-
Seed germination is the first step in the morphogenesis of spermatophyte, which is an important trait in crop production[1−3]. The nutrients of seeds mainly include sugars, fats and proteins, which are the main sources of energy for seed germination and early growth of seedlings[4−7]. Seed germination is a hyper action phase that requires tremendous energy[8]. Fats and proteins are generally first converted into sugars and then utilized by respiration, which provides a large amount of energy required for seed germination[7]. Sugar, especially glucose (Glu), is the main energy substance used by cells to release energy[7,9]. Thus, in the early stage of germination, the respiration substrate of seeds is generally mainly soluble sucrose (Suc) and certain oligosaccharides pre-stored in dry seeds; and during the germination stage, sucrose is degraded into glucose by invertase to provide energy for seed germination[8,10]. The invertases include acidic invertase and neutral invertase[11].
Seed germination is a complex process involving various signal transduction pathways, including phytohormone[12−17]. Of these, the degradation of carbohydrates may be regulated by abscisic acid (ABA) and gibberellin (GA)[8].The ABA and GA antagonistically regulate seed germination, inducing and breaking seed dormancy[18]. ABA and GA can inhibit each other's metabolism and signaling-related genes[19,20]. Recent studies have shown that ABA is involved in the regulation of almost all processes of seed development and germination. The overexpression of Arabidopsis thaliana ABA synthase gene or mutant ABA catabolism key enzyme gene can increase the endogenous ABA content in seeds and delay seed germination; GA mainly acts at the initiation of germination and radicle protrusion[21]. GA and ABA can regulate the germination process of seeds, possibly by regulating the transcription of α-amylase gene[22]. Larger studies showed that the dynamic balance between ABA and GAs, as a signal, is the key to controlling seed germination by regulating the metabolism of nutrients[3,23].
Nutrients, energy, and ABA and GAs for regulating seed germination could be affected by environmental conditions, which include temperature[24]. Poor conditions may delay or inhibit seed germination. Melon is a thermophilic vegetable crop, and it is widely cultivated in winter-spring and autumn[25,26]. During winter and early spring, the low temperature will inhibit melon seed germination, resulting in uneven or failure of emergence for a long time or rotten seeds, which seriously affects later production. Therefore, exploring the physiological mechanism of melon seed germination at low temperature has important theoretical and practical significance for the safe cultivation of seedlings.
Numerous studies have shown that the application of exogenous plant growth regulator could improve the germination rate of seeds under normal or various stress conditions. The application of exogenous amino acids can alleviate the salt stress-inhibited Arabidopsis seed germination[27]. Hydrogen gas increases cucumber seed germination via regulating sugar and starch metabolisms[8]. Priming with exogenous salicylic acid improves the Leymus chinensis seed germination via H2O2 signal under salt-alkali stress[28]. Our previous study showed that exogenous Glu and Suc can partially alleviate the low-temperature damage of melon seedlings[26]. Whether exogenous sugar soak can improve the germination rate of melon seeds under low-temperature conditions is still unclear. Therefore, this study intended to explore the regularity of sugar metabolism during the germination of melon seeds under low-temperature conditions and to clarify the role of exogenous Suc and Glu in the germination of melon seeds.
-
Seeds of Cucumis melo cultivars IVF521 (cold sensitive) and THY (cold tolerant), both thick-skinned, were used in this work. Seeds with full grains that were intact and had no mildew were selected, and they were soaked in 55 °C H2O for 15 min and subsequently at 28 °C for 6 h. The seeds were germinated at 28 °C (normal condition) or 15 °C (low temperature) in petri dishes lined with moistened filter paper. Starch, soluble sugar, and soluble protein contents were measured at 0, 12, 18, 24 and 36 h after normal-temperature treatment, at 0 (L0), 36 (L36), 60 (L60), 84 (L84), and 108 h (L108) after low-temperature treatment, and then followed by normal temperature (28 °C) recovery for 24 h (R24 h). The germination rate was observed at 0, 24, 36, L0, L84, L108 and R24 h. The radicle length and bud fresh weight were observed at L108 and R24 h. The levels of ABA and GAs were measured at L108 h. The contents of Suc, Glu, and fructose (Fru) and the enzyme activities of neutral invertase (NI) and acid invertase (AI) were measured at 0, 24, 36, L0, L36, L84 and R24 h. The expression of CmNIs was analyzed at 0, 12, 24, 36, L0, L36, L60, L84 and R24 h.
To study the roles of exogenous Suc and Glu on the germination of melon seeds at low temperature, the seeds were in 50 mM Suc or 20 mM Glu solutions[26] for 6 h, followed by rinsing with double-distilled water (ddH2O). Then, the seeds were germinated at 28 °C (normal condition) or 15 °C (low temperature) in Petri dishes lined with moistened filter paper. The germination rate was observed after 36, L108, and R24 h.
Evaluation of germination percentage and seed growth
-
Germination percentage = number of germinated seeds/total number of tested seeds × 100%; Germination index (GI) = ∑ (daily germination number/germination days).
The length of the radicle was measured with a ruler and the fresh weight of seed buds was measured using a 1/10,000 scale. Vitality index (VI) = GI × radicle length; Relative GI = the GI at L108 h/the GI at R24 h; Relative radicle length = the radicle length at L108 h/the radicle length at R24 h; Relative VI = the VI at L108 h/the VI at R24 h; Relative bud fresh weight = the bud fresh weight at L108 h/the bud fresh weight at R24 h.
Determination of starch, soluble sugar, Suc, Glu and Fru contents
-
The starch content was measured in accordance with a previous methods[28] using a starch kit from the Nanjing Jiancheng Bioengineering Institute (China). The soluble sugar content was determined using the anthrone method and observed at 625 nm[29].
