-
Fruits and vegetables constitute an essential part of the daily diet. The World Health Organization recommends a daily minimum adult fruit and vegetable consumption of 400 g[1]. As a globally grown vegetable crop, tomato (Lycopersicon esculentum) plays a vital role in people's daily vegetable diets, and its global import and export quantities reached 7,458,437 and 7,773,978 tonnes, respectively, in 2020[2]. Tomatoes contain numerous antioxidant compounds, including carotenoids, ascorbic acid, and phenolic compounds. The most abundant carotenoids are lycopene and β-carotene[3]. As the main vitamin in tomatoes, ascorbic acid is also a potent antioxidant. Although polyphenols are present in tomatoes at lower concentrations, flavonoids (naringenin and quercetin) and hydroxycinnamic acids (chlorogenic acid and caffeic acid) reportedly exhibit considerable antioxidant activity[4]. These antioxidant constituents make tomatoes important in preventing several peroxidation-related diseases[5].
Approximately 75% of tomatoes in the United States are consumed after being processed into products[6], such as ketchup, sauce, and tomato juice. Sterilization and pasteurization are the most critical part of tomato juice processing, and they are predominantly applied to inactivate microorganisms, thereby extending juice shelf-life. A 'best before' (food quality) or a 'use by' (food safety) date is usually printed on the packaging to indicate the shelf-life of food products[7]. Nowadays, consumers and food industries pay more attention to the 'best before' period, also known as quality-stable shelf-life[8], of microbial-stable food products.
High-hydrostatic pressure (HHP) processing has achieved commercial success in the food industry because of its effective inactivation of microorganisms and the relatively lower effects on low-molecular-weight compounds, such as flavors and colors, compared with thermal processing due to the inherent stability of covalent bonds[9]. Furthermore, our previous study proved that more constituents, especially ascorbic acid, quercetin, and carotenoids, were retained in HHP-treated tomato juice than in that treated with high-temperature short-time (HTST) processing from a metabolic profiling perspective[10]. In addition, a previous study found that HHP processing significantly retained higher vitamin C content in tomato puree than thermal processing[11,12]; HHP processing at 300–500 MPa has been shown to increase lycopene extractability from tomato juice and puree[13,14]. Naturally occurring antioxidants are known to be significantly lost during storage, and accumulating evidence suggests that processing significantly affects the nutrient composition of tomato products and the retention of these components during storage[13,15]. HHP processing has been reported to be more effective than thermal processing at maintaining total carotenoid content in tomato juice during storage for 4 weeks at 4 °C[13], 52 weeks at 4 °C[16], and 12 weeks at 20 °C[17]. As a thermal-sensitive vitamin, vitamin C has been found to be more effectively retained in HHP-processed tomato juice than in thermally processed tomato juice[13]. Moreover, Jayathunge et al.[17] found that HHP-treated tomato juice (pH = 3.93) maintained a more favorable total phenolic content than thermally treated tomato juice during 2-week storage at 20 °C. Polyphenols are present in tomato juice in free and bound forms[18]. Bound phenolics in tomatoes have been reported to be easily released during processing[19], and quercetin and ferulic acid content has been found to be reduced during refrigerated storage of commercial tomato juices[20]. However, the effects of HHP processing on polyphenol profiles and the changes during storage in tomato juice have not yet been investigated. In addition, the mechanism by which long-term HHP-treated tomato juice retains all the above-mentioned antioxidants more effectively than HTST-treated tomato juice during safety-stable shelf life remains unknown, implying that the quality-stable shelf life (optimal drink period and antioxidant ingredient perspective) of HHP-treated tomato juice has not yet been elucidated.
Therefore, this study investigated the effect of HHP (550 MPa/10 min) treatment on the antioxidant profiles and capacity of tomato juice and their changes during refrigerated storage. It potentially augments current understanding regarding the benefits of the HHP processing of tomato juice in retaining antioxidant profiles and capacity during storage compared with those of HTST processing and provides evidence for predicting the quality-stable shelf-life of HHP-treated juice from an antioxidant ingredient perspective.
-
Tomato variety 'Heinz Series' samples were obtained and processed according to our previous study[10]. Briefly, tomato samples were washed, sliced, juiced, ground once (20 s), and homogenized (20 MPa/5 min). Then, the juice was treated in an HTST processing system at 110 °C/8.6 s and an HHP unit at pressures of 550 MPa for 10 min under room temperature. Tomato juice samples were packed in brown PET bottles, stored in the freezer (YC-395L, MELNG Co., Ltd., Hefei, China) at 4 °C, and collected weekly during the 4-week storage period.
Microbiological assessment
-
The counting of total aerobic bacteria (TAB) and the viable yeast and mould (Y&M) were counted as described in the previous study[21]. A sterile 0.85% NaCl solution (9 ml) was used to dilute samples (1 ml) in a gradient serially, and 1 mL of each dilution was plated into duplicate plates of appropriate agar. TAB cells and Y & M cells were counted by plate count agar and rose Bengal agar after incubation (37 °C/ 48 h; 28 °C/ 72 h), respectively.
Quantification of carotenoids by HPLC
-
The extraction, identification, and quantification of carotenoids in tomato juice were in accordance with our previous method[10]. Briefly, 1 mL juice and 3 mL extraction solvent (methanol/ethyl acetate/petroleum ether, 1:1:1, containing 0.1 g/L BHA and BHT) were mixed, extracted with 70% amplitude for 30 s (KQ-500E, Kunshang Ultrasound Instrument Co., Ltd., China), centrifuged at 1320 g for 3 min (GR21G, Hitachi Koki Co., Ltd., Tokyo, Japan), then the organic phase was collected and the solid residue was re-extracted with 2 mL extraction solvent until the color disappeared. The organic extracts were combined and dried by nitrogen, then dissolved in tBME and filtered (0.22 μm PTFE membrane, JINTENG, Tianjin, China) into amber vials until HPLC analyses (Waters Corporation, Waters 2695, Milford, MA, USA). The solvent system consisted of eluent A (methanol/tBME/water, 80:18:2, v/v/v) and eluent B (methanol/ tBME/water, 8:90:2, v/v/v), the elution gradient program was as follows: from 0% to 30% B in 5 min, from 30% B to 86% B in 20 min, from 86% B to 100% B in 2 min, from 100% B to 0% B in 4 min, isocratic at 0% B for 4 min.
Extraction and quantification of ascorbic acid by HPLC
-
The extraction and quantification of ascorbic acid in tomato juice were according to our previous method[10]. Briefly, tomato juice and 2.5% metaphosphoric acid were mixed at a volume the ratio of 1:1, then vortexed for 3 min, centrifuged (4,472 g, 10 min, 4 °C), filtered (0.45 μm MCM membrane), stored at 4 °C until further analysis. The solvent system consisted of 90% water (0.1% metaphosphoric acid) and 10% methanol. The first-order kinetic model was built to depict ascorbic acid loss during storage with equation (1):
$ \mathrm{C}=\mathrm{C}_0\mathrm{ }\mathrm{e}\mathrm{x}\mathrm{p}(-\mathrm{k}\mathrm{t}) $ (1) where C0 is the content before storage (t = 0 weeks), k and t are the rate constant (weeks−1) and storage time (weeks), respectively.
