-
Edible film as primary food packaging currently increasingly develops to meet the pursuit of convenient and pollution-free food packaging. Similar to common petro-based packaging, edible film can, to some extent, protect foods from physical, chemical and biological deterioration, enhancing products’ quality and extending their shelf life[1−3]. Collagen becomes an edible film-preparation material in the food industry on account of its excellent biocompatibility and scalability[4, 5]. For example, collagen casing is an exclusive edible protein film available commercially and is extensively used in the meat industry[6, 7].
To better enhance the property of the collagen film, a necessary procedure for production is crosslinking[8], which generally contacts physical and chemical treatments[7]. In the former, treatments such as dehydrothermal treatment (DHT) and ultraviolet (UV) irradiation, are usually believed safe and effective for bio-polymerization. In chemical treatments, common crosslinking agents such as glutaraldehyde (GA) and tannin, that can increase intermolecular crosslinks, are utilized to promote water resistance and enhance the antienzyme of collagen[9, 10]. Wang et al.[6] used UV, DHT and their combination to significantly increase tensile strength. Figueiró et al.[11] prepared GA crosslinked galactomannan–collagen films and evaluated properties. Liu et al.[12] aimed to provide a modified fish skin collagen film (MFCF) for adsorption of tannic acids.
To the best of our knowledge, although various crosslinking methods have been widely developed to prepare edible film, few researchers have studied their effects on nutrition property and edible safety of films. In order to investigate, it is necessary to study the complex digestive processes within the human digestive tract in more detail[13]. In recent years, the in vitro methods of simulating digestion process have already been developed for accurately simulating the complex physicochemical and physiological events in the human gastrointestinal tract[14], and a complete set of standardized protocols have been determined, based on an international consensus developed by the COST INFOGEST network[15], such as the studies on in vitro digestion of soy protein isolate film[16], gelatin film[17], among others. To date, the cell model has been commonly used to evaluate the nutritional performance and safety of food components. Lassé et al.[18] and Vors et al.[19] investigated toxicity and absorption of samples using cell models.
To understand the effect of cross-linking on trophism property and safety of edible films, this study investigated the changes in the physicochemical properties during digestion of the films which were processed by four cross-linking methods including UV irradiation, DHT, tannin treatment, and GA treatment by four analysis methods via dry matter loss, degree of hydrolysis (DH), high performance liquid chromatographic (HPLC) and Fourier transform infrared spectroscopy (FTIR). Plus, the cell model was established to test cytotoxicity and nutritional effects of collagen film.
-
Bovine skin splits were donated by Zibo Huanghelong Bioengineering Co., Ltd. (Zibo, China). Salivary amylase (1,500 U·g−1), pepsin (1:3000, from porcine gastric mucosa) and pancreatin (8× USP, from porcine pancreas) were obtained from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China) in a powder form.
Film preparation
-
Collagen fibers were prepared according to previous methods[20]. And intact collagen fibers were dispersed evenly in distilled water to form 1.0 wt% homogenous collagen slurry. Subsequently, the collagen fiber suspension (40 mL) was poured on a polyacrylic plate (12 cm × 12 cm) and then air-dried at 25 °C for 3 d. The newly formed films were stored in a desiccator (25 °C, 50% relative humidity (RH)).
Crosslinking treatments
-
The films were divided into five groups according different crosslinking treatments as follows: (1) the control film (untreated collagen film); (2) the UV-treated film, which was exposed to a UV lamp (TA 300 DC, NIDEC, JAPAN) at 365 nm with the irradiation intensity of 9.43 mW·cm−2[6]; (3) the DHT film, obtained by dehydrating film at 120 °C for 24 h in a drying oven; (4) the tannin-treated film, prepared by immersed in 5 wt% tannin solution for 12 h obtained after drying[21]; (5) the glutaraldehyde-treated film, achieved by steeping film in 1% glutaraldehyde solution for 24 h after drying. After crosslinking, films were again placed in the same desiccator for storage.
In vitro simulated digestion
-
The simulated digestion of collagen films and preparation of digestive juices were referred to a standardized static in vitro digestion method[22].
Oral and gastric digestion conditions
-
Briefly, the collagen films were immersed in PBS at 1 wt% concentration and then simulated saliva solution (SSF) was then 1:1 mixed in conical flasks and agitated gently. After 2 min, equal simulated gastric fluid (SGF) was added and conical flasks were put in a shaking incubator (HNY-100B, HONOUR, China) for 2 h at 37 °C. Finally, pepstatin was added to stop the digestion.
