-
Melon (Cucumic melo L.) is on the map of temperate zones which may produce great economic value, accounting for 40% of the country's total production[1]. Based on the geographic distribution, it has distinctive characteristics of sweet, juicy, nutritious and delicious aroma in Xinjiang. The characteristics and ripening level of various fruits may be objectively reflected by volatile components, which perform as an important criterion for assessing the quality of fruits[2].
The volatile components of melon are mainly determined by aldehydes, esters, alcohols and alkenes[3−5]. According to previous studies, there are nearly 300 kinds of volatile substances in melon[6]. The different types and concentration of these volatile substances determine the different flavor of melon. Among the amount of volatile substances in melon, esters have the highest content. Melons from different producing areas and different varieties have different proportions of ethyl acetate[7,8]. Jia et al.[9] preformed examination of volatile substances in Jiashi melon and it was discovered that the melon contains compounds such as ethyl acetate, propyl acetate, ethyl butyrate, ethyl caproate, hexyl acetate, and methyl 2-methylpropionate. Ethyl 2-methylpropionate and ethyl 2-methylbutyrate were the main aroma substances of Jiashi melon. Melon can be classified according to the significant differences in aroma characteristics among varieties. Shi et al.[10] analyzed volatiles of 39 kinds of melons and divided them into five groups by principal component analysis.
With the development of molecular biotechnology and bioinformatics, transcriptomics technology has broad application prospects in the study of abiotic stress and disease resistance mechanism of melons. Shan[11] adopted the transcriptome technology to melon fruit with Penicillium sp. infection (0, 48 and 60 h), found a number of pathogen associated molecular receptors (PAMP) and disease resistance related gene expression differences, which more deeply revealed the response mechanism of Penicillium sp. infection. At present, the research on fruit quality has also entered the transcriptome stage. Peng et al.[12] measured the flavor quality of mango in different developmental stages and analyzed the genes causing the flavor differences through transcriptome technology. To explore the key genes for the formation of sugar and acid quality of crown linose in different developmental stages, transcriptome analysis showed that six MYB transcription factors were likely to regulate the accumulation of organic acids in fruits, five genes were important genes for glucose accumulation, and two genes were important bZIP transcription factors for glucose and fructose accumulation in fruits. Transcriptomics technology has been widely used in fruit quality research such as apple[13], fig[14], Lycium ruthenicum Murray[15], grape[16] and apricot[17].
There are many varieties of muskmelon in Xinjiang, but limited reports were related to the volatile components that act as a prime factors in the flavor and quality of different thick-skinned muskmelon. To reveal volatile compounds in nine variaties of melon, we adopted headspace solid phase microextraction and gas chromatography-mass spectrometry (HP-SPME-GC-MS). Cluster analysis and principal component analysis (PCA) were conducted according to various volatile compounds and main volatile substances, respectively, to further explain the differences of volatile components in different varieties of melon. The aim of this study was to provide reference data for the study of the classification and selection of different varieties of muskmelon based on volatile components.
-
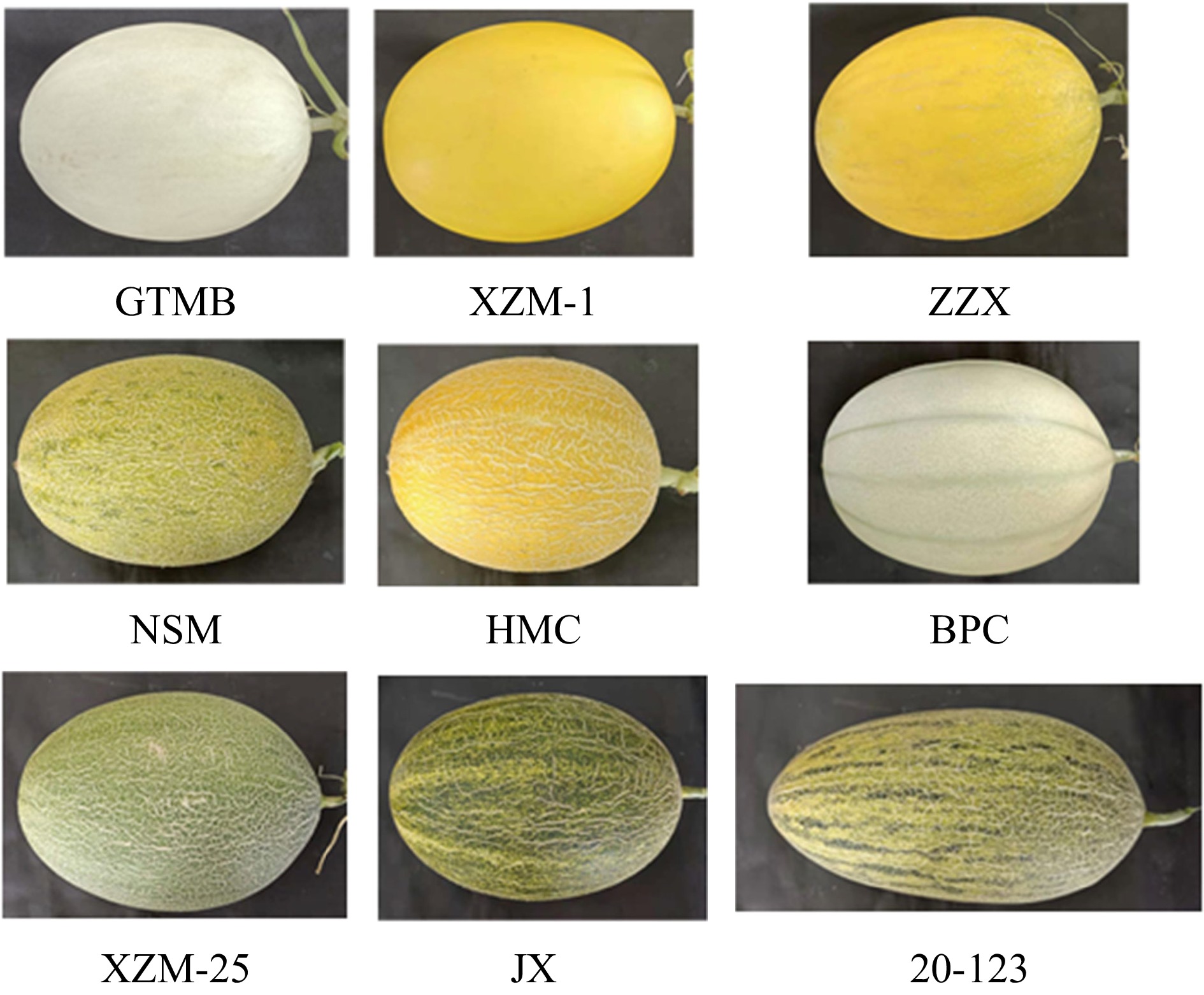
Nine different varieties of muskmelons collected from Turpan, Xinjiang, in June 2021 were used as experiment materials (Fig. 1). Six melons of each cultivar with medium maturity, no diseases and insect pests, were selected randomly and transported to the lab within 24 h. Basic physicochemical characteristics of hardness, longitudinal and transverse diameter, and soluble solids were determined before extraction treatment. Melon samples were first chopped into small pieces (after peeling and seeding) and then frozen with liquid nitrogen. Samples were kept in an environment below 80 degrees until analysis.
