-
Polyploidy is widespread in plants for driving plant evolution, adaptation and biodiversity[1]. At least 50% of angiosperms are polyploid[2], including many important cultivated fruit crops, for instance, kiwifruit (hexaploid)[3], banana (triploid)[4] and strawberry (octoploid)[5]. Among fruit crops, most polyploid plants have properties of large fruit size, good quality and stronger disease resistance[6,7]. Most of the extant polyploid plants were selected from spontaneous mutations in seedlings or clones, with a low mutation rate of 0.3%[8,9]. Artificial induction of polyploid breeding is an efficient strategy to accelerate the breeding process. Polyploid induction efficiency depends on several factors, including materials types, the types and concentration of chemical inducer, and induction time[10,11]. At present, colchicine is the most widely applied and effective chemical inducer to induce polyploidy[12], and it has been successfully applied in many fruit trees as the materials of explants, such as apple[13], banana[14], pomegranate[15] and citrus[16].
Pear belongs to the genus Pyrus in the family of Rosaceae, which is also one of the most economically important fruit crops cultivated in more than 50 countries[17]. Pear fruit is a favorite of consumers for its delicious taste. Since 1982, the chromosome numbers of more than 400 pear cultivars have been observed, and more than 20 polyploid cultivars have been found[18,19]. Compared to diploid plants, tetraploid pear cultivars have larger fruits and most of them are highly productive. Breeding high-yielding pear cultivars is a major goal of pear breeding. Like most fruit trees, pear polyploid breeding is also mainly achieved by explants[20]. Currently, explants of the pear cultivar of 'Fertility' and 'Hosui' have been reported to induce tetraploid pear plants by the treatment of colchicine[20,21]. Compared with explants, the use of seeds as the induced materials have the advantage of producing multi-genotypic homozygous polyploids with multiple heterozygosity and shorter time to obtain polyploidy[22−24]. Recent studies have shown that the tetraploids were successfully obtained from the diploid wild species 'Duli' pear (Pyrus betulaefolia) by chemical mutagenesis[25]. However, the induction efficiency of cultivated species pear polyploidy through the seeds treated with colchicine remain largely unknown.
'Dangshansuli' (Pyrus bretschneideri, 2n = 34), with an annual yield of more than 4 million tons, is the most important ancient local commercial pear cultivar, and it has been cultivated in China for more than 500 years[26]. To establish an effective method for inducing polyploid plants of pear, the seeds from the mature fruits of 'Dangshansuli' were firstly removed of the seed coat, and then treated with colchicine at different concentrations for different temporal gradients. Finally, the chromosome ploidy level of all the germinated seeds was detected by flow cytometry. In order to select abundant variants for domestication and further breeding programs, we also compared the physiological indices of diploids and tetraploids after transplanting.
-
'Dangshansuli' was obtained from the orchard in Gaoyou, Jiangsu, China. All pear trees were managed according to standard horticultural practices (fertilization, pruning, irrigation, and pest and disease control). All mature fruits were picked and transported promptly to the laboratory. Then, all pear seeds were collected.
Polyploidy induction
-
After manually removing the seed coat, the seed kernels were placed in plastic petri dishes with a wet filter paper at the bottom. Each petri dish contained 50 seeds. The plant materials were incubated at 25 ± 1 °C, under a 16/8 h (light/dark) photoperiod. As shown in Fig. 1, the seeds with the seed coat removed were immersed in colchicine solution at final concentrations of 0.1%, 0.5% and 1% (w/v) for 12 h, 24 h and 48 h, respectively. The control groups were treated with sterile distilled water. The seeds were then washed five times with water and cultured on plastic petri dishes for 13 d. Plants were transplanted into mixed soil (organic matter: vermiculite = 1: 1) for subsequent experiments.
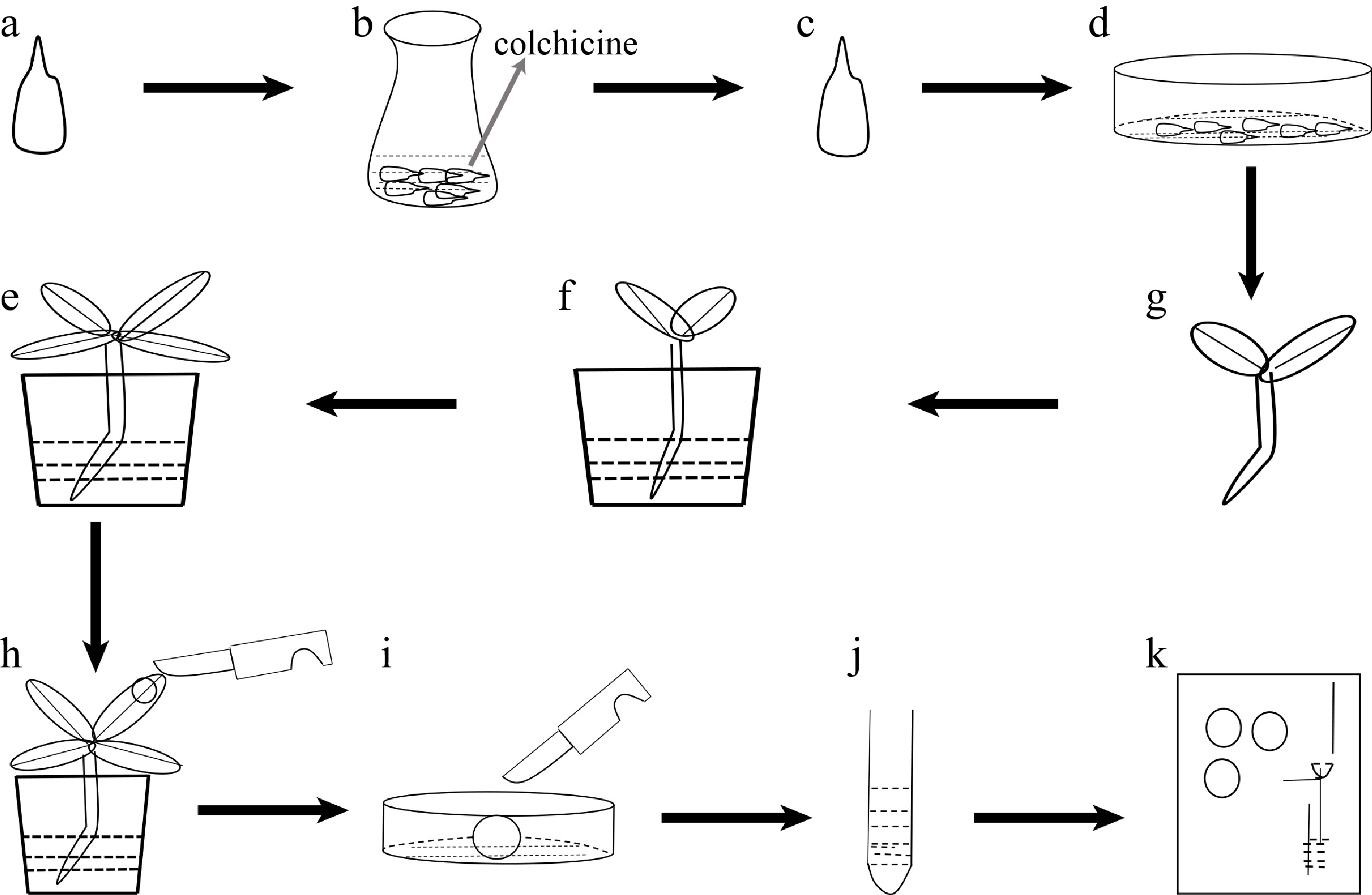
Figure 1.
