-
Angelica sinensis (Oliv.) Diels, a member of the Angelica genus within the Apiaceae family, is commonly known as Danggui in China[1]. This species holds profound cultural significance and is widely used in traditional Chinese medicine[1]. Its use is recorded in one of the oldest Chinese herbal books, Shennong's Classic of Materia Medica[2] dating back to approximately 2,000 years ago (the Han Dynasty). A. sinensis is naturally distributed in shaded, moist, and cool areas at around 2,000 m altitude[3]. The main primary producing regions in China are Mincounty and its neighboring regions, e.g. Tanchang and Weiyuan county, in Gansu province, with scattered distribution in Shaanxi province, Sichuan province, Yunnan province, and Hubei province[4−6]. The main cultivated varieties are Mingui 1 and Mingui 2[4].
Angelica sinensis radix (ASR), is the main organ used as food and medicine in Asian countries, and also as a dietary supplement for women's health in the west[7,8]. Generally, different parts of the root exhibit distinct therapeutic effects: the heads of ASR are primarily used for stopping bleeding, the bodies of ASR for nourishing blood, and the tails of ASR for disrupting blood stasis[9]. ASR has been extensively applied in the treatment of gynecological disorders. ASR extract and its active compounds, e.g. ligustilide, can also be used as an anti-inflammatory and antibacterial substance in personal care products for naturally healing skin conditions such as alopecia, psoriasis, and eczema[10−12]. Due to the application of ASR in multiple industries, the growing demand for A. sinensis and its derivatives has led to an expansion of cultivation efforts throughout China and other Asian countries, resulting in an annual output value of about RMB 10 billion yuan[13].
In recent years, the utilization of multi-omics approaches, including genomics, transcriptomics, proteomics, and metabolomics, has significantly advanced physiological and molecular studies of A. sinensis. Three notable areas of research focus include:
(1) biosynthetic pathways of active compounds, and their underlying regulatory mechanisms;
(2) molecular mechanism of early bolting and flowering which significantly devalues the root;
(3) molecular markers for distinguishing the top-geoherbalism samples of ASR.
This review focuses on the latest research regarding these three topics using multi-omics data in A. sinensis, with a specific emphasis on the genetic and molecular bases of medicinally-active compounds found in this plant. We aim to provide an overview of current progress in molecular studies closely related with the ASR industry, which will shed light on the cultivation, breeding, management, and medicinal use of the species.