HS-SPME extraction
-
Referring to the extraction method outlined by Sun et al.[18], 10 g pulp was deposited in a 20 mL screw-cap brown vial containing 1 g NaCl solution for each extraction. PTFE stirrer bars were added to each vial and the mixture was stirred for 30 min at 30 rpm into a water batch whose temperature is 40 °C. Finally, the SPME fibers were coated with polydimethylsiloxane–divinylbenzene at a thickness of 50 μm, The sample's head space was then exposed for approximately 30 min. This was then placed into the GC-MS 's injector port under spitless mode set at 5 min desorption at 250 °C.
GC -MS analysis
-
Agilent 7890A/5975C was used to analyze the extraction at HP-5 MS capillary column (30 m × 0.25 m × 0.25 mm). Helium with a 1.3 mL/min constant column flow was selected as the carrier gas. A temperature control program were set as follows: Hold 40 °C for 3 min, increase by 3 °C/min until 140 °C, hold 140 °C for 3 min, increase 10 °C/min until 240 °C, hold 240 °C for 3 min. Scan the mass spectra in the m/z range 40–550 amu at 1 s intrervals.
Analysis of volatile substance related enzyme activities
Lipoxygenase (LOX)
-
LOX determination was applied to a melon pulp weighed at 5.0 g and placed in a pestle and mortar[19], 5.0 mL of extraction buffer that had been pre-cooled to 4 °C was introduced. The homogenate was pulverized in an ice bath. Following centrifugation at 12,000× g for a duration of 30 min at 4 °C, the supernatant was collected for LOX activity determination. One hundred μL 0.5% linoleic acid solution was added to 2.7 mL of buffer solution containing 50 mmol/L phosphoric acid at pH 6.8, then held at 30 °C for 10 min. Finally, 200 μL crude enzyme solution was added, mixed, and the absorbance value determined at 234 nm.
Alcohol dehydrogenase (ADH)
-
As a component of the ADH determination process, 5.0 g of melon pulp was measured and deposited into a pestle and mortar[19]. Five mL of extraction buffer, which had been pre-chilled to 4 °C, was introduced. The homogenate was crushed in an ice bath. To determine the ADH activity, the supernatant was subjected to centrifugation at 4 °C for a duration of 30 min at 12,000× g. Two mL 100 mmol/L, pH 6.5 MES buffer, 100 μL a solution of NADH at a concentration of 1.6 mmol/L, 0.2 mL enzyme extract and 200 μL 80 mmol/L acetaldehyde solution were added into the reaction system. After adding acetaldehyde, the mixtures were shaken well and the absorbance value determined at 340 nm.
Alcohol acyltransferase (AAT)
-
Volatile associated enzyme AAT was detected using AAT kit (Beijing Solarbio Technology Co., LTD, Beijing, China) according to the kit instructions.
Analysis of the transcriptome
Extraction of total RNA from melon
-
The RNA prep Pure polysaccharide polyphenol plant total RNA extraction kit was used to isolate total RNA from the melon samples. (Tiangen Biotech (Beijing) Co. LTD, China).
Quality test for RNA
-
The integrity of RNA was detected by QuawellQ3000 ultramicro spectrophotometer (Quawell Technology, Inc, USA). It was calculated that 28 S/18 S was between 1.6−2.0, indicating that the total RNA was excellent, which could be conducted for sequencing experiments.
Statistical analysis
-
Identification of the volatile compounds was conducted by matching the mass spectra of the samples with the NIST 14 data system library. The proportions of each constituent element in the sample were computed according to the area normalization method. To identify groupings and connections between different melon cultivars and their volatile compounds, a technique principal component analysis (PCA) was employed. The data was examined and charted via SPSS 19.0 and Origin 9.0, respectively.
-
For different muskmelon varieties, weight, size, hardness and soluble solids are the basic physicochemical characteristics to reflect the sensory indicators and maturity, which are listed in Table 1. In terms of weight, all the muskmelons were in the range of 1.43−2.85 kg and JX was the heaviest. JX also owned the largest longitudinal diameter (15.6 cm) and the second largest transverse diameter (25.3 cm). Regarding soluble solid content, the highest amount was found in NSM (16.8%), while the lowest content were detected in BPC. The majority of the remaining varieties, 20-123, XZM-1, XZM-25, and HMC, showed soluble solid content varied from 14.0% to 15.0%, respectively.
Table 1. Basic physicochemical characteristics for different muskmelons.
