-
The growth and development process of higher plants is usually divided into three phases: juvenile phase, adult vegetative phase and adult reproductive phase, and each development phase has a series of different morphological and biological characteristics[1]. The juvenile-to-adult phase transition is the most critical phase in the whole life history of plants, which determines whether plants can successfully flower and complete the whole life cycle[2].
The morphological, physiological, and epigenetic characteristics of plant individuals are different in the juvenile and adult phases. Adult plants have flowering ability and can flower under the induction of flowering factors and the external environment, so the identification of the end of the juvenile phase is often marked by whether the plant has flowering ability[3].
Different plants undergo different juvenile phases before reaching their flowering ability, with annual herbs having a shorter phase, perhaps just a few months, while perennial trees have a long and complex phase, often lasting years or decades (Table 1)[4]. After the end of the juvenile phase, perennials transition from periodic vegetative growth to reproductive growth. When a plant is in the juvenile phase, the external leaf morphology, trichome distribution and cell shape are significantly different from those in the adult phase[5,6]. The sugars and hormones in the juvenile phase are also different from those in the adult phase, affecting the duration of the juvenile phase[7]. Sugar is an important energy source produced by photosynthesis for plant life development and plays a key role in various plant life cycles by regulating osmotic pressure in plants[8]. In a mutant of Arabidopsis thaliana with impaired starch synthesis and catabolism, an insufficient supply of starch will prolong the duration of the juvenile phase, while the addition of exogenous sucrose to the mutant at a later period can restore the duration of the juvenile phase to normal, suggesting that sugars play a crucial role in the juvenile-to-adult phase transition[9]. Hormones are important endogenous signals that regulate the transition of plant growth and development and significantly affect the transition time from the juvenile-to-adult phase[10]. Most studies on this topic investigate brassinolide (BR), gibberellin (GA), jasmonic acid (JA), abscisic acid (ABA) and cytokinin (CTK). These genes related to hormone synthesis and signaling pathways are significantly differentially expressed in the juvenile and adult phases, and gene mutants also change the transition time from the juvenile-to-adult phase[10,11].
Table 1. Statistics of time of the juvenile phase in some species (according to the literature).
Species Juvenile phase (years) Reference Lilium spp. 2−3 [21] Piper nigrum 3−4 [22] Malus domestica 4−8 [23] Camellia nitidissima 8 [24] Citrus spp. 5 [25] Eucalyptus globulus 2−5 [26] Jatropha curcas 5 [27] Liriodendron chinense 8−10 [28] Populus spp. 7−10 [29] Pinus tabuliformis 5−7 [30] Ginkgo biloba 15−20 [31] Ziziphus jujuba 1−2 [32] miRNA is involved in many key plant development processes, such as vegetative growth, reproductive growth, seed setting and senescence, and plays a conservative role in regulating plant development[5]. Previous studies have shown that miR156 and miR172 are closely related to the juvenile phase[12]. In the juvenile phase of Arabidopsis thaliana, miR156 maintains a high transcription level and prevents the transition of Arabidopsis thaliana to the reproductive growth phase by inhibiting the expression of its target gene SPL. With the continuous development of the plant, the expression of miR156 is significantly downregulated, which weakens the inhibitory effect on SPL and shortens the juvenile phase[13]. miR172 acts as an SPL target and has the opposite function to miR156, acting on the APETALA2 (AP2) transcription factor family[14]. The AP2 transcription factor family is a widely known regulator of flowering time, and its members include TARGET OF EARLY ACTIVATION TAGGED 1 (TOE1), TOE2, TOE3, AP2, SCHLAFMÜTZE (SMZ) and SCHNARCHZAPFEN (SNZ), which have been widely reported to be involved in various physiological and biochemical processes of plants[15]. TOE1 controls adult traits and negatively regulates the transcriptional levels of GL1, a trichome regulator, delaying the production of Arabidopsis thaliana distal trichomes[16]. Arabidopsis thaliana is primarily regulated during the juvenile phase by the miR156-SPL module, which is influenced by epigenetics in various ways, such as DNA methylation, histone modification, and chromatin remodeling[17]. Epigenetic modification refers to heritable changes in phenotype by altering gene expression without changing DNA sequences[18]. In addition, members of the PEBP family, FT and TFL1, have also been widely reported to be involved in regulating the juvenile-to-adult phase transition[19]. When the PmFT gene from Prunus mume was transferred into Rosa rugosa, the juvenile phase of the rose transgenic strain was significantly shortened, and apical vegetative buds were quickly transformed into flower meristem buds[20].
The juvenile phase is a critical developmental phase in plants. While the external morphology is constantly changing, internal physiological changes are also produced. This phase has a complex molecular regulatory mechanism. This article focuses on the morphological markers, physiological characteristics, influencing factors and molecular mechanisms of the juvenile phase, which will lay a theoretical foundation for future studies on the juvenile phase.