-
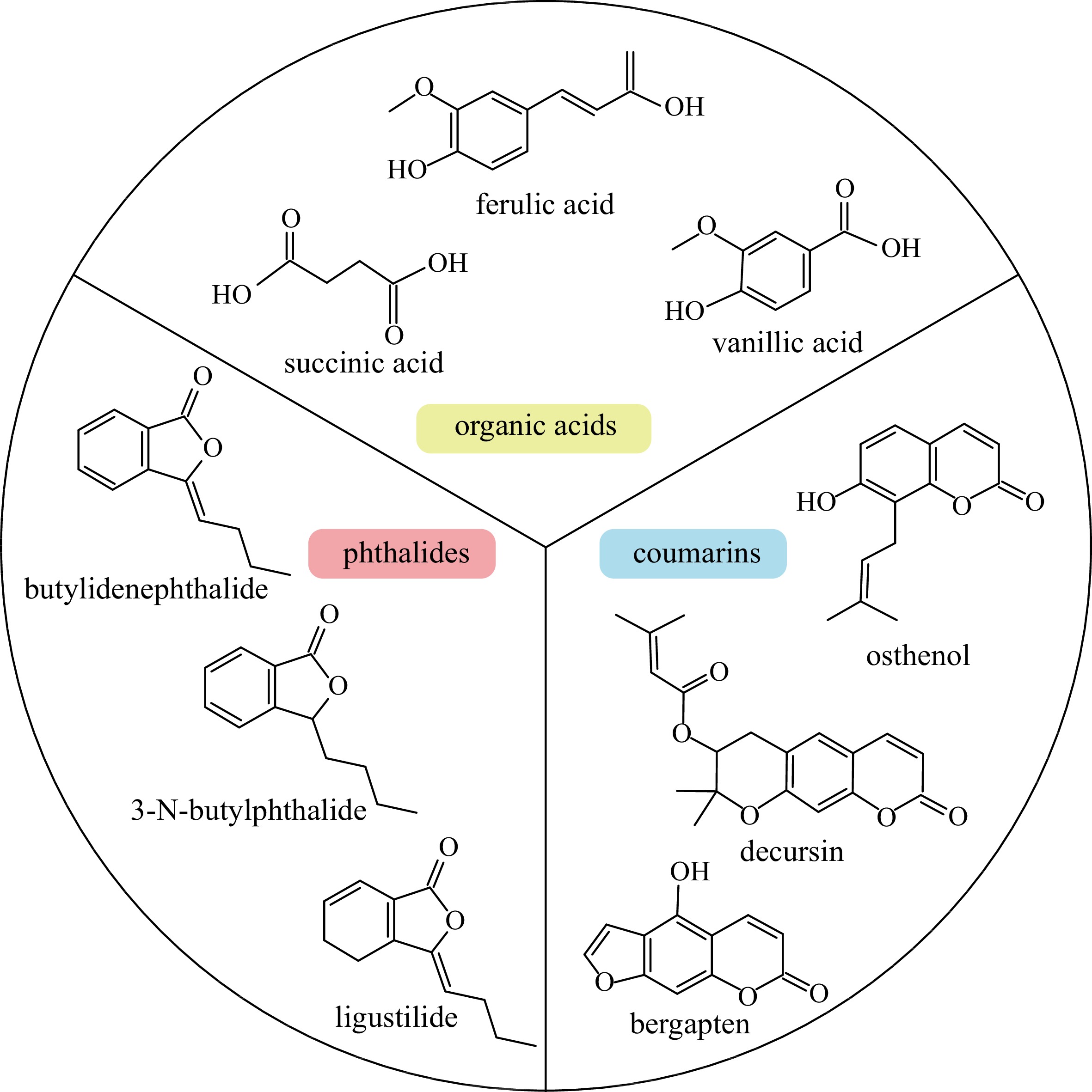
Various active compounds have been identified from A. sinensis, with their content varying among tissues. These reported constituents include organic acids, phthalides and coumarins (Fig. 1). The organic acids in A. sinensis mainly include ferulic acid, succinic acid and palmitic acid[14]. Ferulic acid was the earliest organic acid isolated from ASR and was considered as a control standard to determine quality of ASR in Pharmacopoeia of the People's Republic of China[1,15]. Among all parts of A. sinensis, the root tails exhibited the highest content of ferulic acid, but this varied among different populations of A. sinensis[16]. Ligustilide is the main component of essential oil, another quality control standard, in A. sinensis[17], and it contains a diverse array of phthalide components, with as many as 47 others detected, including butylidene phthalide, 3-N-butylphthalide[17].
In addition to the two types of indicator compounds, A. sinensis contains other compounds with biological activity. Coumarins, which are present at high levels in A. sinensis, can be divided into simple coumarins, furanocoumarins, and pyranocoumarins[18]. Recent research identified 14 coumarins in A. sinensis, including osthenol, isoimperatorin, xanthotoxol, xanthotoxin, isopimpinellin, bergapten marmesin, etc[19]. Biosynthesis and accumulation of these active compounds are influenced by various environmental factors, e.g., light intensity and relative humidity[20,21].
Molecular research related with active compounds
-
With the development of omics technology, the combination of genomics, transcriptomics and metabolomics has been widely used in dissecting the biosynthetic pathways of active compounds in A. sinensis. The correlation between metabolite content and gene expression levels, the co-expression pattern of known and unknown genes in the pathways, phylogenetic trees and potential biosynthetic gene clusters are typical approaches to screening out candidate genes in the biosynthetic pathways. Table 1 summarizes the published literature related to the biosynthetic pathways of active components in A. sinensis, on which we have outlined the elementary framework of the biosynthetic pathways of ferulic acid, coumarins, and flavonoids based on functionally verified biosynthetic genes in other plants, especially model plants (Fig. 2). Only a few genes involved in this process have been cloned, such as 4-coumarate-3-hydroxylase (C3H), O-methyltransferase (COMT), etc.[22,23], and not all genes have been functionally verified in A. sinensis (Fig. 2).
Table 1. Summary of transcriptomics reports from Angelica sinensis.