The Suc, Glu, and Fru contents were determined by high-performance liquid chromatography (HPLC) (waters600E) as described in our previous report[30,31]. In brief, (1) 1 g sample was obtained and added with 5 mL 80% ethanol; the mixture was immersed in a bath at 80 °C for 1 h and stored in a 50 mL centrifuge tube after cooling. (2) After pouring out the ethanol extract, 80% ethanol was added to the test tube, and the mixture was immersed in a water bath at 80 °C for 1 h. The process was repeated 3–4 times, and the extracts were combined. The extracts were transferred to an evaporating dish and evaporated to dryness. (3) The extracts were dissolved with 1 mL of ultrapure water and the supernatant was filtered through an aqueous filter. (4) The filtered extract was diluted, and the liquid phase was used for determination.
Determination of AI and NI activities
-
The AI and NI activities were determined using our previously published method[31]. Briefly, 0.2 mL solution mixed with 1 mL reaction solution (0.1 mol·L−1 Na2HPO4-citric acid/K2HPO4-sodium citrate buffer of pH 4.8/pH 7.2, containing 1% Suc) was incubated at 37 °C for 30 min. The amount of Glu produced was determined using the DNS method.
Determination of ABA and GA levels
-
The ABA and GA levels were measured using HPLC-MS/MS (Zoonbio Biotechnology Co., Ltd, Nanjing, China) in accordance with a previously published method[32].
Determination of soluble protein content
-
The soluble protein content was measured with Coomassie brilliant blue G-250 staining method and determined at 595 nm[33].
Gene expression analyses
-
RNA extraction from seeds was performed following the protocol of TRIzol reagent (CW0581M; Cwbio, Beijing, China). Total RNA was subjected to reverse transcription with a PrimeScript TM RT reagent kit (Takara, Shiga, Japan). Quantitative reverse-transcription polymerase chain reaction was carried out on a Roche LightCycler® 96 instrument (Roche, Switzerland), and Takara TB Green™ Premix Ex Taq™ II (Takara, Shiga, Japan) was used. Actin7 was set as the reference gene. The relative gene expression was calculated in accordance with the method of Livak & Schmittgen[34]. The gene-specific primers used are listed in Supplemental Table S1.
Statistical analysis
-
All experiments were repeated three times. The data were subjected to analysis of variance, which was calculated using SAS software version 8.0 (SAS Institute, Cary, NC, USA). Significant differences among treatments were analyzed using Tukey's test at p < 0.05.
-
The seeds of different varieties may have varying germination rates due to differences in stored nutrients. Therefore, the germination of two different varieties of melon seeds at normal and low temperatures were analyzed. Under normal conditions, the germination of IVF521 seeds was significantly higher than that of THY seeds at 24 h but showed no difference at 36 h (Fig. 1a). The THY seeds germination percentage was 0 before L84 h and germinated starting from L84 h (12% germination percentage), whereas the germinated seeds accounted for 52% of the THY variety and 0% of the IVF521 variety at L108 h. The germination percentage quickly reached 90% in both varieties (no significant difference) at R24 h. The relative GI, VI, radicle length, and bud fresh weight in THY were dramatically higher than those of the IVF521 variety (Fig. 1b).
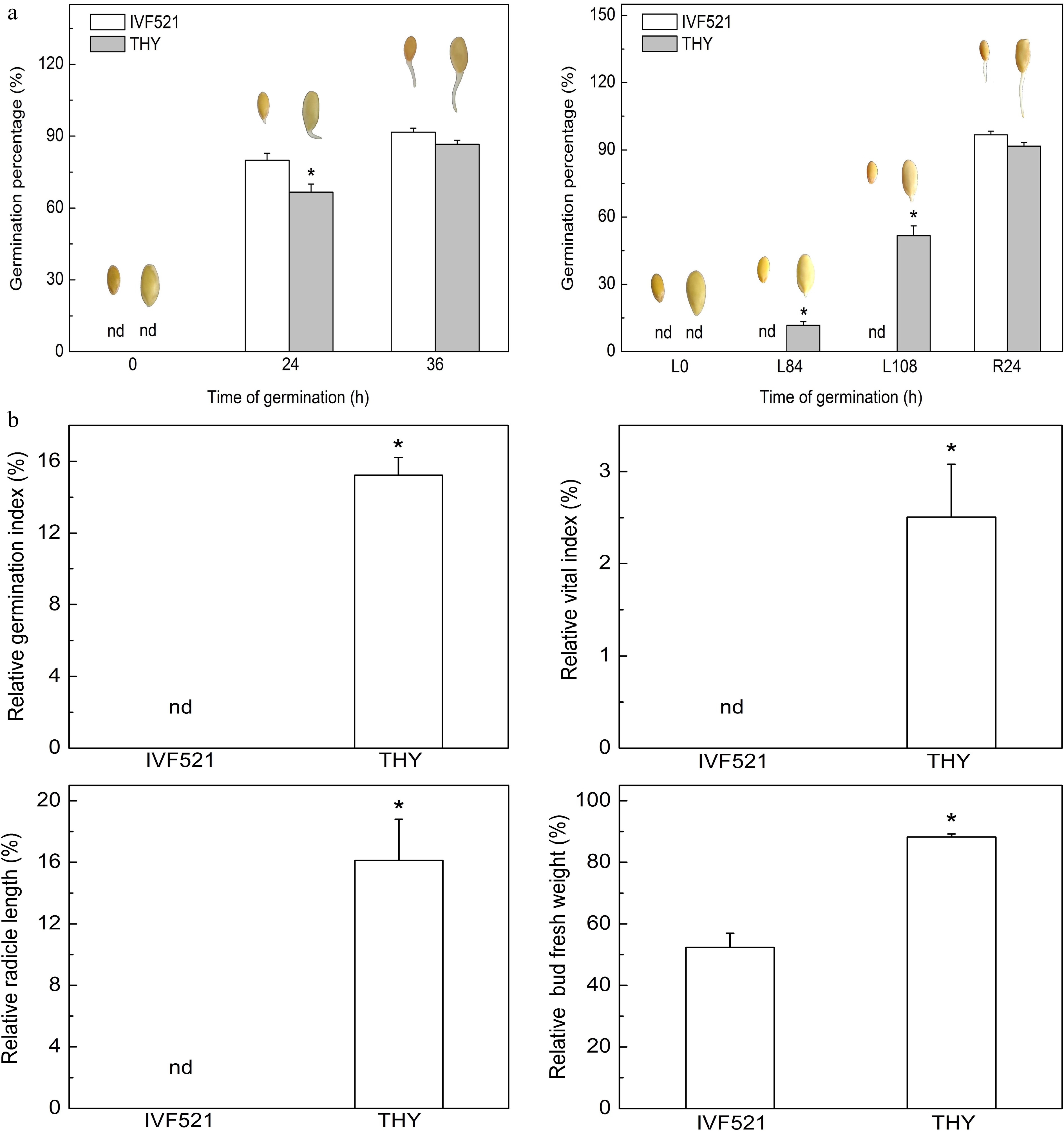
Figure 1.
