-
As global consumers demand and are prepared to pay for new appealing and exotic foods, tropical fruits are now being more intensively investigated. The tropical cropping areas of China and South Asia are considered to be a 'hot spot' for agriculture, with a total area of 48 million hectares. The unique combination of mild temperatures and extended daylight periods make this region one of the best geographical locations in China for the cultivation of tropical fruits[1,2]. Some of the predominant tropical fruits currently grown in China include bananas, pineapples, mango, longan, lychee, coconut, star fruit, papaya, guava, wax apple, and avocado (Fig. 1). Production of these fruits is concentrated in Hainan, Guangdong, Guangxi, Yunnan, Fujian, and Taiwan and six other provinces. The tropical fruit industry is an important source of socioeconomic development and provides an income to approximately ~160 million people in the tropics. Against the backdrop of the internationalization of agricultural economies and the recently established ASEAN-China Free Trade Area, China's tropical fruit industry faces increasingly intense international competition. Consequently, a comprehensive analysis of the industry is required to identify current constraints, compile data on the current tropical fruit varieties (including genetic resources), identify the main challenges and opportunities in the sector, and provide a way forward for the generation of new cultivars which can improve the industry's competitiveness[3−5]. This review aims to cover some theoretical and practical significant information about the tropical crops in Hainan, an area known as the 'Bread-basket of China'.
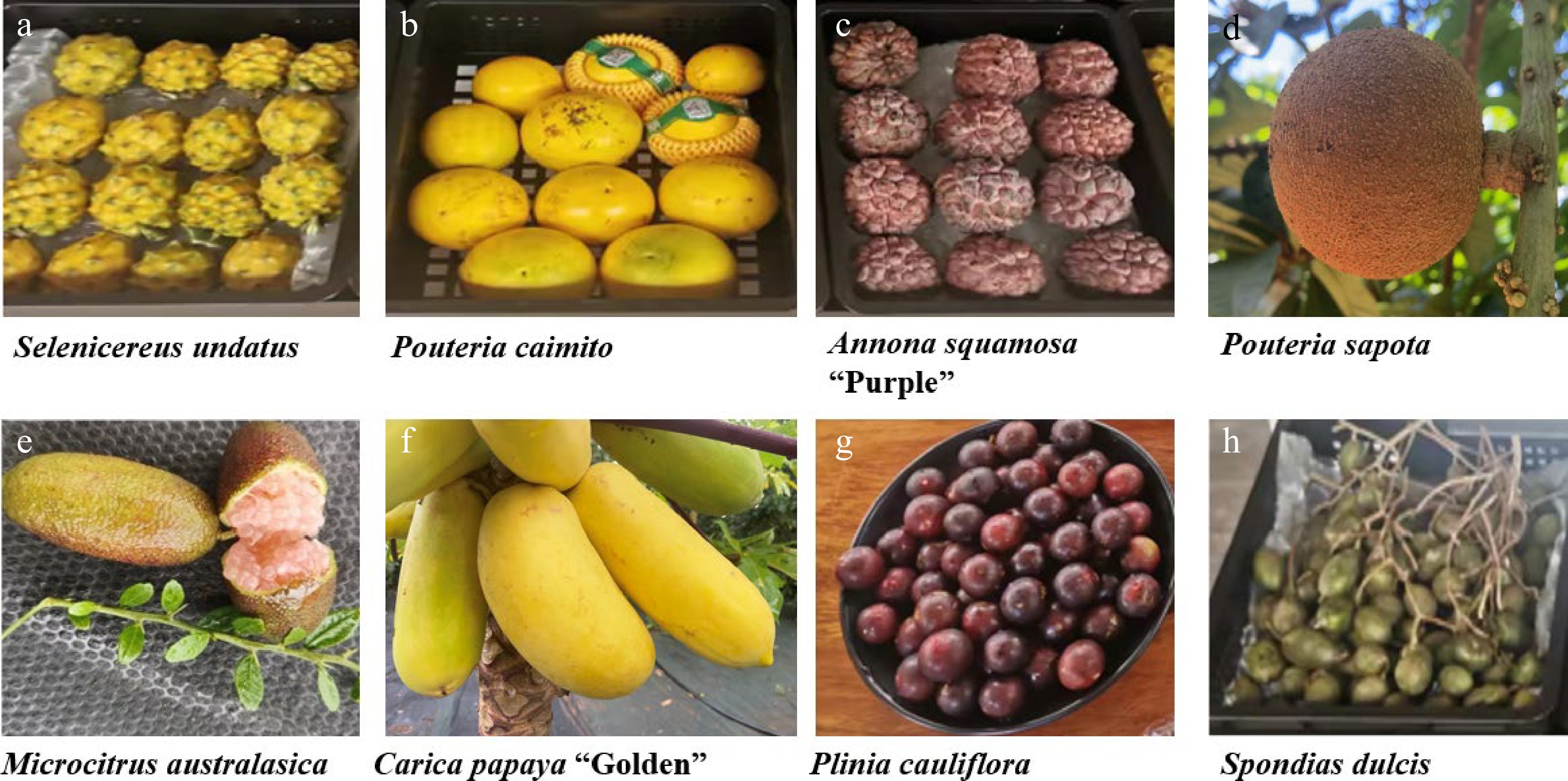
Figure 1.