Intestinal digestion conditions
-
After digestion by the above method, the sample was mixed with simulated intestinal fluid (SIF) at a ratio of 1:1, and further incubated for 2 h. The digestion was terminated by pancreatin inhibitor (1 mM AEBSF).
Treatment after digestion
-
The mixture was centrifuged (J-26 XP, BECKMAN COULTER, USA) at 10,000 g for 30 min. Afterwards, supernatant was removed and stored at 4 °C. The centrifuged deposit was pre-frozen (−80 °C, 24 h) and later lyophilized in a freeze-drier (LGJ 0.5, Thermo Electron, USA). The powder was then stored in the desiccator (25 °C, 20% RH).
In vitro digestion estimation
Determination of dry matter loss
-
The dry matter of collagen films was weighed before and after digestion. The dry matter loss rate is calculated as follows:
${\rm{Rate\; of\; dry\; matter\; loss}} \,({\text%}) = \dfrac{{m}_{0}-{m}_{1}}{{m}_{0}}$ where
$ {m}_{0} $ $ {m}_{1} $ Measurement of the degree of hydrolysis
-
The DH value of the films is measured using the O-Phthalaldehyde (OPA) method[23]. After 10 min reaction of supernatant and OPA/NAC reagent, the absorbance of the mixture was measured at 340 nm with an ultraviolet spectrophotometer (TU-1810, FUSE TYPE T, China). DH value was calculated as using the following formula[24]:
$ DH{\text%}=\frac{n}{N}\times 100 $ Where
$ n $ $ N $ $ n=\frac{({A}_{1}-{A}_{0})\times M\times d}{\epsilon \times \rho } $ Where
$ {A}_{1} $ $ {A}_{0} $ $ M $ $ d $ $ \epsilon $ $ \rho $ Liquid chromatographic determination of hydroxyproline
-
Amino acid derivatization was performed prior to HPLC analysis[25]. Glacial acetic acid- sodium acetate buffer (pH 4.3) and acetonitrile was used to gradient elute with 66:34 (by volume). The wavelength was 265 nm with the flow rate of 1.0 mL·min−1 and sample injections were made every 30 min.
Fourier transform infrared spectroscopy (FTIR)
-
According to a previously published method[26], the FTIR spectra of collagen films and digested freeze-dried powder were respective obtained by FTIR spectrometer (VECTOR 22, Bruker Co., USA) attenuated total reflectance (ATR) mode and the DTGS KBr detector (Thermo, Shanghai, China). Each sample was subjected to 32 scans from 4,000 cm−1 to 400 cm−1.
Cytotoxicity assays
Caco-2 cell line
-
To test the toxicity of crosslinking collagen films to Caco-2 cell lines, 2.0 × 104 cells per well were plated into a 96-well plate and cultured 24 h at 37 °C. The digestion juice with collagen final concentrations of 0.125, 0.25, 0.625, 1.25, 2.5 mg·mL−1, was added and incubated for 24 h. Then 20 μL of the MTT (5 mg·mL−1) was added directly and incubated away from light. After 4 h, 150 μL mixture was sucked out and equal DMSO was added. The plate was shaken for 10 min and then the absorption value was measured at 490 nm with a multi-mode microplate reader (Model 680, BioRad, USA). Untreated cells and cell death inducing 10% DMSO were used as negative and positive controls respectively.
HepG2 cell line
-
The nutritional properties of the film hydrolytes were further estimated with metabolically active HepG2 cell line. The experimental conditions and methods are the same as the above except that cells need to be pre-incubated overnight in the absence of FBS. After that, digestion juice with 0.25 mg·mL−1 collagen was added and incubated for 24 h. Untreated cells (medium without FBS) was used as negative control and the treated cells with complete medium was used as positive control.
Statistical analysis
-
Statistical analysis was performed using the SPSS 24.0 (SPSS for windows, Chicago, IL, USA). Data were analyzed using one-way analysis of variance (ANOVA) at the significance level of 0.05 and Pearson correlation coefficients at the significance level of 0.01. All the results presented in this study were the mean values with standard deviations.