-
Many species exhibit different morphological phenotypes in the juvenile phase, which are mainly reflected in trichome shape, leaf size, leaf shape, leaf base angle and SAM. A trichome is a structure composed of one or more cells in the plant epidermis that resists biological and abiotic stress and is divided into glandular-trichome and nonglandular-trichome according to secretion function[33]. The transformation of trichome morphology is one of the important markers to distinguish the developmental phases of tomato (Solanum lycopersicum). In tomato, there are two types of nonglandular trichomes (III and V) and 4 types of glandular trichomes (I, IV, VI and VII). Vandemiatti et al. showed that tomatoes in the juvenile phase always show type IV glandular trichomes, and the presence of V-type nonglandular trichomes indicates that the tomato is undergoing the juvenile-to-adult phase transition[34]. Arabidopsis has an adaxial trichome in the juvenile phase, which appears on the distal surface of the leaf as it develops into the adult phase[35]. During the vegetative phase transition of Glycine max, the SAM changes from flat to dome-shaped, its size also changes from small to large, the single leaf becomes the alternate compound leaf, and the ratio of leaf width to length also increases significantly[36]. The SAM shape and size of Oryza sativa show the same changes during the transition process, the leaves become elongated, the midvein is significantly clearer, and the leaf type changes from a single leaf to compound leaf[37]. Lawson & Poethig found that the adult epidermal wax of Zea mays is significantly reduced compared with that of the juvenile phase, the cell walls are more closely connected, and the corneum is thickened and blue under toluidine blue staining, while the corneum in the juvenile phase is purple[38].
In the developmental stage of plants, a series of physiological and biochemical reactions occurs[9]. Zea mays contains many sugar substances, such as glucose, in the juvenile phase, which can effectively release energy for plant growth and development[39]. Chuck et al. found that the Panicum virgatum (switchgrass)-converted Zea mays Corngrass1 (Cg1) gene has up to 250% internal starch and increases glucose content in the cell wall[40]. The chlorophyll and soluble protein contents in the seedlings of Vitis labrusca × vinifera 'Kyoho' decrease with increasing node and reach their lowest values during the juvenile-to-adult phase transition[41]. Dihydroflavonol reductase (DFR) is a key enzyme in anthocyanin synthesis, and changes in the activity of this enzyme affect the accumulation of anthocyanins in plant tissues[42]. Studies have shown that the DFR activity of Hedera helix is not present in the adult phase of the plant, while its activity is higher in its juvenile phase, which leads to the difference in anthocyanin accumulation between juvenile and adult plants[43]. The grafted tissue of Sequoiadendron giganteum has a specific membrane-related protein, J16, with a molecular weight of 16 ku, which is conducive to the morphological recovery of the grafted tissue in the juvenile phase[44]. Castanea mollissima contains two specific proteins, 38 ku and 43.6 ku, in adult tissues, but the existence of these two proteins is not detected in tissue culture seedlings in the juvenile phase, so the content of these two proteins reflects the developmental age of the individuals[45]. In Sequoia sempervirens, the relative molecular weights of the specific phosphorylated proteins in the juvenile and adult phases are 31 ku and 32 ku, respectively, and the relative molecular weights of the corresponding phosphorylated protein will become the same as those in the juvenile phase when the adult branches are grafted onto the juvenile rootstock[46]. The protein content of Malus domestica changes during growth and development, which is associated with increased photosynthesis during the juvenile phase, resulting in increased photosynthetic capacity during the juvenile phase. Enzymes involved in protein metabolism and breakdown during the adult phase are elevated, which means that a series of protein-related physiological changes take place in plants during their developmental processes[47].
-
There are significant differences in the duration of the juvenile phase between different plant species and varieties. Usually, annual plants have a shorter juvenile phase, which can end in the same year, and perennial plants have a longer juvenile phase, and the difference among them is greater. Bulb flowers are widely used and have important economic value, but their juvenile phase is long, such as 3−8 years for Narcissus, 4−7 years for Tulipa, and 2−3 years for Hippeastrum, from sowing to flowering[48]. Among them, Lilium has high medicinal, edible and ornamental value, and the juvenile phase of most lily species is approximately 2−3 years, while Lilium × formolongi can grow the stem and bloom in the same year of sowing[21]. The morphological difference between juvenile and adult Rosa is not obvious, so the first flowering is a sign of the end of the juvenile phase. Rosa is mainly divided into two types: the first is a short juvenile phase of only 20 d to 2 months, and the second is a long juvenile phase lasting 1−3 years[49]. The juvenile phase of Citrus generally lasts more than 6 years. Citrus grows vigorously, has thorns and does not flower in suitable conditions in the juvenile phase[25]. Precocious trifoliate orange (Poncirus trifoliata L. Raf.) found in Hubei Province, China, in the 1970s has a shorter juvenile phase, flowering in the second year of germination[50]. Liriodendron chinense is an excellent tree species for landscaping, with a juvenile phase of approximately 8−10 years, and the offspring of the slb1 mutant of the super long blooming 1 gene have a juvenile phase of only 4 months. This mutant not only has ornamental value but also may become a model plant for genetic studies in woody plants[28]. Pear (Pyrus) is a widely consumed fruit, and there are differences between varieties in the juvenile phase. The juvenile phase of hybrids is restricted by parental genetic characteristics. The offspring of Pyrus pyrifolia and Pyrus bretschneideri have a juvenile phase of 4−5 years, the offspring of Pyrus bretschneideri interbreeds have a juvenile phase of 5−6 years, and Pyrus communis has a juvenile phase of 14 years[3,51].