Variety Weight (kg) Vertical diameter (cm) Transverse diameter (cm) Hardness (kg/m3) Soluble solids (%) GTMB 1.75 ± 0.29de 13.9 ± 0.81b 17.1 ± 1.33d 4.10 ± 0.27a 12.2 ± 0.36de XZM-1 1.72 ± 0.17de 12.7 ± 0.58c 17.8 ± 0.61d 2.28 ± 0.11de 14.6 ± 0.46bc ZZX 1.43 ± 0.15e 12.0 ± 0.38c 17.2 ± 0.95d 2.7 ± 0.48bc 16.0 ± 0.79abc NSM 2.03 ± 0.21cd 14.7 ± 0.40ab 19.0 ± 1.5d 2.5 ± 0.14cd 16.8 ± 1.00a HMC 1.76 ± 0.24de 14.8 ± 0.48ab 18.2 ± 1.1d 3.2 ± 0.20bc 14.0 ± 0.76cd BPC 1.63 ± 0.16de 14.0 ± 0.50b 19.3 ± 0.6cd 1.5 ± 0.13f 11.2 ± 1.14e XZM-25 2.25 ± 0.15bc 15.3 ± 0.69a 21.4 ± 0.1c 1.8 ± 0.25ef 14.2 ± 2.25bc JX 2.85 ± 0.40ab 15.6 ± 1.00a 25.3 ± 2.2b 2.1 ± 0.17de 13.5 ± 1.30cd 20-123 2.51 ± 0.33a 14.9 ± 0.39ab 28.3 ± 1.4a 2.2 ± 0.19de 15.0 ± 1.00abc Distinct letters within the same column indicate significant differences (p < 0.05). Investigation of volatile compounds in different melon varieties
-
Findings from this research indicate that HS-SPME-GC-MS analysis detected 170 volatile compounds across nine types of muskmelons. A total of 52 alcohols, 41 esters, 32 ketones, 24 aldehydes, 14 acids and seven volatile phenols were identified. Esters, alcohols, and aldehydes were identified as the primary volatile compounds in muskmelons, and the percentage were 19.18%, 36.06% and 29.66%, respectively. Followed by ketones, acids and phenols the values were 5.63%, 8.56%, and 0.92%, respectively. Table 2 illustrates that volatile compounds ranged from 2.4% to 46.1% for esters, from 13.8% to 47.1% for aldehydes, from 13.2% to 61.4% for alcohols, from 0.1% to 22.9% for ketones, from 0.3% to 30.3% for acids, from 0% to 6.8% for phenols. Similar results were also found for the pocket melon (Cucumis meloL. ssp. dudaim) volatile compounds.[20]
Table 2. Relative content of various volatile components in different melons.
Variety Relative content/% Esters Alcohols Aldehydes Ketones Acids Phenols GTMB 8.2 13.2 18.6 22.9 30.3 6.8 XZM-1 7.3 41.4 47.1 2.5 1.7 0.0 ZZX 46.1 36.8 13.8 0.1 2.9 0.2 NSM 32.5 29.7 37.3 0.2 0.3 0.0 HMC 2.0 57.2 33.4 2.0 5.5 0.0 BPC 42.4 23.7 30.9 1.3 1.5 0.3 XZM-25 2.4 61.4 31.1 1.0 3.7 0.3 JX 25.9 20.6 15.1 16.6 21.6 0.3 20-123 5.7 40.6 39.6 4.1 9.6 0.3 There were significant differences in volatile substance content among different varieties of melon, and their different proportions constituted the flavor characteristics of different varieties of melon. The top ten volatile substances with the highest concentrations were octanal, 2-nonanol, 3-nonanol, bis(2-ethylhexyl)adipate, Acetic acid, octyl ester, 4-Nonanol, benzaldehyde, 2-methylpropanal and acetic acid. Octanal and 2-nonanol were the main common components in the nine kinds of melons. 20-123 had four kinds of special ingredients, including two kinds of alcohols. GTMB had 18 kinds of special ingredients, including six kinds of ketones. There were 15 kinds of unique components in XZM-1, among which seven were alcohols. JX had 10 kinds of unique components. ZZX had 11 unique components, four of which were alcohols. XZM-25 had four unique ingredients, NSM had three kinds of unique ingredients and HMC had four kinds of unique ingredients.
As shown in Supplemental Table S1−S6, among the nine kinds of melons, GTMB, XZM-1, ZZX, XZM-25 and 20-123 all contain octanal, with a relatively higher content compared with other volatile substances, among which XZM-25 has the highest content, reaching 27.6%. Combined with Supplemental Table S1−S6 and Fig. 2, in addition to aldehydes, esters, alcohols, ketones and acids, Individual varieties of thick-skinned melons contain phenolics, but the levels are generally very low. Esters were the most abundant volatile substance in the nine thick-skinned melons, followed by alcohols.