-
Using the INFOGEST method, all film samples were subjected to sequential oral, gastric and intestinal digestion. In view of the poor digestion during the oral stage, only the data of the latter two stages were collected and estimated. Dry matter loss referred to the total content of dissolving and hydrolysis of insoluble collagen during each digestion stage. As shown in Fig. 1a, during gastric digestion, the dry matter loss of UV treated film and DHT film were considerably higher than that of the control film, owing to partial denaturation of collagen. Denaturation-induced crosslinking and degradation coexisted under these physical treatments[27]. Obviously, the positive role of degradation overcame the negative crosslinking in this stage. The glutaraldehyde-treated film had the lowest value of dry matter loss (23.27%), due to irreversible crosslinking.
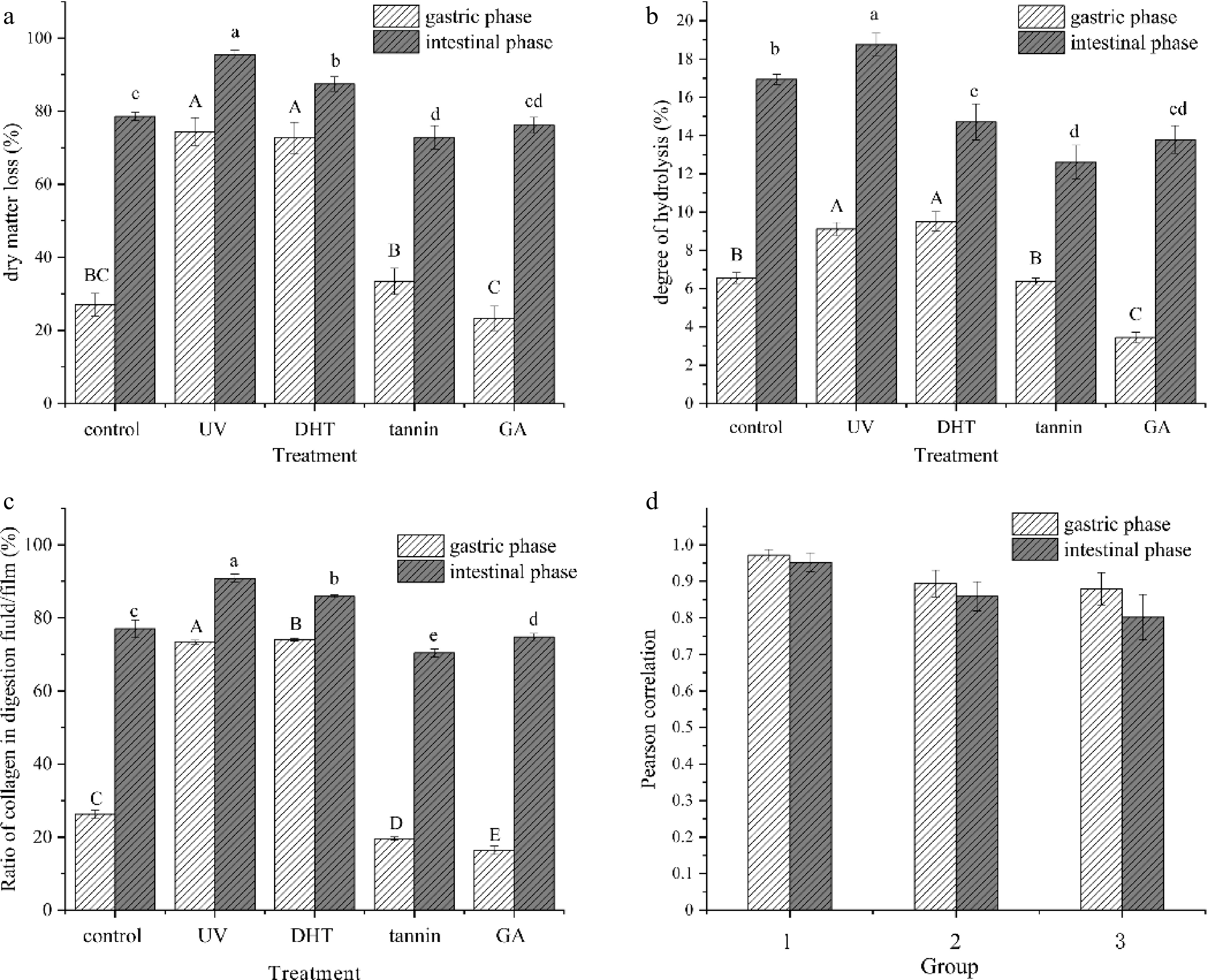
Figure 1.