Quantification of polyphenols content by UPLC-MS
-
Polyphenols were determined according to our previous study method with minor modifications[10]. Tomato juice was freeze-dried (LGJ-25C, Beijing Sihuan Technology Instrument Co., Ltd., China) at −40 °C for 48 h. Tomato powder (100 mg) and 0.6 mL 80% methanol were mixed, extracted at 4 °C overnight, then centrifugated (10,000 g, 10 min, 4 °C), the upper extracts collected, and filtrated (Nylon, 0.22 μm pore size). The sample analysis was according to our previous study[10], and the multiple reaction monitoring (MRM) transitions and crucial compound-dependent parameters were shown in Supplemental Table S1.
Quantification of total phenolic content
-
The Folin–Ciocalteu method was used to determine total phenolic content[21]. Tomato juice and 80% methanol were mixed at 1:1, extracted for 15 min (100% amplitude), centrifuged (12,000 g, 10 min, 4 °C), supernatant collected, the solid residue was re-extracted with the same volume of 80% methanol, combined with supernatant extracts. Volume supernatant (40 μL) , 100 μL Folin–Ciocalteu reagent (1N, 10-fold dilution), and 90 μL sodium carbonate solution (7.5%) were mixed, then set in the dark for 1 h. Then absorbance of the mixture was measured at 765 nm with a spectrophotometer (UV-1800, Shimadzu, Shanghai, China). Total phenolic content was calculated against the GAE standard curve (5–100 μM, R2 = 0.9995).
Enzyme activity assay
-
Phenylalanine ammonia-lyase (PAL) activity was determined according to the method described in a previous study[22]. Juice (1 mL) and 20 mL extraction solution (0.1 M sodium borate buffer (pH = 8) consisting of 2 mM EDTA, 5 mM mercaptoethanol, 0.2 g insoluble PVPP) were mixed, centrifuged (10,000 g, 20 min, 4 °C), collected supernatant. The reaction mixture of PAL activity was 0.1 mL extract and 2.9 mL substrate solution (0.1 M sodium borate buffer (pH = 8) solution including 3mM L-phenylalanine), incubated for 1 h at 37 °C, then measured at 290 nm.
Polyphenol oxidase (PPO) and peroxidase (POD) enzyme activities were determined according to the method described by Liu et al.[23] with minor modifications. 400 mL extraction solution (10 g/L PVPP and 1 mol/L NaCl dissolved in 0.2 mol/L sodium phosphate buffer (pH = 6.5)) was mixed with 1 mL juice, extracted for 1 h (4 °C), centrifugated (10,000 g, 30 min) and collected the supernatant. The reaction mixture for POD and PPO activity was 2.8 mL substrate solution (3 g/L p-phenylenediamine and 1 mL/L hydrogen peroxide dissolved in 0.2 mol/L sodium phosphate buffer (pH = 6.5)) and 0.2 mL extract, 2.5 mL substrate solution (0.07 M catechol dissolved in 0.2 M sodium phosphate buffer (pH = 6.5)) and 0.5 mL extract, respectively. POD and PPO enzyme activities (Abs/min) were calculated by the slope from the linear portion of the ΔA420 and ΔA485 nm reaction curves, respectively.
The percentage of enzyme residual activity was calculated using equation (2):
$ \mathrm{E}\mathrm{n}\mathrm{z}\mathrm{y}\mathrm{m}\mathrm{e}\;\mathrm{r}\mathrm{e}\mathrm{s}\mathrm{i}\mathrm{d}\mathrm{u}\mathrm{a}\mathrm{l}\;\mathrm{a}\mathrm{c}\mathrm{t}\mathrm{i}\mathrm{v}\mathrm{i}\mathrm{t}\mathrm{y}=\mathrm{A}\mathrm{s}/\mathrm{A}\mathrm{c}\times 100 $ (2) here As and Ac were enzyme activities of treated and fresh samples.
Antioxidant capacity measurements
-
Hydrophilic and lipophilic fractions extracted from tomato juice were used in the antioxidant assay. The hydrophilic fraction was obtained as extracts according to the total phenolic content quantification. For the lipophilic fractions, 300 mg freeze-dried tomato powder was mixed with 1 mL extraction solution (n-hexane/methanol, 1:4), then sonicated (60 s), vortexed (60 s), centrifuged (10,000 g, 10 min), and collected supernatant. Extraction solution (1 mL) was used to re-extract solid residue four times, combined with supernatants, evaporated under nitrogen, and then dissolved in methanol of the total amount extraction volume (5 mL).
According to the previous method, 40 μL supernatant extract was mixed with 260 μL 0.1 mM 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) solution, set in the dark for 30 min, and then the absorption was measured at 517 nm[24]. The antioxidant capacity evaluated by DPPH was calculated against the Torlox standard curve (5–100 μM, R2 = 0.9994). According to manufacturers' instructions, the ferric-reducing antioxidant power (FRAP) assay was measured by the BC1310 kit (Solarbio, Beijing, China). The U/mL definition: the antioxidant capacity of the sample is expressed in terms of the standard liquid ion concentration (μmol/mL) required to achieve the same absorbance change value (ΔA).
Statistical analysis
-
The results from the experiments were conducted with one-way ANOVA for more than two groups and unpaired Student's tests for two groups (p < 0.05). Orthogonal partial least squares discriminant analysis (OPLS-DA) and partial least squares (PLS) analysis were performed by SIMCA software (Version 14.1, Umetrics, Sweden). Differential compounds were screened for 0-week and 1-week HHP-treated tomato juice by combining variable importance in project (VIP) values (≥ 1) of the OPLS-DA model and the p-value (≤ 0.05). Bi-plots of PLS and volcano plots were produced by Origin 2019 (OriginLab, Northampton, MA, USA). Graphing analysis and kinetic modelling were conducted with GraphPad Prism7.0 (GraphPad Software, San Diego, USA).
-
Pre-processing TAB and Y&M counts were 7.92 ± 2.61 and 2.97 ± 0.18 log10CFU/mL in tomato juice, respectively (Table 1). HHP and HTST processing reduced TAB to < 2 log10CFU/mL and Y&M to below the detection limit in tomato juice. Furthermore, microbial stability was maintained in treated samples during 4 weeks of refrigerated storage, meeting the standard of the Chinese Beverage National Food Safety standards.
Table 1. Microbial levels in treated tomato juice during storage.
