-
Chrysanthemum indicum L. is a perennial plant belonging to the Chrysanthemum L. genus of Asteraceae. Besides its vast range of applications as decorative plants, C. indicum is a significant medicinal herb used in traditional Chinese medicine (TCM) since the Qin and Han Dynasties, having a first recording in Shengnong’s herbal classic[1]. The dry head inflorescence of C. indicum is referred to as 'Yejuhua' in the Pharmacopoeia of the People's Republic of China (version 2020), which has cold properties, a bitter and pungent flavor, and effects the clearing of heat and detoxifyication through liver and heart channels. Many distinct chemical components, including terpenoids, flavonoids, tannins and glycosides, were isolated from C. indicum. These molecules have a variety of pharmacological activities, such as anti-inflammatory, anti-microbial, antioxidant, and anticancer properties, leading to a great potential for medicinal applications[2].
Both the diploid (2n = 2x =18) and tetraploid cytotypes of the C. indicum were identified in the wild populations[3]. In the high altitude mountains of the Shennongjia Forest region, a variety of C. indicum var. aromaticum Q.H.Liu et S.F.Zhang was described with a natural distribution that only present there[4]. Previous studies revealed several closely related taxa of C. indicum such as C. nankingense (Hand.-Mazz.) X.D.Cui and C. lavandulifolium (Fisch. ex Trautv.) Makino, and gene flow between them is common[5−7]. Notably, C. nankingense have ever been treated as a variety of C. indicum due to their close relationship[6]. In addition to these taxa in China, local varieties such as C. indicum var. albescens and C. indicum var. acuta from Korean and C. indicum var. iyoense and C. indicum var. maruyamanum from Japan were also reported, but their distribution is extremely constrained and the number of related studies is small[8,9]. Therefore, we referred here the C. indicum complex mainly including the different C. indicum cytotype populations and the C. indicum var. aromaticum variety in China. Additionally, in some contexts, the two closely related taxa of C. lavandulifolium and C. nankingense were also listed.
Considering the increasing market demands of C. indicum plant resources, it is theoretically and practically significant to address the origin and phylogeny of the C. indicum complex, including the identification and classification of different ecotypes/cytotypes and varieties. Furthermore, the studies in relation to molecular biology and genomics of C. indicum and its closely related taxa was crucial for our comprehensive understanding of the genetic diversity and population structure present in the complex as well as for tracking the molecular mechanisms underlying the ecological adaptation and diversification of these taxa in a variety of environments. In this review, we summarize the recent progresses in the evolution, genetic variation, and ecological adaptation of the C. indicum complex and suggest potential directions for future molecular biology and genomics studies that may aid in the breeding and therapeutic uses of this important medicinal plant complex.
-
The genus Chrysanthemum, classified within the Asteraceae family, belongs to the Anthemideae tribe as part of the Artemisiinae subtribe[10]. Within the Artemisiinae subtribe, there are two groups: the Artemisia Linn. Sensu stricto, excl. Sect. Seriphidium Bess. group and the Chrysanthemum group[11]. The majority of the species in the Chrysanthemum come from the genera Chrysanthemum, Ajania Poljak., Arctanthemum (Tzvelev) Tzvelev, and Elachanthemum Ling et Y. R. Ling, with Chrysanthemum and Ajania having the closest genetic affinity[9,12−15]. The Chrysanthemum genus has more than 40 species, the majority of which are found in East Asia (China, Korea, Japan and Siberia)[13−15]. According to pollen morphology and both the cpDNA and nuclear gene tree data (e.g., the chrysanthemyl diphosphate synthase (CDS), ITS and ETS genes), Chrysanthemum is not a monophyletic clade, and the Chrysanthemum species native to China can be roughly divided into two groups, the C. indicum group and the C. zawadskii Herbich group[13]. Chrysanthemum species and their associates may have undergone rapid adaptive diversification, and as a result, Chrysanthemum species differentiation appears to be strongly influenced by a variety of geographical and environmental variables[11,13].
Chromosome ploidy levels mostly range from diploid to tetraploid within the C. indicum complex[3,6,16,17]. Additionally, reports of hexaploid C. indicum samples from Japan have been made[9,13]. However, the origin, evolutionary history, polyploid affinities of the diploid C. indicum remain poorly understood. Molecular analysis provided evidence for recurrent origins and lineage recombination in the C. indicum polyploid complex, suggesting that the C. indicum tetraploids found in the Shennongjia mountains are thought to have an autopolyploid origin from the local diploids while those found in other populations may have undergone allopolyploidization[6]. Based on nuclear CDS gene haplotypes, the tetraploid C. indicum from Nanjing has its CDS haplotypes dispersed in far-off evolutionary clades, and the hexaploid sample from Japan likewise harbors divergent CDS haplotypes[13]. Less research has been done on the origin of C. indicum var. aromaticum. Both the cpDNA and CDS gene trees appears to support a tight relationship between C. indicum var. aromaticum and C. lavandulifolium, or C. nankingense[13]. As an alternative, a study using both the rpl16-rpl14 and the GS genes showed that the tetraploid C. indicum var. aromaticum and C. chanetii share a close relationship[18]. Our recent analysis using leaf transcriptome data revealed a strongly evolutionary link between C. indicum var. aromaticum and C. indicum, suggesting that the former underwent local adaptation compared to the latter (Fig. 1).
