-
With the advancement in living standards, people are increasingly demanding nutrient-rich, convenient, fast, flavorful, safe and healthy food. Protein-rich foods (mainly including livestock and poultry meat, aquaculture products, seafood and milk) are essential for human life. Protein-rich foods, due to their high protein, fat and nutrient content, are susceptible to protein oxidation, lipid oxidation and microbial contamination during storage or transportation. The spoilage of such foods not only negatively impacts their flavor, taste and nutritional value but also poses a potential threat to human health by increasing the risk of food-borne illnesses. With the increasing global concern for food quality and safety, it is crucial to adopt appropriate methods to extend the shelf life of food and effectively monitor its freshness.
Packaging plays a crucial role in the food industry as it serves to maintain product integrity and freshness throughout transportation and until consumption. At present, the majority of food packaging films available on the market are plastic products made from synthetic polymers derived from petroleum. These materials account for approximately 70% of all packaging materials[1]. Although plastics and their derivatives are commonly used as packaging materials to preserve food, their function is limited in terms of improving the freshness of food. As a result, the shelf life of food remains relatively short. In addition, the raw materials used in plastic products are non-renewable and non-biodegradable. A significant amount of plastic waste persists in the environment for extended periods, generating toxic residues that pose a severe threat to environmental health. Certain plastic packaging materials that exhibit suspected chemical compound leakage have the potential to pose a risk to food safety during the packaging process, thereby indirectly impacting consumer's health. Therefore, the utilization of degradable packaging materials is a novel trend in food packaging development to conform with the advancement towards green, environmental protection and safety.
Biodegradable packaging materials are natural biopolymers that can decompose into carbon dioxide, water, and small molecules under certain conditions through the action of microorganisms in nature, which poses no harm to the environment. With the continuous economic growth and the increasing awareness of food safety, natural biopolymers such as proteins, lipids and polysaccharides are gradually being selected for use in food packaging preparation. The packaging referred to as edible films or coatings has limited functional activity. To enhance their functionality, they are often utilized as carriers of active ingredients for the preparation of antibacterial and antioxidant active packaging films that can effectively delay food spoilage and extend the shelf life of food products. In addition to prepare active packaging films with excellent properties, the development of intelligent packaging films that can monitor food conditions and provide information on food quality and safety has recently gained increasing attention[2,3], as shown and summarized in Fig. 1. Therefore, selecting suitable natural active substances to prepare packaging films with active and/or intelligent functions has become a hot topic.
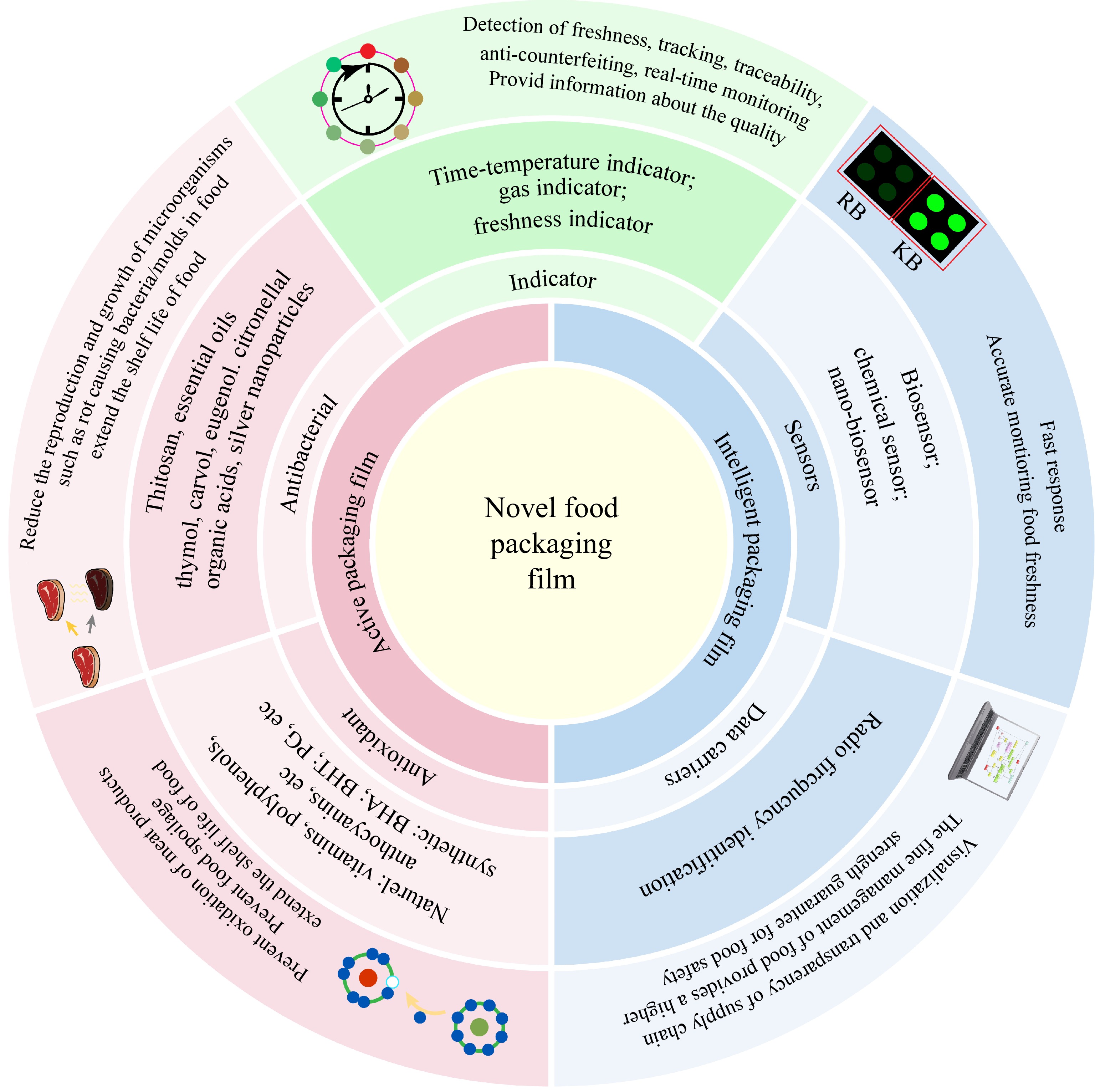
Figure 1.
The interrelationship among novel food packaging films. Note: Butyl hydroxyanisole (BHA), Butylated hydroxytoluene (BHT), Propylene glycol (PG).