Flow-process diagram of inducing polyploidy from pear seeds treated with colchicine. (a) Pear seeds; (b) pear seeds soaking in colchicine solution; (c) seeds treated with colchicine solution; (d) seeds spread in plastic Petri dishes; (e) seedlings; (f) seedlings transplanted into soil; (g) plant growth; (h) collected leaf samples; (i) chopped leaves; (j) samples to be tested after centrifugation; (k) flow cytometry assay.
Flow cytometry analysis
-
Samples of 100 mg fresh leaves were collected, putting them into a glass Petri dish. Then, we added 1 ml of lysate, and chopped the leaves within 2 min. The mixture was filtered into a 2 ml centrifuge tube with a 300 mesh nylon filter sieve and centrifuged at 5,000 rpm for 4 min. One hundred μl of supernatant was obtained, 400 μl of lysate added, then vortexed and mixed, centrifugation was carried out once. Finally, we added 200 μl lysate, 500 μl Na2HPO4 solution, 50 μl RNaseA and 70 μl staining solution to avoid light staining for 5 min, and the ploidy level was detected by flow cytometry (CytoFLEX, Beckman Coulter, Inc., USA).
Investigation of germination rate
-
The germination rate of investigated plants was based on the root growing up to 1 cm, and the germination rate of control and induced plants was calculated respectively. The formula was as follows:
${\rm{ Germination\; rate}}\; ({\text%}) ={\rm{a/b}} \times 100{\text%} $ where, a is the number of seeds with roots exceeding 1 cm and b is the number of all seeds.
Observation of leaf morphological characteristics
-
From the 3rd to 5th leaves at the base of the plant, the length and width of the leaves were measured with a steel ruler and the leaf index was calculated. The formula was as follows:
leaf index = leaf length/leaf width
Observation of stomatal characteristics
-
The upper epidermal leaf flesh of the fresh leaves placed on a clean slide were immediately scraped away with a one-sided blade, and the thin transparent lower epidermis was covered with a coverslip. Stomata were observed with an Olympus inverted fluorescence microscope (OLYMPUS PH0054, Tokyo, Japan). At least 10 view fields were assessed for each sample under 16 × 40 lense for counting stomatal density and measuring length, width and length-width ratio of guard cells.
Determination of chlorophyll content
-
Three pear leaves in the same position from each plant were collected and cleaned of surface dirt. Then, 0.1 g leaves from both sides of the main vein were collected, and cut into pieces and placed in a 10 ml centrifuge tube, 2 ml of 85% ethanol solution was added, and wrapped with tinfoil. When the material completely turned white after 24 h, the chlorophyll content was tested using a Microplate Reader (Cytation3, BioTek, USA) and calculated using the formula from Arnon[27].
Electron microscopy
-
Electron microscopy was performed as described previously[28]. Leaf pieces (4 × 4 mm) from one side of the main vein for each plant were excised using a blade, fixed in 2.5% glutaraldehyde. The dehydrated samples were attached to a sample stage with conductive tape and coated with gold particles using a Hitachi E-1045. The coated samples were examined using a Hitachi 4800 field emission scanning electron microscope.
Determination of cuticular wax
-
The surface area of pear leaf was quantified by ImageJ software[29]. Pear leaves cuticular wax was extracted according to the method of Li et al.[30]. Leaves were soaked in chloroform for 1 min under a fume hood. The wax-containing solvent was transferred to a vial, blow dried with a nitrogen blowing instrument (JHD001S, Shanghai Jiheng Industrial Co., Ltd., Shanghai, China).
The components in the wax extracts were analyzed using the method of Wu et al [31]. The extracted samples dissolved with 1.2 mL chloroform were detected by gas chromatography-mass spectrometry (Bruker 450-GC, Bruker 320-MS) and a column (BR-5MS, 30 × 0.25 × 0.25). The carrier gas was helium at a flow rate of 1.2 mL/min. The parameters were as follows: inlet temperature, 280 °C; MS transfer line temperature, 280 °C; ion source temperature, 250 °C; quadrupole temperature, 150 °C; EI, 70 eV; scanning range, 50−650 m/z.
The temperature increase procedure was as follows: initally50 °C for 2 min. Next, the temperature was increased to 200 °C at a rate of 40 °C/min for 2 min. Finally, the temperature was increased to 320 °C at a rate of 3 °C/min and held for 30 min.