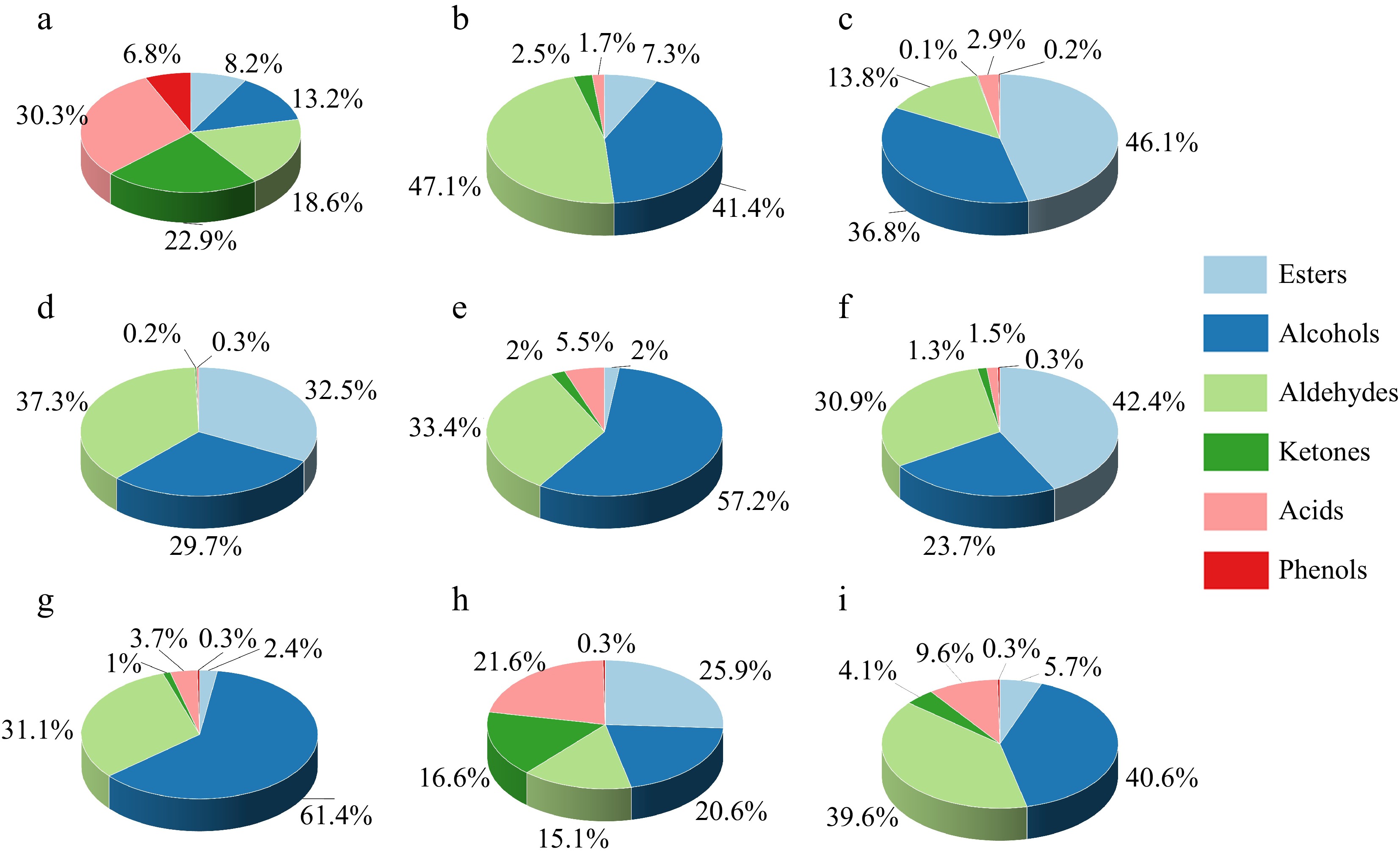
Figure 2.
Types of volatile substances in different muskmelon varieties. (a) GTMB, (b) XZM-1, (c) ZZX, (d) NSM, (e) HMC, (f) BPC, (g) XZM-25, (h) JX, (i) 20-123.
Clustering analysis of volatile substances in different muskmelon varieties
-
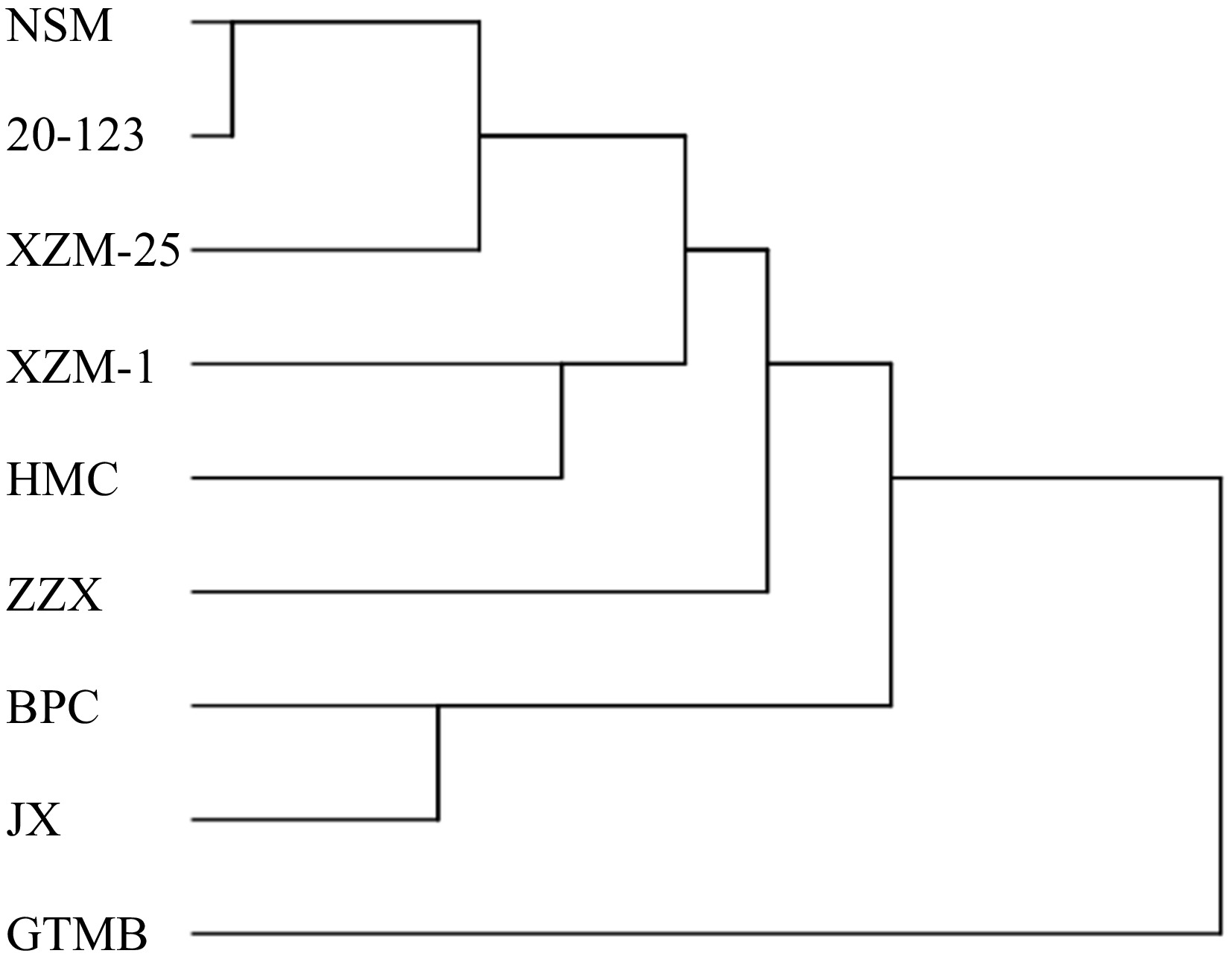
The volatile substances detected in nine kinds of muskmelons were mainly alcohols, aldehydes, esters, ketones and acids. All kinds of volatile substances were clustered. As shown in Fig. 3, they were divided into two categories. The first category was NSM, 20-123, XZM-25, XZM-1, ZZX, HMC, BPC and JX. The second category is GTMB. There was no significant difference among the other eight kinds of melons except GTMB. Ketones and acids in GTMB were higher than those in other varieties, 22.9% and 30.3%, respectively.