External factors
Temperature
-
The different responses of higher plants to environmental temperature during the juvenile phase result in differences in the duration of the juvenile phase. Temperature is one of the main factors driving banana (Musa nana) from colonization to flowering. This species ends the juvenile phase after growing the 7th leaf at a cooler temperature of 16 °C, while the juvenile phase ends when growing the 15th leaf at a warmer temperature of 24 °C[52,53]. After 4 weeks of low-temperature treatment at 15 °C, the expression levels of the SPL9 and SPL15 genes increase, the cell wall of the SAM becomes thinner, starch granules are reduced, and growth is accelerated to start the vegetative phase change (Fig. 1)[54]. However, Narcissus tazetta var. chinensis is sensitive to high temperature, and long-term exposure to a high temperature environment will activate SAM and increase the expression of the flowering factor FT gene to shorten the juvenile phase (Fig. 1)[55]. In Arabidopsis thaliana, warmer temperatures shorten its life cycle, when plants in the juvenile phase are exposed to cold for long periods, the number of rosette leaves and branches increases[56]. Brassica oleracea var. Italica has a short juvenile phase, and the juvenile phase of all varieties of Brassica ends at 3−6 weeks after seed germination. Low temperature can induce curd formation of the two Brassica varieties 'Waltham' and 'Green Mountain' at 4 weeks in the juvenile phase. The curd is composed of an inflorescence meristem and flower bud[57].
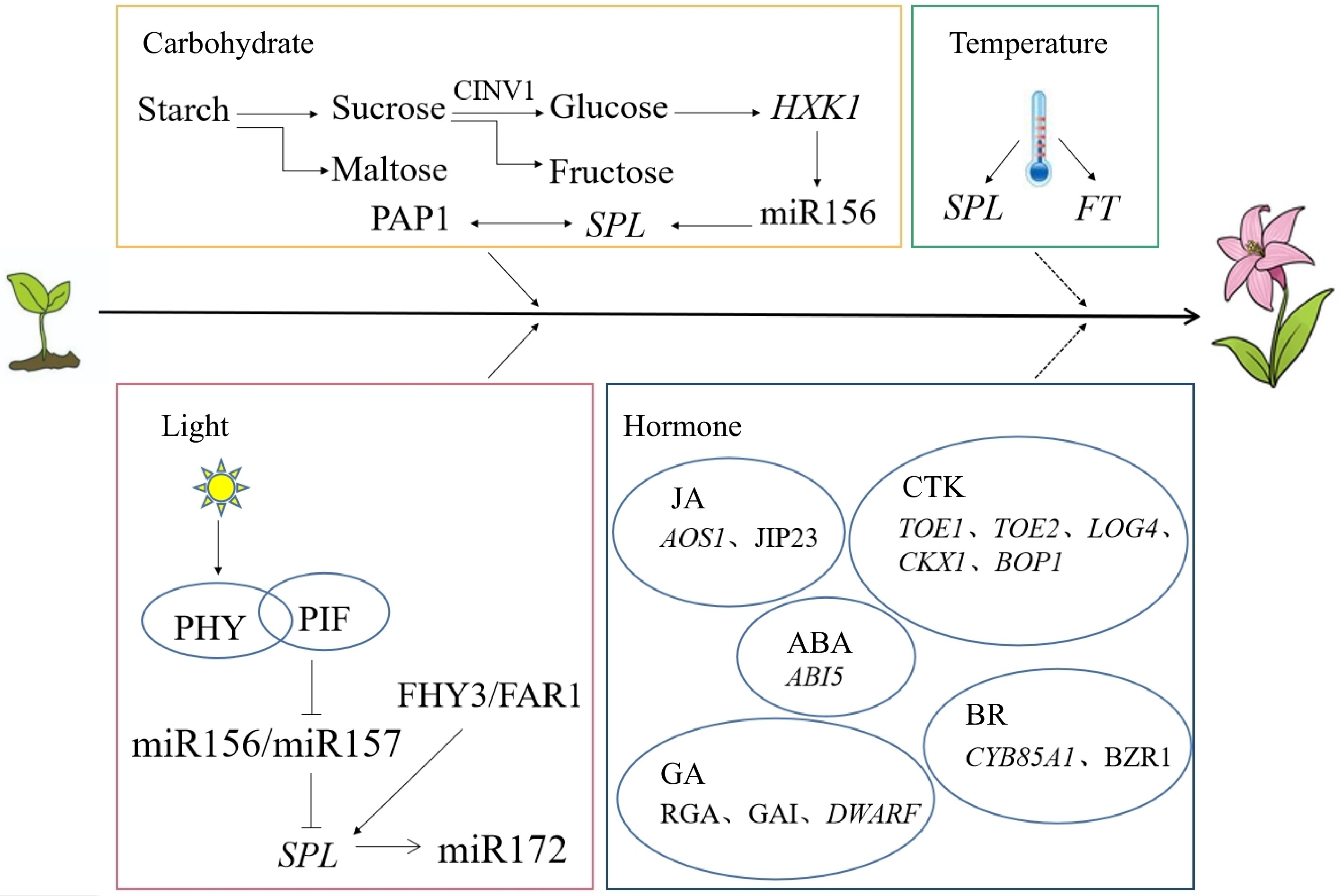
Figure 1.