Statistical analysis
-
The chemical composition of cuticular wax was analyzed using the NIST 2013 Library. The cluster relationships of the wax composition data were determined via principal component analysis (PCA). Significance of the samples was tested using T-test. Data analysis was carried out using SPSS Statistics 25.0 and Microsoft Excel 2016. The data is shown as the means ± SD.
-
The process of inducing polyploidy of pear using seeds could be divided into the following four steps: 1) use colchicine to soak 'Dangshansuli' seeds with seed coat removed (Fig. 1a, b); 2) grow the treated seeds in a Petri dish with wet filter paper at the bottom (Fig. 1c, d); 3) transplant the seedings into the soil (Fig. 1e, f); 4) identify the ploidy of plants (Fig. 1g−k). To find not only the most suitable concentration of colchicine but also the suitable time for the treatment of the seeds, nine experimental groups (0.1% colchicine for 12 h, 24 h, 48 h; 0.5% colchicine for 12 h, 24 h, 48 h; 1% colchicine for 12 h, 24 h, 48 h) were set up, and we found that the most effective induction treatment was 0.1% colchicine for 12 h (tetraploid rate, 6.0%) (Table 1). In addition, we also observed that the survival rate of the colchicine treated seed was significantly lower than that of the control groups. As shown in Fig. 2, all seeds in the control groups survived, and the seeds were germinated and the length of the radicle was rapidly extended within 10 d (Fig. 2a). On the contrary, the germination rate of seeds was significantly decreased (0%−46%) and the radicle was extremely short (Fig. 2e) in the treated groups. The germination rate was only 8% and the radicle was difficult to develop into taproot after treating with 1% colchicine for 24 h (Fig. 2d). When treated with 0.5% and 1.0% colchicine for 12 h, 24 h and 48 h, respectively, compared to the control group, the plant height and the number of true leaves decreased by 56.00%−80.80% and 43.75%−70.88%, respectively (Table 1). The most significant phenotype was obtained by treating with 0.5% colchicine for 48 h, with the shortest plant (1.20 cm) and the lowest true leaf number (2.33) (Table 1). This result indicates that the inhibitory effect of colchicine was not proportional to the concentration and duration of colchicine.
Table 1. Mutagenic effect of colchicine concentration and treatment time on ‘Dangshansuli’ seeds with seed coat removed.
Concentration
(%)Number
of seedsTime
(h)Survival
rate (%)Plant
height/cmTrue leaf
numberNumber of tetraploid Tetraploid
rate (%)Control 50 48 86 6.25 ± 2.45a 8.00 ± 1.26a 0 0 0.1 50 12 28 5.20 ± 1.94a 5.83 ± 1.17b 3 6 50 24 40 6.65 ± 2.22a 7.83 ± 0.98a 0 0 50 48 16 3.28 ± 2.30b 4.00 ± 1.41cd 2 4 0.5 50 12 24 1.72 ± 0.42b 3.83 ± 1.17cd 1 2 50 24 8 2.75 ± 0.96b 4.50 ± 1.29c 1 2 50 48 4 1.20 ± 0.20b 2.33 ± 0.58e 0 0 1 50 12 18 1.38 ± 0.32b 3.33 ± 0.82cde 2 4 50 24 8 2.23 ± 1.21b 3.00 ± 1.41de 1 2 50 48 0 0 0 0 0 Note: Statistical significance was determined using Student's t-test. Different letters indicate significant differences at the 5% level. Control: control group treated with water solution. 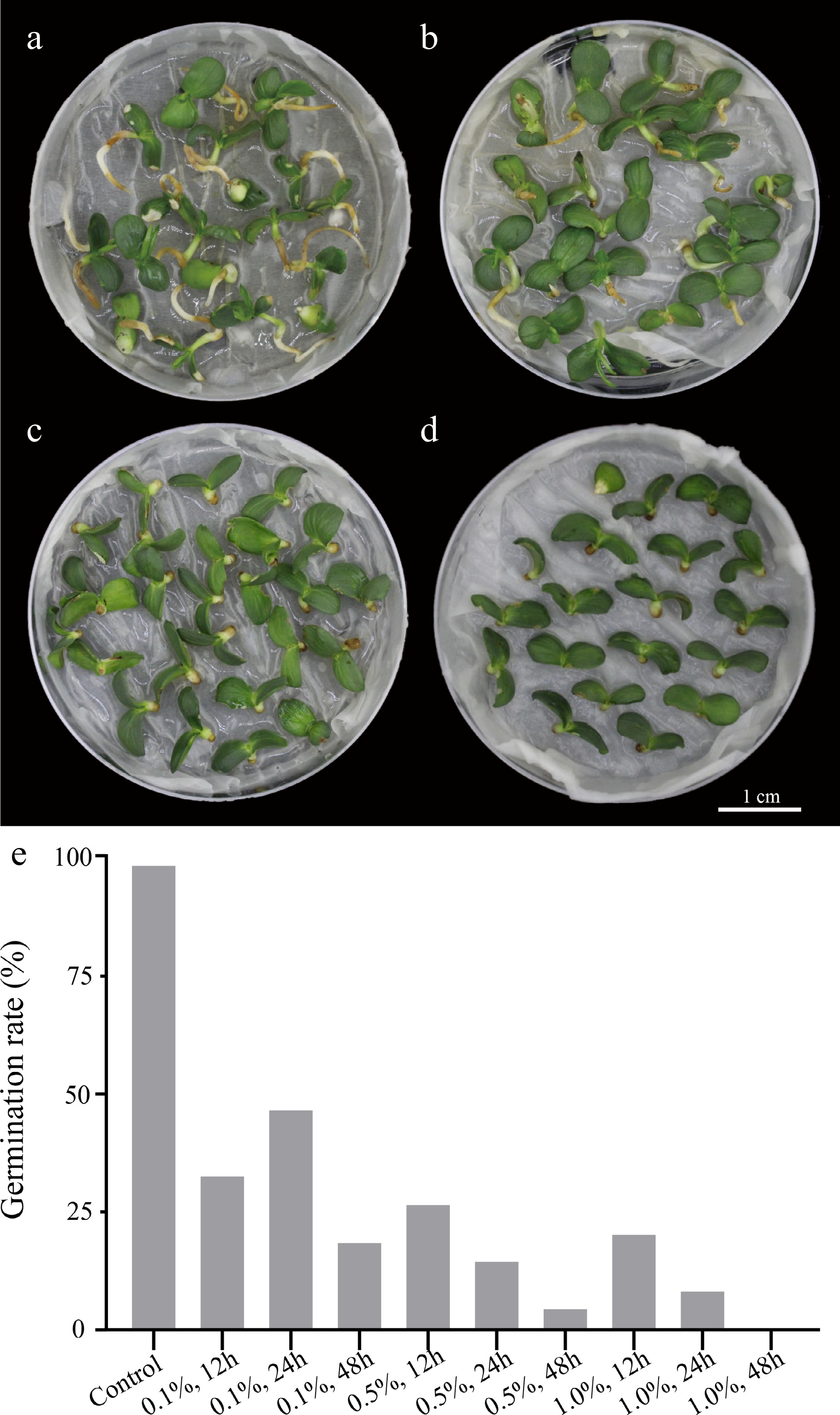
Figure 2.