(a) Dry matter loss, (b) degree of hydrolysis and (c) ratio of collagen in digestion fluid/film of samples in gastric and intestinal. The relations between (a), (b) and (c) were determined by (d) Pearson's correlation coefficient. Control: untreated collagen film; UV: UV-treated film; DHT: film treated with dehydration; Tannin: tannin-treated film; GA: glutaraldehyde-treated film.
After the intestinal phase, the dry matter loss of all films increased expectedly with a relatively narrow range compared with the gastric phase, due to long immersion and strong enzymatic hydrolysis. Among them, the UV treated and DHT films still showed higher values. It was noted that tannin treated film showed a lower value in dry matter loss compared with that of the control. This was presumably due to the effect of tannin on digestive enzymes and collagen molecule configuration which could reduce the digestibility of proteins[28].
Overall, after gastric and intestinal digestion, all treated films showed a relative high dry matter loss. Especially UV treated or DHT film had a higher value. A steep increase of loss of the other three films during the intestinal stage suggested the efficient supplement of intestinal digestion closely associated with its strong hydrolysis.
Degree of hydrolysis
-
The degree of hydrolysis was estimated by the content of free amino acid in the digested films in this study (Fig. 1b). The changes in DH value showed a similar trend as the dry matter loss, while the DH value was lower than dry matter loss during each stage. This indicated that dissolved collagen was not completely hydrolyzed.
Especially, DH of the DHT films was higher in the gastric phase, and lower in the intestinal phase than that of the control, indicating different digestive mechanisms of the collagen. A likely explanation was that lipase in pancreatin preferentially acted on the ester bonds thereby reducing the effect of amide bonds. Compared with the control, a lowed DH value was observed in tannin-treated films after digestion (p < 0.05), further proving that tannin crosslinking could reduce the digestibility of collagen.
HPLC analysis
-
Hydroxyproline is a specific amino acid for collagen, and its content is relatively stable (accounting for 13.4%[29]). Consequently, the determination of hydroxyproline concentration was widely accepted as a method of quantifying the collagen content[30]. Herein, the ratio of collagen in dissolved and hydrolyzed collagen in the gastrointestinal tract were determined (Fig. 1c).
The results were also consistent with the tendency of dry matter loss (Fig. 1a), except the tannin film during the gastric phase, in which the film revealed a lower value than its dry matter loss. A possible explanation was that tannin might react with proteases such as pepsin and then decrease enzyme activity[31]. Another reason is that tannin had a preferential action on collagen, which decreased the collagen’s acid solubility ratio, while the impurity and amorphous part (less hydroxyproline content) were unaffected.
The relations between dry matter loss, degree of protein hydrolysis and absorption of collagen were all positively correlated (p < 0.01), based on Pearson's correlation coefficient. Figure. 1d summarized, that in collagen films, the ratio of dissolved and/or hydrolyzed collagen were highly correlated with the dry matter (p < 0.01), especially in the gastric phase (r = 0.972). Additionally, the degree of protein hydrolysis also affected the absorbability of collagen and dry matter loss (p < 0.01). It showed that the higher the degree of hydrolysis, the easier the structure of collagen was destroyed or even absorbed, and it led to an increase in dry matter loss.
FTIR analysis
-
The structural changes of collagen film at the molecular level during gastrointestinal tract digestion spectroscopy can be shown by FTIR[6]. During digestion, pepsin and pancreatin mainly destroyed the covalent bonds in collagen and this kind of conformational changes often induced secondary structure transitions[32].
As shown in Fig. 2a, after cross-linking, the collagen film’s peak intensity of amide B was significantly reduced as shown in Table 1, crosslinking changes the contents of the α-helix and β-turn in digestion. However, the secondary structure destruction of glutaraldehyde film after digestion was not consistent with the other three, indicating that different cross-linking changed the structure of collagen to different degrees and thus affect digestion. It was noted that the structure of β-sheets of all collagen films was mainly destroyed in digestion, as shown in Table 1. In particular, the random coil of the collagen film from UV treated, DHT and tannin treated were completely digested in the gastric phase while the content increased after intestinal phase. One possible explanation was that all random coil was absorbed completely in the gastric phase, then the rest of the ordered structure was destroyed in the intestinal phase leading to an increase of disordered structure. The peak intensity of amide A increased after gastric digestion and decreased after intestinal digestion and after the intestinal phase, the peak shape of the amide I band changed. These further supported the previous speculation.