Working mechanism of multiple factors regulating the juvenile phase in plants (according to the references). Solid arrows indicate positive interactions, dotted arrows indicate uncertain interactions, and flat lines indicate negative interactions[54,55,68−72,76,79,83,84,86,90,91,93−95,98,99].
Light
-
Light is a key factor regulating the juvenile-to-adult phase transition in plants. Light is the main component of the plant growth environment, and light intensity, photoperiod and lighting modes affect the duration of the juvenile phase[58−60]. Compared with the solar greenhouse, seedling progenies of Lilium regale are easier to obtain through the juvenile phase in the open field with sufficient light and suitable temperature[61]. A long juvenile phase is conducive to enhancing the photosynthetic capacity of plants, which is conducive to the production of high-quality crops. Under short-day conditions, the early flowering time of Glycine max is shortened in the juvenile phase, which limits the vegetative growth of Glycine max and results in a decrease in yield[62]. Studies on off-season production of Rubus idaeus found that the flowering time is earlier under long-day conditions and that the height of the internode flowering sites is lower than that under short-day conditions[63]. Helianthus annuus is a widely cultivated short-day flower. Short-day treatment during the true leaf stage can accelerate flower bud differentiation and development, improve cut flower quality and shorten the juvenile phase[64]. Vaccinium corymbosum seedlings blossom earlier under high light intensity (400 μmol·m−2·s−1) with 8 h of short day than with 12 h of long day[65]. The adaptability of Coffea arabica to light intensity is different in different stages of development. The epidermal cells and cuticles of leaves in the juvenile and adult phases are observed, and the activities of various oxidases are detected. Only leaves in the adult phase can adapt to high light intensity[66]. Increased expression of miR156/miR157 at low light intensity leads to decreased expression of the SPL gene and decreased carbohydrate synthesis, thus prolonging the juvenile phase of Arabidopsis thaliana[67]. When wheat is cultivated, the proportion of blue and red light in the spectrum decreases, the content of assimilates increases, and the time of the juvenile phase is shortened[58]. Plant perception of light is mediated by photophoresis (PHY) as a photoreceptor. Upon encountering light, plants initiate a complex internal process to interact with the transcription factor PHYTOCHROME INTERACTING FACTORs (PIF) to mediate the expression of miR156-SPL, while FAR-RED ELONGATED HYPOCOTYL3 (FHY3) and FAR-RED IMPAIRED RESPONSE1 (FAR1), related to photophoresis, interact with the SPL protein to downregulate miR172 expression and delay flowering time (Fig. 1)[68].
Sugars
-
Sugars can be used not only as nutrients but also as signaling factors to regulate the plant juvenile phase, inhibiting miR156 accumulation to regulate the juvenile phase and weakening the inhibition of the SPL gene to facilitate the juvenile-to-adult phase transition (Fig. 1)[69,70]. Starch levels affect the juvenile phase of Arabidopsis thaliana. When accumulated to a certain threshold, starch maintains a stable supply of maltose and sucrose for the juvenile-to-adult phase transition (Fig. 1). Starch-damaged mutants can also save the late-flowering phenotype by exogenous addition of sucrose and prolongation of the photoperiod[9]. Sucrose is an important source of carbon obtained in plants. Sucrose produced during photosynthesis can indirectly induce the expression of the PRODUCTION OF ANTHOCYANIN PIGMENTS 1 (PAP1) gene of anthocyanin synthesis through miR156-SPL to shorten the juvenile phase. Cytosolic invertase 1 (CINV1) breaks down sucrose into glucose and fructose (Fig. 1)[71]. The HEXOKINASE 1 (HXK1) gene encoding a glucose signaling protein has dual regulatory effects, which can promote glucose degradation and respond to changes in glucose concentration. Low glucose can help reduce the expression level of miR156, and regulating its transcription is conducive to the end of the juvenile phase (Fig. 1)[72]. Trehalose-6-phosphate (T6P) is a signaling sugar in plants, reporting the state of sucrose in the body and balancing sucrose levels as a negative feedback signal. When the expression of the trehalose-6-phosphate synthase (TPS1) gene encoding T6P synthesis is inhibited, the decreasing trend of miR156 with development is inhibited, and the end of the juvenile phase is delayed[73]. In summary, sugars can promote the end of the juvenile phase by reducing the expression of miR156.