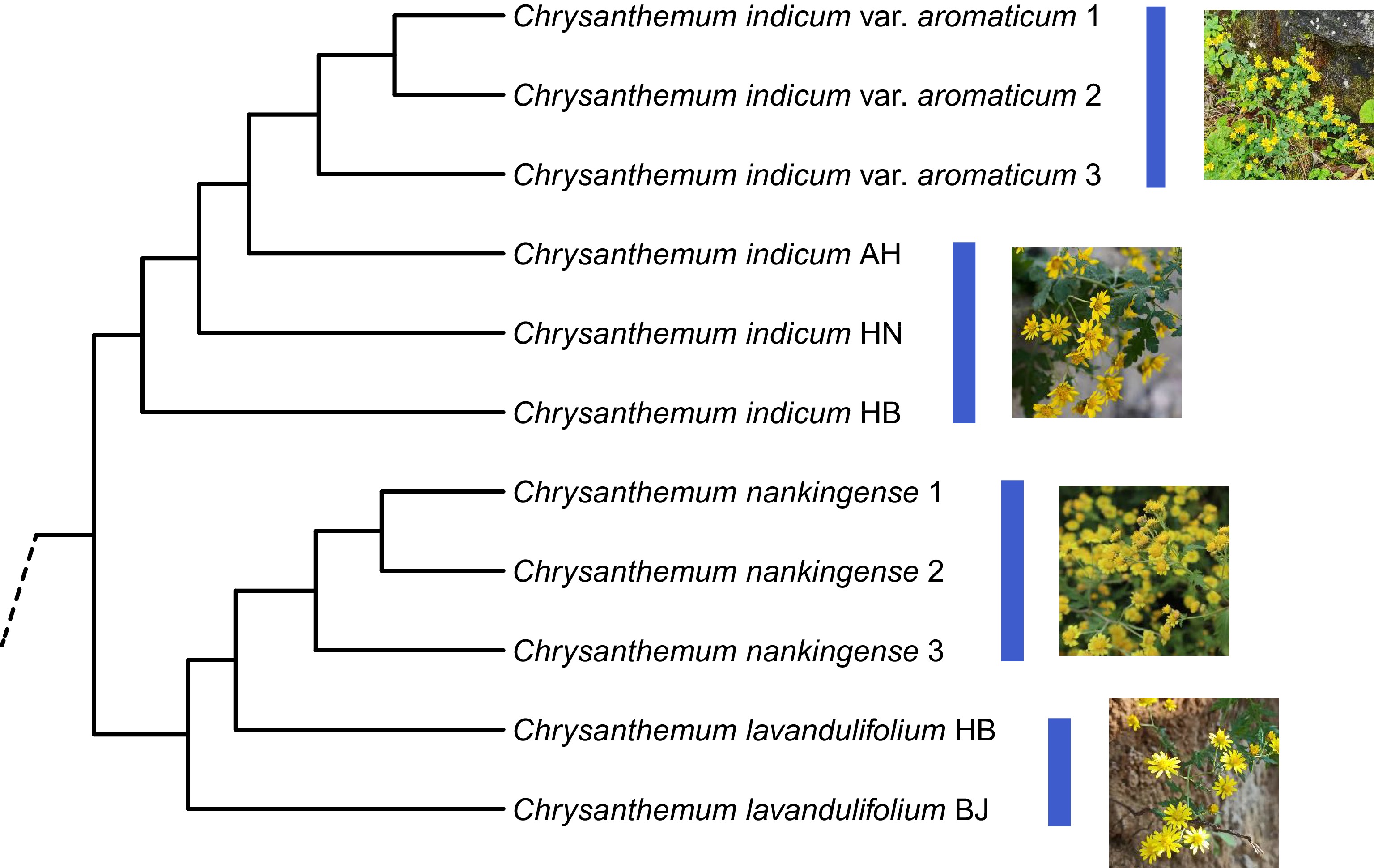
Figure 1.
The transcriptome data-based species relationship of the Chrysanthemum indicum complex. The C. indicum var. aromaticum samples were collected from the Shennongjia Forest region (China), and the C. nankingense samples from Nanjing, Jiangsu (China). AH, Anhui; HN, Hunan; HB, Hubei; BJ, Beijing. The specimens of these samples (HBTCM18101704-HBTCM18101714) were preserved in the Herbarium of Hubei University of Chinese Medicine (China).
Natural hybridization is rampant in the Chrysanthemum genus, which has led to a highly complex species relationship[6,14,19,20]. Some species may have evolved through natural hybridization or have engaged in gene introgression[9]. Previous research that suggested gene flow spontaneously occurred between C. indicum and C. lavandulifolium[6], and we have certainly observed the possible medium form between them in Shennongjia mountains (China). Similarly, both C. indicum and C. indicum var. aromaticum were also possibly involved into natural hybridization in their overlapping populations[21]. When the proper genotype is carefully chosen, reciprocal crosses between Chrysanthemum species, including the C. indicum complex, are possible[22]. The evolutionary history of cultivated chrysanthemums has received a lot of interest. The C. indicum complex, including the closely related taxa such as C. lavandulifolium, C. nankingense, and C. vestitum (Hemsl.) Stapf, was thought to be the primary genomic donors of cultivated chrysanthemums[20,23−26]. Through genetic analysis of C. indicum, C. vestitum and C. vestitum var. latifolium J. Zhou et J.Y. Chen using microsatellite markers and trnL-trnF chloroplast genes, 20 hybrids between these taxa at various ploidy levels were found, providing insights into the hybrid origins of horticultural chrysanthemums[20]. Furthermore, data from chloroplast genomes suggested that the cultivated chrysanthemums were the result of numerous hybridization episodes involving a number of parental species, with an extinct or un-sampled species possibly acting as the maternal parents[23,27].
