-
The Green Revolution in the 1960s was characterized by the widespread adoption of semi-dwarf Green Revolution varieties (GRVs) with enhanced logging resistance[1], leading to increased global cereal production. The generation of the GRVs was closely related to gibberellin (GA), an important plant growth promoting hormone. A defect in GA-biosynthesis in rice (sd1 allele) or the dominant mutation of the GA-signaling gene DELLA in wheat (Rht-B1b or Rht-D1b allele) contributed to the generation of semi-dwarf crops. However, a major problem that accompanied these impressive yield increases was the overuse of the nitrogen-based fertilizers because of the low nitrogen (N)-use efficiency (NUE) of GRVs. It unavoidably threatened both the ecological environment and sustainable agriculture. In rice, DELLA was reported to interact with and suppress the function of OsGRF4 (growth-regulating factor 4), a transcription factor and positive regulator of N assimilation and C fixation, hence reducing the NUE of GRVs[2]. In addition, a recent study reported that NGR5 (nitrogen-mediated tiller growth response 5), a positive regulator of rice tillering in response to increased N-content, directly interacted with GID1 (GA insensitive dwarf1) and then promoted GA-triggered ubiquitination and degradation of NGR5. Interestingly, DELLA can also interact with NGR5, thereby competitively suppressing the GID1-NGR5 interaction and consequently stabilizing NGR5[3]. Although impressive progress has been made in our understanding of the underlying mechanisms of why the GRVs can increase crop yield in response to high nitrogen-fertilizers but with low NUE, all GRVs rely on the GA hormone and DELLA, which has certain limitations to initiate another Green Revolution in the new era. In general, modern GRVs should not only have high yield, but also superior quality and environmently-friendly characteristics. Therefore, there is an urgent need to develop new strategies and mine more valuable novel genetic resources to breed the next generation of GRVs.
Brassinosteroid (BR), another key plant growth promoting hormone, has the potential to launch the next green revolution. BR is a plant-specific polyhydroxylated steroidal hormone with a wide range of roles in regulating important agronomic traits of crops, including plant architecture, grain size, leaf angle, plant height, tillering, seed germination, and response to environmental stimuli[4,5]. To date, great progress has been achieved in identifying the key components of the BR signaling pathway in plants. Thus, an almost complete BR signaling cascade has been established, starting with the perception of BR at the cell surface, which triggers a series of downstream phosphorylation and dephosphorylation events, finally activating several key transcription factors, such as BZR1 (Brassinazole-resistant 1) and DLT (dwarf and low-tillering). In fact, the perception of BR and its subsequent signaling is crucial to regulate normal plant growth and development. Destruction of the BR receptor will cause serious dwarfism and an abnormal phenotype of plants. In more detail, the BR receptor BRI1 (brassinosteroid insensitive 1) and co-receptor BAK1 (BRI1-associated receptor kinase 1) are both leucine-rich-repeat containing receptor-like kinases (LRR-RLKs) localized in the plasma-membrane. BR binding not only activates the two receptors by changing the conformation of their cytoplasmic domain, but also triggers the disassociation of BKI1 (BRI1 kinase inhibitor 1), an inhibitor of BR signaling. However, the appropriate modest reduction of BR biosynthesis or attenuation of BR signaling can generate crops with a dwarf and compact architecture that with enhances lodging resistance and are suitable for high density planting[6,7].
Recently, an innovative strategy was successfully established to develop the new generation of wheat with a semi-dwarf compact architecture, high yield, and improved NUE[8]. The core of this strategy is the attenuation of BR perception via a natural deletion of an r-e-z haploblock. In more detail, a quantitative trait locus (QTL) for high thousand grain weight in a segregating wheat population was dissected, and finally correlated to a ~500 kb DNA fragment deletion. Three high-confidence genes, Rht-B1, EamA-B and ZnF-B, were included in this region, hence they were designated as r-e-z. Interestingly, wheat plants with a deletion of the r-e-z haploblock mimic a similar semi-dwarf plant height to those with the Rht-B1b, EamA-B and ZnF-B alleles, but exhibited higher grain weight, larger spikes, stronger culms, and more compact plant architecture. Field trials indicated that deletion of the r-e-z haploblock increased wheat grain yield in both low density (LD) and high density (HD) planting conditions, with a more impressive increase under HD conditions. The application trial of the r-e-z deletion in a commercial GRV wheat cultivar confirmed its promising effect to further enhance the grain yield. Most importantly, the r-e-z haploblock is highly conserved in both wheat and other plants, implying its wide application potential in generating novel semi-dwarf GRVs. Further evidence from the generated independent mutants of the three genes indicated that ZnF-B and Rht-B1b play antagonistic roles in controlling wheat plant height and grain size.