Effects of temperature on the germination of different varieties of melon seeds. (a) Germination percentage and (b) relative GI, VI, radicle length and bud fresh weight. The seeds were subjected to 28 °C or 15 °C incubator for germination. The germination percentage was counted at 0, 24 and 36 h at 28 °C or L0, L84 and L108 h at 15 °C and R24 at 28 °C. The relative GI, VI, radicle length and bud fresh weight were calculated based on the ratio of these indexes at L108 (15 °C) and R24 h (28 °C). Data were presented as means of three biological replicates ± standard error, and * indicate significant differences at p < 0.05 according to Tukey's test.
Sugar metabolism during seed germination of melon
-
The contents of starch and soluble sugar were higher in IVF521 seeds than in THY seeds (Fig. 2). The starch content of the two cultivars decreased sharply in the early stage of germination, remained at a stable stage before germination, and showed a downward trend after the start of germination (under normal temperature conditions) or the starch content of THY seeds increased, whereas that of IVF521 declined (under low-temperature conditions) (Fig. 2a). The soluble sugar content in IVF521 seeds showed a downward trend at normal or low temperature, whereas that in THY seeds increased in the early stage of germination dramatically decreased at the germination stage, and increased significantly at the later stage of germination at normal or low temperature (Fig. 2b). Under normal or low temperature, Suc showed an overall downward trend, whereas Fru and Glu had no significant change at the early stage of germination but increased at the later stage in both varieties (Fig. 3). No significant difference was observed in Fru and Glu in the late germination between the two varieties under normal conditions, whereas under low-temperature conditions, Fru and Glu in THY seeds were significantly higher than those in IVF521 seeds in late stage of germination (Fig. 3).
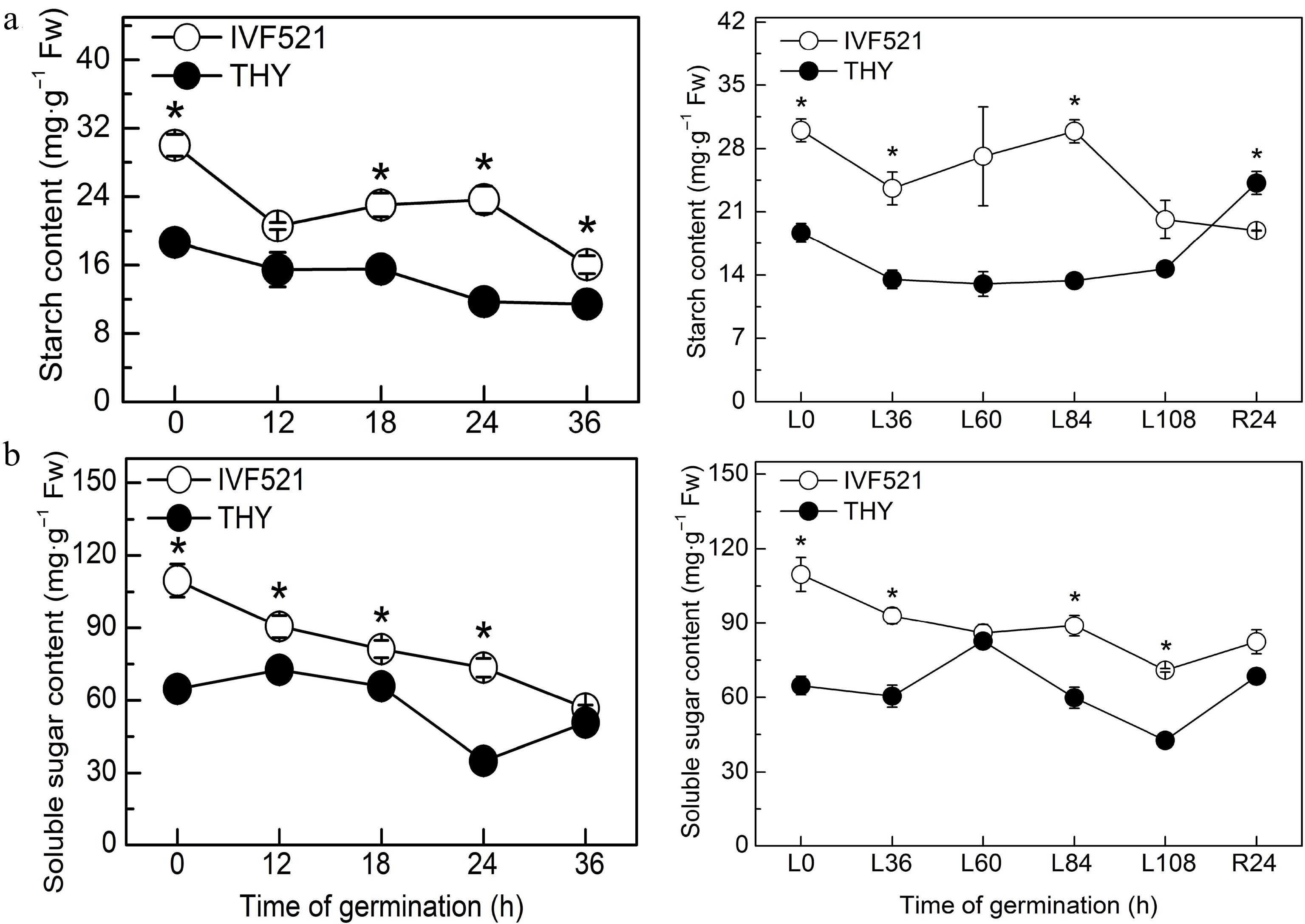
Figure 2.