Principal component analysis of different volatile substances
-
A principal component analysis (PCA) was performed on the nine most prevalent volatile compounds with high levels (≥ 0.01%): octyl acetate, bis(2-ethylhexyl)adipate, benzaldehyde, octanal, 2-methylpropanal, 2-nonanol, 3-nonanol, 4-nonanol and acetic acid. Three components were extracted, and the cumulative contribution rate was 84.68%.
The contribution rate of the first principal component was 39.3%. The volatile substances with higher loading and positive correlation were 3-nonanol, 2-nonanol, 2-methylpropanal, bis (2-ethylhexyl) adipate, octanal, benzoin aldehyde and octyl acetate, with loading values of 0.777, 0.751, 0.692, 0.656, 0.638, 0.633, and 0.535, respectively. The contribution rate of the second principal component was 31.69%, among which bis (2-ethylhexyl) adipate, benzoin aldehyde, 2-methylpropanal and octanal were positively correlated with higher loading values (0.702, 0.635, 0.615, and 0.546, respectively), and 3-nonanol, 2-nonanol and octyl acetate were negatively correlated. The contribution rate of the third principal component was 13.69%. The loading amount of acetic acid was the highest and positive correlation, with the loading value of 0.859, and octanal was negative correlation, with the loading value of −0.552. As shown in Fig. 4, according to the PCA model of nine volatile substances, it can be seen that GTMB and BPC were separated, ZZX and HMC were separated individually, and the other five kinds of melons were closely separated without obvious separation. According to the eigenvalue, contribution rate and load value of PCA, 3-nonanol, 2-nonanol, bis (2-ethylhexyl) adipate and 2-methylpropanal were identified as the volatile substances with high contribution in nine kinds of melon.
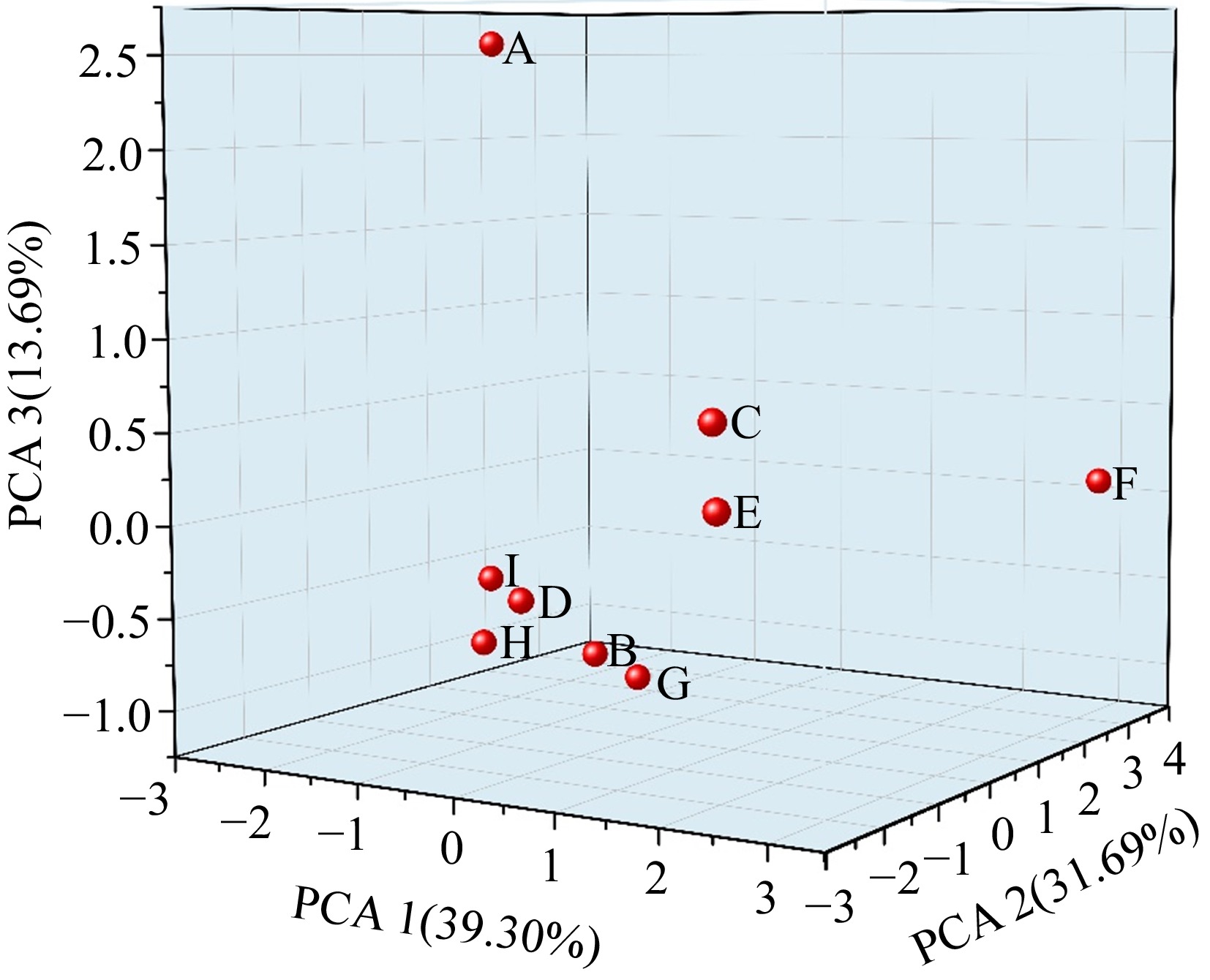
Figure 4.
