMD expression, pivotal constituents of the pyroptosis pathway. PRP treatment leads to lowered levels of IL-1β and IL-18, produced during pyroptosis[56].
In summary, Polygonati rhizoma's anti-inflammatory effects are mediated through the intricate orchestration of multiple signaling pathways, encompassing AMPK, NF-κB, and NLRP3/GSDMD (Fig. 4). This orchestration culminates in the downregulation of an array of inflammatory factors. These studies reveal the complex mechanism of the anti-inflammatory effects of Polygonati rhizoma and provide an important scientific basis for its further clinical applications. By gaining a deeper understanding of the mechanism of action of its active components, Polygonati rhizoma can be better utilized and developed as a potential drug for the treatment of inflammatory diseases. However, despite significant progress, further clinical studies are needed to validate its efficacy and safety for widespread use in clinical practice.
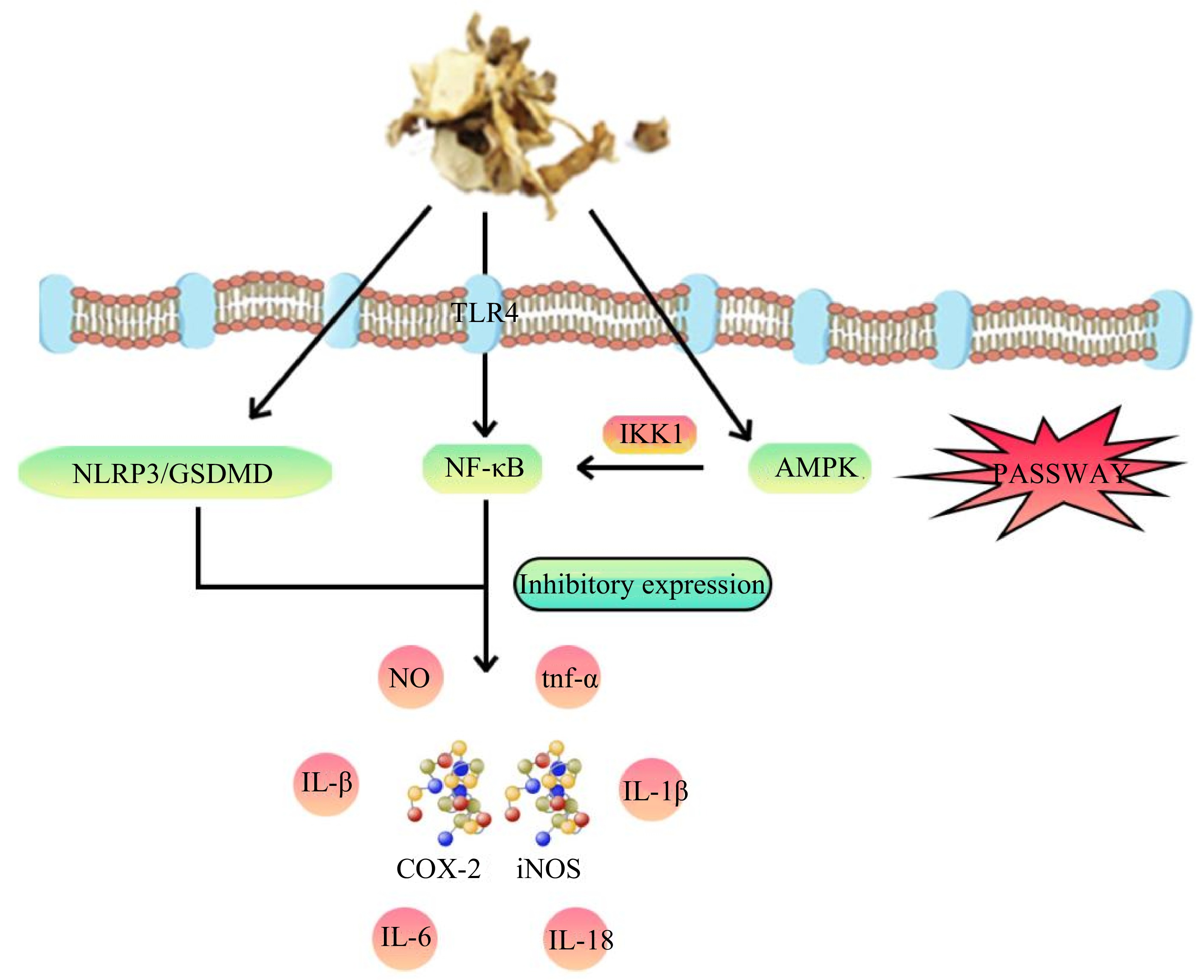
Figure 4.