Effects of temperature on the contents of starch and soluble sugar in different varieties of melon seeds. (a) Starch content and (b) soluble sugar content. The seeds were subject to 28 °C or 15 °C incubator for germination. The contents of starch and soluble sugar were determined at 0, 12, 18, 24 and 36 h at 28 °C or L0, L36, L60, L84 and L108 h at 15 °C and R24 h at 28 °C. Data were presented as means of three biological replicates ± standard error, and * indicate significant differences at p < 0.05 according to Tukey's test.
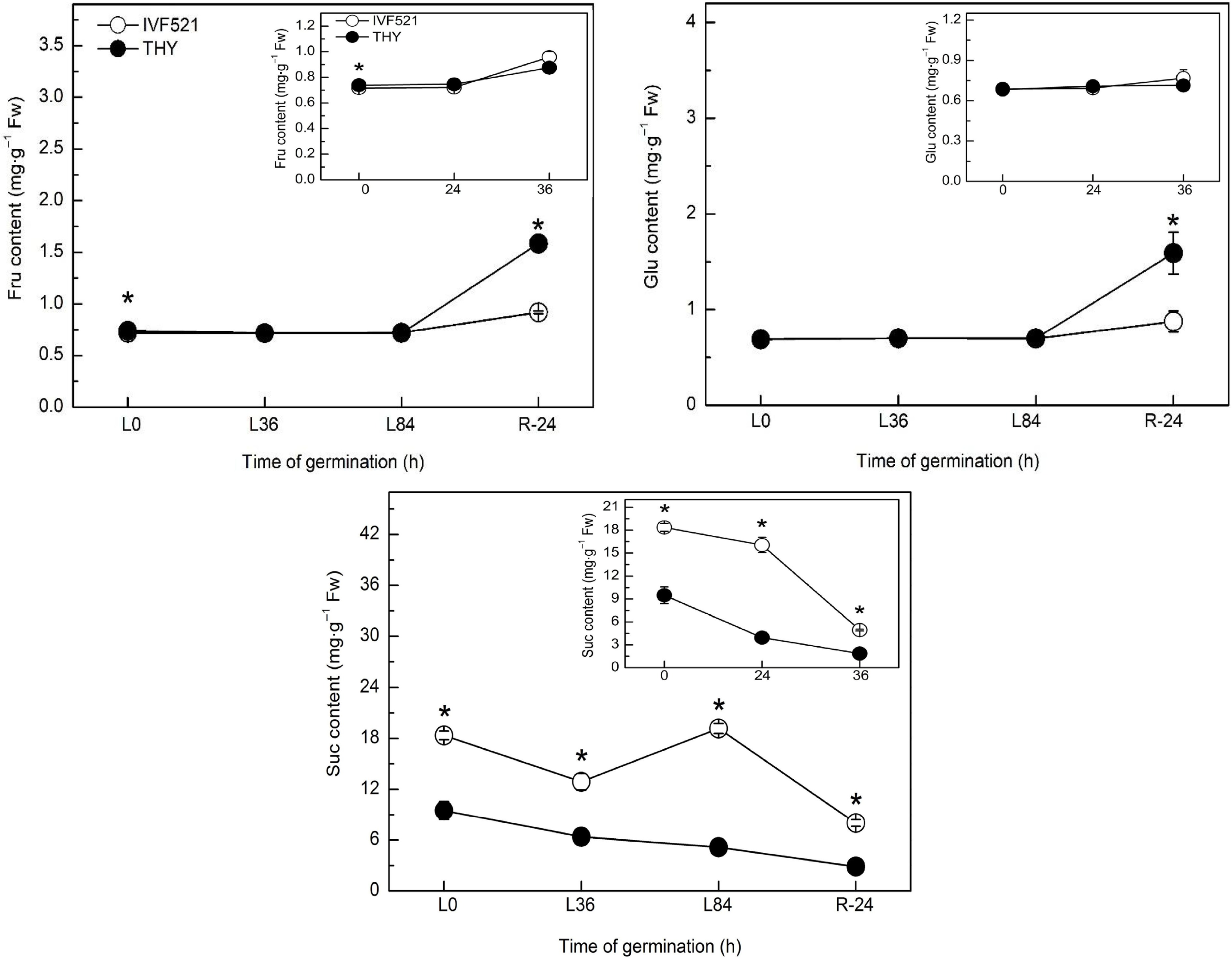
Figure 3.
Effects of temperature on the contents of Suc, Glu and Fru in different varieties of melon seeds. The seeds were subject to 28 °C or 15 °C incubator for germination. The contents of Suc, Glu and Fru were determined at 0, 24 and 36 h at 28 °C or L0, L36, L84 at 15 °C and R24 h at 28 °C. Data were presented as means of three biological replicates ± standard error, and * indicate significant differences at p < 0.05 according to Tukey's test.
Suc is mainly hydrolyzed to Glu and Fru by NI and AI. Results showed that at the early stage of seed germination, the NI activity in THY seeds was significantly higher than that in IVF521 seeds under normal or low temperature, but no significant difference was observed in the NI activity in both varieties at the later stage of germination (Fig. 4). No difference was noticed in the AI activity between the two varieties under normal or low temperatures (except the higher AI activities in IVF521 than in THY at L36 h).