Tissues Research focus Reference Pathway of active compounds Root head and tail Phenylpropanoid biosynthesis pathway and ferulic acid metabolites [26] Root head and tail Phenylpropanoid biosynthesis [27] Root head and tail Metabolites [31] Root Phthalides biosynthetic [32] Leaf and petiole from cultivars Mingui 1 and Mingui 2 Flavonoid biosynthesis [30] Root from cultivated and wild resources Metabolism pathway [3] Leaf from cultivars Mingui 1 and Mingui 2 Flavonoid biosynthesis [33] Root head and tail Ferulic acid biosynthesis [34] Root head, body and tail Ferulic acid biosynthesis [28] Early bolting and flowering Flower bud of early flowering Early bolting and flowering [35] Mixed sample from different growth stages Early bolting and flowering [36] Whole shoot tip and young developing leaf Early bolting and flowering [37] Leaf, stem, seed, and root from early bolting plant Early bolting and flowering [38] Seedling with different vernalization treatments Early bolting and flowering [39] Leaf from normal and early bolting plant Early bolting and flowering [40] Seedling stored at different temperature Early bolting and flowering [41] Seedling GAs biosynthetic pathway [42] Adversity and stress Leaf under UV-B radiation Adaptive mechanism under UV-B radiation [43] 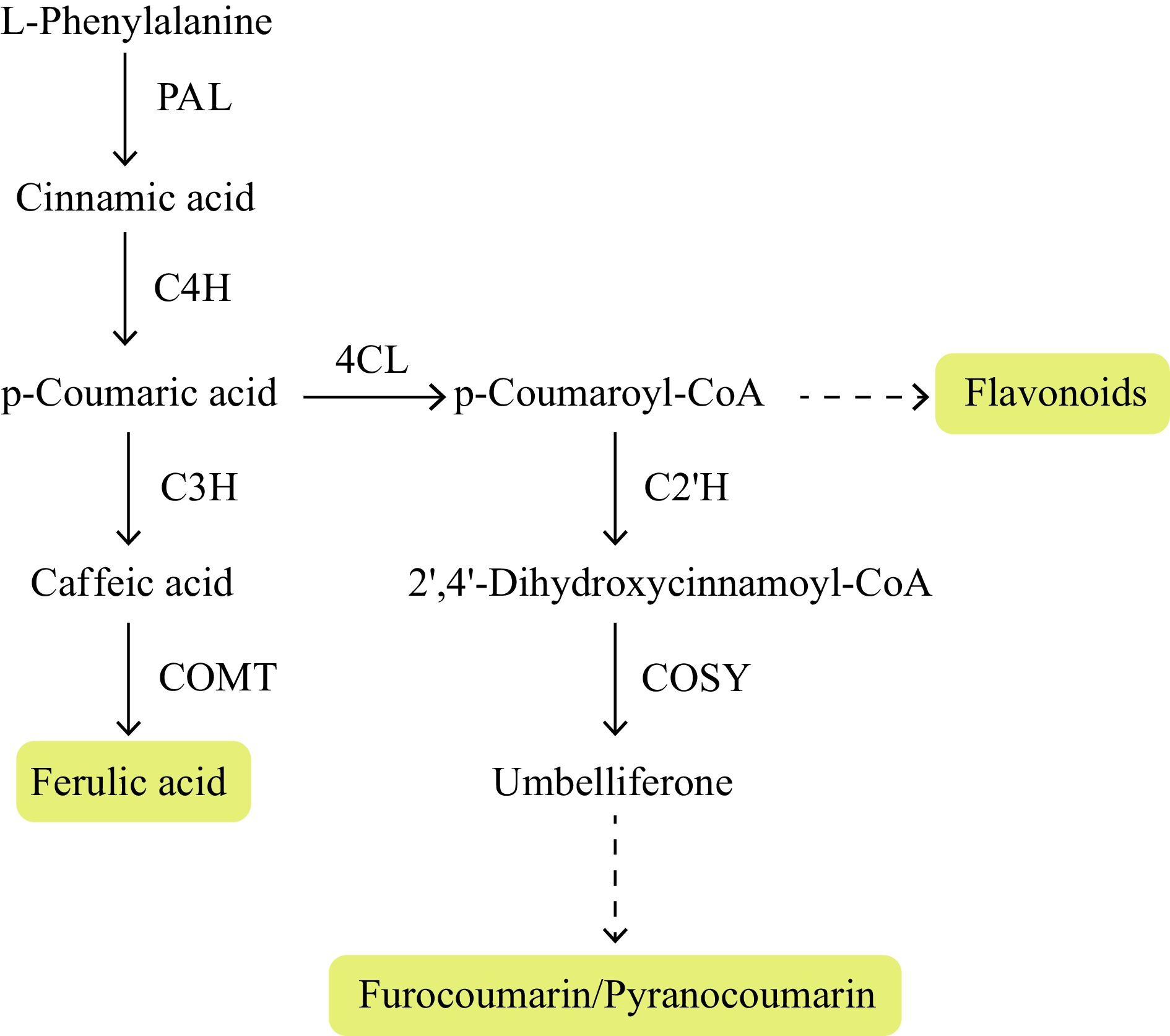
Figure 2.