Plants derived natural colorants refers to the pigment components found in various parts of plants, including roots, stems, leaves, flowers, fruits and bark. These pigments include anthocyanins, betacyanins, carotenoids, chlorophyll and curcumin. Not only are they safe and abundant in sources but they also posses certain functional activities such as bacteriostatic properties, antioxidant effect and pH sensitive[4]. Therefore, the incorporation of natural colorants derived from plants into edible films for the preparation of active and intelligent packaging has garnered increasing attention from researchers and manufactures[5−8]. This integration has significantly expanded the functionality of food packaging while also providing additional uses for fruits and vegetables rich in such natural pigments. Therefore, the aims of this review are to provide a comprehensive overview of biodegradable packaging utilizing natural colorants derived from plants, with particular emphasis on their diverse application in active and/or intelligent packaging films. Moreover, we will highlight their potential use in fresh protein-rich foods while also discussing prospects and limitations.
-
Anthocyanins are a type of phenolic compound that belong to the flavonoid polyphenols. They are natural water-soluble pigments found in a wide range of fruits, vegetables, flowers and cereals, and are responsible for producing various colors such as red, orange, blue and purple[9, 10].
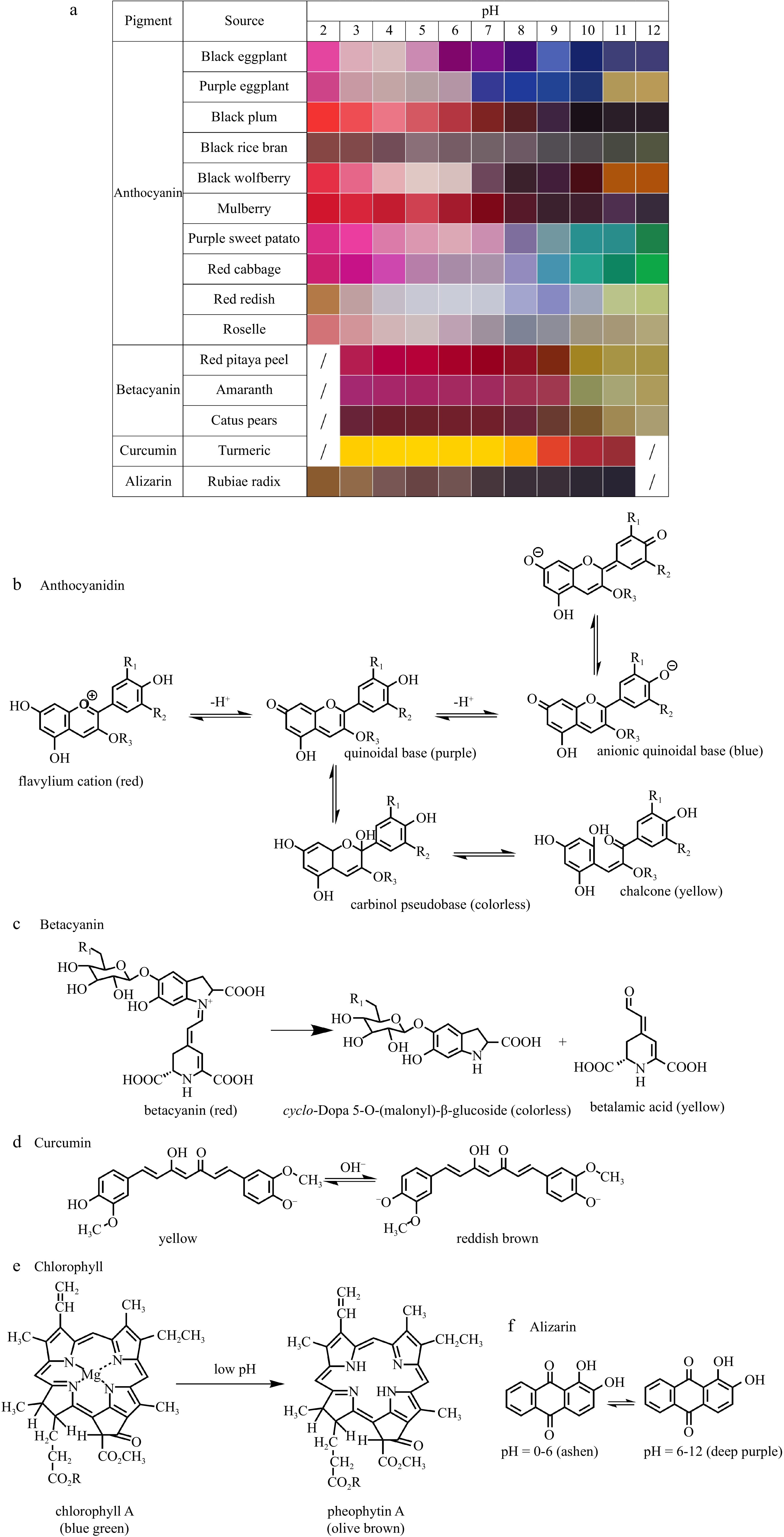
Anthocyanins are pH-sensitive, resulting in alteration to their chemical structure and corresponding shifts in color[11]. The color of anthocyanins solutions undergo a transition from red to pink, purple/blue, and ultimately yellow/green as pH values increase. This change in hue is indicative of their structural transformation (Fig. 2b)[11]. At pH 1.0, anthocyanins mainly exist as the red flavylium cation, which gradually converts to colorless carbinol pseudo base with decreasing H+ concentration. As the pH value reaches 7, the anthocyanins transformed into purple quinonoid bases. Further increase in pH would result in a shift towards blue anionic quinonoid bases. In a highly alkaline environment, anthocyanins undergo rapid self-oxidization, resulting in the formation of yellow degradation products known as chalcones[12−15]. In addition, it has been demonstrated that anthocyanins possess numerous health-benefits and biological properties[13]. Of these, the antioxidant and antimicrobial activities of anthocyanins are especially advantageous for developing active packaging, while their pH-sensitive properties has been widely explored in the development of intelligent packaging.
The stability of anthocyanins varies among different plant species due to differences in their structures. Anthocyanins are highly sensitive to various factors such as pH, temperature, light exposure, enzymatic activity and other flavonoids that can significantly impact their stability. Anthocyanins interact with other molecules, such as amino acids, organic acids, metal ions, flavonoids, polysaccharides, and other anthocyanins to enhance their stability by playing a complementary role in coloration. The stability of anthocyanins can be enhanced by forming complexes with metal ions, among which copper, iron, aluminum, magnesium and potassium are the most commonly used.