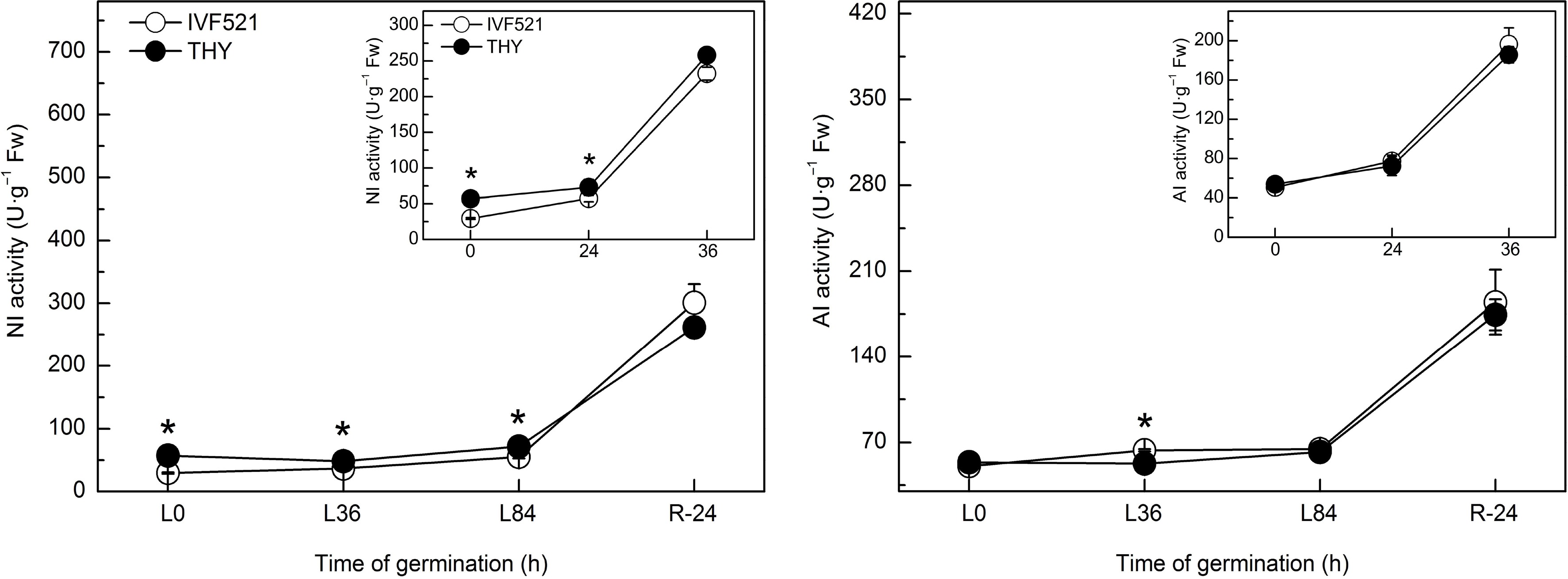
Figure 4.
Effects of temperature on the activities of NI and AI in different varieties of melon seeds. The seeds were subject to 28 °C or 15 °C incubator for germination. The activities of NI and AI were determined at 0, 24 and 36 h at 28 °C or L0, L36, L84 at 15 °C and R24 h at 28 °C. Data were presented as means of three biological replicates ± standard error, and * indicate significant differences at p < 0.05 according to Tukey's test.
The genes encoding NI in melon were further analyzed, and the results showed that under normal conditions, the expression levels of all CmNIs genes in THY seeds were significantly higher than those in IVF521 seeds at 0 h (Fig. 5). The gene expressions of CmCINV1 (at 12 and 36 h), CmCINV2 (at 24 h) and CmCINV4 (at 36 h) were higher in THY seeds than in IVF521 seeds under normal conditions. Under low-temperature conditions, all CmNIs gene expressions in THY seeds were also significantly higher than those in IVF521 seeds at 0 h. The genes expressions of CmCINV1 (at L0, L36, L60 and L84 h), CmCINV2 (at L84 h), CmCINV4 (at L0, L36 and L84 h) and CmCINV5 (at L0 and L60 h) in THY seeds were also significantly higher than those in IVF521 seeds. However, all NI genes in IVF521 were significantly higher than those in THY seeds at R24 h.
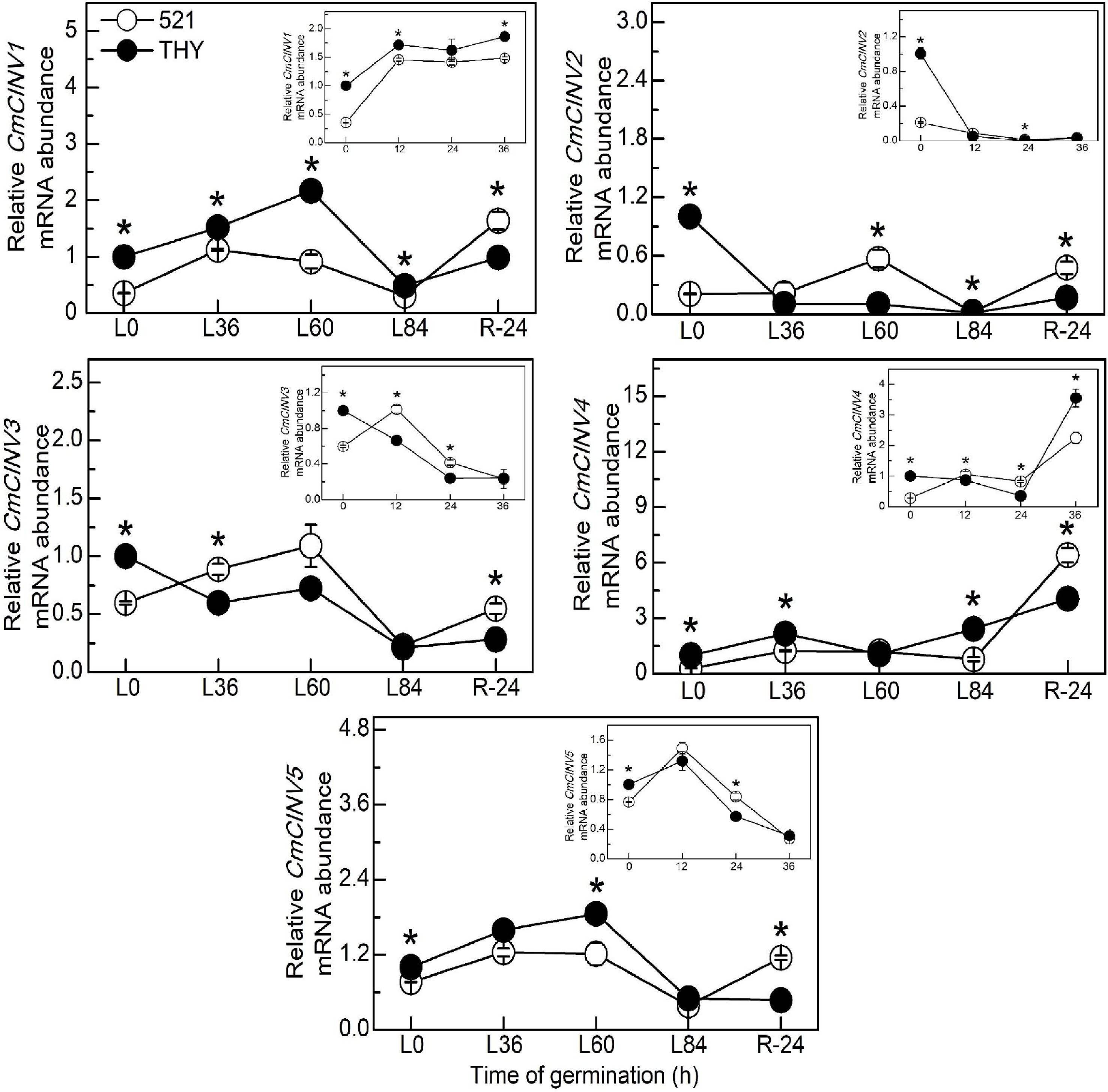
Figure 5.