Hormones
-
BR is a growth-promoting steroid hormone with a 5α-cholestane skeleton, a core ring and side chain consisting of four carbon rings, originally isolated in the pollen of Brassica napus[74]. BR is a plant hormone essential for normal plant growth and development and plays a role in resistance to biological and abiotic stresses, as well as regulating flowering time[75]. The expression of the oxidase gene CYP85A1 of BR is weak in the juvenile phase, and the promoter activity of the CYP85A1 and CYP85A2 genes is strong in the juvenile and adult phases (Fig. 1). Both Solanum lycopersicum and Arabidopsis thaliana contain this gene[76]. BR can regulate the content of cellulose, hemicellulose and lignin in Populus, shorten the juvenile phase and improve wood performance[77]. BR biosynthesis is essential for cell division, and by promoting cell division, the BR synthetic mutant dwarf7-1 (dwf7-1) has a slower rate of callus growth and induction than the wild type[78]. The leaves of Arabidopsis thaliana in BR-deficient and BR-insensitive mutants are rounded, and the transcription factor BZR1 in the BR signaling pathway interacts with SPL9 to regulate downstream gene expression and shorten the juvenile phase (Fig. 1)[79].
GA is an important hormone affecting the juvenile-to-adult phase transition. GA synthesis is mainly promoted through gibberellin 20 oxidase (GA20ox) and gibberellin 3 oxidase (GA3ox) biosynthetases, where GA20ox inactivates its precursor to promote turnover, and GA promotes the binding of the GIBBERELLIN INSENSITIVE DWARF1 (GID1) receptor to the DELLA protein. Thus, the ubiquitination of the 26S proteasome triggers DELLA degradation, promoting the plant's ability to express genes and grow[80]. GA can promote the juvenile-to-adult phase transition, and short-day exposure can also promote the formation of underleaf surface furs during the juvenile-to-adult phase transition in Arabidopsis thaliana. The GA deletion mutants ga1-5 and ga4-1 and the ga-insensitive mutant gai-1 show a delay in trichome production even under long-day conditions[81,82]. REPRESSOR OF GA1-3 (RGA) is the negative regulator of the GA signaling pathway and inhibits the expression of ga1-3 in GA biosynthesis mutants, resulting in a delay in the appearance of distal trichomes[83]. GIBBERELLIN-INSENSITIVE (GAI) and RGA are highly homologous and partially redundant in function, and RGA has a stronger effect on the GA signaling pathway than GAI (Fig. 1). The synergistic effect of the mutant rga-24 and gai-t6 can completely restore the surface type of the ga1-3 mutant and even show the traits caused by excessive GA and advance the time of the appearance of the abaxial trichome[84]. The effects of spraying GA at different times are also different, and the juvenile phase of Kalanchoe pinnata is usually two years. When the juvenile phase is sprayed with GA at 3 months and 9 months, they can bloom, and the former requires a higher content of GA[85]. The dwarf1, dwarf3 and dwarf5 mutations delay the juvenile-to-adult phase transition in Zea mays, the key genes that control GA biological activity (Fig. 1)[86].. Grafted 5-year-old Camelia chrysantha shoots are treated with the gibberellin synthesis inhibitor paclobutrazol (PBZ), flower buds appear early[87].
JA delays the decrease in miR156 expression levels, controls the continuous differentiation pattern, affects the duration of the juvenile phase, and delays the emergence of adult traits in Zea mays[88]. In jasmonic acid-treated seedlings, miR156 expression was twice as high as that in untreated leaves[89]. In Hordeum vulgare, jasmonate-inducing protein (JIP-23) is mainly located in phloem companion cells, and more endogenous jasmonates have been found in some tissues of 6-day-old seedlings (Fig. 1)[90]. The decrease in JA concentration when the gene encoding the key enzyme of JA biosynthesis, allene oxide synthase (OsAOS1), is mutated will shorten the juvenile phase and lead to early flowering, which also proves that JA has a delayed role in the juvenile-to-adult phase transition in Oryza sativa (Fig. 1)[91].
CTK plays a role in the growth and development of plant buds, organs and roots[92]. Studies have shown that CTK exerts a positive influence on the age-dependent pathway of nutrient phase transition in Arabidopsis thaliana, promoting miR172 to regulate the expression of target genes TOE1 and TOE2, thereby regulating the juvenile-to-adult phase transition (Fig. 1)[93]. Overexpression of the CTK synthesis gene LONELY GUY 4 (LOG4) in the epidermis of Arabidopsis thaliana increases CTK synthesis and leads to an earlier juvenile-to-adult phase transition, whereas overexpression of the cytokinin-degraded CYTOKININ OXIDASE 1 (CKX1) gene showed the opposite effect (Fig. 1)[94]. In Physcomitrella patens, CTK treatment downregulates miR534a transcription to enhance temporal and spatial expression of the BLADE-ON-PETIOLE 1 (BOP1) gene associated with cell differentiation, and the juvenile-to-adult phase transition is controlled when the threshold level of BOP1 is reached (Fig. 1)[95].
ABA is involved in many important physiological processes in plants, such as seed germination, seed maturation, vegetative growth, and resistance to stressful environments[96]. In the process of shortening the juvenile phase of fruit trees, many measures, such as pulling branches, sticking skins and spraying PBO solution, are often taken to break the hormonal balance in fruit trees. ABA has different trends but obvious changes under different measures[97]. The expression of ABSCISIC ACID INSENSITIVE 5 (ABI5), a key regulator of the ABA signaling pathway, is increased in the miR159 mutant and acts upstream in the miR156-SPL module to affect the gene expression of this pathway and promote the juvenile phase growth of Arabidopsis thaliana (Fig. 1)[98].