-
Knowledge of the geographical distribution of cytotypes/ecotypes and the associated pattern of population genetic variation is critical to our understanding of the origin and evolution of species complex. Given the variety of cytotypes found in the C. indicum complex, the diploid C. indicum are primarily found in central and northern China, whereas the tetraploid form may have expanded its range southward in the recent Quaternary glacial periods, leading to a currently natural occurrence at both north and south of the Yangtze River[3]. Analysis on both plastid and nuclear ribosomal DNA (rDNA) sequence polymorphism showed that the population genetic diversity of the diploid and tetraploid C. indicum populations was different[7]. The chloroplast haplotypes and ITS types within diploid C. indicum populations were nearly fixed, making it possible to distinguish them from other closely related diploids, while the tetraploid populations had polymorphic chloroplast haplotypes and ITS types, leading to a higher level of genetic diversity in tetraploid populations[7]. A recent demographic expansion, particularly the range extension of tetraploid C. indicum, was compatible with the lack of clearly phylogeographic structure found within the C. indicum complex[7,28].
The populations of C. indicum var. aromaticum also revealed genetic differentiation from those of C. indicum populations, indicating that it is an endemic variety adapted to the high Shennongjia mountain areas (> 2000 m above sea level)[21]. Our genetic analysis using 21 simple sequence repetitive (SSR) markers on six distinct or mixed C. indicum var. aromaticum and C. indicum populations clearly demonstrated genetic differentiation between the samples of both taxa despite a similar level of genetic diversity being observed within the populations of each taxon (Fig. 2)[21]. Local adaptation may also enhance population genetic differentiation, as has been seen between the C. indicum populations in Wuhan and Shennongjia as well as between the three C. indicum var. aromaticum populations found in the Shennongjia Forest region. On the contrary, gene flow can happen simultaneously between samples from nearby populations of each taxon as well as between C. indicum var. aromaticum and C. indicum samples that were both taken from the same site[21].
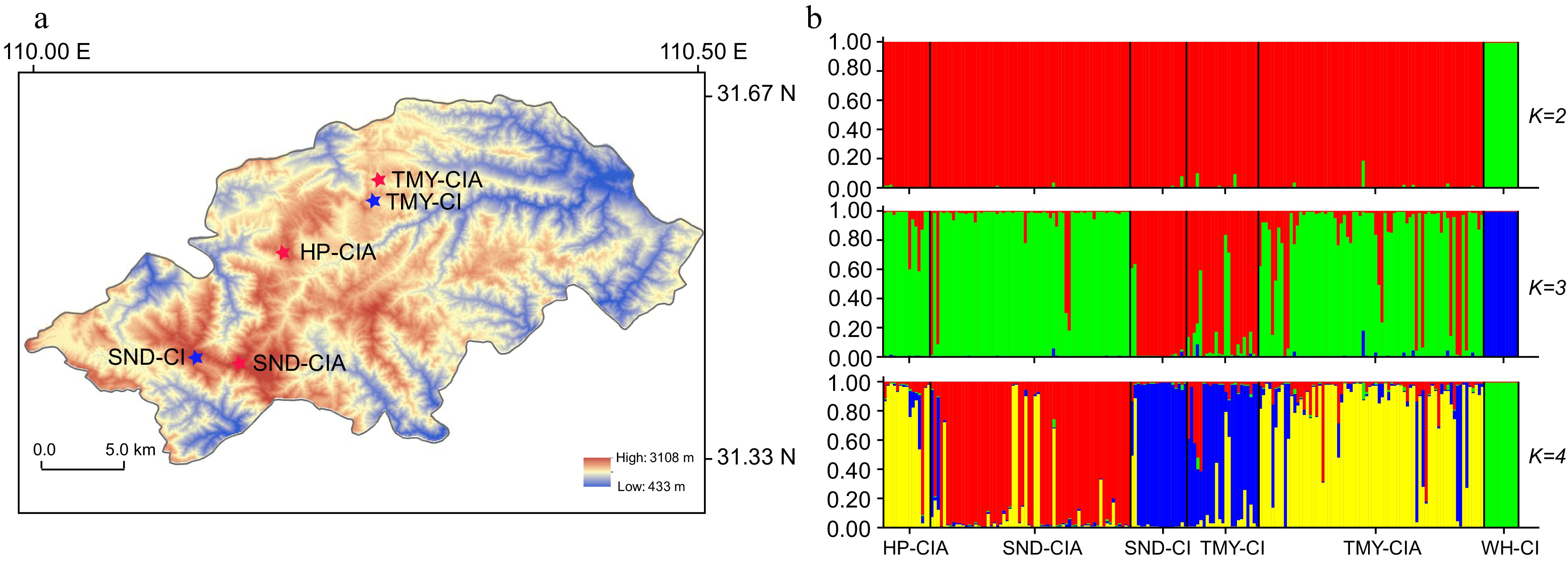
Figure 2.
Genetic structure for Chrysanthemum indicum and C. indicum var. aromaticum populations. (a) Geographical distribution of the populations sampled from the Shennongjia Forest region. (b) STRUCTURE analysis of the sampled populations. CI, C. indicum; CIA, C. indicum var. aromaticum; WH, Wuhan; HP, Hongping, Shennongjia; SND, Shennongding, Shennongjia; TMY, Tianmenya, Shennongjia. When K = 2, the C. indicum samples from Wuhan can be distinguished from those samples in Shennongjia. When K = 3, the C. indicum samples in Shennongjia further showed genetic differentiation from those of the C. indicum var. aromaticum populations in Shennongjia. When K = 4, subdivision was observed within the C. indicum var. aromaticum populations.