Effects of temperature on the genes expression of CmNIs in different varieties of melon seeds. The seeds were subject to 28 °C or 15 °C incubator for germination. The gene expression of NI were determined at 0, 12, 24 and 36 h at 28 °C or L0, L36, L60, L84 at 15 °C and R24 h at 28 °C. The CmNIs levels in the THY seeds at 0 h were normalised as 1. Data were presented as means of three biological replicates ± standard error, and * indicate significant differences at p < 0.05 according to Tukey's test.
Under normal conditions, the germination percentage of IVF521 seeds soaked with exogenous Suc solution was significantly lower than that of seeds soaked with ddH2O, but no significant difference was observed between soaking with exogenous Glu solution and ddH2O soaking (Fig. 6a−c). THY seeds soaked with exogenous Suc or Glu exhibited no significant difference in germination percentage compared with those soaked in ddH2O under normal conditions. At L108 h, the THY seeds soaked with exogenous Suc solution significantly inhibited the germination of seeds compared with ddH2O soaking, whereas the exogenous Glu solution-soaked seeds significantly improved the germination percentage of THY seeds compared with ddH2O soaking (Fig. 6b, c). Soaked IVF521 and THY seeds with Suc solution significantly inhibited seed germination compared with ddH2O soaking, whereas soaking with exogenous Glu solution significantly improved the germination rate of THY seeds compared with ddH2O soaking at R24 h.
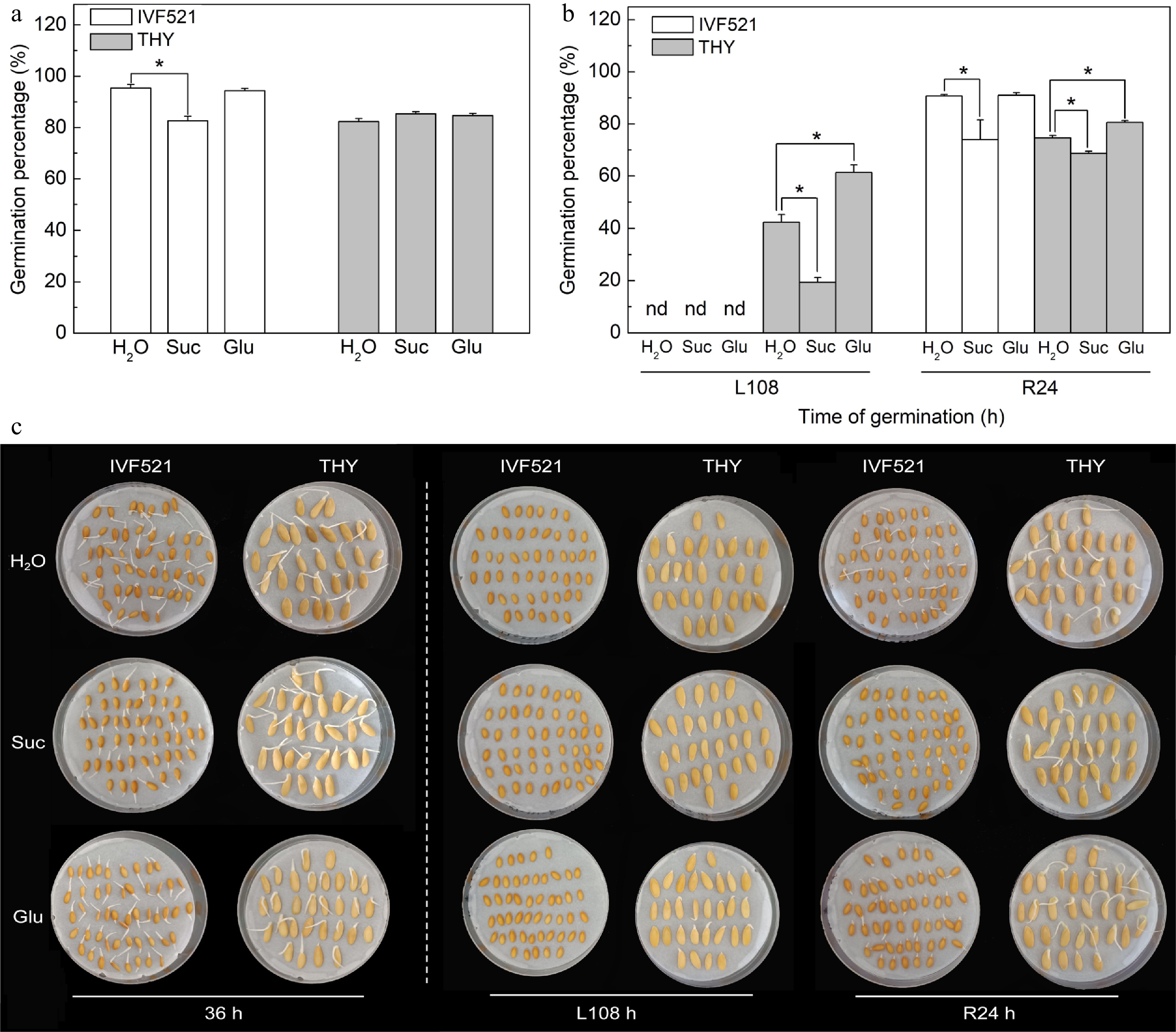
Figure 6.