Treatment Storage time (weeks) 0 1 2 3 4 Total viable count
(Log10cfu/mL)Fresh 7.92 ± 0.13 HHP 1.85 ± 0.02a 1.73 ± 0.02b 1.88 ± 0.11a 1.45 ± 0.02c 1.87 ± 0.03b HTST 1.77 ± 0.16ab 1.55 ± 0.06a 1.86 ± 0.12ab 1.39 ± 0.32ab 1.89 ± 0.09b Yeasts and molds
(Log10cfu/mL)Fresh 2.97 ± 0.18 HHP ND ND ND ND ND HTST ND ND ND ND ND Mean values ± SD (n = 3) with different lowercase letters in the same row indicate significant differences (p < 0.05). ND: below the detection limit. Carotenoid concentration changes in treated tomato juice during storage
-
This study detected 11 types of individual carotenoids in tomato juice. The total carotenoid concentration in fresh tomato juice was approximately 110.11 ± 11.95 μg/g (Table 2), of which lycopene approximated 95% (104.19 ± 11.58 μg/g). Total lutein, lycopene, and carotenoid concentrations significantly increased by 18%, 25%, and 27% after HHP processing, respectively; however, their concentrations merely decreased slightly in quantity after HTST processing.
Table 2. The effect of HHP and HTST treatment on carotenoids, polyphenols, ascorbic acid content, and antioxidant activities in tomato juice.
Treatments Fresh HHP HTST Carotenoids (μg/ml) All-trans-lutein 0.25 ± 0.05a 0.36 ± 0.02b 0.33 ± 0.05ab 13-cis-lutein 0.41 ± 0.04a 0.42 ± 0.04a 0.37 ± 0.02a 13-cis-β-Carotene 0.93 ± 0.13a 1.15 ± 0.10b 0.98 ± 0.02ab 15-cis-β-Carotene 0.31 ± 0.02a 0.21 ± 0.01a 0.34 ± 0.11a All-trans-β-carotene 3.40 ± 0.76a 3.72 ± 0.16a 3.97 ± 0.61a cis-β-Carotene 0.62 ± 0.04a 0.77 ± 0.01b 0.60 ± 0.05a 15-cis-lycopene 1.31 ± 0.02a 1.46 ± 0.03b 1.31 ± 0.02a 13-cis-lycopene 5.87 ± 0.50a 9.81 ± 0.98b 6.06 ± 0.32a 9-cis-lycopene 1.35 ± 0.03a 1.36 ± 0.01a 1.37 ± 0.03a 9,13-dicis-lycopene 3.07 ± 0.42a 4.36 ± 0.35b 3.10 ± 0.24a All-trans-lycopene 92.60 ± 10.74a 113.80 ± 8.20b 89.46 ± 5.84a Total Carotenoids 110.11 ± 11.95a 137.41 ± 7.99b 107.895 ± 6.76a Ascorbic acid (μg/ml) Ascorbic acid 125.09 ± 5.27a 122.92 ± 1.18a 111.07 ± 0.41b Polypenols (μg/g) Cryptochlorogenic acid 47.72 ± 3.03a 48.32 ± 3.81a 44.33 ± 2.39a Caffeic acid 11.45 ± 1.05a 9.80 ± 1.77a 9.86 ± 0.62a p-Coumaric acid 4.10 ± 0.92a 2.82 ± 0.54b 1.34 ± 0.12c Ferulic acid 4.56 ± 0.55a 2.07 ± 0.23b 1.39 ± 0.07b Rutin 9.48 ± 0.95a 12.37 ± 0.64b 17.75 ± 1.70c Quercetin 5.64 ± 0.43a 5.41 ± 0.43a 2.95 ± 0.12b Vanillic acid 0.95 ± 0.03a 0.75 ± 0.04b 0.52 ± 0.01c Sinapic acid 0.26 ± 0.03a 0.39 ± 0.03a 0.28 ± 0.03a Total phenols
(mg GAE /100 g)Total phenols 49.60 ± 2.28a 49.85 ± 2.47a 36.29 ± 2.17b Antioxidant activity DPPHa (mm Torlox /100 ml) 56.78 ± 2.89a 56.41 ± 2.88a 50.85 ± 6.27a FRAPa (U/ml) 17.46 ± 0.07a 14.09 ± 1.48b 13.26 ± 0.83b DPPHb (mm Torlox /100 g) 189.58 ± 17.61a 188.37 ± 1.40a 144.25 ± 6.09b FRAPb (U/g) 39.68 ± 0.53a 45.58 ± 0.97b 30.48 ± 0.28c Mean values ± SD (n = 3) with different lowercase letters in the same row indicate significant differences (P < 0.05). DPPHa and FRAPa represent antioxidant capacity in hydrophilic fractions of tomato juice, and DPPHb and FRAPb represent antioxidant capacity in lipophilic fractions. As shown in Fig. 1, except for 15-cis-β-carotene, all individual carotenoid concentrations depleted substantially in tomato juice irrespective of treatment during 4-week storage. Isomerization and oxidative degradation potentially explain trans-lycopene, trans-β-carotene, and trans-lutein loss in treated tomato juice. During storage, the cis-isomer reduction was due to oxidative degradation, and the 15-cis-β-carotene increase was caused by the isomerized accumulation of trans-β-carotene[25]. Moreover, total lycopene and carotenoid concentrations exhibited no significant differences between the two treated tomato juice after 1-week storage (Fig. 1). A similar phenomenon was observed in HHP-processed (600 MPa, 1 min) and thermally treated tomato juice in the third week of storage[17].
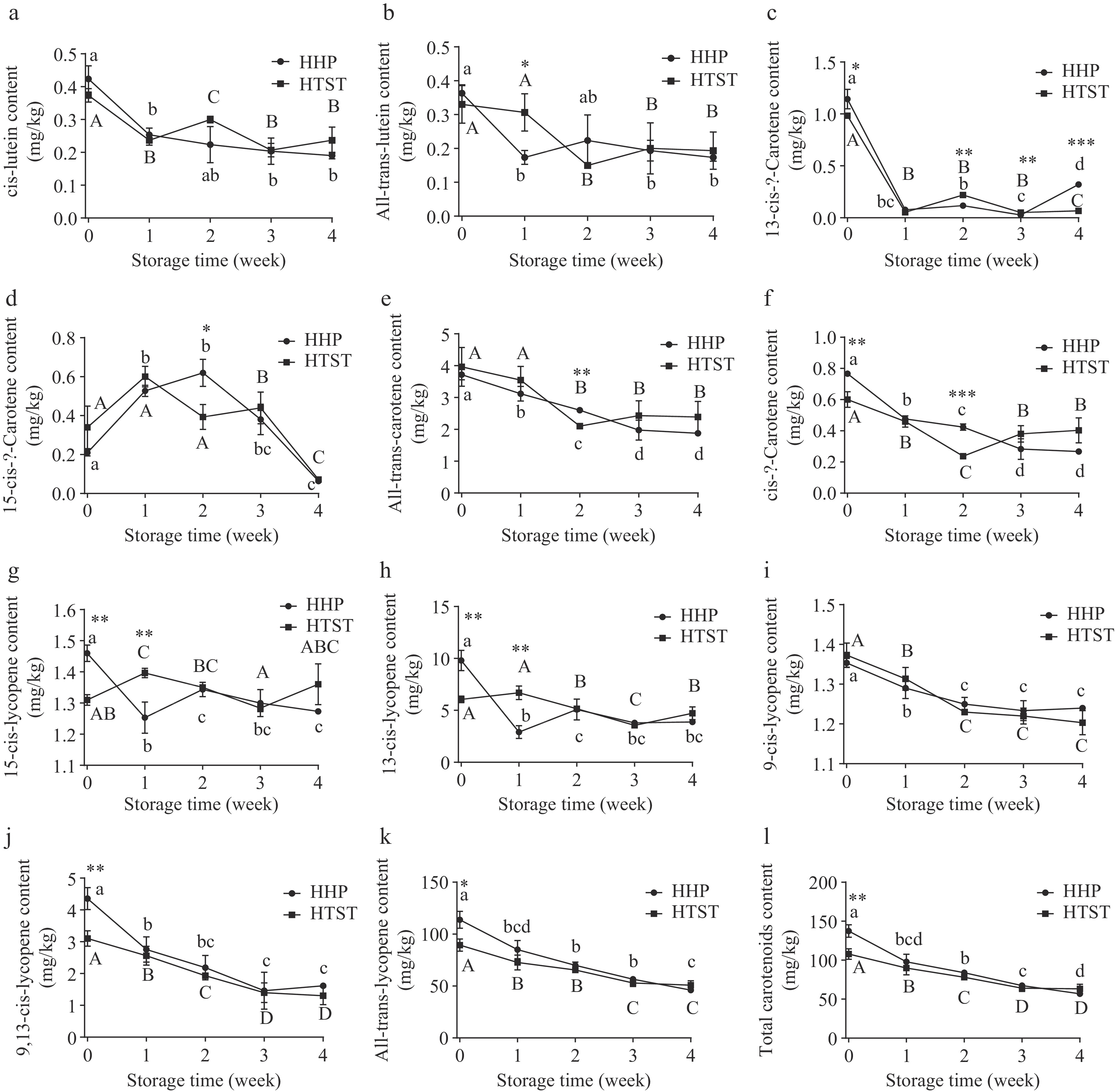
Figure 1.