Less research has been done on the genetic diversity of the closely related taxa of C. indicum[29,30]. A genetic analysis of a population of 30 C. nankingense lines showed consistently high genomic diversity and methylation polymorphism, which may be responsible for the observed morphological variability between these lines[29]. A new genotyping-by-sequencing (GBS) method was developed to assess the genetic diversity of 80 wild chrysanthemum germplasms including C. indicum[30]. In accordance with the findings of morphological observation, an investigation of the genetic diversity of an interspecific F1 hybrid population between C. nankingense and C. lavandulifolium revealed that the hybrids were more similar to their female parent in terms of heredity[31]. Using specific-locus amplified fragment sequencing (SLAF-seq) technology, a high-density genetic map of a F1 population of the cultivated chrysanthemum (C. morifolium) was also constructed, yielding the identification of seven QTLs related to flower-type features[32].
-
Reconstruction of historical biogeography for genera within the subtribe Artemisiinae suggested that the Chrysanthemum group originated in western or central Asia, and that there was a gradual northeasterly movement into western China and Mongolia. Chrysanthemum then migrated eastward into eastern Asia, where Ajania underwent in situ diversification[15,33]. On a scale within the genus, the C. indicum group and C. zawadskii group exhibited modern distribution division, with the former often distributed in southern China and the latter typically found in northern China, although their distribution regularly overlapped in central China[33,34]. The geographic structure of the lineages in the Chrysanthemum group is associated with niche differentiation, with mountains and altiplanos acting as boundaries that facilitated the emergence and evolution of new taxa, resulting in many Chrysanthemum species which are narrowly distributed and habitat-specific (Fig. 3)[13,15,33].
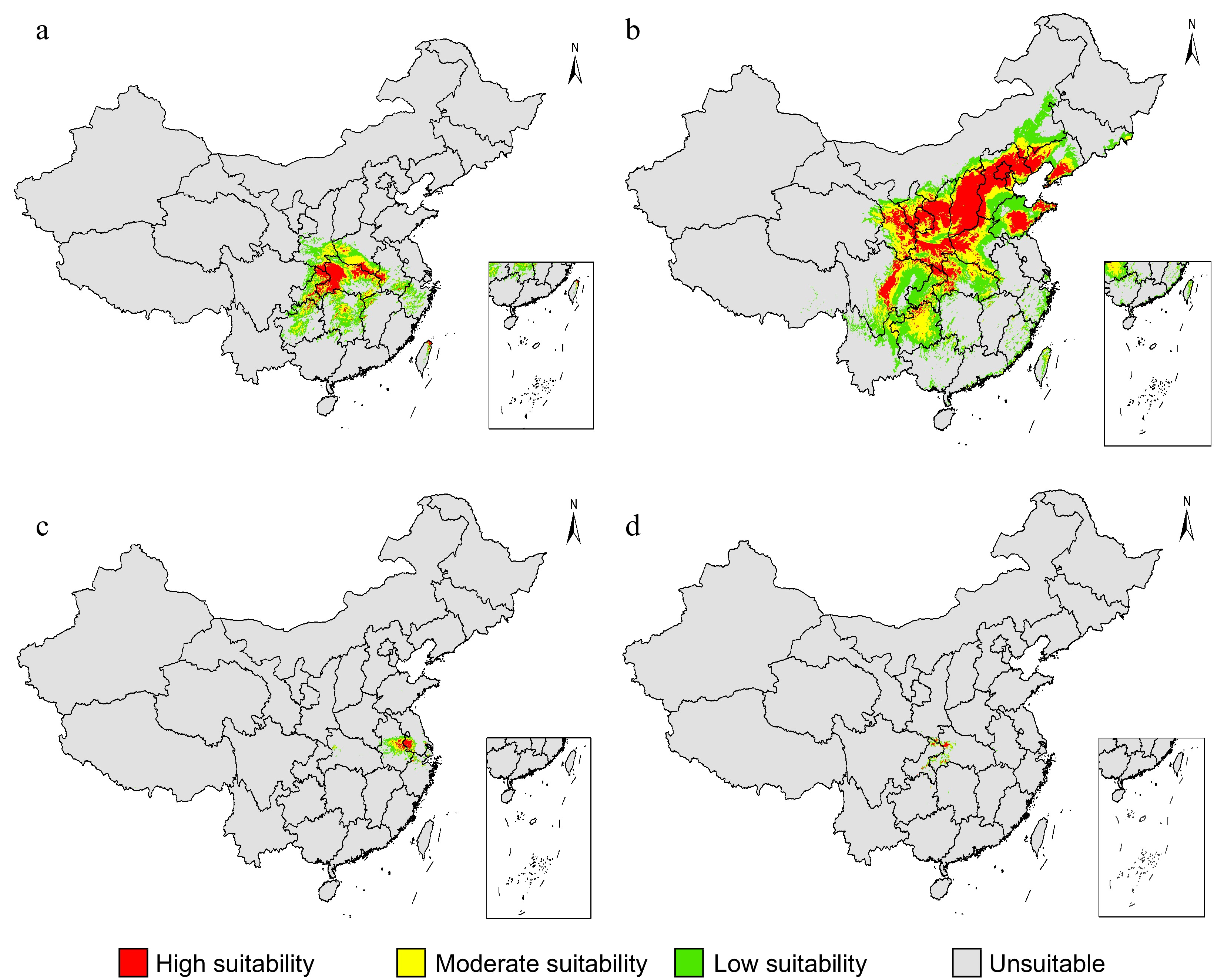
Figure 3.