Proposed ferulic acid, coumarin and flavonoid biosynthesis pathways in Angelica sinensis. Note: PAL, phenylalanine ammonia-lyase; C4H, cinnamate 4-hydroxylase; C3H, 4-coumarate-3-hydroxylase; COMT, O-methyltransferase; 4CL, 4-coumarate CoA ligase; C2'H, p-coumaroyl CoA 2'-hydroxylase; COSY, coumarin synthase.
The genome of A. sinensis (2n = 22) is 2.23 Gb in size, with low heterozygosity of 0.2%[24]. The genome contains 45,567 protein-coding genes and is characterized by a high percentage of repetitive sequences (77.74%)[24]. The A. sinensis genome shows expansion of gene number involved in phenylpropanoid biosynthesis. Combining transcriptomes and metabolomes, 81 genes were identified encoding enzymes responsible for the biosynthesis of ferulic acid and coumarins[24]. Among them, one, and three genes encode coumarin synthase (COSY), and COMT, respectively, showing gene family expansion and significant correlation with coumarin content.
Ferulic acid is an essential active compound in ASR, and a commonly used marker to detect and determine quality. The synthesis of ferulic acid occurs through COMT (Fig. 2), which is a crucial step in phenylpropanoid biosynthesis[25]. Experimental and clinical data revealed an uneven accumulation of ferulic acid and flavonoid content in different parts of the root and different cultivars of A. sinensis[26−28]. Metabolites in A. sinensis vary across different root parts, heads, bodies, and tails. Analysis of the root heads and tails identified 1,336 metabolites with significantly different content, involved in multiple metabolic pathways, including phenylpropanoid biosynthesis, isoquinoline alkaloid biosynthesis and plant hormone signal transduction[29]. Root tails contained higher levels of several metabolites than other root parts, especially ferulic acid and umbelliferone, while root heads showed higher levels of ligustilide[28].
Differences in the metabolites possibly reflect differential expression of key genes in biosynthetic pathways of plant secondary metabolites (Table 1). To identify such genes, Yang et al. identified transcripts isolated from the root heads and tails of A. sinensis using a high-throughput sequencing approach (Illumina HiSeq 2000), and identified 3,359 differentially-expressed genes, of which 15 were involved in phenylpropanoid biosynthesis and ferulic acid metabolism[26]. In other research with root head, body and tail samples, 28 unigenes involved in ferulic acid metabolism were identified, with 17 unigenes highly expressed in the root tails[28]. Transcriptome analysis of different cultivars also revealed significant differences between cultivars Mingui 1 and Mingui 2 (Table 1), with 2,210 differentially-expressed genes identified[30]. These genes were shown to be involved in organ-specific functions, and 29 of them were annotated as related to flavonoid biosynthesis, consistent with the abundance of flavonoid metabolites in these cultivars[30].
-
Growth and development of the cultivated A. sinensis plant is usually complete within three years, with seed cultivation (seedling period) in the first year, vegetative growth (tuberous root preparation period) in the second year, and reproductive growth (flowering period) in the third year[4].
However, in the practical production process, early bolting and flowering occurs in some plants in the second year of the vegetative growth period due to environmental factors[44]. This ultimately results in root lignification and block of secondary metabolite formation, with the root becoming hollow and losing its medicinal value[4]. Early bolting occurs in over 30% of plants in Gansu province, and this leads to lignified roots and economic losses of ~ 40 million Yuan per annum[4]. Hence, early bolting and flowering is one of the most serious problems in the production of A. sinensis.