Mechanistically, a series of experiments demonstrated that ZnF, a plasma membrane-localized E3 ubiquitin ligase, functions as a positive regulator of the BR pathway. Moreover, ZnF can directly interact with both TaBRI1 and TaBKI1, and BR promoted ZnF-TaBKI1 interaction, but suppressed ZnF-TaBRI1 interaction. Most importantly, ZnF specifically ubiquitinates only TaBKI1 in vitro and in vivo for its consequent degradation via the 26S proteasome pathway. Further evidence indicated that ZnF-mediated TaBKI1 degradation only occurred on the plasma membrane[8]. Therefore, the newly revealed strategy for generating new GRVs via application of r-e-z haploblock deletion exhibits a better yield potential and sustainability of wheat production than traditional GRVs, which mainly relied on modulating the GA pathway. Therefore, this study in wheat provides an impressive and representative demonstration of the potential contribution of BR to accelerate the realization of the next green revolution.
In addition to wheat, similar studies were reported in the staple crops rice and maize. In rice, the mutation of OsDWARF4, a rate-limiting enzyme in BR biosynthesis, led to slight defects in either BR content or rice morphology. In more detail, the height of the osdwarf4 rice mutant decreased only a little but had notably erect leaves, without any other abnormal phenotypes related to major agronomic traits. Under dense planting conditions, the panicle number and estimated grain yield were significantly increased, even without extra fertilizer[9]. Hence, appropriate genetic modulation of BR biosynthesis can enhance rice yield with less fertilizers, representing a more sustainable and environmentally-friendly agriculture. To solve the contradiction between crop production and the detrimental effects on ecosystems caused by overuse of fertilizers, scientists made great efforts to identify more key genes to improve the NUE of crops. Tillering is a key trait that correlates tightly with rice NUE. Recently, OsTCP19 (Teosinte branched Cycloidea and PCF 19), a modulator of rice tillering, was identified via genome-wide association study. OsTCP19 is responsive to nitrogen and a 29-bp indel in its promoter remarkably affected its transcription and the consequent tillering response to nitrogen. Moreover, OsTCP19 directly inhibits the expression of DLT, which encodes an essential transcription factor in the BR signaling pathway with positive roles in regulating rice tillering. Therefore, the OsTCP19-DLT module integrated the nitrogen signal and BR pathway to coordinate rice growth and development in response to different soil environments[10]. Hence, the identified natural valuable allele or regulatory module can be applied to improve the NUE of modern rice cultivars, and ultimately increase grain yield, with only moderate or even low nitrogen fertilizer input. Recently, an efficient and practical method was developed to precisely downregulate target gene expression by editing its upstream open reading frames (uORFs). Due to the importance and application potential of DLT in coordinating rice NUE and yield, the DLT gene was chosen as a target to verify the new approach. The results showed that a series of rice plants with different tiller numbers and heights were obtained by editing the 5′ terminus of the untranslated region of DLT[11], hence producing ample valuable DLT alleles for future precise molecular breeding of elite rice varieties. In maize, UPA1 and UPA2, two major QTLs conferring upright plant architecture, were successfully cloned and dissected. The target gene of UPA1 is brd1, encoding brassinosteroid C-6 oxidase1, the key enzyme controlling BR biosynthesis. UPA2 was regulated by a 2-bp indel polymorphism controlling the expression of a downstream transcription factor encoding gene ZmRAVL1 (encoding related to ABI3/VP1RAV‐Like 1). DRL1 (DROOPING LEAF1) not only exhibited differential binding to different UPA2 alleles, but also directly interacted with LG1 (LIGULELESS1) and suppressed LG1 activation of ZmRAVL1. By contrast, ZmRAVL1 controlled the expression of brd1, hence modulating endogenous BR biosynthesis and the leaf angle. Application of the wild ancestral UPA2 allele in modern hybrid maize and editing ZmRAVL1 could increase maize yields under high-density planting conditions[12].
In conclusion, a number of impressive studies in the major crops have demonstrated that BR is a potentially ideal biotechnological target to produce the next generation of GRVs (Fig. 1). However, there are still a series of challenges to overcome before the new Green Revolution comes true. For example, the pleiotropic effects of BR make it difficult to fully exploit its advantages and avoid the negative side effects during crop breeding practice. In addition, further research efforts are required to reveal more existing natural recipes, like the r-e-z haploblock in wheat, or generate ample artificial recipes, for future precise and efficient molecular design breeding of ideal crop varieties. We believe that by appropriate genetic modulation of BR biosynthesis or signaling, as well as harmonious integration of BR and other regulatory pathways, the overall performance of new GRVs, including their yield, quality, resistance, and environmentally friendly characteristics, will be strikingly improved.