Seed germination performance of Dangshansuli’ in control and colchicine treatment groups. (a) Control group treated with water for 24 h; (b) 0.1% colchicine treatment for 24 h; (c) 0.5% colchicine treatment for 24 h; (d) 1.0% colchicine treatment for 24 h; (e) germination rate of 'Dangshansuli' seeds treated with colchicine at different concentrations and times.
Ploidy identification
-
To identify the chromosome ploidy of each plant, we examined the peaks at different fluorescence intensities by flow cytometry (Table 1). The result showed that diploid plants only had a single peak at fluorescence intensity 200, whereas 10 plants treated with colchicine had a single peak at fluorescence intensity 400, indicating that colchicine can induce chromosome doubling of pear seeds, and these 10 plants should be tetraploid plants (Fig. 3a−d, Table 1).
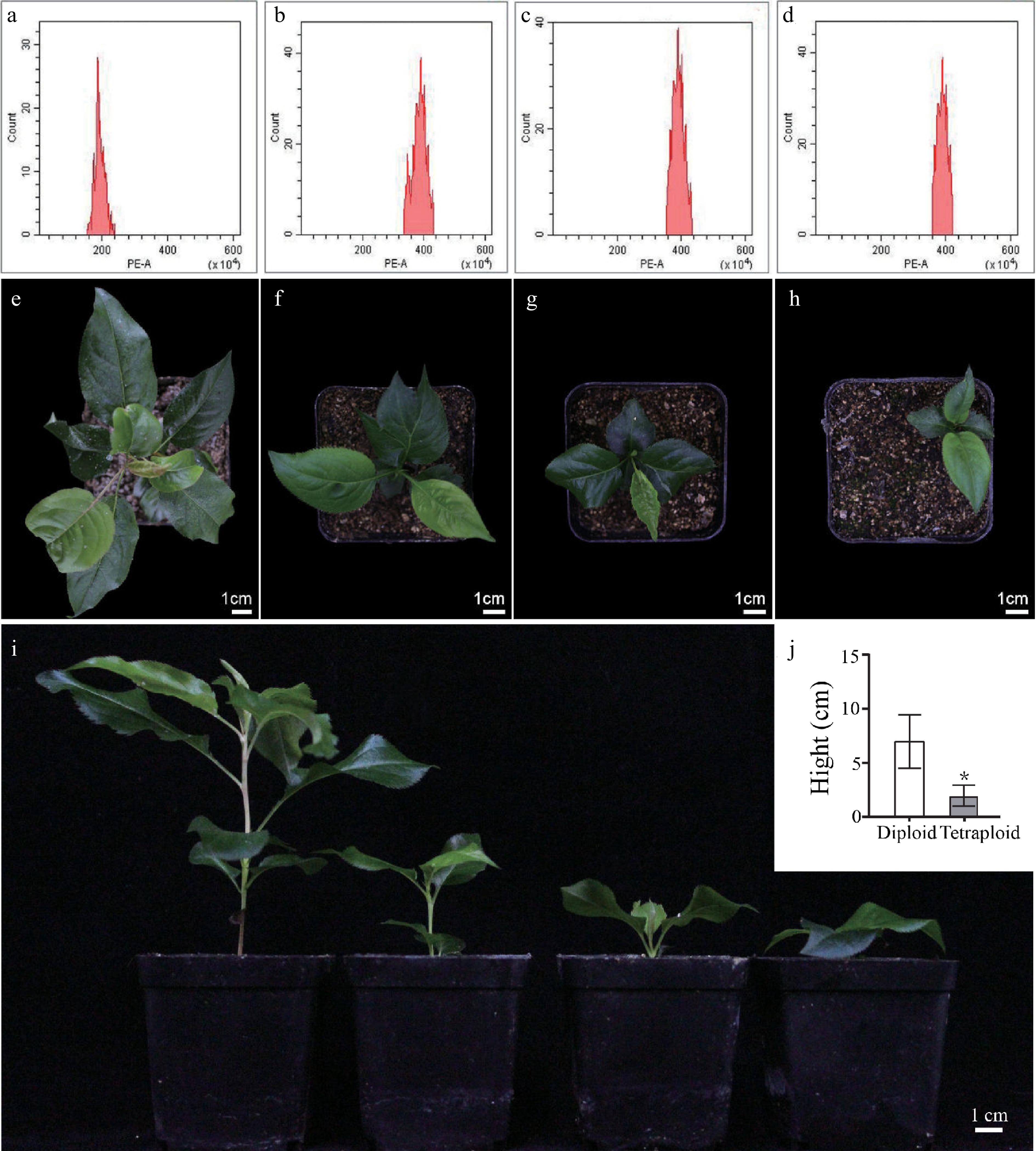
Figure 3.