Molecular mechanism of early bolting and flowering
-
A multi-omics approach has been used to investigate the content of secondary metabolites and the pattern of gene expression in early bolting. Compared to normal plants, early bolting plants have lower content of soluble sugars and proteins but higher content of free amino acids and activity of peroxidase and polyphenol oxidase[45]. More importantly, early bolting and flowering of A. sinensis seriously affects the quality of the roots and accumulation of secondary metabolites[46,47]. Ferulic acid, total flavonoids and total phenol content of non-bolting plants were 15.5, 2.4 and 3.0 times of that of early-bolting plants, respectively[46,47].
Li et al.[36], Gao et al.[37] and Wang et al.[40] conducted transcriptome sequencing of early- and non-bolting plants and found differentially-expressed genes involved in plant hormone signaling and flowering pathways. Vernalization is a phenomenon in which the flowering process is facilitated through extended exposure to cold treatment, either applied to a fully hydrated seed or a growing plant. Without vernalization, plants exhibit delayed flowering or continue to remain in a vegetative state. Luo et al. conducted transcriptome analysis of A. sinensis, involving comprehensive sampling under various vernalization treatments, including uncompleted, completed and avoided vernalization, to investigate the gene regulatory networks regulating flowering[39]. This network included the vernalization pathway (e.g., flowering locus C (FLC), flowering locus T (FT), suppressor of overexpression of constans 1 (SOC1), LEAFY (LFY)), the autonomic pathway (e.g., flowering time control protein FCA (FCA), flowering time control protein FPA (FPA)), the age pathway (e.g., sucrose synthase (SUS), sucrose-phosphate synthase (SPS)), the gibberellic acid (GA) hormone pathway (e.g., histidine kinase 3 (AHK3), auxin transporter-like protein 2 (LAX2)) and circadian clock (e.g., cold-regulated protein (COR27)). Meanwhile, 272 long non-coding (lncRNAs) were identified, which demonstrated positive or negative regulation of flowering of A. sinensis[41].
-
Top-geoherbalism, known as 'Daodi' in China and 'provenance' or 'terroir' in Europe, pertains to the cultivation of traditional herbs in specific native areas where they exhibit superior quality and efficacy compared to those grown in other regions[48]. It is closely associated with folklore, geographic, climatic, and soil conditions, as well as genetic factors. Min county and the surrounding areas in Gansu province in China are the top geoherb areas of A. sinensis. Multiple varieties of A. sinensis from both top- and non-top-geoherbalism regions and possibly its close relatives with lower medicinal values exist on the market, which largely affects the standardization of the traditional Chinese medicine (TCM) market[49,50].
The molecular mechanism of top-geoherbalism in A. sinensis
-
DNA molecular markers identified in radix from different Angelica species or from different production regions of A. sinensis have been widely used in identifying top-geoherbalism of ASR, as they have stable genetic traits and high genetic polymorphism. At present, DNA molecular markers, such as RAPD (random amplified polymorphism DNA), AFLP (amplified fragment length polymorphism), ITS (internal transcribed spacer), SSR (single sequence repeat) and ISSR (inter simple sequence repeat), have been applied in A. sinensis.
RAPD utilizes short random primers to amplify DNA fragments and has been applied to distinguish three medicinal plants, A. gigas, A. sinensis and A. acutiloba[51,52]. AFLP markers were effective in distinguishing wild and cultivated samples and different varieties (purple and green stem) of A. sinensis, which indicated the two varieties of A. sinensis may have different genotypes[53]. ITS sequences were utilized as a DNA barcode to differentiate Angelica plants, with the phylogenetic tree of species in the genus based on ITS demonstrating strong bootstrap support and clear phylogenetic relationships[54,55], however the effectiveness of ITS sequence in distinguishing different varieties of A. sinensis was not consistent. Zhang et al. found that there was no difference in ITS sequence among wild and cultivated A. sinensis or different phenotypes (green and purple stem)[56]. However, Chu et al. found that ITS sequences can be recognized as effective molecular markers of A. sinensis, and both 586 site base and 589 site base of the ITS sequence of A. sinensis can be regarded as identification bases of the top-geoherbalism in ASR[57]. Using SSR markers, 18 microsatellite loci from A. sinensis have been isolated to date, which have been useful in identifying top-geoherbalism and to investigating genetic diversity and population structure in this medicinal plant[58].