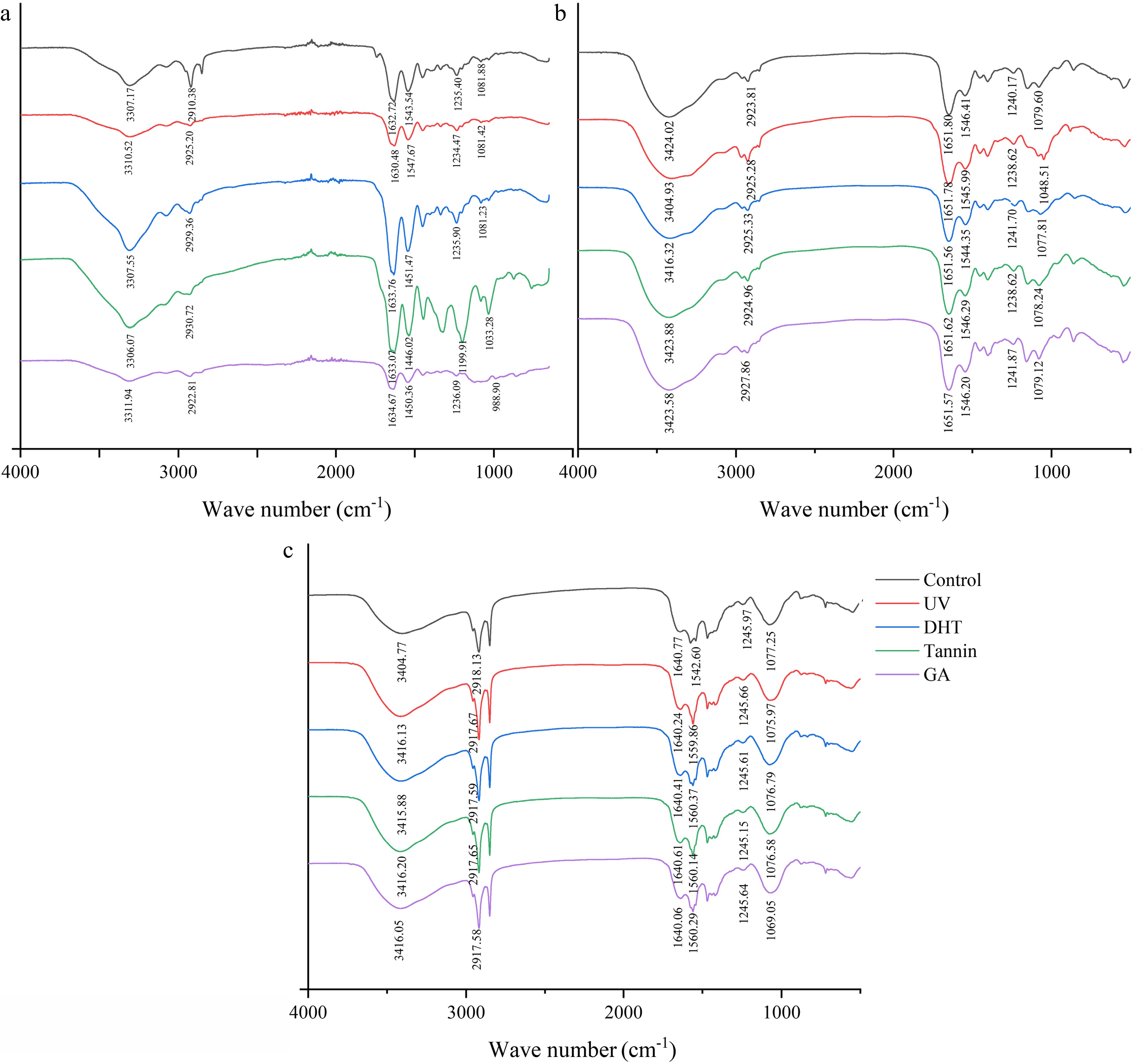
Figure 2.
The FTIR spectra of (a) collagen films, (b) freeze-dried powder after gastric digestion and (c) freeze-dried powder after intestinal digestion.
Table 1. The secondary structure analysis of amides I region obtained by deconvolution.