Mechanism model diagram illustrating the anti-inflammatory effects of Polygonati rhizoma. The anti-inflammatory attributes of Polygonati rhizoma predominantly hinges upon its adept modulation of a multitude of signaling pathways, notably encompassing AMPK, NF-κB, and NLRP3/GSDMD. This orchestrated modulation effectively curtails the expression of inflammatory factors, alongside COX-2 and iNOS, thereby culminating in the achievement of its potent anti-inflammatory effect.
-
The intricate maintenance of biological homeostasis in vivo is orchestrated by the interplay of vital regulatory mechanisms within the nervous, immune, and metabolic systems[57−59]. Polygonati rhizoma root extract emerges as a notable contender in enhancing sleep quality and cognitive function among mild insomnia patients[60]. Additionally, it navigates the realm of cognitive health by influencing synaptic plasticity in aging rats through the BDNF-TrkB signaling pathway[61]. Intriguingly, a monomeric polysaccharide, PRP-1, isolated from Polygonati rhizoma has exhibited the potential to counteract synaptic loss and restore gut microbiota composition in 5xFAD mice, suggesting its potential to alleviate cognitive deficits by modulating gut microbiota[62]. These preliminary findings underscore Polygonati rhizoma's efficacy as a safe and effective therapeutic avenue, paving the way for its integration into health foods for neurological enhancement and aging mitigation. Sleep quality studies attest to Polygonati rhizoma root extract's capacity to substantially enhance sleep quality over a four-week period, evidenced by improvements in Athens Insomnia Scale scores, extended sleep duration and altered perfusion levels in default mode network brain regions.
Furthermore, Polygonati rhizoma rhizome aqueous extract displays sleep-inducing properties in rodent models, attributed to its constituents, oligopeptidic acid, and glycerol mono-linolenic ester. This extract notably reduces sleep latency, and prolongs sleep duration, thereby substantiating its potential as an efficacious and secure treatment for sleep quality enhancement and nervous system protection[63]. The sleep-promoting effect of Polygonati rhizoma extract was accompanied by changes in sleep structure, including an increase in non-rapid eye movement sleep (NREM) and a decrease in rapid eye movement sleep (REM). In the caffeine-induced recovery model, Polygonati rhizoma root aqueous extract increased total sleep time and NREM sleep time, while upregulating protein and mRNA expression levels of GABA(A)-R2 and 5-HT1A receptors involved in sleep regulation. These results provide preliminary evidence to support the use of Polygonati rhizoma rhizome extract as a safe and effective treatment for improving sleep quality and its protective effect on the nervous system. This suggests that Polygonati rhizoma has the potential to be a functional health food for improving nerve function and combating aging.
Rooted in traditional Chinese medicine, Polygonati rhizoma's reputed effects encompass Qi invigoration, Yin nourishment, spleen fortification, and kidney tonification. It's capacity to modulate metabolism and bolster immunity is acknowledged, although the precise mechanisms underpinning immune enhancement remain elusive[64]. Evaluating the immunomodulatory effects of Polygonati rhizoma ethanol extracts, PSE30 and PSE75, unveiled the augmentation of immunoglobulin G (IgG) and immunoglobulin M (IgM) serum levels in cyclophosphamide-induced immunosuppressed mice, aligning with the capacity of PRP to elevate serum protein levels in cyclophosphamide-induced immunosuppressed chick models[59]. These investigations shed light on Polygonati rhizoma's diverse mechanisms for enhancing immune function. Notably, the regulatory effect of Polygonati rhizoma on the immune organ, the spleen was confirmed, with PSE30 and PSE75 enhancing natural killer (NK) cell activity and mRNA expression of Th1 and Th2 markers in spleen cells. Additionally, PRP exhibited the potential to impede apoptosis in immune organ cells, thus upregulating IL-2, IL-6, and IFN-γ expression by steering immune cells toward S phase and G2/M phase. This orchestration is intricately linked to B cell activation, proliferation, and differentiation, instrumental in IgG and IgM production. Strikingly, PSE75 demonstrated the ability to restore cyclophosphamide-disrupted gut microbiota, paralleling PRP-1's restoration of gut microbiota composition in mitigating cognitive deficits in 5xFAD mice[62].