-
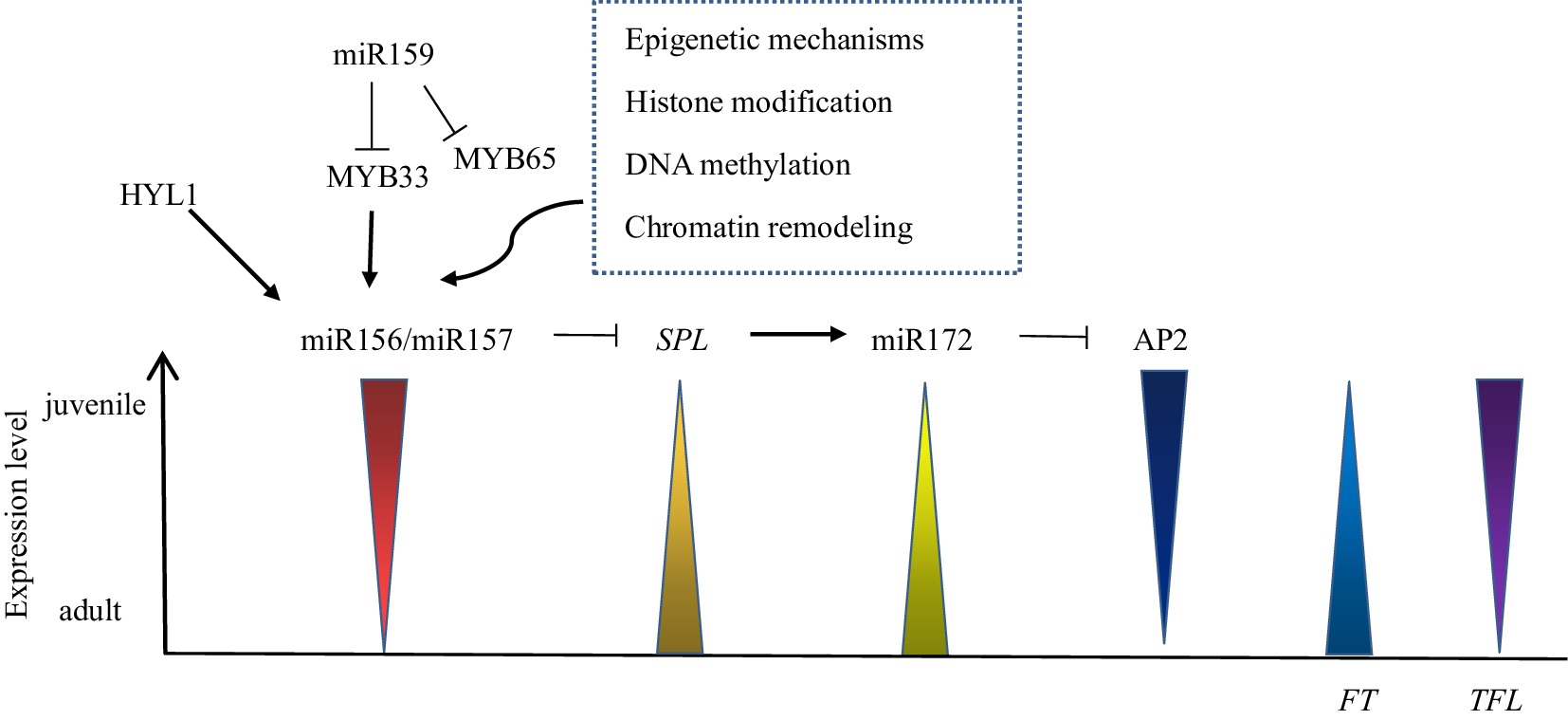
miRNAs in plants are single-stranded endogenous noncoding RNAs that regulate gene expression, with a size of approximately 21 nt. miRNA is formed by treating transcripts composed of 70−200 nucleotides in length[100]. miR156 is the first miRNA to be identified and is conserved in land plants[101], miR156 is highly expressed in the juvenile phase and decreases with increasing plant age, such as Arabidopsis thaliana, Zea mays, Acacia confusa, Eucalyptus globulus, Hedera helix, and Quercus acutissima, which can be detected in this change (Fig. 2)[102]. Overexpression of miR156 in Arabidopsis thaliana can restore some of the juvenile phase traits, such as nonserrated leaf margins, leaf hair loss on the lower epidermis of leaves, normal flowering time, and adventitious root growth[103]. The miR156-SPL module is a major regulator of the plant juvenile phase, and the expression of the SPL gene is the negative feedback target of miR156 (Fig. 2)[104]. High expression of the SPL gene can shorten the juvenile phase of Lilium, in which SPL9 is an important miR156 target gene member, and other SPL members also have functional redundancy in juvenile phase regulation[54]. HYPONASTIC LEAVES 1 (HYL1) is an important protein involved in the production and processing of miR156, which indirectly regulates the expression level of the SPL gene to control the juvenile phase (Fig. 2), and the adult traits of Arabidopsis thaliana will appear prematurely when it is mutated[105]. In addition, miR159 can inhibit the expression of target MYB33 and MYB65 in Arabidopsis thaliana, while MYB33 directly affects two genes, MIR156A and MIR156C, which control the expression level of miR156. Therefore, overexpression of miR159 can indirectly inhibit the overactivation of miR156 and shorten the juvenile phase (Fig. 2)[98]. miR157 may also exist in other species. The difference between miR156 and miR157 is that they have three nucleotide differences, but they have the same target. The expression levels of redundant miR157 and miR156 decrease with aging. With the decrease in miR156/miR157 expression in plant development, SPL gene expression is enhanced, and SPL translated mRNA also increases (Fig. 2)[106]. The SPL gene targets miR172, and the target of miR172 regulation is the AP2 transcription factor family with two DNA-binding domains, including TOE1, TOE2, TOE3, AP2, SMZ and SNZ. The expression level of miR172 increases with plant development, inhibits the expression of AP2 family members and accelerates the end of the juvenile phase (Fig. 2)[5]. In Arabidopsis thaliana, miR156/7 genes are activated de novo in each generation through sexual reproduction, embryogenesis and seed germination to ensure that the juvenile phase does not shorten in each generation[107].