Betalains
-
Betalains are nitrogen-containing pigments that are soluble in water, and they can commonly be found in sources such as beetroot[16,17], amaranth[18], prickly pear[19] and pitaya[20]. Structurally speaking, betalains can be classified into two categories: red betacyanins and yellow betaxanthins[8]. Betacyanins are the main structure of betalains. In the pH range of 3−7, betalains mainly exists in the stable structure of betacyanins (red), but they gradually convert to cyclo-Dopa 5-O-(malonyl)-β-glucoside (colorless) and betalamic acid (yellow) and change color from red to yellow, under alkaline conditions (Fig. 2c)[8, 21, 22]. Studies have demonstrated that betalains exhibit antioxidant, antimicrobial, antitumor, lipid resistance and anti-diabetic properties[23,24]. Subsequently, it is suggested for employment in the production of active and intelligent packaging. Until now, there have been limited studies on the development of active and intelligent packaging based on betalains[8, 21, 25, 26].
Betalains are subject to various external factors, including temperature, light, pH, metal ions and more. Increasing its concentration can enhance its stability while acylation and glycosylation levels as well as beet pigments in dark and cold environments contribute to its high quality.
Curcumin
-
Curcumin is a natural compound extracted from the rhizomes of Curcuma, and it represents a rare diketone pigment in the plant world[27]. In general, curcumin appears as an orange-yellow crystalline powder that exhibits poor solubility in water. The structural change of curcumin in response to environmental pH alteration leads to a diverse range of coloration[14, 27]. In the pH range of 3−7, the curcumin solution presents a vivid yellow hue. When the pH level exceeds 8, the solution gradually turns orange to red before ultimately developing a reddish-brown hue as alkalinity increases (Fig. 2d)[27]. Curcumin exhibits high photosensitivity while poor stability under conditions of light, heat, enzyme exposure, and alkaline pH. It is particularly vulnerable to degradation in humid environments when exposed to light. However, curcumin has several biological activities, such as antioxidation[28], anti-inflammatory[29], antibacterial[28] and antineoplastic activities[30]. Based on these factors, certain researchers have developed edible films containing curcumin in order to improve their potential for practice use[31−33]. Therefore, the antioxidation, antibacterial and pH-sensitive properties of curcumin make it a promising candidate for the development of active and intelligent packaging[34, 35].
Chlorophyll
-
Chlorophyll, the most prevalent green plant pigment in nature, is a derivative of pyrrole. The primary structure of chlorophylls consists of a symmetrical cyclic tetrapyrrole known as a porphyrin ring with a central magnesium atom and a hydrophobic phytol group. Usually, it is mainly classified into two distinct types: chlorophyll a and chlorophyll b. The former is characterized with a methyl group (-CH3) on the porphyrin ring, while the latter features an aldehyde group (-CHO) at the same position as chlorophyll b. Chlorophyll is susceptible to degradation and color change due to various factors, including temperature, oxygen, acidity, light exposure and enzymatic activity. Under accelerated temperature and/or acidic conditions, the degradation rate is accelerated, resulting in a loss of color (Fig. 2e). The chlorophyll becomes pheophytin (olive green) upon losing magnesium ions[36].
The stability of chlorophyll can be improved by inactivating unfavorable enzymes, such as through blanching pretreatment. The underlying principle is to inactivate enzyme activity while mitigating the adverse effects of acid. Alkaline substances, such as potassium hydroxide and sodium hydroxide, are usually employed for acid neutralization. During preservation, chlorophyll is usually stored at low temperature and shielded from light to effectively reduce the deleterious effects of ultraviolet radiation on pigment stability[37]. In addition, substitution of magnesium with other metal ions in chlorophyll results in the formation of a more stable chlorophyll metal salt.
Alizarin
-
Alizarin, a natural dye derived from the roots of the madder plant, is widely used for data recording and as an analytical reagent. The alizarin solution exhibited a bright golden-brown hue which transitioned to red upon raising the pH level to 11. Alizarin is an anthraquinone molecule with hydroxyl groups that are modifiable at positions 1 and 2 (Fig. 2f)[7]. Under acidic conditions, alizarin molecules possess phenolic hydroxyl groups; whereas under alkaline conditions, they exist as deprotonated hydroxyl anions. Variations in electron configuration caused by proton transfer lead to diverse electron conformations of alizarin dye molecules, resulting in a range of colors. As the pH changes, two hydroxyl groups of alizarin form intramolecular hydrogen bonds with carbonyl oxygen atoms, resulting in a color shift from yellow to purple[38]. Furthermore, the release of volatile chemicals due to microbial deterioration can cause an raise in pH levels. Therefore, the pH response characteristics of alizarin can be employed as a reliable indicator for assessing the quality of packaged food products.
-
pH-sensitive natural colorants based active packaging films were usually made with biopolymers and a certain number of plasticizers as film forming substrate. At present, commonly utilized biopolymers include protein (such as gelatin, soybean protein isolate, corn protein), polysaccharide (including starch, cellulose, chitosan, pectin, alginate, κ-carrageenan, agar, and tara gum) and other polymers. Among these, gelatin and chitosan are the most commonly utilized film-forming materials due to their superior properties[40]. Notably, blending different types of biopolymers is a notable approach to overcome the limitations of single film-forming substrates and to develop pH-sensitive natural pigments-based active packaging films.
Plasticizers are polymer material additives that enhance the plasticity, elasticity, toughness and other properties of biopolymers. By modifying the properties of biopolymers, it can improve the mechanical and water vapor/gas barrier properties of films. In the selection of plasticizer, standards include safety and non-toxicity, stability, good compatibility, not-volatility, migration and diffusion in biopolymers, wide availability and low cost[41]. Glycerol and sorbitol are common food grade plasticizers in film-making processes. Among them, glycerol is the most popular choice due to its stability and compatibility with hydrophilic biopolymeric packaging chains.
Preparation of films based on pH-sensitive natural pigment
-
The performance and functional properties of pH-sensitive natural pigments and biopolymers-based active packaging film depend on the chemical properties of both the substrate and the film-making methods. The natural pigments, once dissolved, diffuse into the film-forming substrate and establish full contact with it. In addition, through molecular interactions, these natural pigments are uniformly integrated within the film-forming substrate via slow migration and release to achieve functional applications.