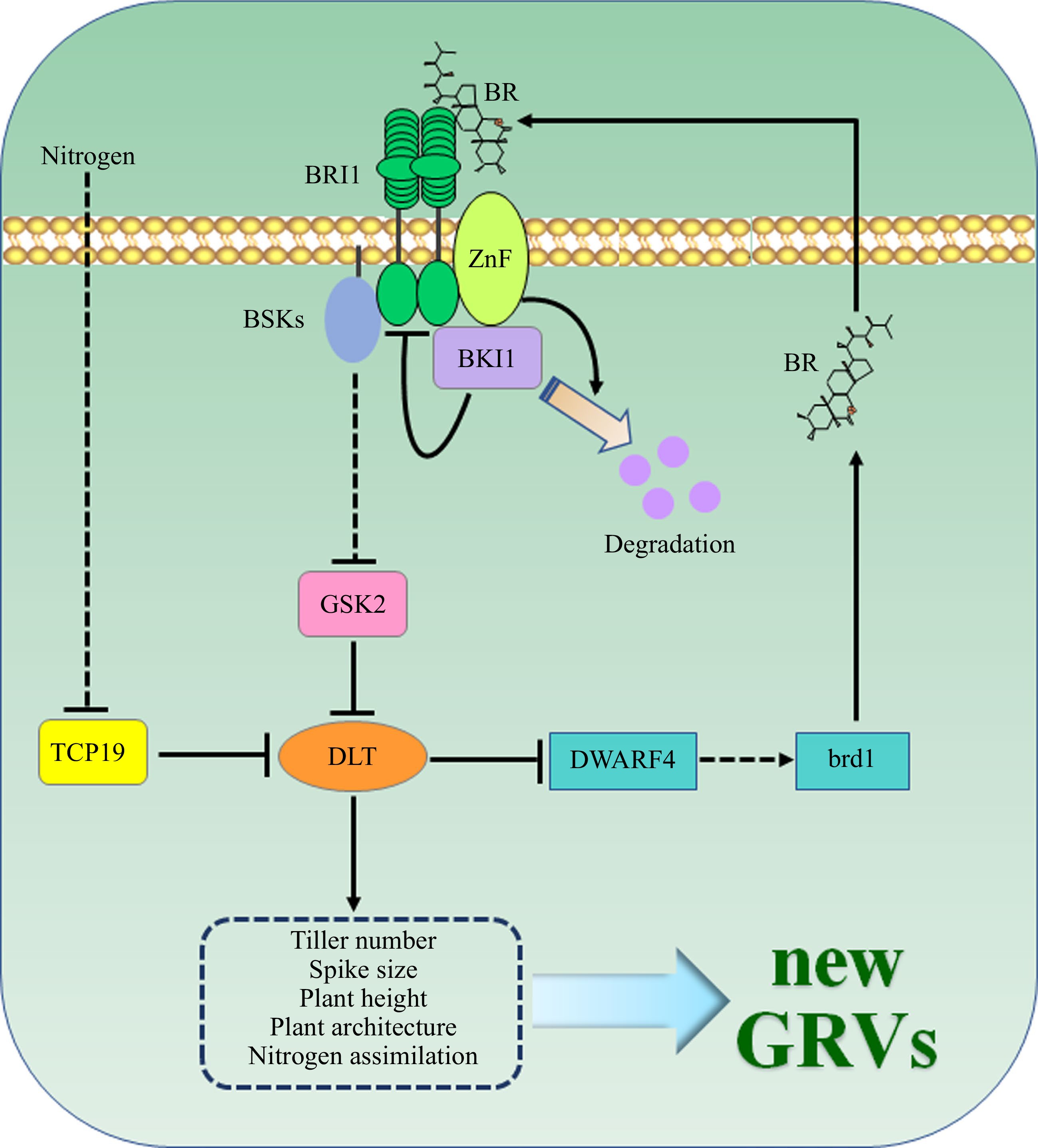
Figure 1.
Schematic illustration of brassinosteroid (BR) mediated regulation of key agronomic traits in major crops, including tiller number, spike size, plant height, plant architecture, and nitrogen assimilation, to generate new Green Revolution varieties (GRVs). ZnF, a newly identified E3 ubiquitin ligase, functions as a positive regulator of the BR pathway via triggering the degradation of BKI1, an inhibitor of BR signaling. ZnF mutation combined with the Rht-B1 mutation can lead to a similar semi-dwarf plant height as the GRVs, but without detectable negative side effects on other key agronomic traits of the wheat plants. Mutation of the key genes in BR biosynthesis, such as DWARF4 or brd1, can slightly alter endogenous BR content and reduce leaf angle, hence suitable for high density planting. Moreover, the OsTCP19-DLT regulatory module coordinates the nitrogen signal and BR pathway to harmonize the growth and development of crops.
HTML
This work was supported by the National Natural Science Foundation of China (32270575), and the Programs from Jiangsu Province Government (BK20200045, JBGS[2021]001, SWYY-154 and PAPD).
-
Qiaoquan Liu is the Editorial Board member of Seed Biology. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and his research groups.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Hainan Yazhou Bay Seed Laboratory. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Li QF, Gao Q, Yu JW, Liu QQ. 2023. Brassinosteroid, a prime contributor to the next Green Revolution. Seed Biology 2:7 doi: 10.48130/SeedBio-2023-0007 |