Effects of Suc and Glu on germination percentage of different varieties of melon seeds under normal or low temperature. The seeds were pre-soaked in 50 mM Suc or 20 mM Glu solutions for 6 h, followed by rinsing with double-distilled water (ddH2O). Seeds were subjected to 28 °C or 15 °C incubation for germination. The germination percentage was counted at (a) 36 h at 28 °C or (b) L108 h at 15 °C and R24 at 28 °C, and (c) the photos of seeds germination were taken. Data were presented as means of three biological replicates ± standard error, and * indicate significant differences at p < 0.05 according to Tukey's test.
ABA/GAs and soluble protein contents during seed germination under low temperature
-
The balance of ABA and GAs plays an important role in seed germination[3]. Under low-temperature conditions, the ABA content of IVF521 seeds was significantly higher than that of THY seeds, whereas the level of GAs was significantly lower than that of THY seeds (Fig. 7). ABA/GAs in IVF521 seeds were significantly higher than those in THY seeds.
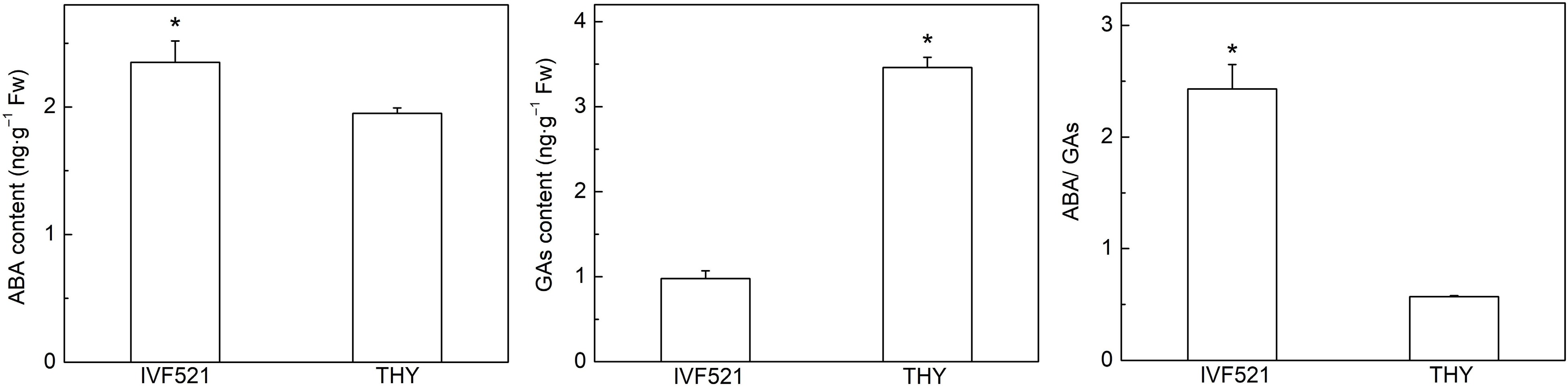
Figure 7.
Contents of ABA and GAs (GA9, GA15 and GA53) in different varieties of melon seeds under L108 h. The seeds were subject to 15 °C incubator for germination. The contents of ABA and GAs were determined at L108 h under 15 °C. Data were presented as means of three biological replicates ± standard error, and * indicate significant differences at p < 0.05 according to Tukey's test.
In the seeds of two melon varieties, the soluble protein contents of the IVF521 variety was significantly higher than that of the THY variety at 12 and L60 h. At other times, no significant difference was noticed in the soluble protein content between the two varieties (Supplemental Fig. S1).
-
The process of seed germination is accompanied by the metabolism and conversion of carbohydrates, fats, and proteins[7,35−37]. There are differences in nutrient contents among different species or varieties of the same species, thus resulting in differences in germination rate and speed[37,38]. In addition, the germination of seeds is affected by environmental conditions[39,40], especially temperature[41]. In this study, IVF521 seeds contain more nutrient substances than THY for seed germination, therefore, the germination percentage of THY seeds at normal temperature were lower than that of IVF521 seeds (Figs 2 & 3). IVF521 seeds may be more sensitive to environmental temperature. Therefore, IVF521 seeds more easily sense environmental factors and regulate the metabolism of stored substances for germination at normal temperature. IVF521 may be more sensitive to temperature than THY and was thus more dramatically inhibited by low temperature. Therefore, under low-temperature conditions, THY seeds can still maintain the metabolism of stored substances, provide nutrients and energy required in the process of seed germination and promote germination, and embryo growth (Figs 1−5).
In this study, the main energy sources of different melon varieties during the germination period may be sugars instead of proteins and amino acids (Supplemental Fig. S1). Therefore, starch, soluble sugar, and Suc showed a decreasing trend at the seed germination stage (Figs 2, 3). The difference in sugar levels in THY and IVF521 seeds was mainly due to the genetic characteristics of different melon varieties.
Sugar is the main energy-producing substance for seed germination[7,9]. Under normal-temperature conditions, the decomposition rate of starch and soluble sugar of IVF521 seeds at the early stage of germination was significantly higher than that of THY varieties; they were then decomposed into Suc, which was easily used by seed germination (Figs 2, 3). This result may be the reason for the fast germination speed and high germination percentage of IVF521 (Fig. 1). α-Amylase, the main enzyme of starch decomposition, may be regulated by ABA/GAs[8,42], and the ratio of ABA/GAs in THY varieties was significantly lower than that in IVF521 varieties (Fig. 7). Therefore, under low-temperature conditions, starch slowly decomposed into soluble sugars, and the decomposition in THY seeds was relatively fast (Fig. 2). This may be the reason that low temperature inhibited the decomposition of starch into soluble sugars in IVF521 seeds compared with that in THY seeds. Thus, THY seeds could partially germinate under low-temperature conditions (Fig. 1).