Metabolites can also be used as molecular marker to identify top-geoherbalism of A. sinensis. A metabolomics study of ASR from top geoherb regions, Min county and Weiyuan county, Gansu province and non-top geoherb regions, including Qinghai province, Sichuan province, Hubei province, Ningxia province, Xizang province, and Yunnan province identified 51 volatile organic compounds[59] and 14 inorganic elements[60]. Among these, volatile organic and inorganic elements, β-ocimene, α-pinene, 3-methylbutanal, heptanes, butanal were reported as biochemical markers for identification of the top-geoherbalism[59]. Seven secondary metabolites, including chlorogenic acid, ferulic acid, xanthotoxin, coniferylferulate, Z-ligustilide, decursin and decursinol angelate, were considered as biomarkers of radix in A. sinensis, A. gigas and A. acutiloba[61,62]. Moreover, untargeted/targeted metabolomics and glycomics in ASR and A. acutiloba radix (AAR) revealed that the type and abundance of secondary metabolites and carbohydrates differed in ASR and AAR[63]. Additional metabolomic studies of A. sinensis will provide us insights into metabolite accumulation under different conditions and identify further agricultural management practices to improve the content of target metabolites.
-
With the rapid development of sequencing technology and analysis methods, researchers have focused on the genetics of A. sinensis due to its medicinal value, leading to the investigation of various biological processes. In-depth studies of the molecular mechanisms underlying biological processes, such as the accumulation of active compounds, the molecular mechanism of early flowering and the genetic basis of top-geoherbalism are now feasible. In recent years, numerous breakthroughs have taken place in the molecular study of A. sinensis[26,30], including the release of its chromosome-level genome, which paves the way for genetic improvement of important quality traits of A. sinensis and future genomic research[24]. Genomic data will inform studies of accumulation and regulation of active components, and identify molecular markers for selective breeding of A. sinensis for particular high-value traits, notably high content and variety of coumarins[19].
In the following paragraphs, we will present the current limitations in molecular studies of A. sinensis and propose future prospects that aim to address these limitations.
Domestication and top-geoherbalism
-
Owing to its long cultivation history, A. sinensis is an ideal example to investigate the domestication history of medicinal plants. The long-term intense artificial selection of cultivated individuals has probably resulted in a severe genetic bottleneck, and the genetic diversity of cultivated varieties is significantly reduced compared to their wild ancestors[64]. The current A. sinensis reference genome is from the widely cultivated variety, Mingui 1[24], which obviously cannot represent the rich genetic diversity of other germplasms and its wild relatives. A lack of whole-genome resequencing of A. sinensis germplasms and its wild relatives limits deep understanding of the genetic mechanism of the domestication of A. sinensis, especially the genetic changes underlying variation in secondary metabolite contents.
The variation in genotypes among populations in different regions and differences in environment, such as climate and soil, can lead to the variation in chemical composition[65]. In the long process of evolution, medicinal plants adapt to the environment to produce special secondary metabolites, which are often the active compounds. Abiotic stress is expected to trigger the accumulation of secondary metabolites and it is believed that adversity may be more conducive to top-geoherbalism of TCM[66]. The top-geoherbalism region of A. sinensis lies in the south-western part of Shanxi province, generally high altitude regions with harsh conditions, i.e., intense UV radiation and low temperatures. The genetic causes of top-geoherbalism and adaptation to harsh environments have not yet been reported.
The genetic mechanism of domestication and top-geoherbalism in A. sinensis awaits thorough exploration based on a comprehensive sampling of the diverse germplasms of A. sinensis, including cultivated varieties and wild species. The content of significant secondary metabolites and other morphological traits, such as the volume and weight of tuberous roots and flowering time, should be collected on germplasm planted in the same common garden to avoid the influence of environmental factors. Genetic markers such as single nucleotide polymorphism (SNP), copy number variant (CNV), and structural variation (SV) should be utilized to explore the genetic basis of key trait changes during the domestication process.