Carotenoid content was evaluated in treated tomato juice during storage. Lowercase and uppercase letters refer to the HHP group and HTST group, respectively, and different letters indicate significant differences (p < 0.05). *p < 0.05, ** p < 0.01, *** p < 0.001 HHP vs HTST at the same storage time.
Changes in ascorbic acid concentration in treated tomato juice during storage
-
The ascorbic acid content of our fresh tomato juice was 125.09 ± 5.27 μg/g (Table 2). It was found to be stable after HHP processing and decreased after HTST processing due to high temperature. In addition, a decreased ascorbic acid content was also detected after 4-week storage in both treated tomato juices (Fig. 2), and this was attributed to non-enzymatic browning reaction and aerobic oxidation in HHP- and HTST-treated samples, respectively[26]. The behavior of ascorbic acid loss in treated tomato juice during refrigerated storage followed the first-order (K0) kinetic model. Furthermore, the K0 was 0.350 ± 0.060/week and 0.106 ± 0.009/week in HTST and HHP-treated tomato juice, respectively, indicating a lower ascorbic acid loss rate was found in HHP-treated tomato juice than in HTST-treated tomato juice. Moreover, the ascorbic acid content of HHP-treated tomato juice was 41.27 mg/mL higher than that of HTST-treated tomato juice after 4-week storage. Hsu et al. also observed a similar phenomenon in HHP-processed tomato juice, which exhibited greater ascorbic acid retention than heat-processed samples during storage at 4 °C[13].
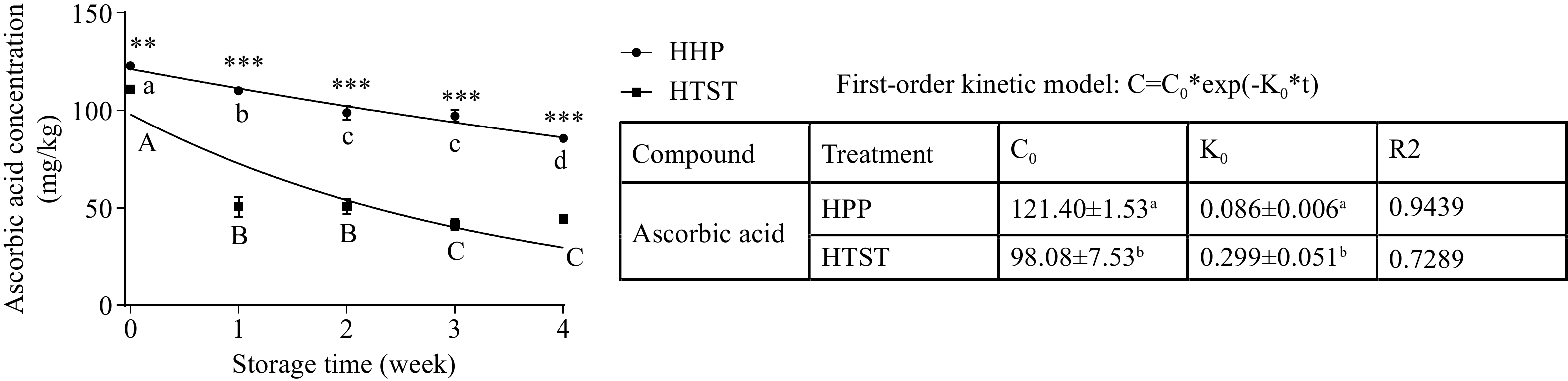
Figure 2.
Ascorbic acid content and kinetic model evaluated in treated tomato juice during storage. Lowercase and uppercase letters refer to the HHP group and HTST group, respectively, and different letters indicate significant differences (p < 0.05). * p < 0.05, ** p < 0.01, *** p < 0.001 HHP vs HTST at the same storage time.
Changes in polyphenol concentration in treated tomato juice during storage
-
Cryptochlorogenic acid, caffeic acid, and rutin were the most abundant polyphenols detected in our samples, followed by ferulic acid, quercetin, p-coumaric acid, vanillin, and sinapic acid (Table 2). No significant differences in caffeic acid, cryptochlorogenic acid, and sinapic acid concentrations were noted between the two tomato-juice processing methods. However, the ferulic acid, vanillic acid, and p-coumaric acid concentrations decreased, while rutin content increased considerably. In addition, retained quercetin and total phenolic concentrations were found to be 40% and 37% higher in HHP-treated than in HTST-treated tomato juice, respectively, indicating that HHP treatment has less influence on quercetin oxidation than HTST treatment.
The amount of vanillic acid decreased, while that of caffeic acid, quercetin, ferulic acid, and p-coumaric acid increased in both treated tomato juices during the storage at 4 °C (Fig 3). In addition, caffeic acid, quercetin, ferulic acid, and p-coumaric acid concentrations were 8.31, 4.77, 1.86, and 6.84 μg/g higher in HHP-treated than in HTST-treated tomato juice at the end of 4 weeks of storage, respectively. (Fig 3). Cryptochlorogenic acid and sinapic acid concentrations in HHP-processed tomato juice seemed stable during storage time. Sinapic acid and rutin concentrations were more abundant in HTST-treated than in HHP-treated tomato juice during most of the 4-week refrigerated storage period. As regards total phenolic content, no difference was noted between the two treated tomato juices during the storage.