Tropical fruits cultivated by Qionghai Shengda Modern Agricultural Development Co. Ltd., Qionghai city, Hainan province, China. Tropical fruits are currently grown in the region and contributing to socio-economic development including the following: (a) Selenicereus undatus, (b) Pouteria caimito, (c) Annona squamosa (Purple variety), (d) Pouteria sapota, (e) Citrus australasica, (f) Carica papaya (Golden variety), (g) Plinia cauliflora, (h) Spondias dulcis.
Opportunities in the Hainan tropical fruit industry
-
Hainan island is located in the northern band of the tropics, at a latitude of approximately 19° N. Consequently, it has a tropical monsoon climate, with a long summer, mild winter and a reputation as a 'natural greenhouse'. The average annual temperature is 22−27 °C, with a minimum temperature of 17−24 °C in January and an accumulated annual temperature of 8,200 degree-days over 10 °C. In total, this region receives 1,750−2,650 hours of sunshine every year, with a light rate of > 50%[6−8].
There are 29 families, 53 genera and over 400 kinds of cultivated and wild fruit trees found on the island of Hainan, many of which are rare in other parts of the world. Some of the tropical fruit tree species include longan, lychee, banana, myrtle, chestnut, olive and bayberry. Tree species introduced from the Nanyang Islands and other places include durian, popular fruit, cashew, avocado, guava, sweet peach, jackfruit, mango and mangosteen. In 2020, 4.95 million tons of fruit were harvested from an area of 195.3 thousand hectares. This mainly comprised bananas, mango, lychee, pineapple and longan. Lychee is mainly produced in Haikou, Wenchang and Chengmai in northern Hainan; while mango is grown in Sanya, Ledong, Dongfang and Lingshui and other southern parts of Hainan; pineapple is cultivated in Wanning; and longan in Tunchang, Wenchang and Ding'An counties. With continued governmental supporting policies, Hainan's tropical fruit production has steadily increased over the last decade (Table 1). The tropical fruit industry has become an economic pillar for industrial growth in Hainan Province, currently accounting for one-third of the total agricultural output value (Table 1). In addition to providing employment opportunities and increasing farmers' income, this has also driven the development of food processing industries in surrounding regions.
Table 1. Value of total agricultural production and fruit crops in Hainan between 2005 and 2019 (Values given in 10,000 CNY; data taken from Hainan Agricultural Yearbook 2020).
Year 2005 2010 2014 2015 2016 2017 2018 2019 Farm output value 179.63 337.89 555.72 598.7 676.56 707.42 729.51 819.58 Fruit crops − 120.55 151.03 157.96 180.96 200.69 201.78 256.54 In addition to Hainan's rich tropical resources and biodiversity, the construction of the Hainan Free Trade Zone and Free Trade Port provides a historic opportunity. More than ever, it is an ideal time to increase the volume and quality of tropical fruit production in Hainan. In order to do this, it is important to develop optimal tropical fruit genotypes which are suited for commercial production in Hainan.
An important step in this process is the introduction of leading, novel fruit cultivars from around the globe. Combined with other technological developments (such as creating a highly efficient and technology-driven agriculture system, and developing closed-loop industries for adding value to these fruits), this will help build the Hainan tropical fruit brand and drive Hainan's economic growth[9−11].