PCA analysis based on main volatile substances in different varieties of melon. A: GTMB, B: XZM-1, C: ZZX, D: NSM, E: HMC, F: BPC, G: XZM-25, H: JX, I: 20-123.
Analysis of volatile substance related enzyme activities
-
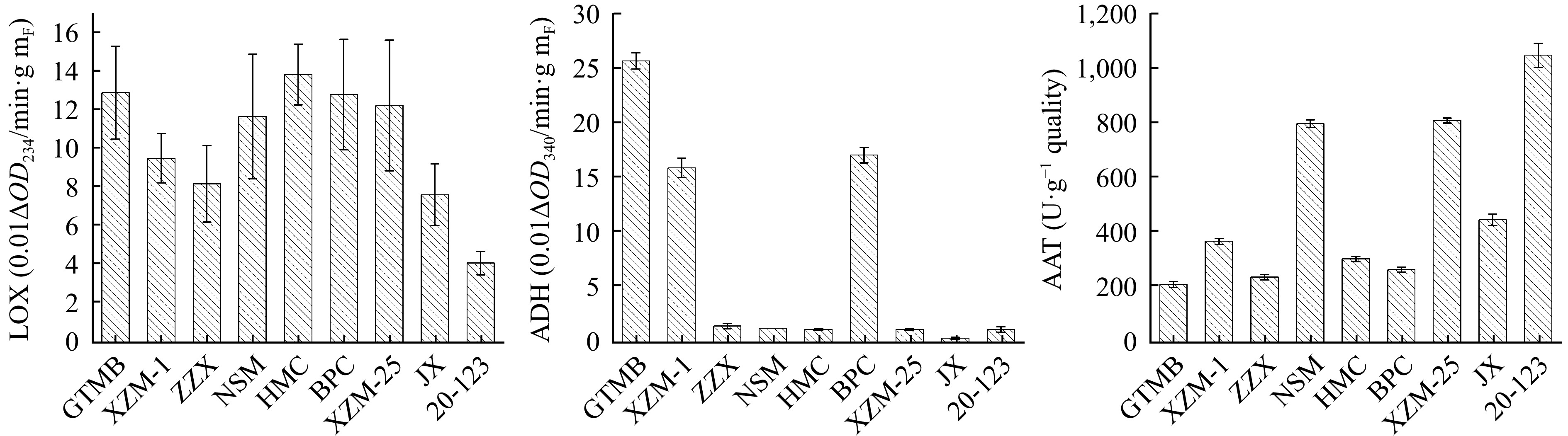
As shown in Fig. 5, LOX activity of HMC was the highest, followed by GTMB and BPC, and the lowest was 20-123. The results of ADH activity of GTMB, XZM-1 and BPC were more prominent. The highest activity was GTMB, followed by XZM-1 and BPC, and the lowest activity is JX. 20-123 had the highest AAT activity in melon, followed by XZM-25 and NSM, and GTMB had the lowest activity.
Transcriptome-based volatile substances related differential genes (DEGs) analysis in muskmelon cultivars
-
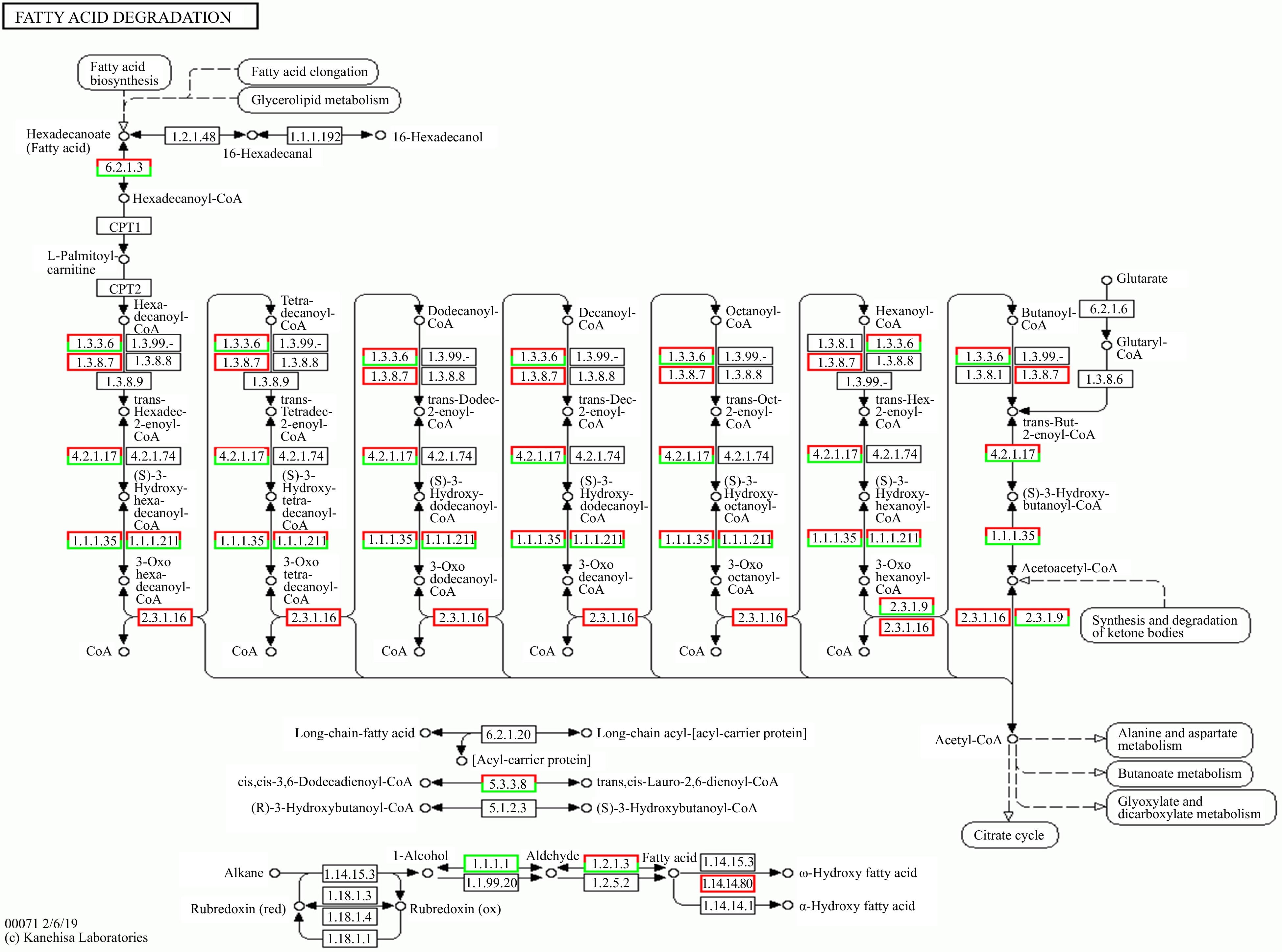
Two kinds of melons with large differences in volatile substances, GTMB and XZM-25, were analyzed using transcriptome sequencing, to compare the DEGs in the two kinds of melons. Based on the screening standard of Q-value ≤ 0.05 and |Log2FC| ≥ 1.5, a complete sum of 10,721 DEGs were screened, comprising of 5,385 up-regulated genes and 5,336 down-regulated genes. Breaking down of fatty acids, production of fatty acids, extension of fatty acids, formation of unsaturated fatty acids, fatty acid metabolism, pyruvate metabolism were annotated by KEGG Pathway, among which, pyruvate metabolism pathway and amino acid metabolism pathway had an important contribution to the odoriferous compounds in melon. As depicted in Fig. 6, in the fatty acid degradation pathway, hexadecyl alcohol was dehydrogenated to hexadecaldehyde, which in turn generated hexadecenoic acid under the action of dehydrogenase, and then entered the fatty acid degradation system. Coenzyme A, as an important participant, run through the whole fatty acid degradation process. Seven genes are responsible for encoding long-chain acyl-CoA synthetase [EC:6.2.1.3] (LOC103482821, LOC103483722, LOC103486958, LOC103487586, LOC103489907, LOC103493094 and LOC103500501). There were five down-regulated genes and two up-regulated genes between GTMB and XZM-25. Acyl-CoA oxidase is encoded by six genes. [EC:1.3.3.6], including two down-regulated genes and four up-regulated genes, among which, GTMB and XZM-25 exhibited markedly distinct expression levels of LOC103494416 and LOC103496003, with difference folds of 1.17 and −0.86, respectively. The expression levels of acetyl-CoA acyltransferase, which is encoded by LOC103483502 1 [EC:2.3.1.16] in GTMB and XZM were 202.173 and 403.843, respectively, with significant differences. In GTMB and XZM melons, LOC103500505 encoding acetyl-CoA-c-acetyltransferase [EC:2.3.1.9] was expressed 421.9 and 911.7 times, respectively.
-
The taste profile of melon is determined by its soluble sugars, organic acids and volatile substances. The volatile aroma compounds found in muskmelons are considered crucial indicators of their overall quality. This study carried out the detection of volatile substances for different varieties of muskmelon in Xinjiang, China. Among them, 170 kinds of volatile substances were identified, including aldehydes, esters, alcohols, ketones, and acids for nine main volatile substances, moreover, seven kinds of phenols were also detected. Ma et al.