Polygonati rhizoma demonstrates significant potential in addressing metabolic conditions such as diabetes, osteoporosis, obesity, hyperlipidemia, and hypertension by modulating key metabolic processes. MicroRNAs (miRNAs) play a pivotal role in orchestrating cell proliferation, differentiation, lipid metabolism, and hormone secretion. In the realm of bone metabolism disorders, Polysaccharide-Protein Complex (PRP) propels osteoblast generation while impeding osteoclast formation through the Wnt/β-catenin signaling pathway (Fig. 5). Further investigations have illuminated PRP's inhibition of osteoclast formation via the Mir-1224-based Hippo signaling pathway, offering novel insights into its potential for enhancing bone health and thwarting osteoporosis[65]. Regarding other aspects of metabolic regulation, such as lipid metabolism, PRP exerts a profound influence on high-fat diet-induced obesity in rodents by curtailing body weight and lipid levels. It achieves this through the activation of the AMP-activated protein kinase (AMPK) signaling pathway, thereby inducing lipid-lowering and anti-inflammatory effects[53]. In the domain of lipid metabolism regulation, PRP wields its impact on hyperlipidemic mice by modulating the expression of key proteins implicated in lipid processing. Notably, PRP elevates the levels of peroxisome proliferator-activated receptor alpha (PPAR-α) and peroxisome proliferator-activated receptor beta (PPAR-β), while concurrently suppressing the expression of peroxisome proliferator-activated receptor gamma (PPAR-γ), sterol regulatory element-binding protein-1c (SREBP-1c), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α)[53]. PRP's ability to enhance high-density lipoprotein cholesterol (HDL-C) while diminishing low-density lipoprotein cholesterol (LDL-C) further showcases its potential in averting hyperlipidemia, managing obesity, and alleviating osteoporosis stemming from oophorectomy[66].
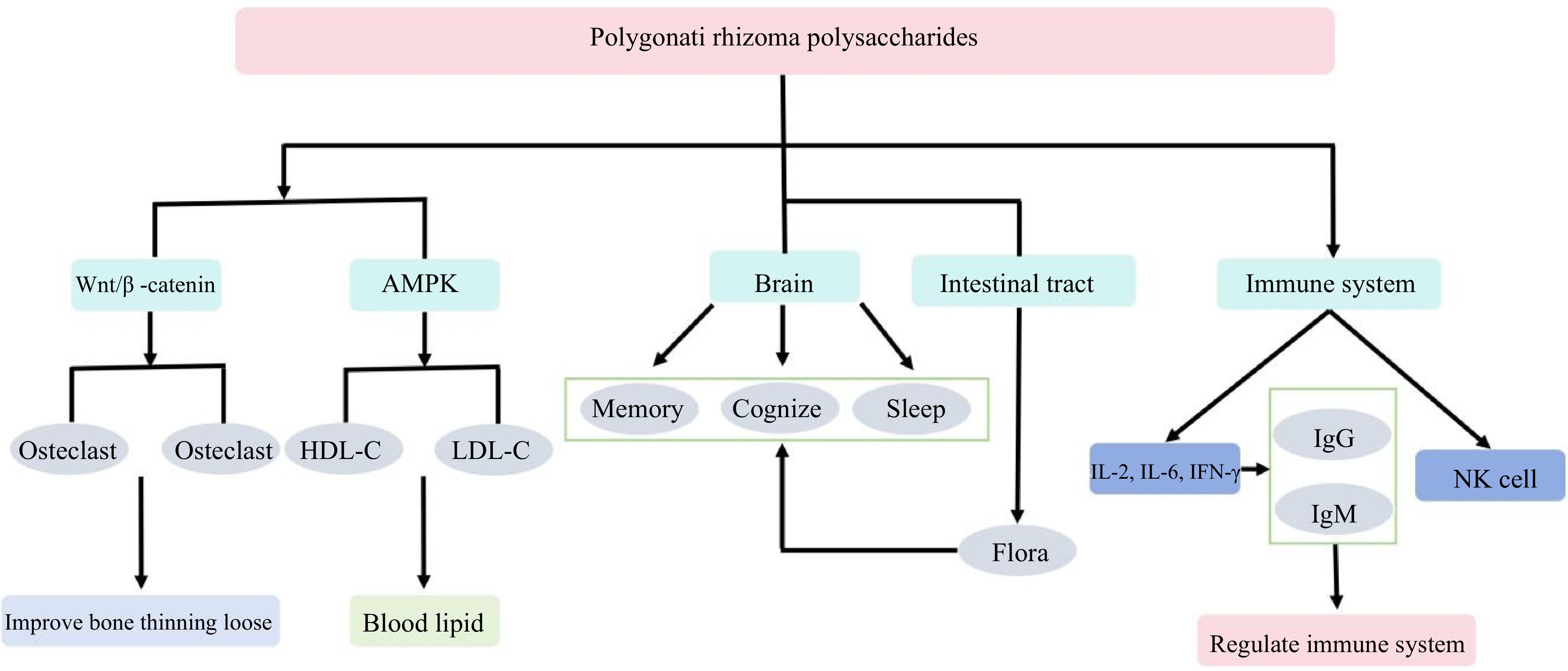
Figure 5.