Interaction of natural pigments and film-forming substrates
-
The molecular interaction between anthocyanin and the film formation matrix has been demonstrated to be dependent on the charged properties of the biopolymer within the matrix. Neutral biopolymers (such as agar, Artemisia sphaerocephala Krasch. gum, starch, and konjac glucomannan) interact with anthocyanins through hydrogen bonding[9,42−44]. Biopolymers like carboxymethyl cellulose, chitosan, κ-carrageenan, zein, gellan gum, soy protein isolate and gelatin take charge of the electrostatic and hydrogen bonding interactions with anthocyanins[45−48]. Curcumin can be fixed in polymer film formation matrix by chemical bonding. Liu et al.[49] studied the interaction between curcumin and chitosan molecules through molecular simulation, revealing the presence of hydrogen bonds between them. Govindaraj et al.[50] used molecular dynamic simulation to clarify the interaction between curcumin and polyacrylonitrile, which also indicated interaction between curcumin and polyacrylonitrile through hydrogen bonds. Research has indicated that betalains interact with the film formation matrix through hydrogen bonds in a similar manner[8, 22].
Preparation methods of active packaging film
-
There are typically two methods for natural pigments to combine with film substrate: direct addtion of the natural pigments to the substrate, or coating/adsorption of the pigments onto the surface of the substrate[10]. Generally, pH-sensitive natural pigment-based films are usually prepared through extrusion, casting, knife-coating and coating. During extrusion, casting and knife-coating processes, natural pigments are intimately mixed with the film substrate. Meanwhile, physical and chemical methods are usually employed to coat or fix natural pigments onto the surface of biopolymers film during coating.
Extrusion is a highly prevalent method for industrial production of biopolymer-based film. The extruder employs high temperature and high shear force to melt the biopolymer matrix and natural pigments. Therefore, full consideration should be given to the thermostability of natural pigments during the film-making process, regardless of whether they will degrade or not.
Casting is a straightforward method for producing films, commonly employed in laboratory settings and for small-scale film production. This method involves the application of a film-forming solution onto a flat, non-stick surface, followed by the removal of the resulting film after it has dried[51]. Biopolymers are usually dissolved in suitable solvents, with or without heating, while pH-sensitive natural pigments are blended with biopolymers and plasticizers to prepare film-forming solutions at room temperature. Hence, there are minimal adverse effects on natural pigments when preparing films through casting[6, 8, 52].
The knife-coating process is a continuous industrial film-making procedure that has gained increasing popularity in recent years[53]. It overcomes the limitation of a batch procedure. With this technique, a precise layer of film-making solution is spread onto a surface that moves beneath a knife. Micrometer adjustments are available to regulate the knife height above the surface, thereby precisely controlling the thickness of the film. This method enables effective control of thickness and spreading velocity on a suitable support, which can be selected for each biopolymer solution utilized. The significant advantage of knife-coated films lies in their enhanced uniformity, which is achieved through strict thickness control. This results in improved reproducibility of properties, especially in tensile testing[54].
The coating process involves the application or fixation of natural pigments onto the surface of a film substrate. In comparison to other film-making methods, this technique disperses natural pigments within the film layer without requiring heat or mechanical action, resulting in minimum activity loss during substrate combination.
Electrospinning is a process that involves passing a polymer solution through a high voltage electric field to create nanoscale strands. Under mild conditions, electrospinning is a straightforward and versatile approach for producing nanoscale fiber films with high specific surface area[55]. The electrospinning film exhibits higher porosity and greater sensitivity to the pH of its environment. Additionally, electrospinning technology can compensate for the low sensitivity of hydrophobic indicators. Polyvinylidene fluoride is a crystalline polymer made up of C-F bond molecular chains that exhibit high hydrophobicity[56]. Therefore, the resulting film exhibits a high sensitivity towards volatile ammonia.
By precisely controlling the layer-by-layer deposition of materials, 3D printing technology is capable of fabricating solid or semi-solid objects in a variety of shapes[57]. To achieve precise control over the size, shape, and thickness of films, 3D printing technology leverages computer science, precision engineering, numerical control techniques, and material science[58]. Therefore, the utilization of 3D printing technology can expand the application range of edible film in food products.
Characteristics of films based on pH-sensitive natural colorants
Mechanical characteristics
-
Table 1 displays the mechanical characteristics of the natural pigment-based films. The intramolecular interaction between natural colorants and other elements of the film matrix enhances their dispersion within the matrix. Within a suitable range, the flatness of film increases proportionally with the increase in natural pigment content. The water vapor permeability and hydrophilic group utilization of the film are both reduced due to the strong intermolecular interactions between natural colorants and the film-forming matrix, which also lead to decrease in the film's affinity for water vapor. Natural colorants also form hydrogen bonds with the film matrix, which improves the uniformity of the film and reduces its crystallinity. Additionally, adding colorants impart corresponding colors to the film. Small amounts of natural colorants have no impact on the film thickness, however, some natural colorants such as anthocyanins and betalains contain higher levels of solid dry matter which results in an increase in film thickness with a corresponding increase in pigment content. Natural colorants generally function as cross-linking agents within the film-forming matrix, thereby enhancing the tensile strength of the film. However, their addition may also result in a decrease in the density of the film-forming matrix and consequently lower its tensile strength, in some cases. Natural colorants exhibit a plasticizing effect on the film, thereby weakening intermolecular force between adjacent macromolecules and promoting polymer chain mobility. Consequently, natural colorants may enhance the elongation at break of the film.
Table 1. Impact of incorporation of plant extract pigments on the structural characterization, physical and functional properties of film.