The predicted suitable areas of the Chrysanthemum indicum complex under current bioclimatic conditions. The respective prediction for each taxon of the C. indicum including (a) both diploids and tetraploids, (b) C. lavandulifolium, (c) C. nankingense and (d) C. indicum var. aromaticum were showed. The predictions were conducted using the MaxEnt model based on 27 bioclimatic variables retrieved from the Worldclim website.
Chrysanthemum species like C. indicum and C. zawadskii have a discontinuous distribution around the world, indicating that the genus has undergone varying degrees and directions of evolutionary radiation[34]. The Hengduan-Qinling Mountains in China have been postulated as a potential secondary radiation and evolution center of Chrysanthemum species and its allied genera because of the numerous refuges created by mountain topography throughout previous glacial-interglacial cycles[34]. In the Hengduan mountains, for instance, ecological islands of refuges in connection to rocky topographies and mixed forest marginal communities played a crucial role in the evolutionary divergence between C. indicum and its coexisting Ajania species[35]. A key characteristic that can separate C. indicum from Ajania taxa is the elongation and evolution of marginal female florets, which may be driven by specialized habitat conditions including low light intensity and moderate humid environments[35]. Two regions in and around China have been identified as diversity hotspots of Chrysanthemum, and many species, like the C. indicum complex, can be found there frequently, consistent to the multiple evolutionary radiation of these taxa[6,34].
-
Because of the frequent natural hybridization and polyploidy, which significantly contribute to the large genome size and high repeat content of the Chrysanthemum genomes, it is still relatively challenging to analyze their genomes[14,36]. Despite the fact that the C. indicum genome has not yet been released, it has been reported that the assembled genomes of its closely related species have increased[37−42]. The genome of C. nankingense was sequenced using the Oxford nanopore long-read technology, and it is also the first genome assembled in the Chrysanthemum genus[37]. Chrysanthemum nankingense is currently used as a model species for genetic and breeding studies within the genus. The draft genome of C. nankingense demonstrated evolutionary complexity, as seen by the large total size of about 3 Gb and the repetitive sequences making up 69.6% of the genome assembly[37]. The repetitive DNA classes displayed an atypical structure with a large percentage of transposable elements (40%) and a relatively low percentage of tandemly repeated sequences (1.02%)[36]. Through comparative genomic analysis with other plant genomes, a total of 56,870 protein-coding genes were predicted in C. nankingense, of which 1,939 gene families containing 8,009 genes specifically found in it[37]. In particular, genes related to terpenoid biosynthesis were expanded and the TS/CYP gene clusters contributed to the terpenoid diversity in chrysanthemum[37]. Using the same nanopore long-reads, a complete mitogenome of C. nankingense was assembled and exhibited diversity in terms of the highly rearrangeable and variable chrysanthemum mitogenomes[43].
Our knowledge of the genomic landscape of Chrysanthemum species was furthered by the report of several chromosome-scale genome assemblies[40−42]. The diploid species C. lavandulifolium is generally used to study the diverse capitula types in chrysanthemum[44]. A 2.60 Gb chromosomal-level genome of C. lavandulifolium was obtained using a combination of sequencing reads from both the PacBio RS II platform and Oxford Nanopore sequencing technologies, and a total of 64,257 protein-coding genes and 1.76 Gb of repetitive sequences were identified[41]. The transcriptomic profiling of six developmental stages of the C. lavandulifolium capitulum further exhibited that gene families such as the MADS-box, TCP, NAC and LOB were involved in the differentiation of the disc and ray floret primordia[41]. The E-class MIKCc-type MADS-box genes of C. lavandulifolium may undergo subfunctionlization because they have significantly expanded and diverse expression patterns, while the C-class gene are highly expressed in the disc floret corolla, offering hints to explore the morphological variations between disc and ray floret corolla[45]. Chrysanthemum seticuspe (Maxim.) Hand. is a diploid species belonging to the C. indicum group in Japan. A pure line Gojo-0 bred from the self-compatible mutant of C. seticuspe was sequenced and assembled into a 3.05 Gb chromosome-level genome[40]. The recent segmental duplication and retrotransposon expansion were found to significantly contribute to the relatively large genome size of C. seticuspe[40]. Similarly, the genome of another Japanese species C. makinoi Matsum. et Nakai was recently sequenced and assembled into nine pseudochromosomes with a total size of 3.1 Gb[39].
Genomic study of the cultivated chrysanthemum (C. morifolium Ramat.) is particularly difficult due to its complicated hexaploid structure. A chromosome-scale genome assembly of C. morifolium (8.15 Gb) has been constructed using a haploid line (n = 3x = 27) generated from the pot variety ‘Zhongshanzigui’, taking use of recent advancements in long-read sequencing technologies[42]. Chrysanthemum morifolium has a complicated palaeopolyploid history, including a very recent polyploidization event (~3 Mya) and a lineage-specific whole-genome duplication (WGD) event (WGT-2, ~6 Mya) shared by all Chrysanthemum species[42]. Moreover, the inflation of the Chrysanthemum genomes was significantly fueled by the amplification of LTR/Copia elements. Phylogenomic analysis showed that C. rhombifolium (Y. Ling & C. Shih) H. Ohashi & Yonek. and tetraploid C. indicum (from Nanjing) may be more closely related to cultivated chrysanthemum, and extensive and multiple genomic introgression have contributed to complex reticulate evolutionary history of Chrysanthemum species[42]. Since C. morifolium is polyploid, as expected, its genome contains more MADS-box genes than those of other Asteraceae plants, especially those belonging to the SEP and SVP clades. Moreover, numerous artificial hybridization-based introductions of the carotenoid cleavage dioxygenase (CmCCD4a) have increased the floral color diversity of modern cultivated chrysanthemums[42].