Analysis of ploidy levels and growth morphology of 'Dangshansuli' pear. Flow cytometry histograms for (a) diploid and (b), (c), (d) tetraploids. Top view of phenotype of (e) diploid plants and (f), (g), (h) tetraploid plants planted in soil for 60 d. (i) Lateral view of phenotype of diploid plants (first from the left) and tetraploid plants (second to fourth from the left) planted in soil for 60 d. (j) Plant height in diploid and tetraploid 'Dangshansuli' seedlings.
Morphological characteristics of leaf and stomata
-
It has been reported that the induced tetraploid could change the morphological characteristics of leaves[32]. Here, we also detected the differences of morphological characteristics of leaves and stomata between diploid and tetraploid 'Dangshansuli' seedlings. Our results showed that the size of tetraploid 'Dangshansuli' seedlings were significantly smaller than that of diploid seedlings (Fig. 3e−i). Compared to diploid plants, tetraploid plants have shorter stem nodes, fewer number of true leaves and slower growth (Fig. 3i, j). The stomata of tetraploid 'Dangshansuli' seedlings were larger than those of diploid (Fig. 4a−d). The stomata length, width and length to width ratio increased by 90.43%, 48.15% and 29.43% in the induced tetraploids (Fig. 4e, f), which indicating that the guard cells in tetraploid 'Dangshansuli' seedlings are longer and narrower in appearance (Fig. 4a−d). In contrast, stomatal density, leaf length, leaf width and leaf shape index of tetraploid 'Dangshansuli' seedlings were reduced by 67.86%, 50.30%, 38.55% and 19.80% (Fig. 4g, h, i). Specifically, the leaf shape of tetraploid plants is oval with leaf shape index of 1.6, and the leaf shape of diploid plants are ellipse with a leaf shape index of 2.1 (Fig. 4i).
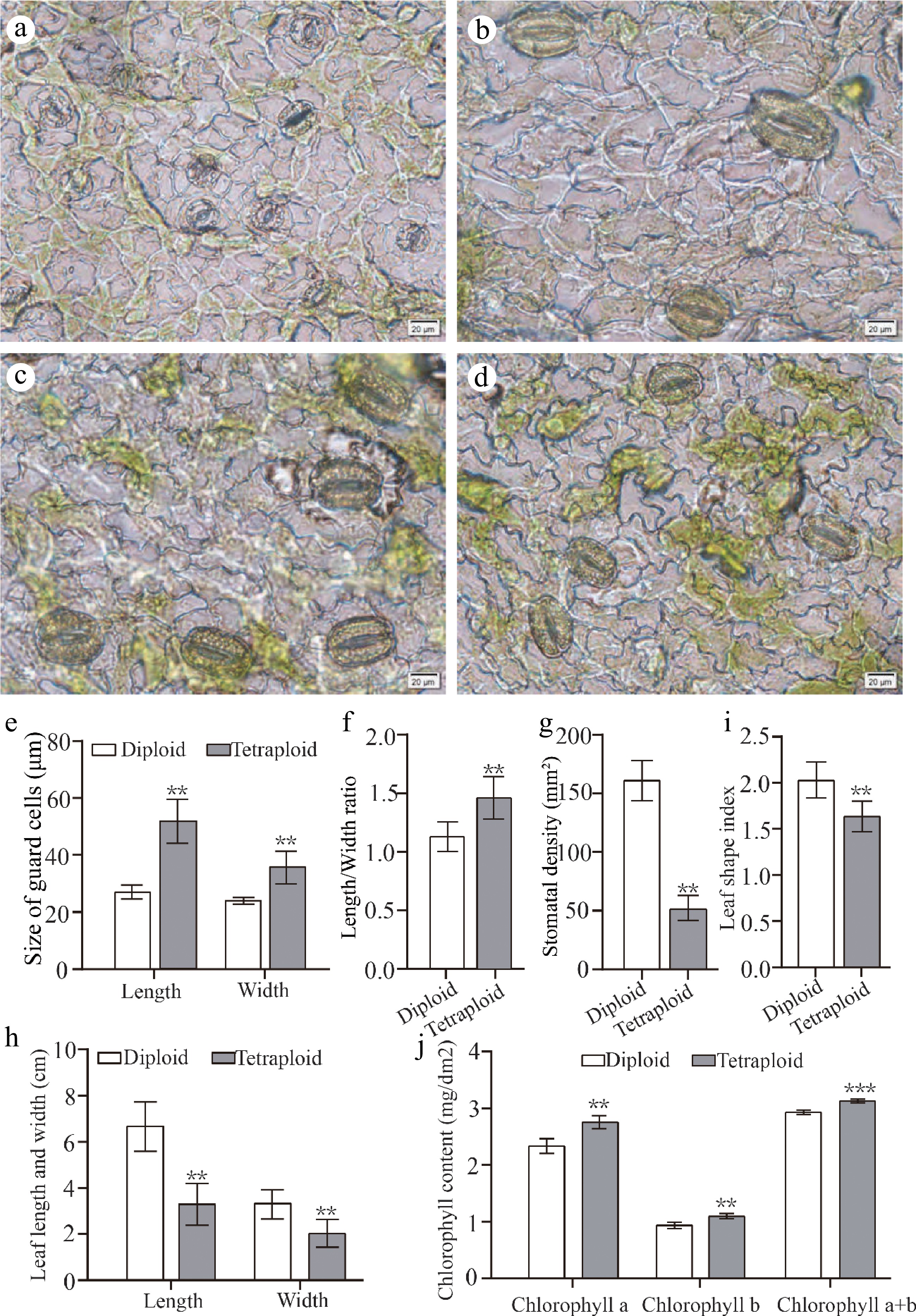
Figure 4.
Leaf morphology and stomatal characteristics of diploids and tetraploids 'Dangshansuli' seedlings. Abaxial stomata in leaves of (a) diploid and (b) − (d) tetraploid 'Dangshansuli' seedlings; (e) the length and width of guard cells in diploid and tetraploid 'Dangshansuli' seedlings; (f) length/width ratio in diploid and tetraploid 'Dangshansuli' seedlings; (g), stomatal density in diploid and tetraploid 'Dangshansuli' seedlings; (h) length and width of leaf in diploid and tetraploid 'Dangshansuli' seedlings; (i) leaf shape index in diploid and tetraploid 'Dangshansuli' seedlings; (j) chlorophyll content in diploid and tetraploid 'Dangshansuli' seedlings. Data are the means ± SD of three replicates. P values were determined by student's two-tailed t-test (***P < 0.001; **P < 0.01; *P < 0.05).