Film properties Effects Explanation Structural characterization Cross-section Increases with more plant extract pigments. Pigments can be introduced into the substrate and interact with it. Crystalline character Decreased gradually with an increase in the concentration of plant extract pigments. The plant-derived pigment formed hydrogen bonds with the membrane matrix, thereby enhancing film uniformity and reducing crystallinity. Physical properties Surface color Deepens with more plant extract pigments. This is due to the dispersion of pigments extracted from plants into the film matrix, which imparts color to the films. Thickness Increase in the presence of pigments. The internal structure of the matrix is modified by plant-derived pigment. Moisture
contentIn most cases, there is a reduction in the presence
of the pigments.Plant extract pigments, which interact with each other to form more complex matrices. Water
solubilityIncreases with increasing the concentration
of plant extract pigments.Plant pigments can reduce the interactions between polymeric chains, facilitating the dissolution of the film matrix in water. Decrease in the presence of pigments. Plant pigments can form hydrogen bonds with the film matrix, thereby reducing the number of free hydroxyl groups. Light barrier
abilityThe UV–vis light transmission and the transparency of the films decreased proportionally with an increase in plant extract pigments content. The presence of multiple unsaturated bonds in the plant extract pigments enables them to absorb UV–vis radiation. Water vapor barrier ability Decrease in the presence of pigments. The intermolecular interactions between natural pigments and substrates reduce the membrane's hydrophilicity. Mechanical properties Tensile strength: increased with the increasing content of plant extract pigments. Increase, which was because plant extract pigments play a cross-linking effect on the film matrix. Elongation at break: increased with the increasing content of plant extract pigments content. Pigments enhance the mobility of polymer chains by reducing the intermolecular interactions that bind adjacent macromolecules together. Functional properties Antioxidant activity Increased with the increasing content of plant extract pigments. Mainly due to the antioxidant activity of plant extract pigments. Antimicrobial activity Increased with more plant extract pigments. Pigments can affect the permeability of cell membranes in food-borne pathogens, leading to their eventual death. pH-sensitive Sensitive when films were immersed in different
pH buffer solutions or exposed to ammonia.Films can alter their coloration due to the plant extract pigments in response to varying pH conditions. Functional properties
-
In addition, the incorporation of pH-sensitive natural colorants or their extracts exhibits favorable antioxidant, antibacterial and pH-sensitive properties. Natural colorants exhibit pH-dependent color changes, while films containing these natural pigments display varying hues when exposure to different buffer solutions or ammonia. Free radicals are implicated in the pathogenesis and progression of numerous diseases, as well as contributing to the spoilage and degradation of nutritional quality in packaged foods. Natural colorants, such as anthocyanins and betalains, exhibit excellent radical scavenging activity due to the presence of multiple phenolic hydroxyl groups and other hydrogen donors. The incorporation of natural colorants significantly improves the film's ability to scavenge DPPH radicals, and as the concentration of natural pigment increases, so does the film's scavenging ability towards DPPH radicals. In addition, natural colorants have the potential to disrupt cellular structure and alter membrane permeability, leading to microbial death or inhibit microbial growth. Therefore, the antimicrobial activity of the films was found to increase proportionally with the pigment content in plant extracts. Similarly, an increase in natural colorants content resulted in a corresponding enhancement of the antimicrobial capacity of these films.
-
In recent years, there has been an increasing focus on intelligent packaging that can monitor the condition of packaged food throughout storage, transportation, and sale to provide valuable information regarding its quality. Microbial growth and metabolism result in the formation of volatile compounds, which subsequently alter the pH value of the atmosphere within a hermetically sealed food package during storage. pH-sensitive indicators are utilized for monitoring food quality, as illustrated in Fig. 3. Chemical indicators are generally unsuitable for monitoring food freshness due to their potential health hazards when used in food packaging. Therefore, the quest for a pH-sensitive, non-toxic and eco-friendly indicator derived from nature sources to monitor food freshness during storage has emerged as a prominent research area.
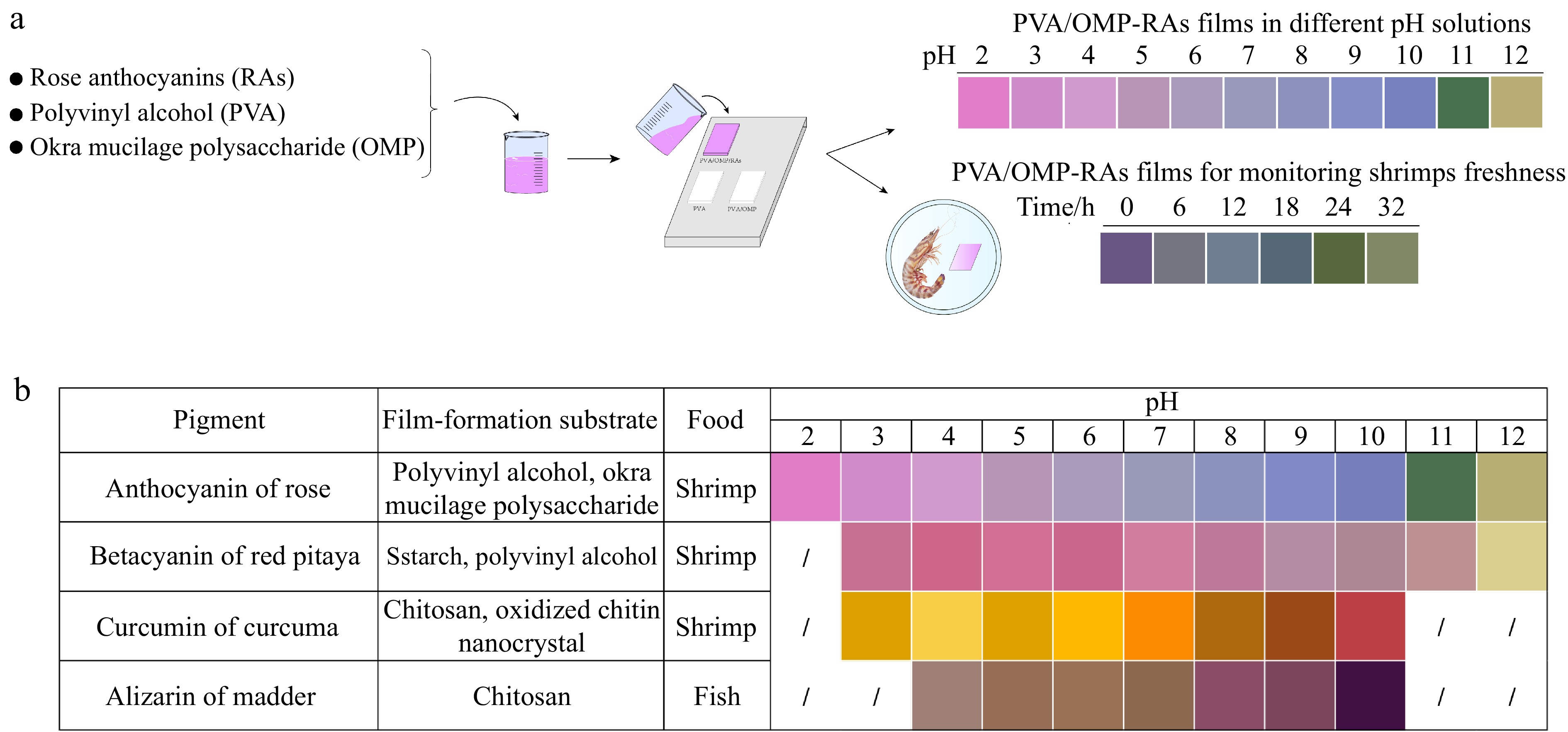
Currently, pH-sensitive natural pigments extracted from plants, such as anthocyanins, betalains and curcumin, are utilized in intelligent packaging to monitor the freshness of foods, especially protein-rich foods including fish, shrimp, chicken, pork, milk and cheese. They are summarized in Table 2. As an illustration, Chi et al.[59] incorporated grape skin powder containing anthocyanins into a ĸ-carrageenan/hydroxypropyl methylcellulose base film and applied it for preserving chilled fresh pork. The results indicated that the TVB-N value of pork increased from 14.63 mg/100 g to 15.25 mg/6 g after a storage period of 23 h at 8 °C, accompanied by a color change in the indicator film from purple to green. Therefore, the indicator film can effectively monitor pH changes and spoilage progression in stored pork samples. Liu et al.[27] utilized a curcumin-rich film to monitor the changes in freshness of pork and shrimp during storage. Their results showed that the control group (without adding curcumin) exhibited no significant color alteration during storage, while the indicator films in the experimental group changed from an initially yellow to red with the pork or shrimp stored for 3 d. Wu et al.[35] utilized a novel intelligent film incorporating curcumin into a chitosan/chitosan oxide nanocrystal matrix to monitor seafood freshness. During the storage of shrimp, the film's color gradually transitioned from yellow (day 0) to orange-red (day 5), indicating its potential for detecting alkaline substances produced during meat preservation and thus serving as an indicator of food freshness. Qin et al.