Multi-target regulatory mechanism of Polygonati rhizoma on physiological function. Polygonati rhizoma's multifaceted regulatory actions encompass diverse physiological domains, including bone health enhancement, blood lipid level reduction, memory and cognitive function improvement, sleep enhancement, and immune system modulation. These intricate mechanisms collectively contribute to the potential therapeutic impact of Polygonati rhizoma across various aspects of human health.
Additionally, Polygonati rhizoma demonstrates efficacy in mitigating blood glucose and lipid levels in type 2 diabetic mice, underscoring its role in managing diabetes[20]. In addressing metabolic hypertension, Polygonati rhizoma ultrafine powder, enriched with PRP and Polysaccharide-Sulfate Complex (PRS), exhibits a remarkable capacity to ameliorate hypertension and lipid metabolism disruptions. This is achieved through modulation of diverse mechanisms, encompassing intestinal flora composition, intestinal barrier fortification, and vascular endothelial function[67]. These collective findings underscore Polygonati rhizoma's potential to foster a balanced and beneficial intestinal flora composition, which in turn exerts a positive impact on metabolic functioning. This regulatory effect holds promise in sustaining harmonious digestion and absorption processes, enhancing immune function, and ultimately offering potential avenues for the prevention and treatment of diverse metabolic disorders.
In summary, Polygonati rhizoma has multiple regulatory effects that provide pathways for improving sleep quality, cognitive function, immune function, and metabolic health. Its mechanism of action involves intricate signaling pathways and regulation of cellular processes in various physiological systems. In essence, Polygonati rhizoma emerges as a formidable contender in metabolic regulation, with its diverse mechanisms affording potential therapeutic strategies across a spectrum of metabolic conditions. The complex interplay between Polygonati rhizoma and metabolic pathways positions it as a promising agent in enhancing overall metabolic well-being and health.