The determination of photosynthetic pigment content showed that chlorophyll a, chlorophyll b, and total chlorophyll content increased by 16.06%, 16.00% and 6.38% in the induced tetraploids, respectively. (Fig. 4j). Next, we observed the leaf surface of the plant by scanning electron microscopy. The epidermal cells with irregularly or polygonal-shaped in tetraploid are more densely than those of diploid plants (Fig. 5).
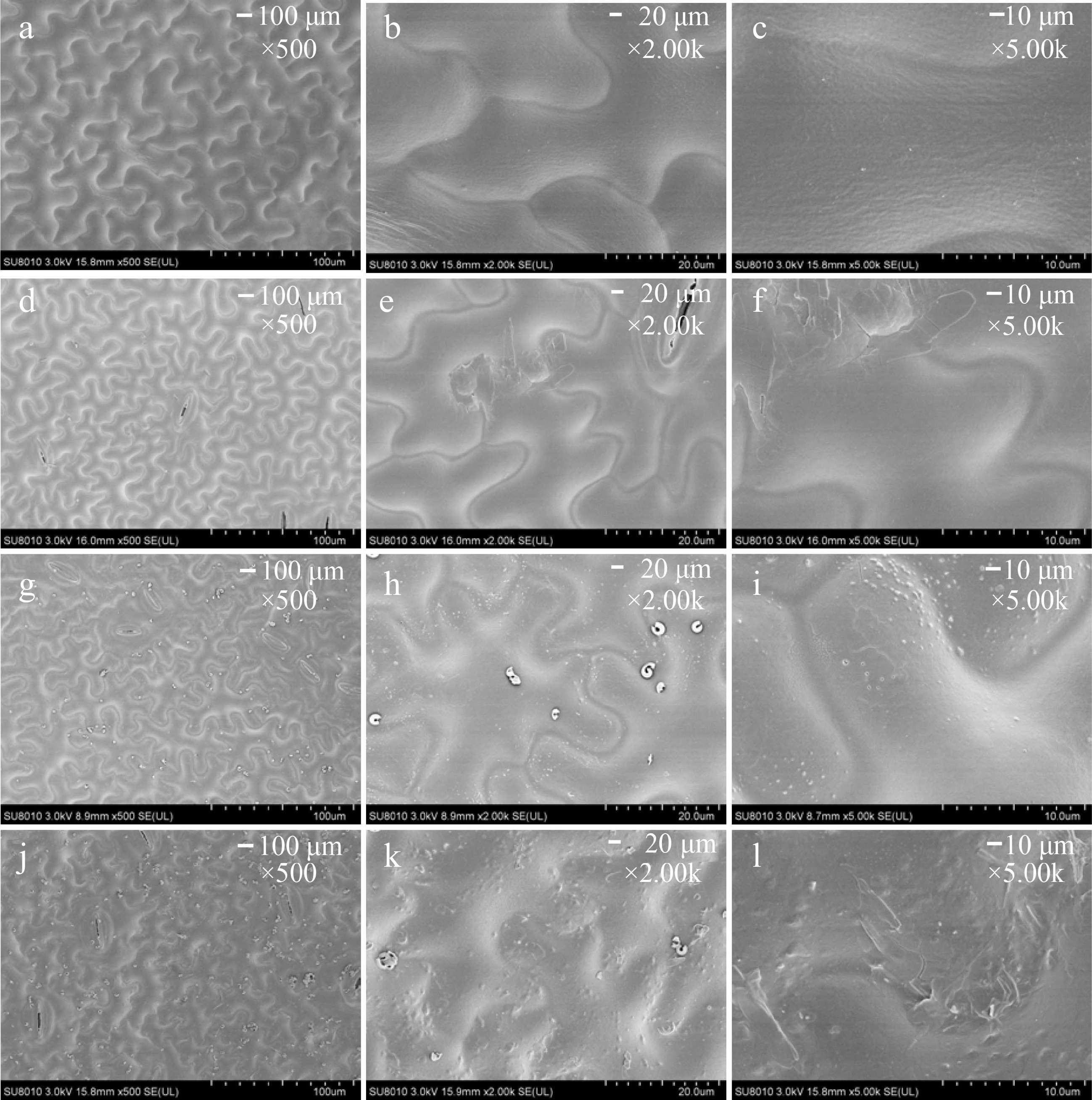
Figure 5.
The magnification series of the scanning electron microscope images of leaf morphology in (a) − (c) diploid and (d) − (f), (g) − (i), (j) − (k) tetraploid ‘Dangshansuli’ seedlings. Scale bars represent 100 μm (magnification 500×) in (a), (d), (g), (j), 20.0 μm (magnification 2.00 K×) in (b), (e), (h), (k) and 10.0 μm (magnification 5.00 K×) in (c), (f), (i), (l).