[21] also detected two furans in the 'gold queen' melon, which exhibited a fruity and charred aroma. Among the nine kinds of melons, ZZX had the strongest fragrance. As a result of the tests, ZZX had the greatest combined levels of esters and alcohols, accounting for a total of 82.9% of these compounds. It was found that 3-hexene acetate, 2-methyl butyl acetate, butyl acetate and butyl acetate increased at the maturation stage of melon according to Fallik et al.[22]. Tang[23] found aldehyde matter content was rich in immature 'shannon golden 1' melon, and after maturity, the volatile substance was given priority to acetic acid esters and alcohols, which was consistent with our results. Kourkoutas et al.[4] tested the volatile substances of musky melons and found that there were more sulfur-containing esters, which was somewhat different from the results of our study.
In this study, results showed that alcohols, aldehydes and esters had important effects on the volatile substances in melon, and the contribution of esters and alcohols was particularly prominent. Li et al.[24] evaluated the volatile components in the three kinds of towel gourd and the results showed the volatile matter content is higher for benzaldehyde, nonyl aldehyde and linalool. Luo et al.[25] found esters were the main volatile substances for respiratory jump type melon, rather than the non-respiratory jump type melon, in which volatile substances is given priority to C9 alcohols and C9 aldehydes. Qian et al.[26] found that the ethyl acetate of thin-skinned melon accounted for the main part, while the volatile aroma substances of thick-skinned melon were mainly alcohols and aldehydes, which was consistent with the present study.
Analysis of volatile substance related enzyme activities
-
The volatile components in melon are complex, and the enzymes involved are mainly LOX, ADH and AAT[27,28]. Straight-chain esters are produced by the LOX enzyme using fatty acids as a substrate, while aldehydes are converted to alcohols by the ADH enzyme and alcohols are further converted to esters by the AAT enzyme[29]. By catalyzing linoleic acid and linolenic acid, LOX can produce C6 and C9 compounds (hexanal, hexene aldehyde, nonyl aldehyde and nonene aldehyde, etc.). ADH catalyzes aldehydes into the corresponding alcohols, which provides the metabolic precursors for the synthesis of esters. The main function of this enzyme is to oxidize fatty acids, leading to the formation of acetic acid, butyric acid, and caproic acid. These compounds are subsequently transformed into esters by another enzyme[30]. Within the context of this research, the highest levels of compounds were observed for esters, alcohols, and aldehydes, and LOX activity were all high among the nine kinds of melons, illustrating the LOX catalytic produced large amounts of aldehyde substances. The activity of ADH was only outstanding in GTMB, XZM-1 and BPC, however, the high content of alcohols in the nine melons indicated that the effect of ADH on the production of alcohols was not outstanding at the mature stage, which maybe because the high content of alcohols inhibited the activity of ADH in reverse. The activity of AAT and the content of esters in the nine kinds of melons were all high, which suggested that the aldehydes generated in the development process of melons were converted into alcohols, and the activity of AAT was higher, converting alcohols into esters, thus making the content of esters in melons higher, The research results of Defilippi et al.[31] and Shalit et al.[32] indicate a positive correlation between AAT activity and ester content, which is consistent with our research findings.