[8] incorporated red pitaya peel extraction into a starch/polyvinyl alcohol film matrix, and found that the resulting film containing 1 wt% extract was highly responsive to ammonia, making it suitable for monitoring shrimp freshness with visible color changes[8]. Ai et al.[60] developed real-time intelligent films using betacyanins derived from pitaya and glycerol as film-forming substrates for detecting pork freshness. The color of film changed significantly from reddish-purple or light pink to yellow during pork deterioration. Maciel et al.[37] found that films containing chlorophyll and chitosan have the potential for generating color within the colorimetric temperature range of 50−75 °C. The system will undergo an irreversibly color change from green to yellow when exposed to this temperature range. Alizarin was incorporated into chitosan-based films to develop innovative indicator films for evaluating seafood freshness[7]. The color of chitosan/alizarin films underwent changes over time, exhibiting a khaki hue initially, followed by yellow at 24 h and light brown after 48 h. Intelligent indicator films incorporating a complex blend of natural pigments are more effective in indicating the freshness of foods compared to those containing only a single pigment. For example, Chen et al.[14] utilized curcumin, anthocyanin and their combination to develop a novel colored-pH sensitive film for monitoring the freshness of Bighead carp. A optimal ratio of curcumin to anthocyanin in the films was found to be 2:8 (v/v), which exhibited excellent indicative performance. The film presented three distinct colors corresponding to fresh, slightly spoiled, and fully spoiled carp, allowing for easy visual differentiation. Qin et al.[25] prepared several types of film, the film containing a mixture of anthocyanins and betacyanins in a weight ratio of 1:3 exhibited significant color changes when utilized to monitor pork freshness. Therefore, it is recommended to utilize multiple natural colorants, such as anthocyanins, betacyanins and curcumin in the preparation of intelligent films for achieving more precise detection results of protein-rich food freshness.
Table 2. Implementation of natural pigment-based pH-sensitive intelligent packaging films in food industry.
Natural pigments Source Film formation substrate Food Application effects Reference Source Species Anthocyanins Mulberry Natural Sodium carboxymethyl starch, κ-carrageenan Fish The color of films is subject to variation based on the freshness of fish (red-blue-yellow). [61] Black rice bran Gelatin, oxidized chitin nanocrystals Shrimp and hairtail Films can be utilized to monitor the spoilage of shrimp and hairtail. The films undergo a color change from purple to gray blue or brown. [62] Black carrot Starch Milk The changes in hue of the label demonstrated a strong correlation with the physical, chemical, and microbiological alterations that occurred during milk spoilage, indicating the indicator's ability to distinguish between fresh, moderately fresh, and spoiled milk. [63] Black carrot Bacterial nanocellulose Rainbow trout, common carp At the stages of fresh fish (dark red), best edible fish (attractive pink), and rotten fish (jelly bean blue and khaki), recognizable color variations are exhibited by the pH indicator film. [64] Echium amoenum flowers Bacterial cellulose Shrimp The color of the film may undergo substantial changes over time, exhibiting fresh (purple), utilized young (gray), and ruined (yellow) shrimp. The TVC and TVB-N values of shrimp are consistent with these color variations. [65] Grape skin κ-carrageenan, hydroxypropyl methylcellulose Pork When the TVB-N level in pork reaches 14.63 mg/100 g (pork is considered spoiled when TVB-N exceeds
15 mg/100 g), the film changes from purple to green, indicating its effectiveness in monitoring pork freshness.[59] Rose Sodium alginate, sodium carboxymethyl cellulose Shrimp The color of the film changes from pink to pale yellow to yellow-green during shrimp storage at 4 °C, indicating that its chromatic response is influenced by pH or TVB-N content. [66] Purple cabbage Watermelon peel pectin Mutton The antioxidant and antibacterial activities of the films were proportional to the anthocyanin content. Furthermore, as the anthocyanin content increased, the film color intensified. [67] Blueberry residue Cassava starch Orange juice, corn oil and chicken pieces The film successfully established a correlation between the color of samples submerged in buffer solutions with varying pH values, simulants and food product. [68] Rose Synthetic and natural Polyvinyl alcohol, okra mucilage polysaccharide Shrimp The target film is capable of real-time monitoring of shrimp freshness, with color changes that can be easily distinguished by the naked eye. [15] Roselle Hydroxypropyl methylcellulose modified polyvinyl alcohol Shrimp The color of the film changed from rose-red to light green on day 4, indicating the onset of spoilage. Subsequently, it turned yellow on day 8 when the shrimp was severely spoiled. [69] Roselle Starch, polyvinyl alcohol, chitosan Pork When utilized for assessing the freshness of pork stored at 25 °C, the film transitioned from red to green prior to the gradual increase of TVB-N value in pork reaching its rejection threshold (15 mg/100 g) after 36 h. [70] Purple cabbage Polyvinyl alcohol, cellulose nanocrystals Shrimp Anthocyanin-containing films exhibit exceptional pH (2−13) and volatile ammonia sensitivity, rendering them ideal for detecting shrimp freshness. [71] Grapes Polyvinyl alcohol, starch Pork The resulting film exhibits color variations within the pH range of 2 to 12, enabling real-time monitoring of pork freshness in the package. [72] Mulberry Polyvinyl alcohol, chitosan nanoparticles Fish The film's color transitioned from red to green due to fish spoilage. [73] Betalain Amaranth Natural Quaternary ammonium chitosan, fish gelatin Shrimp After 24 h of storage, the TVB-N value of the shrimp slightly exceeded the limit, and the color of the films turned yellow as an indicator of decreased freshness. [21] Amaranth Chitosan, gelatin Fish Films enriched with amaranth anthocyanins exhibit active and intelligent properties, including antioxidant, antibacterial, and