-
Overwhelming studies on Polygonati rhizoma have revealed a spectrum of pharmacological benefits encompassing anti-diabetic, anti-tumor, and anti-inflammatory properties, alongside its capacity for physiological regulation. Additionally, Polygonati rhizoma has emerged as a reservoir of anti-aging and antioxidant effects. Polysaccharides sourced from Polygonati rhizoma have demonstrated notable distinctions in their physicochemical properties. Polysaccharide composition and molar ratios differ markedly between polysaccharides obtained from wine-processed (PRPW) and crude (PRPC) forms[68]. Employing the 'nine-steaming and nine-drying' procedure, a method entailing meticulous extraction from wine, not only elevates Polygonati rhizoma's medicinal potential but also enhances its therapeutic value. Remarkably, diverse cooking durations of PRP exhibit the potential to ameliorate memory impairment in D-galactose-induced mice, an effect attributed to heightened antioxidant activity. These observations further accentuate Polygonati rhizoma's versatility as a versatile medicinal agent, particularly concerning cognitive enhancement and anti-aging properties[69]. In addition, PRP has been shown to confer anti-aging effects in D-galactose-induced rats by enhancing learning and memory, while concurrently counteracting pathological kidney changes. Subsequent inquiries have demonstrated PRP's potential to elevate Klotho expression, reduce FOXO3a and p-FOXO3a expression, and suppress FGF-23 protein expression in the femur[53]. This orchestration results in reduced oxidative stress and equilibrium in calcium and phosphorus metabolism. Moreover, PRP exhibits the capability to mitigate D-galactose-induced cardiac aging and damage. This is achieved by mitigating ROS and MDA levels in cardiac tissue, bolstering SOD levels, and curbing oxidative stress-induced DNA damage and lipid peroxidation[70], reminiscent of PRP's ability to curtail lipofuscin accumulation, which aligns with studies illustrating that heightened resistance to oxidative stress augments lifespan in nematodes[71].
Furthermore, Polygonati rhizoma unveils potential in alleviating menopausal symptoms by rejuvenating vaginal epithelial thickness via upregulation of estrogen receptors ER alpha (ESR1) and ER beta (ESR2), and downregulation of serotonin receptor 1A (5-HT1A) and glucocorticoid receptor. This orchestrated modulation culminates in a surge in serotonin concentration[66]. This amelioration extends to symptoms like hot flashes and vaginal dryness. PRP also exhibits promise in mitigating menopausal manifestations such as obesity and bone loss, achieved through lipid level regulation and osteoporosis improvement. Moreover, it offers the potential to mitigate the adverse effects associated with hormone replacement therapy, thus opening novel avenues for Polygonati rhizoma as a natural menopause treatment. Transcriptomic analysis has unraveled the intricacies of PRP's anti-aging effects. It is revealed that PRP significantly modulates a range of circular RNAs, miRNAs, and mRNAs, closely linked to synaptic activity[66].
In conclusion, PRP plays an anti-aging and antioxidant role by regulating gene expression, modulating estrogen receptors, balancing calcium and phosphorus metabolism, and reducing oxidative stress. These findings provide a new theoretical basis for its use as a natural anti-aging agent and a direction for the development of related drugs or nutraceuticals.
-
Polygonati rhizoma, an esteemed medicinal and culinary plant with an enduring history in China, showcases a diverse array of biological activities encompassing immunoregulation, anti-fatigue, antioxidant, hypoglycemic, and anti-inflammatory effects. The diverse beneficial properties of Polygonati rhizoma make it promising for applications in drug discovery, cosmetics, and health food innovation. While significant progress has been made in the research of polysaccharides, saponins, and aqueous extracts, there is still a need to expand the research of lectins, lignans, flavonoids, and other smaller biomolecules.
Polygonati rhizoma, as a functional food, has been the subject of relatively few structural-functional and resource utilization studies in food products, with low extraction rates of active ingredients and relatively simple processing technologies. Even though PRP is the most important class of medicinal active ingredients in Polygonati rhizoma, there are fewer polysaccharide preparations used in clinical practice as well as corresponding types of products on the market. In addition, the structure of PRP is complex, and the pharmacological mechanism of PRP with different molecular compositions has not been thoroughly investigated. Meanwhile, there are relatively few studies on the changes in polysaccharide structure during Polygonati rhizoma preparations as well as the pharmacological effects after structural modifications. Therefore, in future studies, PRP with different compositions can be isolated and purified to further elucidate the pharmacological activities of different PRP and their mechanisms of action, to promote the development and application of novel therapeutic agents, functional foods or adjuvants of PRP, and to provide the necessary scientific basis for the development and utilization of Polygonati rhizoma products. The two types of bioactive components, PRP and PRS, in Polygonati rhizoma function through complex multi-target, multi-pathway, and multi-effect mechanisms. Undeniably, progress has been made in understanding the bioactivities of PRP and PRS, but challenges remain in the specific regulatory mechanisms. At the same time, the vast majority of current studies related to the pharmacology of Polygonati rhizoma have not been refined in depth. Therefore, in future studies, specific refinement should be made to specific monomer components in a particular class of compounds.