Biosynthetic pathway of active compounds
-
One type of omic data is not sufficient to unravel the biosynthetic pathways of active compounds. Compared to gene expression profiles at the mRNA level, protein expression is a more direct and reliable reflection of the function of key enzymes in these biosynthetic pathways. Correlating the proteome profiles of various tissues of the same plant and the content of active compounds could reveal the candidate genes involved in the biosynthesis of active compounds in medicinal plants. For example, by combining metabolomic, transcriptomic, and proteomic data, the authors found a number of differentially produced/expressed metabolites, transcripts, and proteins that are involved in the biosynthesis of suberin, phenylpropane, cutin, and waxes[67]. One putative gene, PbHHT1, which encodes ω-hydroxypalmitate-O-feruloyl transferase, was experimentally confirmed to be involved in russeting in sand pear fruit[67]. However, no proteome data is currently available for A. sinensis. It is imperative to integrate such data to dissect the biosynthetic pathways and regulatory mechanisms of active compounds in A. sinensis. Additionally, the biosynthetic pathways of some secondary metabolites, such as pyrancoumarins and phthalides, have not yet been fully elucidated and warrant further investigation.
The biosynthetic pathway of active compounds and related enzymes in A. sinensis can be clarified by cutting-edge multi-omics technology. The application of single-cell and spatial transcriptome technology is promising in dissecting the detailed map of biosynthetic pathway of active compounds. For example, Li et al. used a complementary multi-omics approach to interrogate the monoterpene indole alkaloid (MIA) biosynthetic pathway in Catharanthus roseus[68]. In this study, the combination of single-cell transcriptome and metabolome led to the discovery of sequential cell-type-specific partitioning of the leaf MIA biosynthetic pathway and the identification of a reductase producing the bis-indole alkaloid anhydrovinblastine. Such technology will benefit the identification of genes or enzymes in involved in the biosynthesis of active compounds in A. sinensis. Meanwhile, proteomics data will also promote our understanding of the translational regulation of secondary metabolites and their biosynthetic pathways.
Early bolting and flowering
-
Studies on early bolting and flowering of A. sinensis are limited to identifying candidate genes involved in bolting and flowering[37,39,40]. However, key genes and/or master regulators have not yet been experimentally verified.
Studies on key genes related to early bolting and flowering of A. sinensis, such as FLC, FT, SOC1 and LFY, require further in vivo or in vitro functional verification. Recently, a potential transformation system for A. sinensis was reported[69], which will benefit functional experiments of flowering time genes in A. sinensis. In addition, various gene editing methods, i.e., CRISPR/Cas9, RNA interference (RNAi), and virus induced gene silencing (VIGS), are prospective to be constructed for A. sinensis to either reduce expression of targeted genes by gene knockout or inhibition, or increase expression by gene overexpression technologies.
-
The authors confirm contribution to the paper as follows: writing and figure preparation: Han X; review and editing: Li M, Yuan Q, Lee S, Li C, Ren Y, Garth M; conceptualization and supervision: Wang L. All authors reviewed, read, and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This work was supported by the National Natural Science Foundation of China (Grant No. 32070242), the Natural Science Foundation of Guangxi (Grant No. 2020GXNSFAA159151), the National Key Research and Development Program of China (Grant No. 2020YFA0907900), the Shenzhen Science and Technology Program (Grant No. KQTD2016113010482651) and special funds for science technology innovation and industrial development of Shenzhen Dapeng New District (Grant Nos RC201901-05 and PT201901-19).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Han X, Li M, Yuan Q, Lee S, Li C, et al. 2023. Advances in molecular biological research of Angelica sinensis. Medicinal Plant Biology 2:16 doi: 10.48130/MPB-2023-0016
Advances in molecular biological research of Angelica sinensis
- Received: 15 June 2023
- Accepted: 22 September 2023
- Published online: 10 November 2023
Abstract: Angelica sinensis (Oliv.) Diels belongs to the Apiaceae family. The root of A. sinensis, is used in traditional Chinese medicine for its antioxidant and immune regulation properties. The main active compounds in A. sinensis include organic acids, phthalides and coumarins, and their biosynthetic pathways are the focus of international attention. A. sinensis is prone to early flowering and bolting, which negatively impacts production for several reasons, including germplasm degradation and quality instability in artificial cultivation. The identification of top-geoherbalism of A. sinensis has also become the focus of recent research, as it would allow selection for breeds with excellent medicinal quality and remarkable curative effects. Advances in sequencing technology and bioinformatic methodologies have enabled extensive molecular and genetic studies in A. sinensis. In this review, we summarize the latest molecular research advances related to A. sinensis, including biosynthetic pathways and regulation of active compounds, and molecular underpinnings of early bolting and flowering and top-geoherbalism. We discuss limitations of the current research and propose prospective topics in need of further exploration.