pH-responsive capabilities. [74] Bougainvillea glabra Choisy flowers Potato starch Fish A starch film containing 15% betacyanin was capable of visually detecting the changes in Caspian sprat quality during cold storage by undergoing a color change from pink to yellow, coinciding with microbiological and chemical alterations in the fish samples. [75] Cactus pears Synthetic and natural Quaternary ammonium chitosan, polyvinyl alcohol Shrimp The films containing 2 and 3 wt% of betalains exhibited a color change from purple to orange when shrimp was not fresh. [22] Amaranth Polyvinyl alcohol, gelatin Fish and chicken The film showed discernible color change from red to yellow on spoilage. [52] Red pitaya peel Starch, polyvinyl alcohol Shrimp The film containing 1.00 wt% of the extract presented visible color changes due to the accumulation of volatile nitrogen compounds during shrimp spoiling. [8] Curcumin / Natural κ-carrageenan, gelatin, zein Grass carp fillets The film indicates a deterioration in freshness through a color change from yellow to red as storage time increases. [76] / Chitosan, oxidized chitin nanocrystal Hair tail and shrimp The color of the films gradually shifted from yellow (day 0) to orange-red (day 5), which may be attributed to the increase in TVB-N levels in seafood samples during storage. [35] / Zein, chitosan Blueberry The reactive film exhibits excellent UV resistance and displays a sensitive pH response to discoloration. [77] / κ-carrageenan Pork and shrimp The color of the film clearly changed from yellow to red on the 3rd day for pork and shrimp storage. [27] / Synthetic and natural Pectin, sulfur nanoparticles Shrimp The film exhibited a pH-responsive color transition that was highly distinctive, changing from yellow to orange in response to variations in shrimp quality. [78] / Chitosan, polyvinyl alcohol Pork and shrimp With the prolongation of storage time, the color of the indicator film shifts from yellow to orange, which serve as an effective means for assessing pork freshness. [79] / Tara gum, polyvinyl alcohol Shrimp The color of the film exhibited a slight yellow hue, which transitioned to an orange-red tint after 3 d due to the increasing pH levels of the stored shrimp. [34] Chlorophyll / Natural Chitosan / Color can be changed from green to yellow by using films containing chlorophyll and chitosan at temperatures between 50 °C and 75 °C. [37] Alizarin / Natural Chitosan Fish The film exhibited a discernible shift in color from khaki to light brown upon the onset of fish spoilage. [7] / Cellulose-chitosan Minced beef The color of the indicator changed from brown to purple as the TVB-N reached a critical level. [39] Anthocyanins
/CurcuminPurple sweet potatoes Synthetic and natural Starch, polyvinyl alcohol Fish The incorporation of anthocyanins and curcumin into the film enabled the packaging of fish with three distinct colors, each indicating a different level of freshness: high, medium, and spoiled. [14] Saffron petals Natural Bacterial cellulose nanofiber substrate Fish The nanofiber loaded with curcumin-anthocyanin exhibited a distinct color change upon exposure to spoiled fish meat in a transparent plastic package. [80] Anthocyanins
/BetacyaninLycium ruthenicum, red pitaya peel Synthetic and natural Starch/polyvinyl alcohol Pork The film, which contained a mixture of anthocyanins and betacyanins in a weight ratio of 1:3, exhibited noticeable color changes when employed as an indicator for monitoring the freshness of pork. [25] Red cabbage, red pitaya Polyvinyl alcohol/sodium carboxymethyl cellulose Pork The diverse colors of CPA-3A1B film can be utilized to distinguish the states of freshness, medium freshness, and deterioration of pork. [81] Antioxidant and antimicrobial active packaging films
-
The objective of active packaging is to preserve the quality and prolong the shelf life of food products by mitigating oxidation and inhibiting growth of spoilage microorganisms, which are major contributors to food deterioration, particularly in protein-rich foods. Protein-rich foods are typically excellent sources of both proteins and lipids, though the oxidation of these compounds is the main cause of food deterioration, including changes in flavor, color, texture, and nutritional value. Furthermore, this process may lead to the formation of toxic compounds. Microbial contamination is also a significant factor contributing to a shortened shelf life for many types of food. In the process of microbial reproduction, not only biogenic amines, organic acids and other substances would be produced, but also lipid oxidation and other oxidation processes may be accelerated, resulting in an unpleasant odor that is un acceptable to consumers. Therefore, incorporating natural colorant extractives from certain plants, known for their antioxidant and antimicrobial properties, into biopolymer-based films is a viable approach to developing active packaging films.
Natural antioxidant and antimicrobial-based films have garnered significant attention as a means to delay or prevent oxidation, inhibit the growth of spoilage microorganisms, and extend the shelf life of food products. Natural colorants can be combined with various polymer substrates to achieve bacteria-inhibiting effects through molecular migration. This not only inhibits the growth of microorganisms but also reduces lipid oxidation in food products. The antioxidant and antimicrobial performance of packaging films depends on many factors, such as the molecular interaction between natural pigments and polymer substrates, the quantity of added natural pigments, structural modifications, and environmental conditions. Generally, the antibacterial and antimicrobial properties of films increase with the rise in natural pigment content. In comparison to coated films, active packaging films require a higher amount of antimicrobial actives to achieve an equivalent level of antimicrobial efficacy. Antimicrobial agents present in coated films are situated on the surface of the package, thereby increasing their reactive surface area and enhancing their antimicrobial efficacy. Conversely, antimicrobial agents found in active packaging films are located within the package and may have limited migration and contact with food. However, an excessive amount of natural pigments would also have a negative impact on the flavor of food, it is very important to determine the appropriate amount.