Based on the existence of these problems and challenges mentioned above, we believe that the chemical composition of Polygonati rhizoma can be analyzed by various means, such as modern fingerprinting, bibliometric analysis, and network pharmacology so that we can use the individual components as the starting point for research, and then explore the mechanisms of its pharmacological and nutritional functions in vivo and ex vivo using animal experimental studies based on clarifying the pharmacodynamic substances. This will provide a positive reference for the development and utilization of Polygonati rhizoma in medicine and food, and better promote the healthy, stable, and sustainable development of Polygonati rhizoma industry.
-
The authors confirm contribution to the paper as follows: study conception and design, funding acquirement: Li N, Yang G; draft manuscript preparation, literature review: Yang G, Jiang D, Huang LJ; manuscript revision and language editing: Huang LJ, Chen B, Huang J. All authors reviewed the results and approved the final version of the manuscript.
-
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation, to any qualified researcher. Requests to access these datasets should be directed to Guoqun Yang (gqun@csyft.edu.cn).
This work was supported by Training Program for Excellent Young Innovators of Changsha (kq2009016), National Natural Science Foundation of China (31901345), Natural Science Foundation of Hunan (2021JJ31141), Education Department of Hunan (20A517 and 22B0260).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Guoqun Yang, Dong Jiang
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Yang G, Jiang D, Chen B, Huang J, Huang L, et al. 2024. Biochemical compounds and pharmacological functions of a traditional Chinese medicinal herb Polygonati rhizoma. Medicinal Plant Biology 3: e012 doi: 10.48130/mpb-0024-0009
Biochemical compounds and pharmacological functions of a traditional Chinese medicinal herb Polygonati rhizoma
- Received: 05 January 2024
- Revised: 18 March 2024
- Accepted: 10 April 2024
- Published online: 27 June 2024
Abstract: Polygonati rhizoma (Huangjing in China) is a widely utilized medicinal and edible plant within traditional Chinese medicine, displaying significant potential for medicinal application, agricultural value, and societal importance. Enriched with polysaccharides, saponins, flavonoids, alkaloids, and other bioactive ingredients, it serves as both a nutrient-dense supplementary food and an alternative therapeutic agent for prevention. Although Polygonati rhizoma's purported anti-aging, anti-tumor, anti-diabetic, and physiological regulatory properties have been postulated, the specific ingredients and underlying mechanisms remain largely elusive. Due to its notable bioactive effects on human health, Polygonati rhizoma has garnered considerable attention and has undergone extensive research. In recent years, modern research with multidimensional approaches has achieved an understanding of the biochemical nature and pharmacological mechanism of Polygonati rhizoma. It is anticipated that Polygonati rhizoma holds the potential to emerge as a pivotal resource for the development of novel pharmaceuticals and therapeutic strategies targeting various human ailments. This review synthesizes the latest research on Polygonati rhizoma, centering on its effects related to anti-aging, anti-tumor, anti-diabetic, and physiological regulation aspects. Furthermore, we evaluate the specific mechanisms through diverse perspectives and provide insights into often overlooked facets of Polygonati rhizoma's pharmacological potential, aiming to contribute valuable suggestions that could advance its incorporation and practical utilization within the framework of modernized traditional Chinese medicine.
-
Key words:
- Polygonati rhizome /
- Bioactive components /
- Pharmacological mechanisms /
- Huangjing