Figure 3.
Polyphenol content was evaluated in treated tomato juice during storage. Lowercase and uppercase letters refer to the HHP group and HTST group, respectively, and different letters indicate significant differences (p < 0.05). * p < 0.05, ** p < 0.01, *** p < 0.001 HHP vs HTST at the same storage time.
Changes in PAL, PPO, and POD enzyme activity in treated tomato juice during storage
-
Polyphenols not only exist in a free form in a plant but also in the form of glycosides, and specific glycosidases can cause the glycoside polyphenols to release free polyphenols. Moreover, PAL is the first enzyme that catalyzes phenylalanine to produce phenolic acids, anthocyanins, and flavonoids through the phenylpropanoid pathway[27]. POD and PPO catalyze phenolic-compound oxidation to generate quinones[28]. In this study, the enzyme activities of PAL, PPO, and POD were mainly determined to help understand polyphenol changes after HHP and HTST processing during storage.
PAL activity was inhibited to 76.79% after HHP processing, nonetheless, it was activated to 131.76% after HTST processing (Fig. 4). In addition, PPO and POD activities were both significantly inhibited by HTST processing, decreasing to 6.63% and 18.46%, respectively. However, POD and PPO activities in tomato juice appeared substantially stable after high-pressure processing, retaining 81.17% and 97.60% of activity, respectively, indicating that phenolic-compound synthesis and oxidation may both exist in the juice after processing, and phenolic-compounds content depends on which reaction is predominant during tomato juice processing.
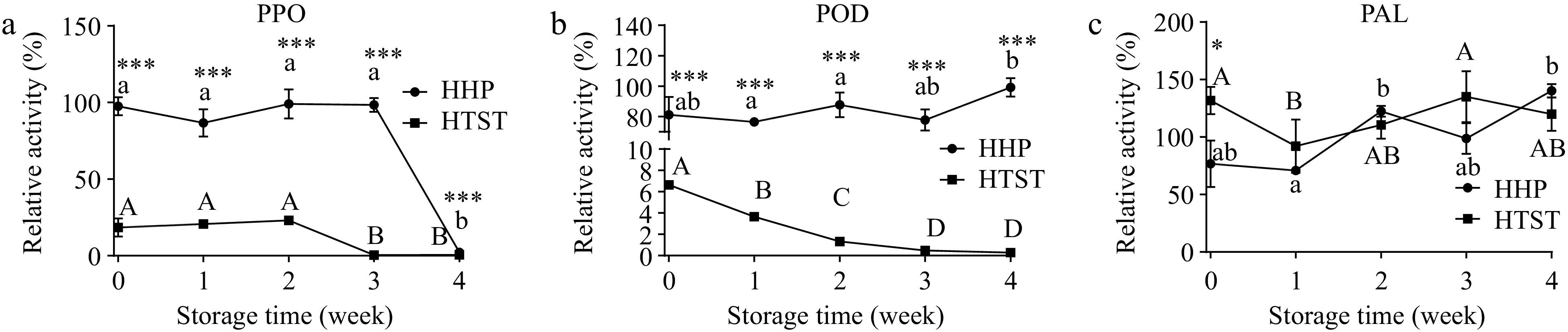
Figure 4.
POD, PPO, and PAL enzyme activity were evaluated in treated tomato juice during storage. Lowercase and uppercase letters refer to the HHP group and HTST group, respectively, and different letters indicate significant differences (p < 0.05). * p < 0.05, ** p < 0.01, *** p < 0.001 HHP vs HTST at the same storage time.
Post-processing PAL activity was kept considerably stable in tomato juice during storage at 4 °C. However, PPO activity decreased sharply to 0.41% in the third week in HTST-treated tomato juice and 2.18% in the final week in HHP-treated tomato juice (Fig. 4). Furthermore, during the entire storage period, POD activity exhibited a decay in HTST-treated tomato juice and remained stable in HHP-treated tomato juice (Fig. 4). PPO- and POD-activity loss during storage can be partly explained by polyphenolic- product accumulation. For example, Le Bourvellec et al.[29] found that caffeoylquinic acid, procyanidins, and epicatechin oxidation products inhibited apple PPO activity. Furthermore, o-quinones can form protein-bound phenols by reacting with enzyme functional groups[30], thus potentially inhibiting these enzymes' activities.
Changes in antioxidant capacity in treated tomato juice during storage
-
Regarding hydrophilic fractions, FRAP-measured antioxidant capacity in tomato juice decreased significantly to 14.09 ± 1.48 U/mL and 13.26 ± 0.83 U/mL after HHP and HTST processing, respectively. Furthermore, no statistically significant differences were observed among these fractions (Table 2). In contrast, DPPH-assayed antioxidant capacity demonstrated no changes after treatment (Table 2). In addition, greater antioxidant-capacity retention was observed in the hydrophilic part of HHP-treated tomato juice during most of the refrigerated storage time than in that of HTST-treated tomato juice (Fig. 5). This phenomenon was consistent with the changes in ascorbic acid and vanillic acid concentrations during storage.