Analysis of wax compounds
-
To further compare the difference between diploid and tetraploid plants, we detected compounds of cuticular wax in the leaf epidermis and performed principal component analysis (PCA). In the PCA score plots, PC1 (46.7%) and PC2 (19.5%), explained 66.2% of the variation (Fig. 6a). Six samples were clearly separated into two groups according to different ploidy levels along PC1, which indicated that, compared to diploid plants, the coverage of cuticular wax compounds of in tetraploid plants substantially differed (Fig. 6a). Then, we focused on the analysis of 10 components of cuticular wax with significant differences between diploid and tetraploid, including four alkane components, two ester components, and four terpenoid components. Alkanes are important aliphatic compounds in the cuticular wax of pear leaves. Compared with diploid plants, the content of three odd carbon atom alkanes (pentacosane, 0.14 ± 0.05 μg/cm2, P = 0.007; heptacosane, 0.21 ± 0.05 μg/cm2, P = 0.009; and hentriacontane, 0.22 ± 0.03 μg/cm2, P = 0.030) decreased significantly in the leaf wax of tetraploid plants and the content of one even carbon atom alkane (hexacosane, 0.19 ± 0.03 μg/cm2) was increased (P = 0.043) significantly in the leaf wax of tetraploid plants (Fig. 6b). In addition, arachic acid benzyl ester (0.25 ± 0.03 μg/cm2, P = 0.004) and cycloartenol acetate (2.51 ± 0.78 μg/cm2, P = 0.006) content were also significantly increased in the tetraploid than diploid, while lanosteryl acetate (0.20 ± 0.09 μg/cm2, P = 0.009) and β-Sitosterol acetate (1.47 ± 0.22 μg/cm2, P = 0.009) content were significantly decreased than in diploids (Fig. 6b). Notably, specific wax compounds were only detected in tetraploid plants, such as 1-monolinolein (0.23 ± 0.05 μg/cm2) and γ-tocopherol (0.09 ± 0.02 μg/cm2) (Fig. 6b).
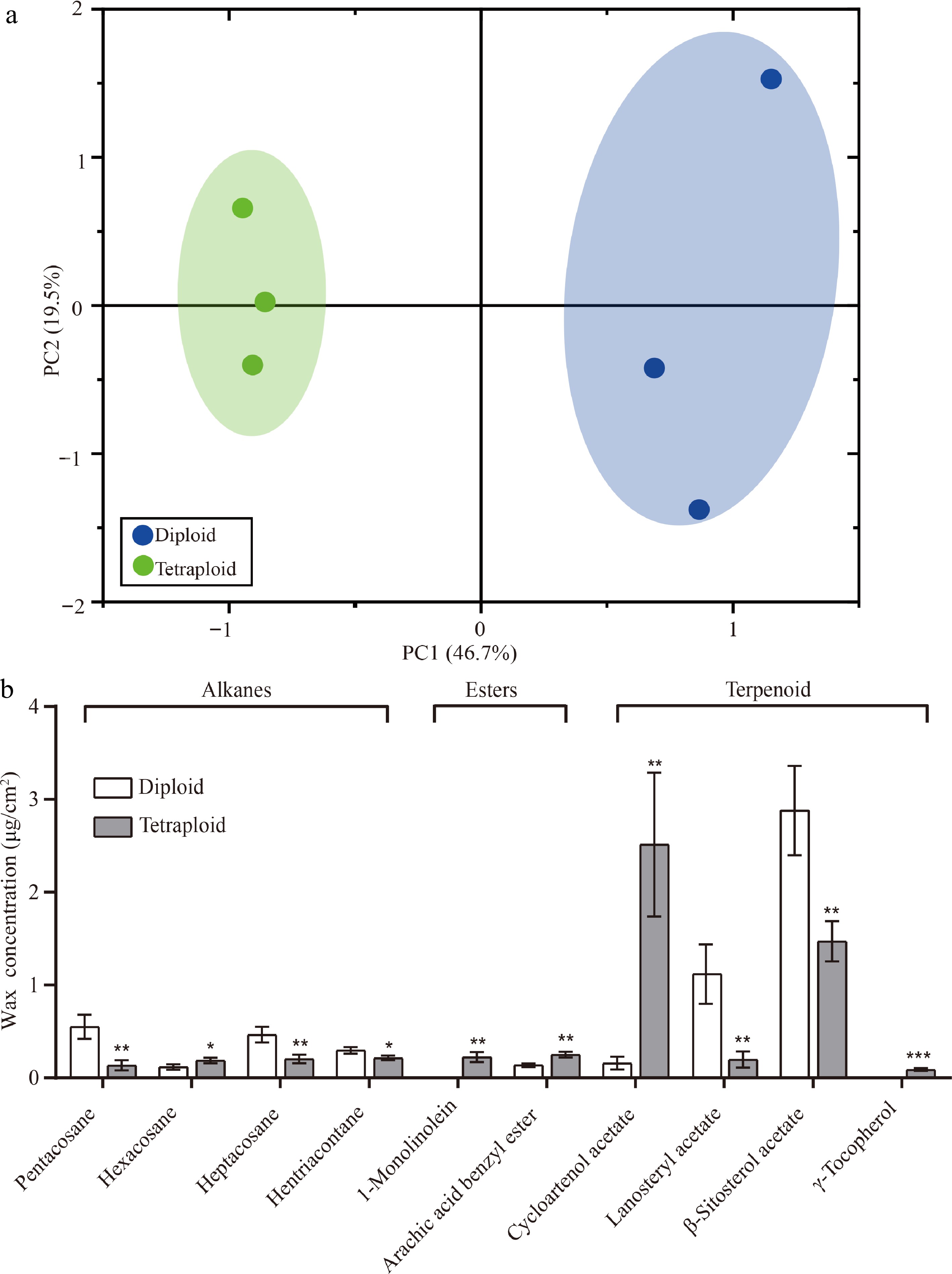
Figure 6.
PCA and 10 differentially expressed wax components in diploid and tetraploid 'Dangshansuli' seedlings. (a) PCA of the chemical compositions of the cuticular wax in diploid and tetraploid 'Dangshansuli' seedlings; (b) differentially expressed wax components in diploid and tetraploid 'Dangshansuli' seedlings. Data are the means ± SD of three replicates. P values were determined by student’s two-tailed t-test (***P < 0.001; **P < 0.01; *P < 0.05).