Transcriptome-based volatile substances related differential genes (DEGs) analysis
-
Straight-chain aliphatic compounds, aldehydes, and ketones are primarily products of fatty acid metabolism, whereas branched-chain aliphatic alcohols are produced via amino acid metabolism. Additionally, the breakdown of amino acids results in the formation of aldehydes, ketones, and terpenoids.[7,33]. The aroma compounds in melons are produced via fatty acid metabolism. During this process, acetic acid, butyric acid, and caproic acid are generated from the breakdown of fatty acids. These compounds are subsequently converted into alcohols that contribute to the distinctive aroma of melons[34]. In the presence of acyl-CoA, fatty acids are esterified into esters by acyltransferase[35]. Acetyl transferase is a critical enzyme involved in the synthesis of esters. Esters present in melons such as hexanoate, benzyl acetate and E-2-hexenyl acetate are catalyzed by different acyl transferases[36]. Results in this study showed that esters content in GTMB (5.8%) was more than XZM-25 (0.1%), and the transcriptome results of GTMB vs XZM-25 showed that there were five genes encoding long chain acyl-CoA synthetase down-regulated and two genes up-regulated, indicating that this enzyme synthesized hexadecenoic acid to palmitoyl-CoA at the beginning of the fatty acid degradation pathway, which provides an important CoA for ester formation. The majority of genes responsible for encoding acyl-CoA oxidase and acetyl-CoA acyltransferase 1 were up-regulated and negatively correlated with ester content, indicating that the high expression of these two enzyme genes did not play a key role in mature melon. The gene encoding acetyl-CoA-c-acetyltransferase was significantly up-regulated and exhibited a positive correlation with ester levels, indicating that the formation of esters was closely related to the expression of multiple acyl-coenzyme genes, which was consistent with the results of El-Sharkawy et al.[36].
-
Aroma substance is one of the important indexes to measure the quality of melon. In this study, nine varieties of melon were found to contain a collective total of 170 different volatile substances. The contents of ketones and acids were the highest in GTMB. The results of PCA showed that 3-nonanol, 2-nonanol, bis (2-ethylhexyl) adipate and 2-methylpropanal contributed significantly to the flavor of melon, and 3-nonanol was the volatile substance with the highest contribution rate in Xinjiang muskmelon. The transcriptome sequencing analysis of two kinds of melons GTMB and XZM-25 with great difference in nutritional quality revealed a total of 10,721 DEGs, with 5,385 showing increased expression (up-regulated) and 5336 showing decreased expression (down-regulated). The DEGs of fatty acid degradation pathway were analyzed in KEGG metabolic pathway. The quality difference observed between the two types of melons can be attributed to the significant role played by long chain acyl-CoA synthase and acetyl-CoA-c-acetyltransferase genes.
This study was funded by China Agriculture Research System of Modern Agro-industry Technology Research (CARS-25), public welfare scientific research institutes of Xinjiang (KY2021118 and KY2020108) and China Postdoctor (No. 299580).
-
The authors declare that they have no conflict of interest.
-
These authors contributed equally: Yingying Fan, Binxin Jia
- Supplemental Table S1 Volatile component of esters in 9 varieties of muskmelon.
- Supplemental Table S2 Volatile component of aldehydes in 9 varieties of muskmelon.
- Supplemental Table S3 Volatile component of alcohols in 9 varieties of muskmelon.
- Supplemental Table S4 Volatile component of ketones in 9 varieties of muskmelon.
- Supplemental Table S5 Volatile component of acids in 9 varieties of muskmelon.
- Supplemental Table S6 Volatile component of phenols in 9 varieties of muskmelon.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Fan Y, Jia B, Cao X, Yang J, Li X, et al. 2023. Comparative analysis of volatile compounds in different muskmelon cultivars in Xinjiang based on HS-SPME-GC-MS and transcriptomics. Food Innovation and Advances 2(3):217−224 doi: 10.48130/FIA-2023-0023
Comparative analysis of volatile compounds in different muskmelon cultivars in Xinjiang based on HS-SPME-GC-MS and transcriptomics
- Received: 25 April 2023
- Accepted: 07 July 2023
- Published online: 29 August 2023
Abstract: Volatile flavor has prompted a great amount of influence in acceptance and view points in fruit products. Melon (Pyrus communis) is an aroma-dense fruit, thus, the evaluation of volatile flavor is crucial to melon-breeding. The volatile compounds present in nine varieties of Xinjiang muskmelons were identified and analyzed using the headspace solid-phase microextraction and gas chromatography-mass spectrometry methods. In addition, transcriptomics were used to discover the differential genes in fatty acid degradation pathways. It was found that a total of 170 volatile substances, including 52 alcohols, 41 esters, 24 aldehydes, 32 ketones, 14 acids and seven phenols, were identified in the nine melons. Results of PCA showed that 3-nonanol, 2-nonanol, bis (2-ethylhexyl) adipate, and 2-methylpropanal contributed more to the flavor of melon. It was verified that high activities of acyl-coenzyme A cholesterol acyltransferase (AAT) promoted the conversion of alcohols to esters, so that the melons have a high content of esters. Four genes of long-chain acyl-CoA synthetase were mainly responsible for the large difference in volatile substances. This practice may further undermine the primary rationale for the breeding and promotion in different cultivars of muskmelon.