-
Flowering and its related mating system are two of the most important factors affecting the ecological adaptation of plants as well as environmental variables can also influence plant flowering[46]. In chrysanthemum, the production of flowering usually requires the regulation of day length[47]. With the development of a UV-visible reporter-assisted CRISPR/Cas9 gene editing system in C. indicum, several mutants related to TERMINAL FLOWER 1 (TFL1) genes were generated and the edited plants displayed varying degrees of early flowering[48]. Chrysanthemum lavandulifolium is an obligate short-day plant. The CONSTANS (CO) and FLOWERING LOCUS T (FT) homologous genes in C. lavandulifolium have roles in controlling its flora transition[49,50]. Moreover, the Cryptochrome2 (CRY2) genes in C. lavandulifolium can regulate the expression of genes related to the circadian clock, such as the CONSTANS-like genes (COLs) and FTs, revealing a crucial role for floral transition, while both CRY1 genes (ClCRY1a and ClCRY1b) in C. lavandulifolium could control flowering time via different pathways[51,52]. N plays an important role in flowering of chrysanthemum[53]. In the presence of an ideal nitrate environment, RWP-RK transcription factors such as NIN-like proteins (NLPs) from C. lavandulifolium can regulate the flowering time by increasing the transcription level of the ClCRYs[54]. Temperature shift can initiate and produce flowers of C. lavandulifolium through a unique mechanism in which mitotic arrest deficiency 1 (MAD1) interacts with Suppressor of FRIGIDA 4 (SUF4) to alter Flowering Locus C (FLC)-mediated floral transition[55]. Other genes, including a LFY/FLO homologue DFL in C. lavandulifolium was found to be involved in the first step of the transition from vegetative to reproduction development under photoperiodic induction, and an AP1/FUL-like transcription factor gene ClM8 in C. lavandulifolium can upregulate FT and LFY expressions in Arabidopsis[56,57]. Evolutionary shift for Chrysanthemum flowerheads is important for the diversification of species’ mating system, and it has been demonstrated that ABCE-class and CYC2-like genes are essential for the formation of Chrysanthemum capitula[44]. Further research revealed that the evolutionary shift of the Chrysanthemum group from radiating to disciform flowerheads was caused by CYC2g gene dysfunction[58].
Genes involved into stress responses are greatly related to the geographical distribution of plants. Some highly expressed ClHSP70 genes in C. lavandulifolium roots may have functions for the responses to heat stress[59]. The overexpression of C. lavandulifolium ClCBF1 gene in C. morifolium ‘White Snow’ raised the level of salinity and drought tolerance, while the overexpression of an apetala 2.4 gene ClRAP2.4 in chrysanthemum improved tolerance to cold stress[60,61]. In C. nankingense, a TCP transcription factor named CnTCP4 may play a negative role for its responses to cold stress[62]. Reduced salt tolerance and drought tolerance was observed in transgenic C. indicum plant that overexpressed the microRNA396 (miR396) gene[63]. Soil waterlogging significantly limits the growth of many medicinal plants including chrysanthemums. According to the gene expression analysis of CgACO in both C. nankingense and C. zawadskii, higher CgACO expression may have contributed to higher ethylene accumulation in the waterlogging-tolerant species C. zawadskii[64].
Secondary metabolites such as terpenoids are important functional components for plants that regulate development and mediate environmental interaction and adaptation[65]. Terpene synthases (TPSs) are important enzymes in plants that produce terpenoids[66]. In C. indicum var. aromaticum, monoterpenes and sesquiterpenes are both main constituents in the production of aromatic volatiles[67]. The overexpression of the diphosphate synthase (GPS) and farnesyl diphosphate synthase (FPS) genes of C. indicum var. aromaticum in tobacco can alter trichome formation and terpenoid biosynthesis[68]. Using C. indicum var. aromaticum as the experimental material, a CiTPS gene from the TPS-b subfamily was further cloned with potential function of monoterpene synthase[69]. Transcriptome analysis for C. indicum var. aromaticum flowers at three different development stages showed a large number of differentially expressed genes (DEGs) involved into the biosynthesis of volatile terpenes and at least 20 transcription factors from MYB, bHLH, AP2/EFR, and WRKY families were possible regulators affecting the expression of floral scent-related genes[70]. Similarly, a CiMYC2 transcription factor was speculated to be a key regulator for terpenoids biosynthesis in C. indicum var. aromaticum[71]. Given the different volatile terpenoid profiles between the two cytotypes of C. indicum, four TPS genes (named CiTPS5-8) and their homologous were respectively cloned in the diploid and tetraploid C. indicum, which showed various tissue expression patterns in relation to produce different monoterpenes and sesquiterpenes[72]. A comparative transcriptome analysis further showed that 14 genes from the TPS/CYP gene clusters revealed higher expression level in C. indicum var. aromaticum than those in C. indicum or C. nankingense, which is compatible with the difference in the accumulation of their aromatic ingredients[73].