Treatment α-helix β-sheets β-turn Random coil Film Control 23.92% 11.5% 9.32% 12.01% UV 22.82% 19.01% 7.75% 23.37% DHT 12.85% 16.85% 8.10% 22.79% Tannin 11.75% 23.76% 6.75% 23.22% GA 11.53% 20.66% 7.99% 22.56% Gastric Control 18.27% 30.70% 12.24% 6.02% UV 24.75% 32.96% 6.51% — DHT 21.97% 33.69% 7.97% — Tannin 24.32% 32.98% 5.64% — GA 10.43% 30.09% 6.54% 17.98% Intestinal Control 19.49% 43.21% 2.99% 11.26% UV 17.52% 40.39% 5.33% 9.05% DHT 19.32% 41.13% 5.57% 10.24% Tannin 18.56% 46.91% 1.74% 9.57% GA 10.39% 38.44% 7.84% 13.11% Cell toxicity
-
Caco-2 cells were treated with digested juices of different crosslinking methods to study the effect of collagen films after digestion on cell viability (Fig. 3). All treated films were significantly higher (p < 0.05) than the toxic 10% DMSO control. Importantly, only the glutaraldehyde-treated collagen film digestion fluid was cytotoxic. When the concentration was 2.5 mg·mL−1, the cell survival rate was the lowest (73.13%). It might be attributed to the residual of GA. This was further identified by the results that the four commercial collagen casing didn't show obvious negative effect on cell viability (data not shown). When GA was cross-linked as a processing aid, it was removed completely through a set of procedure until no GA residual was detected in the final product. However, with the increase of concentration, the other collagen films slightly promoted Caco-2 cell growth. When the concentration up to 2.5 mg·ml−1, the survival rate of cells significantly reduced (p < 0.05), which is possibly due to the high concentration[33].
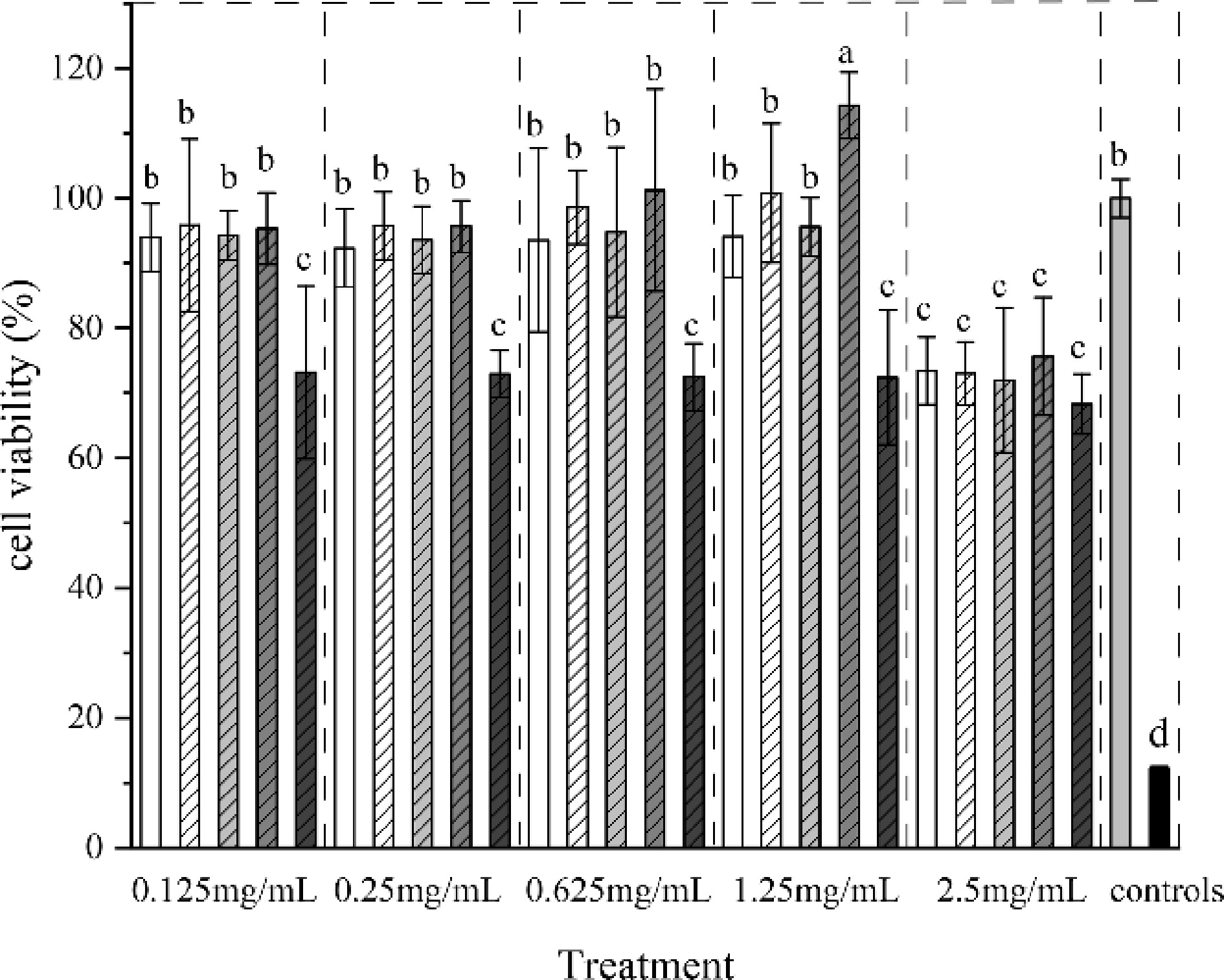
Figure 3.