-
The efficiency and toxicity of chemicals used for genome doubling are the successful key to induction of polyploidy. The polyploidy induction of seeds treated by colchicine solution has been reported in many flower crops, including Taraxacum kok-saghyz[32], Lilium rosthornii[33], Rosa chinensis[34] and Lavandula angustifolia[35]. In this study, we also successfully obtained polyploidy plants from pear seeds treated with colchicine in vitro. Our results showed that 0.1% colchicine treatment for 12 h was the best treatment condition for pear seeds, which can create the highest induction rate of 6%. The colchicine induction system varied among different species. For example, the seeds of Lilium rosthornii treated by 0.05% colchicine solution for 36 h could obtain the highest induction rate of 27.78%[33]; and the highest efficiency (56.6% induction rate) was obtained by treating the seeds with a concentration of 0.1% colchicine solution for 48 h in Taraxacum kok-saghyz[32]. In addition, we found that in a certain range, with the increase of colchicine concentration, as well as exposure time, the induction efficiency was also positively increased, whereas when the threshold was exceeded, the survival rate of pear seedlings was significantly reduced (Table 1). Similar results have also been identified among other species, such as Taraxacum kok-saphyz[32], kiwifruit[36] and wolfberry[37]. This could be attributed to the highly toxic effect of colchicine in blocking spindle fiber production.
Numerous reports have shown that the morphological and physiological characteristics, including stomatal cell size, stomatal density, leaf index, etc. have a relationship with the ploidy levels in many plant species[38,39]. In this study, significant morphological differences were observed between diploid and tetraploid plants. The first visible effect of early tetraploid plants induced by colchicine was delayed growth[40]. Similar to the results reported in potato and citrus, tetraploid plants have shorter plant height than diploid plants[41]. This may be due to the effect of somatic cell doubling or colchicine residues damaging new buds[42].
The stomatal trait has been widely used as a ploidy selection trait. Similar to the results of ploidy level and stomatal characteristics reported in Lilium rosthornii[33], Buddleja lindleyana[43] and Hemerocallis[24], our study also found that stomatal size is positively correlated with ploidy level, whereas the stomatal density is negatively correlated with ploidy level. In addition, leaf length, leaf width and leaf shape index were decreased in tetraploid plants than those of diploid 'Dangshansuli' seedlings (Fig.4h, i). Similar to the results identified in Pyrus communis[1,20]. Previous studies have found that the leaves of the confirmed tetraploid plants were thicker and darker green compared to diploid plants, which was likely to be accompanied by an increased chlorophyll content[44]. By detecting the chlorophyll content of the leaves of diploid and tetraploid plants at the same stage, and found that the chlorophyll content of leaves of tetraploid plants was significantly higher than that of diploid plants, similar to results identified in Lycium ruthenicum and Persian poppy[45,46], which can also explain that tetraploid plants have stronger photosynthetic than diploid plants. Many studies show that a positive correlation between higher ploidy levels and secondary metabolite content has been established in artificially induced tetraploid plants. Similar with the results of Lilium rosthornii[33], Pfaffia glomerata[47], Dracocephalum moldavica[38] and Hyoscyamus reticulatus L.[48], we found that there were significant differences in wax composition between diploid and tetraploid in pear. Qualitative analysis results showed significant changes in the content of 10 wax components, including alkanes, esters, and terpenoids (Fig. 6). Previous studies have shown that changes in wax components and content affect plant stress tolerance. For example, increasing the content of C27, C29 and C31 alkanes enhances plant tolerance to drought[49]. Certain wax components, such as long-chain alkanes, were possibly used for host selection by insects[50,51]. Adding long-chain alkanes to sinigrin and cabbage homogenates can stimulate oviposition by the diamondback moth Plutella xylostella[52]. Therefore, we speculate that there are differences in resistance abilities such as insect resistance and drought resistance between tetraploid and diploid plants, which needs to be verified in subsequent studies.
-
This study reported that the pear tetraploids were successfully induced from seeds of diploid 'Dangshansuli' pear with seed coats removed by colchicine treatment Appropriate colchicine concentration and exposure time are essential to increase the tetraploid induction rate. Compared to diploids, tetraploids show significant changes not only in morphoanatomical characteristics but also in secondary metabolites. For example, tetraploid plants have shorter stem nodes, fewer true leaves, larger stomata, longer and narrower guard cells, higher chlorophyll content, and denser irregular or polygonal-shaped epidermal cells than diploid plants. Ten components of cuticular wax with significant differences between diploid and tetraploid. These tetraploids can be used as useful materials for innovative germplasm resources.
This research was supported by the Natural Science Foundation of Jiangsu Province (BK20221011), The National Natural Science Foundation of China (32202411), the China Postdoctoral Science Foundation (2022TQ0160), This research was supported by the Jiangsu Funding Program for Excellent Postdoctoral Talent (2022ZB338).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Hao Yin, Xiaohua Wang
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Yin H, Wang X, Shi X, Chen Y, Qi K, et al. 2023. Induction and characterization of tetraploid pear from the seeds of 'Dangshansuli' (Pyrus bretschneideri Rehd.). Fruit Research 3:14 doi: 10.48130/FruRes-2023-0014
Induction and characterization of tetraploid pear from the seeds of 'Dangshansuli' (Pyrus bretschneideri Rehd.)
- Received: 17 March 2023
- Accepted: 21 May 2023
- Published online: 14 June 2023
Abstract: Pear is one of the most economically important fruit crops widely distributed around the world. To enrich the pear germplasm resources for obtaining the polyploid plants, the seeds from pear cultivar of 'Dangshansuli' (Pyrus bretschneideri) were treated with colchicine solution under concentration gradients of 0.1%, 0.5% and 1.0% for 12-, 24- and 48-h, respectively. Our results showed that colchicine could inhibit seed germination, and the inhibitory effect was positive with the treated concentration of colchicine. We detected that the 0.1% colchicine solution for 12 h was the most effective treatment for inducing pear polyploidy. Furthermore, compared with the wild diploid pear plants, a significant morphological variation, such as shorter plants, smaller and oval leaves were observed in the induced tetraploid pear plants. Significant changes in leaf anatomy, including larger and sparse stomata and the epidermal cells with irregularly or polygonal-shape were also detected in tetraploid pear plants. In addition, the contents of 10 cuticular wax compounds from the leaves of diploid and tetraploid pear plants were also detected with significant difference. Taken together, this study lays the foundation for polyploid breeding of pear for contributing diverse characteristics.
-
Key words:
- Pear /
- Colchicine /
- Flow cytometry /
- Tetraploid /
- Physiological characteristics