At the early stage of germination, the two varieties of melon seeds mainly provided energy for germination through the decomposition of Suc, and at the later stage, through the glycolysis pathway with Glu as the substrate. Therefore, the Suc content of the two varieties continuously decreased under normal-temperature conditions, whereas those of Glu and Fru gradually increased (Fig. 3). Under low-temperature conditions, the NI activity of IVF521 seeds was partially inhibited (Fig. 4). Thus, the Suc content showed no significant decrease before L84 and the Fru and Glu contents also did not increase significantly. Meanwhile, the NI activity of THY seeds was significantly higher than that of IVF521 seeds. Therefore, the Suc content decreased all the time, whereas Glu and Fru levels began to increase at the late stage of low-temperature germination (Figs 3, 4). Thus, the decomposition of Suc during the germination of melon seeds may be mainly due to the action of NI enzymes (Fig. 4). It has been reported that there are five neutral invertase coding genes in melon (CmCINV1~ CmCINV5)[11]. In this study, the CmCINV1, CmCINV4 and CmCINV5 may play key roles (Fig. 5). Interestingly, AI may not play a major role in sucrose degradation during seed germination of melon seeds. However, the factors regulating these genes and their specific molecular mechanisms still need to be further explored in the future.
The above findings showed that Suc and Glu may play important roles in seed germination, and the germination of seeds becomes more dependent on Glu under low-temperature conditions. Therefore, whether Suc can be decomposed into Glu normally under low-temperature conditions is the key to seed germination. Soaking seeds with Suc solution may inhibit the normal decomposition of endogenous Suc into Glu, which is significant in the cold-sensitive seed IVF521. Thus, the germination rate of IVF521 seeds under normal-temperature conditions was significantly lower than that with ddH2O soaking (Fig. 6). However, the reasons and related mechanisms of sucrose inhibiting seed germination need to be further studied. Under low-temperature conditions, the decomposition of endogenous Suc in seeds was partially inhibited, resulting in low amounts of Glu and Fru, whereas seed soaking with Glu solution can increase the level of endogenous Glu and provide energy-generating substrates for seed germination. Thus, soaking seeds with Glu solution significantly improved the germination rate of THY seeds (Fig. 6). Whether Glu acts as an osmotic regulator or a signal molecule in regulating melon seed germination under low-temperature conditions, and whether there is an interaction between Glu and ABA and GAs signals? These still need to be further studied.
In conclusion, the contents of starch and soluble sugar in IVF521 seeds were higher than those of THY varieties. Therefore, under normal-temperature conditions, IVF521 seeds germinated quickly and had a high germination percentage. Meanwhile, the NI activity of THY seeds was less inhibited by low temperature and thus maintained the decomposition of Suc into Glu, which provided the energy substrate for seed germination of THY. Based on this finding, Glu may play roles in the germination of melon seeds at low temperature.
-
The authors confirm contribution to the paper as follows: experiments design and and writing the manuscript: Liu T, Qi H; performing the experiments: Liu T, Zhang A, Zhang Y, Shao L, Xia H, Miao M; data analysis: Zhang Y, Liu T. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article and its supplementary information files.
We thank Professor Huaisong Wang, from Institute of Vegetables and Flowers Chinese Academy of Agricultural Sciences (IVF·CAAS) for providing melon seeds. This work was supported by grants from the Project funded by the Basic Research Project of Liaoning Provincial Department of Education (JYTZD2023117), the National Natural Science Foundation of China (U20A2044) and the China Agriculture Research System of MOF and MARA (CARS-25).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Tao Liu, Aixin Zhang, Yujie Zhang
- Supplemental Table S1 Gene-specific primers used for quantitative real-time PCR analysis.
- Supplemental Fig. S1 Effects of temperature on the contents of soluble protein in different varieties of melon seeds. The seeds were subject to 28 °C or 15 °C incubator for germination. The contents of protein were determined at 0, 12, 18, 24 and 36 h at 28 °C or L0, L36, L60, L84 and L108 h at 15 °C and R24h at 28 °C. Data were presented as means of three biological replicates ± standard error, and different letters indicate significant differences at p < 0.05 according to Tukey's test.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Liu T, Zhang A, Zhang Y, Shao L, Xia H, et al. 2024. Sucrose catabolism play vital roles in seed germination of melon at low temperature. Vegetable Research 4: e020 doi: 10.48130/vegres-0024-0020
Sucrose catabolism play vital roles in seed germination of melon at low temperature
- Received: 05 April 2024
- Revised: 17 May 2024
- Accepted: 29 May 2024
- Published online: 02 July 2024
Abstract: Seed germination is a vital stage in the morphogenesis of spermatophyte. Seed germination is regulated by storage substances and temperature. Melon is an important economic crop and often encounters sub-low temperature environment at the seed germination stage in winter-spring, which limits the normal germination of melon seeds and affects melon production. Therefore, it is of great significance to explore the physiological mechanism of seed germination at low temperature. Here, two melon varieties, IVF521 (cold sensitive) and THY (cold tolerant), were used to study the germination and relative sugar metabolism at normal (28 °C) and low temperature (15 °C). Results showed that THY seeds exhibited a higher cold tolerance but contained lower starch and soluble sugar than IVF521 seeds. Under normal conditions, IVF521 seeds germinated quickly and manifested a high germination percentage. Under low-temperature conditions, the neutral invertase of THY seeds were less inhibited by low temperature, which contributed to the sustained decomposition of sucrose into glucose (Glu), providing an energy substrate for seed germination. In addition, soaking with exogenous Glu can improve the germination rate of melon seeds at low temperature. Overall, sucrose catabolism may play key roles in melon seed germination under low temperature.
-
Key words:
- Seed germination /
- Low temperature /
- Sucrose /
- Glucose /
- Melon