Cell viability of digested juices of collagen film treated with different crosslinking methods.
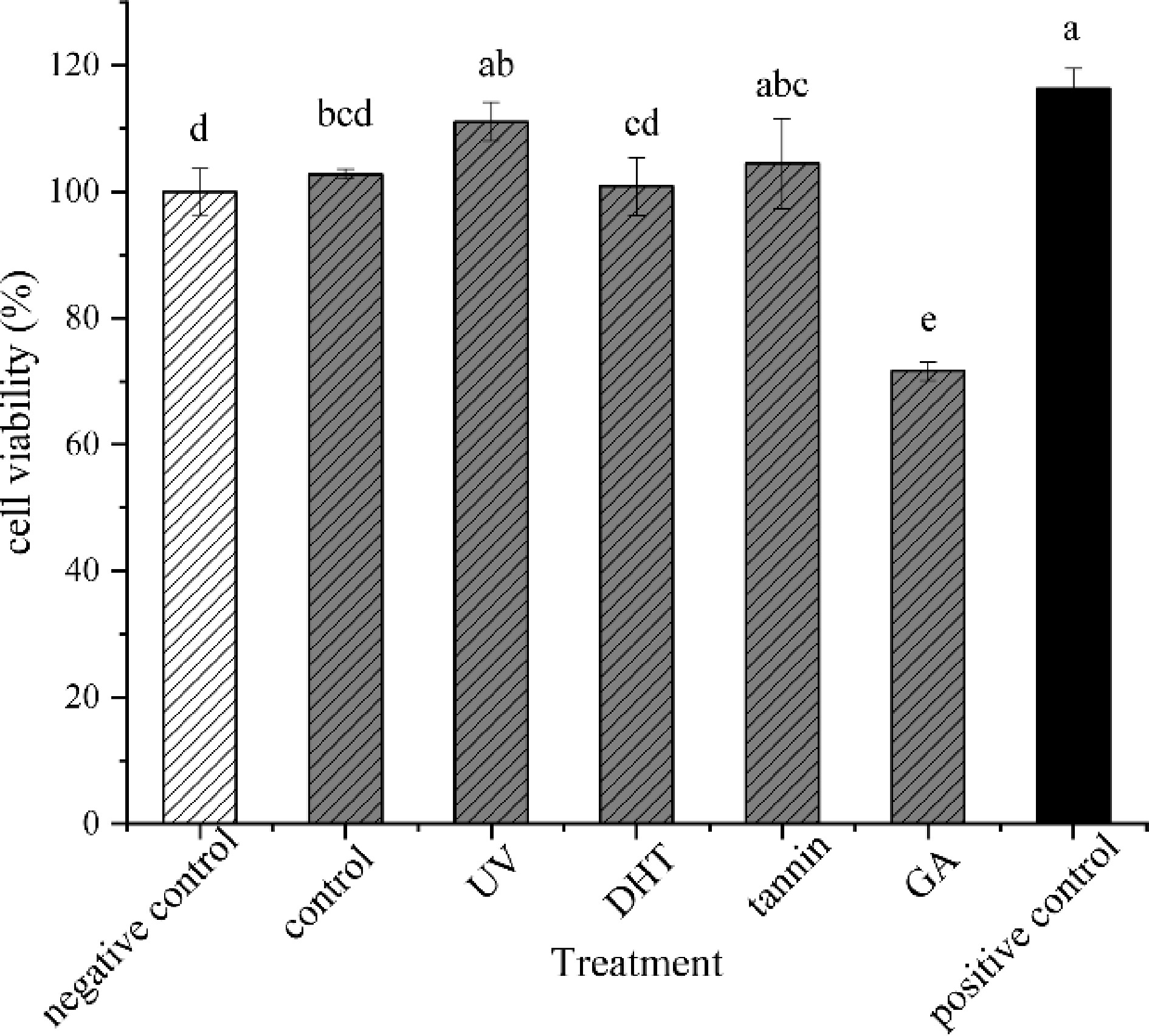
To explore whether collagen films can provide nutrients as a source to promote cell growth, a further study was carried out. Giving its great need of nutritional substance, HepG2 cells were used as a model. Cells were cultured overnight under FBS-free conditions (protein starved) in order to remove the nutritional effects of the FBS to understand whether the collagen films had the function of being the protein supplementation of the cells. The results revealed that all samples could provide nutrition for cells (Fig. 4). Especially UV and tannin-treated films which had no significant difference from the positive control (p > 0.05). It benefited from the higher DH of UV-treated collagen film after digestion, which was more easily absorbed by cells. And in lower concentrations, tannins could be used for the purpose of accelerated recovery of cells[34]. It could be noticed, that although several treated collagen films provided nutritional effects, it was less but still promote cell growth slowly. Overall, various collagen film treatments had significant effects on cell growth (p < 0.05) except the one treated with GA treated, suggesting that different cross-linking treatments had a certain effect on the bio-availability of collagen.
-
In order to estimate the effect of crosslinking on the nutritional and safety of collagen film after digestion, in vitro digestion and cell culture models were adopted. The results of dry matter loss, degree of hydrolysis and absorption of collagen had positive correlation (p < 0.01). Therein, UV treatment and DHT had high digestive performances while tannin and GA were the opposite. The results of FTIR likely showed that crosslinking affects the structure changes in digestion and absorption and pepsin could mainly cleave the disordered structure of collagen.
Only glutaraldehyde treated film reduced cell viability to 73.13%, due to the incomplete removal of glutaraldehyde. The others had no negative effect and could even provide nutrition for HepG2 cells under starvation conditions, especially that UV and tannin treated films possess excellent nutritional properties. Therefore, except for glutaraldehyde, the other crosslinking methods could be used as safe approaches to promote film properties in terms of cell viability tests. These treatments had somewhat positive or negative effects on digestion.