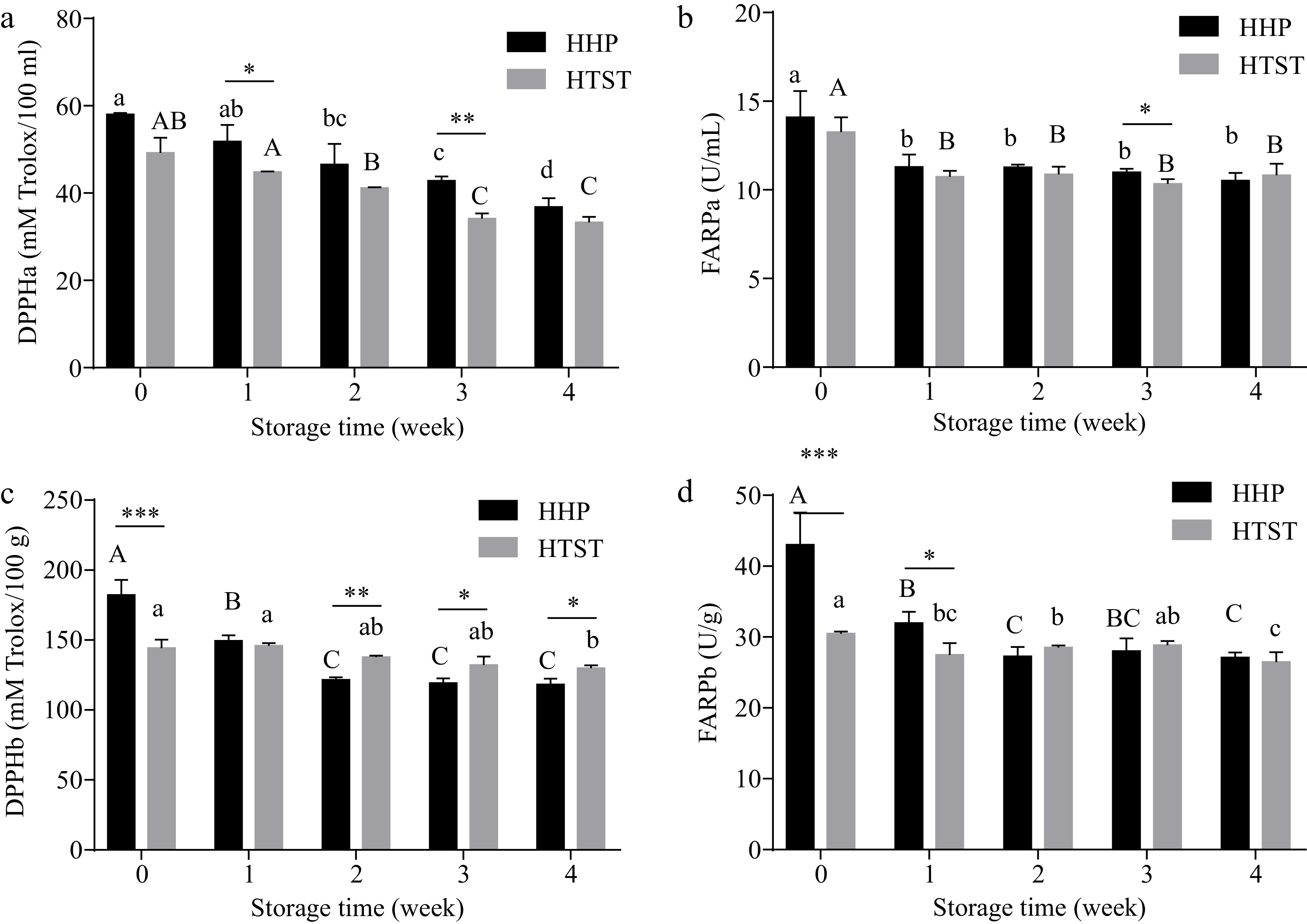
Figure 5.
Antioxidant compacity was evaluated of hydrophilic and lipophilic fractions from tomato juice treated by HPP and HTST processing after storage. DPPHa and FARPa represent antioxidant capacity in hydrophilic fractions of tomato juice, and DPPHb and FARPb represent antioxidant capacity in lipophilic fractions. Lowercase and uppercase letters refer to the HPP and HTST groups, respectively, and different letters indicate significant differences (p < 0.05). * p < 0.05, ** p < 0.01, *** p < 0.001 HHP vs HTST at the same storage time.
Regarding lipophilic fractions, DPPH- and FRAP-assayed antioxidant capacities (expressed as DPPHb and FRAPb in Table 2, Fig. 5 & 6) were 189.58 ± 17.61 mm Torlox/100 mg and 39.68 ± 0.53 U/g, respectively, in fresh tomato juice. The antioxidant capacity was retained and even enhanced after HHP processing, whereas a significant decline in antioxidant capacity after HTST processing. The total lipophilic antioxidant capacities measured by DPPH and FRAP were 30% and 50% higher in tomato juice treated with HHP than in that treated with HTST, respectively (Table 2). These results were consistent with the post-processing lycopene and total carotenoid concentration changes, and they were responsible for the lipophilic antioxidant capacity in tomato juice. However, no significant difference in FRAP-measured antioxidant capacity was noted between the two treated samples during 2–4 weeks of storage.
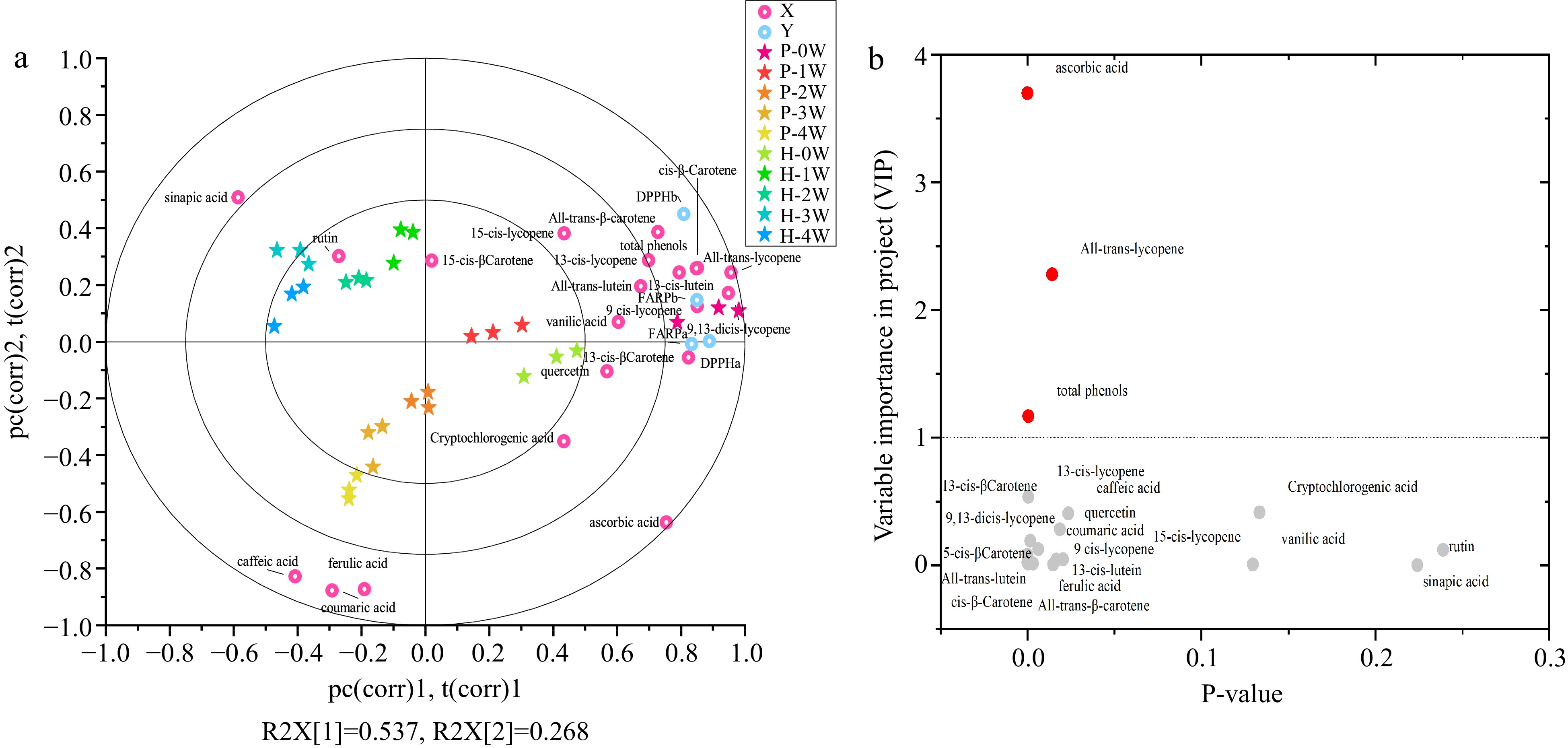
Figure 6.