PEPB gene family
-
The PEPB gene family is evolutionarily conserved in eukaryotes and plays an important role in various plants. Family members include FT and TFL1, which have conserved PEPB domains, and the amino acid similarity is 71% because a change in one amino acid residue causes the gene to function in reverse[108,109]. TFL1 in the FT/TFL1 family of Dendrobium catenatum is expressed in the juvenile phase and has low transcription levels in the adult phase (Fig. 2)[110]. In Populus, which has a longer juvenile phase, increased FT transcription levels lead to an earlier juvenile phase end[29]. The difference in the expression of FT in the adult and juvenile SAM of Malus domestica controls the juvenile phase of Malus domestica[111]. The FT gene from plums was transformed into Rosa rugosa Tao White, a 3-year-old young rose, and the transgenic line showed increased cell lignification and a very early-flowering phenotype, demonstrating that the constitutive expression of FT can shorten the juvenile phase of Rosa[20]. The FT gene in Arabidopsis thaliana is transferred into Gentiana triflora, and the transgenic strain can form flower buds within 4 months. The acquisition of a transgenic strain is conducive to the development of a new gentian variety with a short juvenile phase, which greatly shortens the breeding and cultivation time[112]. Most orchids, including Dendrobium, undergo a vegetative phase of 1 to many years, in which the early flowering trait occurs when the TFL gene is mutated[113]. At present, FT/TFL1 has been identified and isolated from a variety of plants and plays an important role in shortening the juvenile phase.
Epigenetic modification
-
Epigenetic regulation is a change in gene function that does not involve a change in DNA sequence and regulates the miR156-SPL module in the juvenile-to-adult phase transition (Fig. 2)[114]. Epigenetic modification plays a role in the whole genome, including DNA methylation, histone modification and chromatin recombination. DNA methylation is a nongene silencing marker in plants and animals and often occurs in the environment containing three sequence structures of CG, CHG and CHH. The three sequences are maintained at methylated levels by different mechanisms in mammals and plants[115]. In Prunus persica, the level of nuclear DNA methylation in adult SAM is higher than that in juvenile SAM , and the distribution pattern is different, indicating that DNA methylation may be involved in the regulation of bud development[116]. Unlike inherited DNA methylation, histone modification resets with each generation, and modifying histones and their associated markers again eliminates the effects of epigenetic markers[115]. Histone modifications can change dynamically under certain circumstances to affect gene expression levels, histone acetylation increases in Arabidopsis thaliana, and leaf traits in the juvenile phase become similar to those in the adult phase[117]. GENERAL CONTROL NONREPRESSIBLE PROTEIN 5 (GCN5, also known as HAG1) and PROPORZ1 (PRZ1), catalysts of the acetyltransferase complex in Arabidopsis thaliana, maintain high levels of acetylation throughout juvenile development, resulting in high transcriptional output of SPL downstream of miR156[118]. Histone deacetylase 9 (HDA9) binds to the chromatin remodeling complex PICKLE (PKL) in the Chromodomain-Helicase/ATPase-DNA-binding domain (CHD) family and erases acetylation of histone 3 lysine 27 (H3K27ac) of MIR156A/MIR156C to promote miR156 expression and accelerate the end of the juvenile phase[119]. The polycomb group (PcG) is an evolutionarily conserved epigenetic regulatory protein that stably binds to two protein complexes, PcG repressive complex 1 (PRC1) and PRC2, in Arabidopsis thaliana[120]. The components of PRC1, EMBRYONIC FLOWER1 (EMF1) and B lymphoma Mo-MLV insertion region 1 homolog (BMI1), coordinate the control of the juvenile phase by labeling histone H3 lysine 27 trimethylation (H3K27me3) with histone modification and negatively regulating miR172 and miR156 expression, respectively[121]. In addition, BRAHMA (BRM) in the DNA-dependent ATPase switch/sucrose non-fermentable 2 (SWI2/SNF2) subgroup competes with PcG proteins to bind the H3K27me site, reduce H3K27me levels and promote miR156 expression[114].