A summary of active films combined with natural pigments for maintaining optimal food quality is presented in Table 3. The peroxide value of olive oil sealed with gelatin film rich in anthocyanin was significantly lower than that of olive oil sealed with plain gelatin film, indicating the effective inhibition of lipid oxidation by the anthocyanin-rich film[82]. It was reported that the anthocyanin based zein film can reduce the total number of bacteria, mold and yeast, thus maintaining the overall quality and sensory properties of cheese[3]. The gelatin film enriched with curcumin exhibits excellent antioxidant activity, effectively inhibiting lipid oxidation in pork and prolonging its shelf life[83]. The application of betalain-rich polyvinyl alcohol-gelatin films in the preservation of frozen fish/chickens results in delayed microbial growth and minimized oxidative rancidity[52]. Therefore, incorporating natural pigments into edible films can serve as an active packaging solution to preserve the quality, safety, and sensory properties of food products. Although natural pigment-based indicator films offer benefits such as antioxidant and antibacterial properties, their application in the food industry is also limited. Natural pigments are prone to structural changes and loss of color variation due to environmental factors, which may result in inaccurate color representation. Additionally, the use of color-based indicator films in food packaging requires consideration of consumer acceptance.
Table 3. Implementation of natural pigment-based active packaging films with antioxidant and antimicrobial properties in food.
Natural pigments Source Film formation substrate Food Application effects Reference Source Species Anthocyanins Amaranth Natural Hydroxypropyl methylcellulose Salmon oil The films provided superior protection against lipid oxidation by enhancing their barrier properties against light and oxygen. [82] Cranberry Gelatin Olive oil The film inhibited the oxidative deterioration of olive oil. [50] Grape pomace Guar gum Pomegranate arils The developed films exhibited notable antimicrobial activity against diverse food-borne pathogens. [83] Grape pomace Cassava starch Sunflower oil The impact of films on the inhibition of sunflower oil oxidation (peroxide value). [84] Grape pomace Cassava starch Olive oil The incorporation of anthocyanins in the packaging material can effectively inhibit the oxidation of EVOO, thereby prolonging its shelf life. [85] Pomegranate peel Zein Himalayan cheese The utilization of film as a packaging material for kalari cheese effectively inhibited oxidation reactions and microbial spoilage during storage. [4] Prunus maackii juice κ-Carrageenan/
hydroxypropyl methylcelluloseLard The films exhibit greater efficacy in retarding the oxidation of lard. [86] Red rice flour Starch Sunflower oil The film exhibited a noteworthy photoprotective effect of sunflower oil and retarded its oxidation process. [87] Betacyanin Amaranthus leaf Synthetic Polyvinyl alcohol /gelatin Fish/chicken The films exhibited activity in the preservation of chilled fish/chicken by delaying microbial growth and minimizing oxidative rancidity. [21] Curcumin Turmeric Natural Gelatin Ground pork The developed films were applied to fresh ground pork to prolong its shelf life, demonstrating favorable antioxidant activity and effectively preventing lipid oxidation of pork. [32] -
The active and intelligent packaging films, prepared using natural colorants derived from plants, play an important role in preserving food quality, ensuring food safety and providing consumers with valuable information. It is not only a research hotspot in the field of food packaging, but also a major trend in the future of the packaging industry. However, there are some issues that need to be considered as follows:
(1) In terms of active and intelligent packaging, there is a lack of perfect regulations, appropriate assessment methods, and clear regulatory measures. The safety of active components in active packaging needs to be verified, as well as the accuracy, reliability, and scientific nature of the evaluation system for intelligent packaging.
(2) The intricacy and high degree of specialization in technology result in elevated production costs, rendering it challenging to satisfy the market's demand for mass-produced goods, thereby impeding its promotion.
(3) Consumers' limited awareness of active and intelligent packaging results on a reduced acceptance of its effectiveness and safety. For example, certain ingredients may impart distinct odors or colors to the packaging, thereby impacting its marketability.
Although active and intelligent packaging labels still face challenges in terms of safety, reliability, and other problems, the continuous development of technology and increasing consumer awareness will make active packaging and smart tags an essential component of product packaging. They will also become a driving force for technology progress in the packaging industry, leading to new opportunities for profit growth. Its future development trends include the followings: Active packaging utilizing single or compound natural active components, especially those can come into direct contact with food, is readily embraced by consumers due to its enhanced food safety properties; An active intelligent packaging system, consisting of both active packaging and an intelligent label, is being developed. This integrated packaging not only effectively extends the shelf life of food and improves its quality but also independently indicate the freshness of packaged products and provides other valuable information. The deep integration of intelligent packaging labels, information technology, and smart living terminals is poised to become a new trend. With the gradual popularization of the 4G mobile network, cloud storage, big data platforms, and smart homes, package labels will not only serve as a means for preserving product information and preventing counterfeiting, but also provide interactive functions. An intelligent label with an information interaction function can transmit data on freshness, remaining storage period, packaging integrity and other relevant details to consumers' smart terminals such as mobile phones through receiving devices in storage areas. This timely reminder helps reduce food waste and economic losses.
-
With the improvement in living standards, the expansion of product logistics supply chains, and consumers' increasing attention to food quality and safety, higher demands have been placed on novel food packaging. This provides a broad market space for future application of active and intelligent packaging labels. If the packaging industry seizes the opportunity to move towards functionalization, intelligence, and information while continuously improving cost performance, quality reliability, and product safety in packaging, it will undoubtedly make a significant impact on technology upgrades within the industry.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Lv Y, Ai Y, Fang F, Liao H. 2023. Development of active packaging films utilized natural colorants derived from plants and their diverse applications in protein-rich food products. Food Innovation and Advances 2(3):203−216 doi: 10.48130/FIA-2023-0022
Development of active packaging films utilized natural colorants derived from plants and their diverse applications in protein-rich food products
- Received: 22 April 2023
- Accepted: 31 July 2023
- Published online: 29 August 2023
Abstract: With the increasing demand for environmentally friendly, safe, preservative and intelligent food packaging, there is a growing trend towards using plant-derived natural colorants that posses green, non-toxic, antioxidant, antibacterial, and pH-sensitive properties. As a result, the development of active intelligent packaging films containing plant-derived natural colorants has become a research priority in the realm of food packaging. As a novel packaging approach, it can serve as an active and intelligent packaging system to prolong shelf life and monitor food quality. On the basis of introducing several widely used natural colorants derived from plants, this review examines the preparation, structural characterization, physical properties, and functional aspects of these plant-derived pigments. The preparation procedures of various film forming substrates and natural pigment based films are also comprehensively discussed. Furthermore, the utilization of natural pigment-based films as active and intelligent packaging materials in food is discussed in depth, providing valuable insights into the future development of this cutting-edge research area.