Chrysanthemums contain diverse flavonoids that play significant roles in ecological adaptation and diversification[2]. In the Pharmacopoeia of the People’s Republic of China (version 2020), the flavonoid linarin is employed as the C. indicum quality control standard substance. In the C. indicum complex, including both the tetraploid C. indicum and the diploid C. indicum and C. nankingense, the contents of flavonoids like flavone glucosides and flavone rutinosides in their flowers are substantially different, and their biosynthetic pathways remain unknown (Fig. 4)[74]. By characterizing genes encoding rhamnosyltransferases (RhaTs) and their biochemical functions from eight accessions of the C. indicum complex, at least six Chrysanthemum 1,6RhaTs were identified and four of them catalyzed acacetin-7-O-glucoside, apigenin-7-O-glucoside, and diosmetin-7-O-glucoside to acacetin-7-O-rutinoside, apigenin-7-O-rutinoside, and diosmetin-7-O-rutinoside, respectively[74]. Comparatively, these 1,6RhaTs revealed higher substrate specificity for acacetin-7-O-glucoside than others, consistent with the higher accumulation of linarin in diploid C. indicum[74]. The chalcone synthase (CHS) catalyzes the first committed step in the biosynthesis of plant flavonoids and flavone synthase (FNS) is a key enzyme for flavone synthesis and provides precursors for acacetin and linarin biosynthesis[75]. Sixteen CnCHS genes were identified from the C. nankingense genome and they have extensive patterns of expression in various tissues (e.g., in disc floret petals and pistils) and stages of flower development, suggesting their potential roles involved into petal color diversity[76]. In C. indicum, a flavone synthase II gene CiFNSII was identified which related to flavones biosynthesis[75].
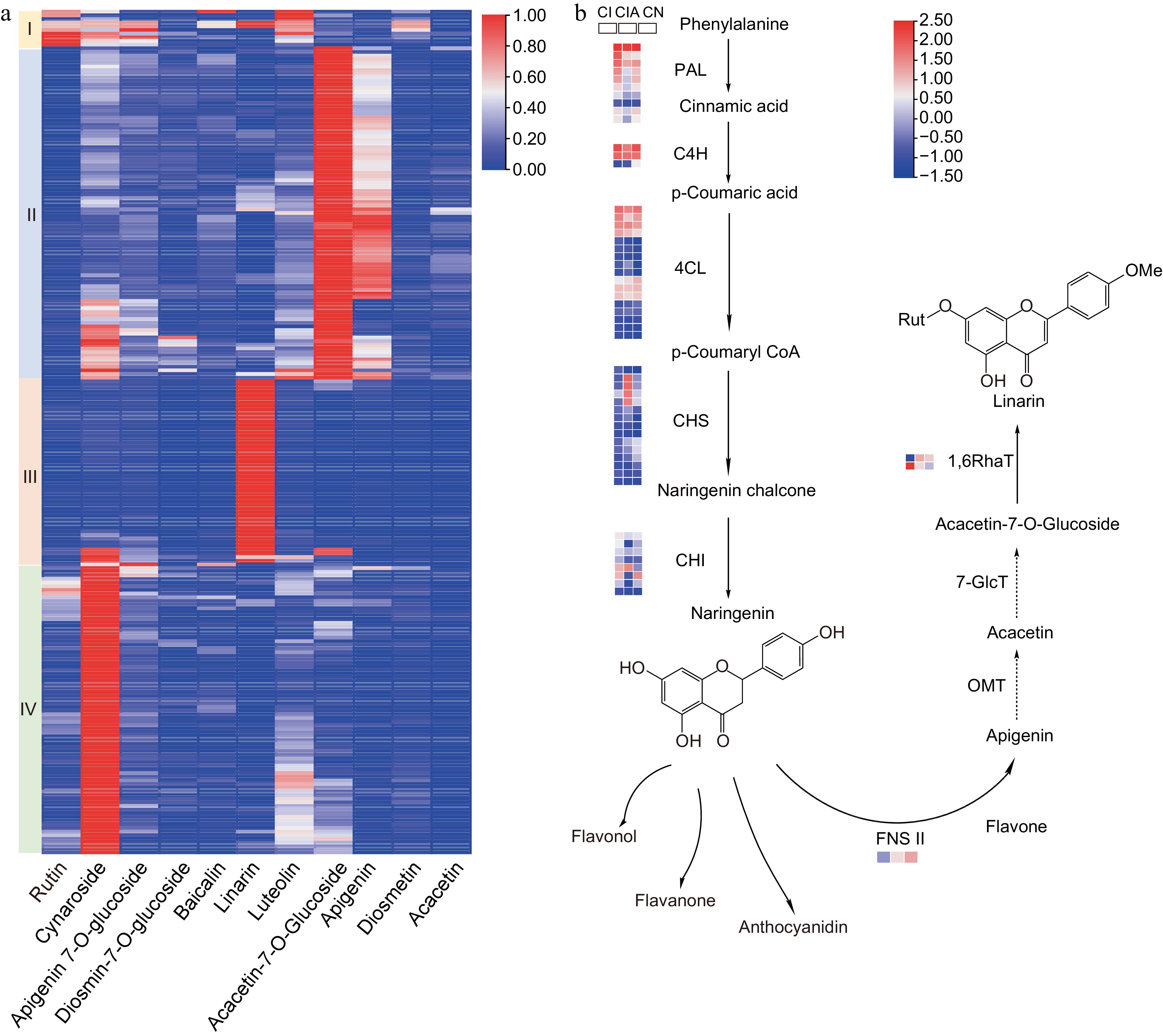
Figure 4.
The main flavonoids presented in the Chrysanthemum indicum complex populations and the speculated biosynthesis pathway of linarin. (a) The C. indicum populations revealed differential distribution of main flavonoids. (b) The possible biosynthesis pathway of linarin. The expressions of potential pathway genes from C. indicum (CI), C. indicum var. aromaticum (CIA) and C. nankingense (CN) were also revealed. PAL, phenylalanine ammonia-lyase; C4H, cinnamate-4-hydroxylase; 4CL, CoA ligase; CHS, chalcone synthase; CHI, chalcone isomerase; FNS II, flavonoid synthase II; OMT, O-methyltransferase; 7-GlcT, flavonoid-7-O-glucosyltransferase; 1,6RhaT, flavonoid-7-O-glucoside-1,6-rhamnosyltransferase.