-
Under natural conditions, higher plants go through different juvenile phases. The juvenile phase is an important phase of growth where the plant grows to a certain size, and once the nutrition has been reasonably distributed, and the external form and internal material for the plant to enter the adult phase is ready, the juvenile phase can end. An accurate grasp of the duration of the juvenile phase helps to ensure the yield and quality of plants, as well as their adaptability to the environment. Many studies have shown that the juvenile phase is jointly regulated by the external environment and internal hormones, and light, temperature, sugars, hormones, etc., have an impact on the juvenile phase. At present, there are many molecular mechanism-focused studies on the juvenile phase. Genes involved in the juvenile phase have been cloned and verified. The duration of the juvenile phase is mainly controlled by the miR156-SPL module, which integrates external and internal signaling functions. DNA methylation, histone modification, and chromatin recombination in epigenetic modifications affect miR156 function by not altering its sequence. In addition, PEPB family members also show differential expression of FT/TFL1 during the juvenile phase, mainly accumulating in the SAM to control the plant juvenile phase. However, the specific molecular mechanism of the juvenile phase is not comprehensive.
There are still some questions about the juvenile phase that need to be addressed. A long juvenile phase seriously hinders the process of plant breeding, and short juvenile phase plants are conducive to the smooth progress of research. Therefore, how to select the mutant with a short juvenile phase needs to be considered. There is a lack of systematic research on the factors influencing the juvenile phase in plants. In woody plants, measures such as branch pulling, skin sticking, ring cutting and grafting can be used to shorten the juvenile phase of fruit trees, while in perennial herbs, there are few studies on what measures to adopt. Although studies have shown that hormones such as JA, ABA and CTK have an effect on the juvenile phase, the specific molecular mechanism regulating the juvenile phase is still unclear. How hormonal crosstalk regulates the juvenile phase process also needs further research. At present, flowering ability is often the mark of the end of the juvenile phase in fruit trees such as rugosa rose, but the criteria for the end of the juvenile phase in herbaceous plants need to be studied[49].
For bulbous flowers with high economic value, Narcissus, Tulipa, Hippeastrum and Lilium[48], the long juvenile phase seriously restricts the breeding of new plant varieties, the study of plant stress and immune responses, and the utilization of resources. The genetic transformation efficiency of Lilium is steadily improving. The application of transgenic technology in the study of the juvenile phase will be beneficial to the selection and breeding of short juvenile phase varieties[122]. However, in the long juvenile phase of woody ornamental plants with large genomes, several genes may have functional redundancies and there is tissue-specific expression. Ectopic expression in model plants may not reflect gene function. Therefore, the successful establishment of an in vitro regeneration system and a genetically stable and efficient transformation system for woody plants will lay a favorable foundation for juvenile phase research. It is necessary to comprehensively understand the mechanism of various plant juvenile phases, combine molecular technology systems with cultivation technology and environmental conditions to reseach the juvenile phase, which can shorten the breeding cycle and save manpower and material resources.
-
The authors confirm contribution to the paper as follows: original manuscript preparation: Pan T and Sun H; data analysis: Fan X. All authors have read and agreed to the published version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This work was financed by LiaoNing Revitalization Talents Program (XLYC2002052), Shenyang Innovation Program of Seed Industry (21-110-3-12), and the earmarked fund for CARS (CARS-23).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Pan T, Fan X, Sun H. 2023. Juvenile phase: an important phase of the life cycle in plants. Ornamental Plant Research 3:18 doi: 10.48130/OPR-2023-0018
Juvenile phase: an important phase of the life cycle in plants
- Received: 16 August 2023
- Accepted: 06 November 2023
- Published online: 21 November 2023
Abstract: The juvenile phase of plants refers to the period from seed germination to the period in which they gain flowering ability. The phase is vital for the breeding of new plant varieties, the study of plant stress and immune responses, and the utilization of resources. The trichome characteristics, leaf size, leaf shape, leaf base angle, and shoot apical meristem (SAM) are different in the juvenile and adult phases, and the types and contents of starch, protein, and polypeptides are also different. The duration of the juvenile phase varies greatly among plants, and woody plants usually have a juvenile phase lasting several years or decades. The duration of the juvenile phase is affected by species, temperature, light, sugars and endogenous hormones. The expression of microRNA 156 (miR156) is higher in the juvenile phase and decreases with aging, and the expression of its target gene, SQUAMOSA promoter binding protein-like (SPL), is the opposite. Overexpression of miR159 can shorten the juvenile phase by indirectly inhibiting miR156 expression. Moreover, both miR157 and miR156 shorten the juvenile phase. In contrast, miR172 facilitates the juvenile-to-adult phase transition. Epigenetic modifications also affect the gene function of miR156. In addition, Flowering Locus T (FT) and Terminal Flower 1 (TFL1) are members of the phosphatidylethanolamine-binding protein (PEBP) family, which play important and opposite roles in the juvenile-to-adult phase transition. In this article, the application prospects and existing problems of the juvenile phase are discussed to provide ideas for future research and regulation of the juvenile phase.
-
Key words:
- Juvenile phase /
- Characteristic /
- Sugars /
- Hormones /
- miR156