(a) The PLS biplot shows the correlation of the antioxidant compounds variations with antioxidant compacity of treated tomato juice during storage. (b)Volcano plots for differential compounds in P-1W juice samples compared with P-0W samples. Each point in the volcanic plot represents an antioxidant compound. The red dots represent differential antioxidant compounds, and the grey dots represent antioxidant compounds that did not change significantly.
Changes in antioxidant compounds and capacity in treated tomato juice during storage: a multivariate analysis approach
-
As demonstrated, vitamin C, polyphenol, and carotenoid concentrations determined the antioxidant capacity of tomato juice. Multivariate analyses were conducted to further elucidate the connection between these antioxidant compounds and antioxidant capacity as well as their changes during storage in tomato juice to determine the quality-stable shelf-life for HHP-treated tomato juice. A partial least-squares (PLS) model was produced based on the concentrations of 21 antioxidant compounds in tomato juice as the X variables and the DPPH and FRAP values from the hydrophilic fractions and lipophilic fractions as Y variables, according to the literature[31], and the PLS biplots are shown in Fig. 6.
The trends in horizontal directions are shown in Fig. 6. PC1 largely separated all points in all the HHP-treated tomato juice samples, except for the 3- and 4-week points in all the HTST-treated tomato juice samples. This indicated that the gradual changes in these antioxidant compounds in each treated tomato juice sample weakened after 3-week refrigerated storage in HTST-treated tomato juice. In addition, a distinct gap existed between storage weeks 0 and 1 in HTST-treated tomato juice, and two distinct gaps between weeks 0 and 1 and weeks 1 and 2 of storage were observed in HHP-treated tomato juice. Notwithstanding, an increasingly gradual evolution was observed in the subsequent storage process, which revealed that the effects of the HHP and HTST treatment on tomato juice's antioxidant compounds weakened after 2-week and 1-week refrigerated storage, respectively. In addition, a significant distance was observed between the initial HHP-treated tomato juice (P-0W) and initial HTST-treated tomato juice (H-0W), whereas a minor distance was observed between a week of storage for HHP-treated tomato juice (P-1W) and that for initial HTST-treated tomato juice (H-0W), highlighting that the HHP processing of tomato juice was advantageous in that it retained antioxidant compounds for 1 week compared with HTST processing. Moreover, total phenolic content, all-trans-lycopene, and ascorbic acid were discriminated as differential antioxidants between weeks 0 and 1 in HHP-treated tomato juice samples, which indicates total phenolic content, all-trans-lycopene, and ascorbic acid can be chosen as the primary sensitive antioxidants during the first-week storage in HHP-treated tomato juice.
Regarding the antioxidant capacity of HHP-treated tomato juice, the samples were initially clustered closer to DPPHa, FRAPa, DPPHb, FRAPb, all carotenoids (except 15-cis-β-carotene), quercetin, vanillic acid, total phenolic content, cryptochlorogenic acid, and ascorbic acid, thus indicating a highly positive correlation among them. Moreover, the distance between carotenoids and antioxidant capacity (i.e., DPPHa, FRAPa, FRAPb, and DPPHb) was near the distance between ascorbic acid and antioxidant capacity, thus revealing that carotenoid antioxidant capacity exceeded that of ascorbic acid in HHP-treated tomato juice. Hence, the higher ascorbic acid retention in HHP-treated tomato juice during refrigerated storage was probably related to the protective effect of carotenoids, thus resulting in carotenoid retention challenges in HHP-treated tomato juice during storage (Fig. 1). Caffeic acid, 15-cis-β-carotene, p-coumaric acid, sinapic acid, ferulic acid, and rutin were clustered farthest from the pre-storage antioxidant capacity, displaying a negative correlation between these compounds and antioxidant capacity in tomato juice. This implies that the antioxidant capacity decline during storage was predominantly due to carotenoid and ascorbic acid loss rather than that of caffeic acid, rutin, p-coumaric acid, sinapic acid, and ferulic acid. Furthermore, this indicated that the antioxidant capacities of caffeic acid, ferulic acid, p-coumaric acid, and sinapic acid were weaker than those of carotenoids, ascorbic acid, and vanillic acid in both treated tomato juices during storage.
-
HHP processing has gained commercial success in the juice industry over the past few years due to its practical sterilization effect and nutrient retention[32,33]. Although microbial stability and more effective carotenoid, lycopene, and ascorbic acid retention have long been associated with the HHP processing of tomato juice[11,13,17,25,34, 35], no study has investigated the quality-stable (antioxidant profiles) shelf life of HHP-treated tomato juice. This study established a 1-week quality-stable shelf life for HHP-treated (550 MPa, 10 min) tomato juice compared with that for HTST-treated (110 °C, 8.6 s) tomato juice during refrigerated storage, indicating enhanced antioxidant-component retention and a higher antioxidant capacity of HHP-treated tomato juice than that of HTST during the first-week of refrigerated storage.
Commercial fruit juices require a careful assessment of microbiological risks. In this study, the number of microorganisms in HHP- and HTST-processed tomato juice remained stable and consistent with national safety regulations after 4 weeks of storage. Hsu et al.[13] also found that a 4-week microbially stable tomato juice could be produced at a pressure ≥ 400 MPa for 10 min, and this may be related to the low pH of tomato juice inhibiting microbial growth. Therefore, antioxidant-component changes were further detected to evaluate the quality of tomato juice during 4 weeks of storage.
As the essential antioxidants in tomato juice, the lycopene and total carotenoid concentrations significantly increased after the HHP treatment of tomato juice. A similar phenomenon was also reported in persimmon fruit purees, as plant carotenoids can be bound in protein-carotenoid complexes. It was probably related to protein denaturation after high pressure, rendering lycopene and other carotenoids more accessible during extraction[36]. As a heat-labile vitamin, the ascorbic acid content is generally considered to decrease after thermal processing but remains stable after HHP processing[13,17,37]. High ascorbic acid retention (94%) has also been observed in HHP-treated (600 MPa, 15 min) tomato paste[12]. In contrast, Jayathunge et al.[17] found a 33% ascorbic acid depletion after HHP processing (600 MPa, 1 min). Different pressure intensities and treatment times can partly explain the inconsistencies.