This work was supported by the National Nature Science Foundation of China (32102052, 32172249), Special project of Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-KJGG-004).
-
The authors declare that they have no conflict of interest.
-
These authors contributed equally: Yinglu Zhang, Kaixuan Zhao
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang Y, Zhao K, Guo Y, Teng A, Li S, et al. 2023. Effects of physical crosslinking methods on digestibility in vitro and safety of edible packaging: A primary study on collagen films. Food Materials Research 3:4 doi: 10.48130/FMR-2023-0004
Effects of physical crosslinking methods on digestibility in vitro and safety of edible packaging: A primary study on collagen films
- Received: 12 November 2022
- Accepted: 24 February 2023
- Published online: 17 March 2023
Abstract: Crosslinking is often used to improve the mechanical strength and barrier properties of edible films. However, the effects on films’ digestibility and edible safety are still unknown. Herein, four crosslinking methods were utilized to crosslink collagen films, including UV irradiation, dehydrothermal treatment (DHT), tannin and glutaraldehyde. The crosslinked samples were then researched via in vitro digestion and cell culture model. With the INFOGEST method, the results of dry matter loss, degree of hydrolysis and absorbed collagen content have significantly correlated relations and showed excellent digestive ability of UV treated and DHT films. The results of the FTIR suggest that the crosslinking affects the structure of digestion and absorption. The toxicity and nutrition of the hydrolytes of collagen films were measured in Caco-2 and HepG-2 cells, and the collagen films, especially UV treated and tannin treated films, can promote cell growth while the glutaraldehyde treated one reduced cell viability presumably due to its residual in the films.
-
Key words:
- Collagen /
- Edible films /
- Crosslinking /
- in vitro digestion /
- Nutrition /
- Safety