-
Our understanding of the major evolutionary transitions in plants, such as the emergence of novel adaptative characteristics or metabolism, is supported by an accurate phylogenetic tree[77]. The complicated relationship between the C. indicum complex and the other related Chrysanthemum species created by multiple evolutionary mechanisms such as natural hybridization and polyploidization making phylogenetic reconstruction on these taxa is particularly difficult. A recent study on the backbone phylogeny of Asteraceae plants showed the origin of Asteraceae at ~83 million years ago (MYA) in the late Cretaceous and the family underwent a series of dramatic radiations during the Eocene[78]. However, it is still unclear for the divergence time of the C. indicum complex from other Asteraceae members. With advances of the abundance of high-quality genome assembly that have benefited from the ease and low cost of genome and transcriptome sequencing, building a phylogenetic tree using whole genome data may reveal its power for resolving complex evolutionary scenarios in plants, including both hybridization and polyploidization[79]. In addition, for the identification and assessment of diverse chrysanthemum resources, it is urgently necessary to develop more powerful molecular markers based on genome-wide phylogenetic signals.
The production of secondary metabolites from medicinal plants has obviously spatiotemporal effects, involving complex transcriptional regulatory networks at the genetic and epigenetic levels[80]. Taking linarin from the C. indicum complex as an example, diverse diploid and tetraploid C. indicum accessions collected from different geographical locations can reveal various accumulations of linarin contents, including the variation of those related ingredients from the corresponding pathways[74]. However, the transcriptional regulation which directly control gene expressions affecting the linarin biosynthesis is far from resolved, which can be partially attributed to the lack of genomic data of C. indicum. Different environmental factors can work together to affect the biosynthesis of active ingredients via hormone signal-transcription regulatory networks, leading to a multilayered mechanism of transcriptional regulation[81]. At this point, future study on the C. indicum complex should place a focus on the molecular interactions between the environmental factors and its genetics, as these interactions are crucial for maintaining the quality of the C. indicum therapeutic materials.
Breeding of medicinal plants is a challenging task because most of them have complicated genomic backgrounds (e.g., the high heterozygosity and polyploidy) and are generally perennial plants. Without changing the composition of the medicinal active ingredients, developing new C. indicum cultivars with novel characteristics such as plant architectures, flowering related traits, postharvest quality, and biotic and abiotic stress tolerance in a time- and cost-efficient pattern is particularly important[82]. With the advance of high-throughput technologies producing substantial amounts of omics data, accelerating the breeding of chrysanthemum is possible by integrating omics data with phenotypic information. For instance, genomic selection (GS) can help facilitate the rapid selection of superior genotypes and speed up the breeding cycle by using both genotypic and phenotypic data from a training population to predict breeding values[83]. In future, smart breeding of medicinal plants might be accomplished by a compressive combination of big data, artificial intelligence and integrated genomic-environmental prediction[84]. At the same time, genome-wide data can help formulate conservation guidelines of wild C. indicum resources. Given the great variation present between different local populations in relation to variable ecotypes or varieties such as C. indicum var. aromaticum within the complex, conservation strategies should be made to maximize the preservation of genetic and metabolic diversity, in terms of breeding and medicinal applications.
-
The authors confirm contribution to the paper as follows: study design: Liu Y; data collection: Lei D, Wang X, Zhang J; figure processing: Zhu M, Zhang H, Liao J; data analysis & draft manuscript preparation: Wang X, Lei D, Liu Y; manuscript revision: Liu Y. All authors approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This work was supported by the Natural Science Foundation of China (32270231) and the Special Scientific Research Projects for Double First-Class Construction of Hubei University of Chinese Medicine.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang X, Lei D, Zhu M, Zhang H, Liao J, et al. 2023. Phylogeny, genetics and ecological adaptation of the Chrysanthemum indicum complex. Medicinal Plant Biology 2:17 doi: 10.48130/MPB-2023-0017
Phylogeny, genetics and ecological adaptation of the Chrysanthemum indicum complex
- Received: 19 September 2023
- Accepted: 20 October 2023
- Published online: 22 November 2023
Abstract: Chrysanthemum indicum L. is a member of the Asteraceae family and its dried capitulum has been utilized for thousands of years in traditional Chinese medicine. The C. indicum complex contains both diploid and polyploid (mainly tetraploid) cytotypes and also has a variety of C. indicum var. aromaticum Q.H.Liu et S.F.Zhang which is endemic to the Shennongjia Forest region. Additionally, two species of C. nankingense (Hand.-Mazz.) X.D.Cui and C. lavandulifolium (Fisch. ex Trautv.) Makino exhibit the strongest evolutionary connections to C. indicum, and natural hybridization between them is common. Due to the extensive natural range of the C. indicum complex in China including many eco-geographic populations, there is a significant amount of variation in its morphology and metabolic makeup. Here, we reviewed the advances in phylogeny, population genetics, and ecological adaptation of the C. indicum complex, as well as those studies involving genomes and functional genes that may help us better understand the molecular mechanisms underlying the complex’s phenotypic variation and biogeographic distribution. To enhance the medicinal applications of the C. indicum complex resource, future study should place a greater emphasis on resolving the complicated species relationship, dissecting the transcriptional regulatory network of active components, and enhancing omics data-assisted breeding.
-
Key words:
- Chrysanthemum indicum /
- Phylogeny /
- Genome /
- Ecological adaptation /
- Secondary metabolites