Few studies have been reported on polyphenols in HHP-processed tomato juice. However, previous studies found that chlorogenic acid to be stable after thermal pasteurization (93 °C) in tomato paste and HHP treatment (550 MPa, 15 min) in tomato purée[38,39]. This is consistent with our results wherein HHP processing (550 MPa, 10 min) had a minimal effect on the cryptochlorogenic acid content. Moreover, we found that HHP processing (550 MPa, 10 min) significantly increased the amount of quercetin in tomato juice. Roldán-Marín et al.[40] highlighted that the total quercetin content in onion after HHP processing (100 or 400 MPa) at low temperature (5 °C) was 8% higher than that after HHP processing (100 MPa) at high temperature (50 °C), indicating that low-temperature treatment plays a vital role in retaining polyphenols. Furthermore, Jez et al.[38] specified that the quantities of all phenolic compounds (including sinapic acid, caffeic acid, rutin, and ferulic acid) studied in their tomato purée significantly decreased after HHP processing, partially contradicting our results. These differences can be explained in part by the tomato variety[38] and tomato products with different processing procedures. Moreover, PAL, PPO, and POD enzyme activities could not be inactivated after HHP processing, thus partially explaining ferulic acid, vanillic acid, and p-coumaric acid decrease, and rutin increases after HHP processing in our study. Similarly, Hernández et al.[41] also observed that the enzyme activities of PPO and POD remained residual after HHP processing of tomato puree.
Ascorbic acid and polyphenols are responsible for hydrophilic antioxidant capacity, while carotenoids are responsible for lipophilic antioxidant capacity in tomato juice[34]. Jayathunge et al.[17] found that the oxygen radical absorption capacity of the hydrophilic fraction in tomato juice was significantly reduced by 60% after both HHP and thermal processing, exhibiting consistency with our FRAP-determined hydrophilic fraction results. Hence, the post-processing declines in ascorbic acid, total phenolic content, and some of the polyphenols potentially justify the weakened antioxidant capacity. In addition, another important reason for the reduced antioxidant capacity could be the formation of primary Maillard products with a pro-oxidant capacity[12]. The different alterations in antioxidant capacity revealed by the FRAP and DPPH assays were due to the various principles on which they are based, and the existence of non-carotenoid compounds that can provide H but cannot change Fe3+-tripyridyltriazine to Fe2+-tripyridyltriazine may be the reason.
Although the above results indicate that HHP can more effectively retain antioxidants and sustain antioxidant capacity than HTST processing, how long these advantages can be maintained during storage remains unknown, as antioxidants decay. Hsu[34] reported higher total carotenoid and lycopene retention in HHP-treated (300–500 MPa, 10 min) tomato juice than in thermal-treated tomato juice during the entire storage period. However, Jayathunge et al.[17] found that lycopene and total carotenoids content in the HHP-treated (600 MPa, 1 min) tomato juice decayed to a similar level in HTST-treated tomato juice after 3 weeks of storage. Nevertheless, the advantages of HHP-processed tomato juice in terms of lycopene and total carotenoid retention were evident in just 1 week, indicating that HHP processing conditions (time and pressure), storage temperature, tomato variety, and packaging materials are important factors affecting changes in total carotenoid and lycopene content during storage. As demonstrated, carotenoids were the most important compounds affecting the antioxidant capacity of lipophilic fractions in tomato juice[34], and storage time potentially narrowed the gap in the antioxidant capacity of lipophilic fractions caused by the different processing methods in our study.
However, higher antioxidant-capacity retention was observed in the hydrophilic component of HHP-treated tomato juice than in that of HTST-treated tomato juice during most of the refrigerated storage time partly because of higher retention of ascorbic acid and vanillic acid. Interestingly, the quercetin content increased during storage, and this was also noticed in industrial tomato paste during storage at room temperature[37]. This may be explained by the hydrolysis of conjugated glycosidic-bond polyphenols[41]. For example, the naringenin content increased during storage owing to the conversion of naringin to naringenin by the retained naringinase activity in grapefruit juice after HHP (300–600 MPa, 5 min)[42]. In addition, HHP treatment resulted in 8.31, 4.77, 1.86, and 6.84 μg/g higher caffeic acid, quercetin, ferulic acid, and p-coumaric acid concentrations in tomato juice than HTST treatment during storage, indicating that the maintenance of greater hydrolase activity potentially causes the further conversion of these conjugated polyphenols to free forms in HHP-treated tomato juice during storage[43]. In this study, the stable total phenolic content during storage was found to be related to the complex dynamic balance of formation, oxidation, and hydrolysis reactions.
-
This research compared the changes in antioxidant compounds and capacity of HHP- and HTST-treated tomato juice during 4-week storage at 4 °C. Our results highlight that HHP processing (550 MPa/ 10 min) could be more effective at retaining ascorbic acid, carotenoids, and antioxidant activity in tomato juice than HTST processing (110 °C/8.6 s) under the same microbiological stability at the beginning of storage. However, HHP processing did not inactivate PAL, PPO, and POD enzyme activity, thus leading to multiple alterations in individual polyphenols during storage. In addition, higher ascorbic acid retention and a lower decay rate during storage in HHP-treated than in HTST-treated tomato juice were noted. Nonetheless, carotenoid and lipophilic-fraction antioxidant activity in HHP-treated tomato juice declined at the same rate at the end of storage compared with that in HTST-treated tomato juice. Multivariate analysis results suggest that HHP pasteurization technology is a practical approach to enhancing antioxidant components and capacity retention in tomato juice compared with HTST during the first week of refrigerated storage, thus providing new insight into the evaluation of HHP products' shelf stability.
This work was supported by National Key Technologies R&D Programs, China (grant numbers: 2017YFD0400705 and 2018YFC1602202).
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 MRM transitions and mass spectrometer parameters.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang X, Dong L, Ma C, Wang Z, Hu X, et al. 2023. Impact of high-hydrostatic pressure and thermal processing on the antioxidant profiles and capacity of tomato juice during storage. Food Innovation and Advances 2(2):124−134 doi: 10.48130/FIA-2023-0016
Impact of high-hydrostatic pressure and thermal processing on the antioxidant profiles and capacity of tomato juice during storage
- Received: 27 September 2022
- Accepted: 21 April 2023
- Published online: 30 May 2023
Abstract: While high-hydrostatic pressure (HHP) has successfully been applied to the pasteurization of fruit and vegetable juice beverages, their quality-stable shelf life during storage has not been fully elucidated. Therefore, we investigated the effect of HHP (550 MPa/10 min) treatment on polyphenols, carotenoids, ascorbic acids, and antioxidant capacity in tomato juice and their changes during 4-week refrigerated storage. High-temperature short-time (HTST, 110 °C/8.6 s) treatment was used as a control. The results revealed a significantly greater presence of polyphenols, carotenoids, ascorbic acid content, and antioxidant capacity in tomato juice after HHP processing than after HTST processing. However, the total carotenoids and total phenolic content in HHP-treated tomato juice decreased dramatically and approached that in the HTST-treated tomato juice after 1 week of storage. Therefore, HHP’s advantage in maintaining antioxidant compounds and capacity was only evident during the first week of storage in tomato juice. Nevertheless, the post-storage caffeic acid, quercetin, ferulic acid, and p-coumaric acid concentrations were 8.31, 4.77, 1.86, and 6.84 μg/g higher in the HHP-treated than in HTST-treated tomato juice, respectively. This study provides a new perspective for predicting HHP products' quality-stable shelf life.
-
Key words:
- Antioxidant profiles /
- Tomato juice /
- High-hydrostatic pressure /
- Antioxidant capacity /
- Storage