Qionghai, Hainan province is a key location to unlock the expansion of the tropical fruit industry in Hainan, as it is home to the 'Boao Forum for Asia' and has established its global reputation as a leader in the agricultural sector. Qionghai would be ideal for the establishment of a tropical fruit tree germplasm resources center, which would collect, preserve, evaluate and distribute new germplasms, as well as develop new cultivars through breeding and distribute them as per Chinese law. Currently, germplasm resource branches and a variety of cultivation sub-centers have been established in Dong-fang city in western Hainan and Wuzhishan city in central Hainan. The advantage of Qionghai will come from the combination of germplasm resource banks with more practical focuses, such as developing and promoting standardized planting patterns and cultivation procedures, and optimizing the industrial processing technology used for these fruits. In addition to supplying the domestic tropical fruit market, the products and technologies will radiate through other countries via the 'Belt and Road' initiative, with the export-oriented Hainan tropical fruit industry at the core[10].
Undoubtedly, new germplasm resources have been a fundamental condition for the growth and development of the global fruit industry[10]. At present, the development and utilization of new and unique tropical fruit germplasm resources, the breeding and uptake of high-yield, high-quality, and disease-resistant fruit cultivars, and the acceleration of industrialization are important means to promote the development of the fruit industry. This can be evidenced in the global tropical fruit industry; discussed in the following section.
The tropical fruit industry abroad
-
Development of the tropical agriculture sector has accelerated in the past half-century. Of the total tropical land area of 530 million hectares, 13.8 million hectares were utilized for tropical crops in the year 2013, with a total production of 2.896 billion tons[10, 12]. The main producers are Asia, India, China, Pakistan and the ten ASEAN countries, followed by the USA, Africa, Brazil, Nigeria, Mexico, Colombia and Oceania.
Generally, tropical crops are grown in small, concentrated areas with suitable agronomic and weather conditions[8]. As previously mentioned, Asia, Africa, Latin America and the Caribbean are all important tropical fruit producers; however, Southeast Asia is particularly important in the production and marketing of tropical fruits. Thailand, Philippines, Indonesia, Malaysia, Vietnam and neighboring ASEAN countries are world-famous for being the 'hometown of tropical fruits'. In these areas, favorable climatic conditions allow dry tropical fruits (such as banana, pineapple, mango, papaya and cashew) to be produced across all four seasons. They also grow a large number of rare tropical fruit cultivars, including durian and mangosteen[10]. The total tropical fruit output of ASEAN countries currently stands at 27.46 million tons per year, accounting for 20.6% of the world's total output. This is grown across a planting area of 2.7 million hectares, accounting for 24.3% of the world's total harvest area. Consequently, the ASEAN region remains one of the most important tropical fruit producing areas in the world, leading the way in both production area and output of major tropical fruit crops[10].
-
China is one of the world's leading producers of tropical fruit, including banana, pineapple, longan, mango, lychee, jackfruit, heart fruit, carambola, papaya, guava, wanpee, wax apple, avocado, coconut, lime and mangosteen. The main growing regions encompass Hainan, Guangdong, Guangxi, Yunnan, Fujian, Hunan, southern Sichuan and Guizhou[8]. However, due to a combination of the rapid development of ASEAN free trade and the existing constraints of natural and labor resources, market pressure on China's tropical fruit industry has increased significantly in recent years. In 2017, a total of 4.4 million hectares of land was planted to tropical crops in China, with a total output value of US
${\$} $ ${\$} $ Table 2. Overview of tropical fruit production in Hainan from 2008 to 2018.
Year Planting
area (mu)New planted area
that year (mu)Harvest
area (mu)Total output
(10,000 tons)2008 256.62 32.30 190.71 246.12 2009 255.90 42.52 204.60 267.90 2010 261.80 40.90 208.38 285.35 2011 269.37 48.78 221.60 308.17 2012 269.68 39.96 227.09 334.63 2013 256.65 31.67 226.89 342.54 2014 248.05 33.47 210.16 311.35 2015 243.30 23.14 204.22 296.68 2016 240.30 18.89 205.53 291.53 2017 251.30 23.77 210.60 303.81 2018 256.08 26.88 225.71 322.12 mu = 667 m2 With the improvements in agronomic practices in recent years, the increasing output of tropical fruits in China has been primarily driven by an increase in yield per unit area. Between 2008 and 2015, there was a small, steady increase in tropical fruit production area (Fig. 2). From 2016, the statistics of tropical fruits in China excluded those pertaining to citrus, lemon and other Rutaceae fruits; thus explaining the sharp fall in 2016. However, over the period 2007−2017, the average yield grew dramatically from 8.4 to 13.9 t ha−1. According to FAO data, China accounted for 28.1% percent of the world's total tropical fruit production in 2007.
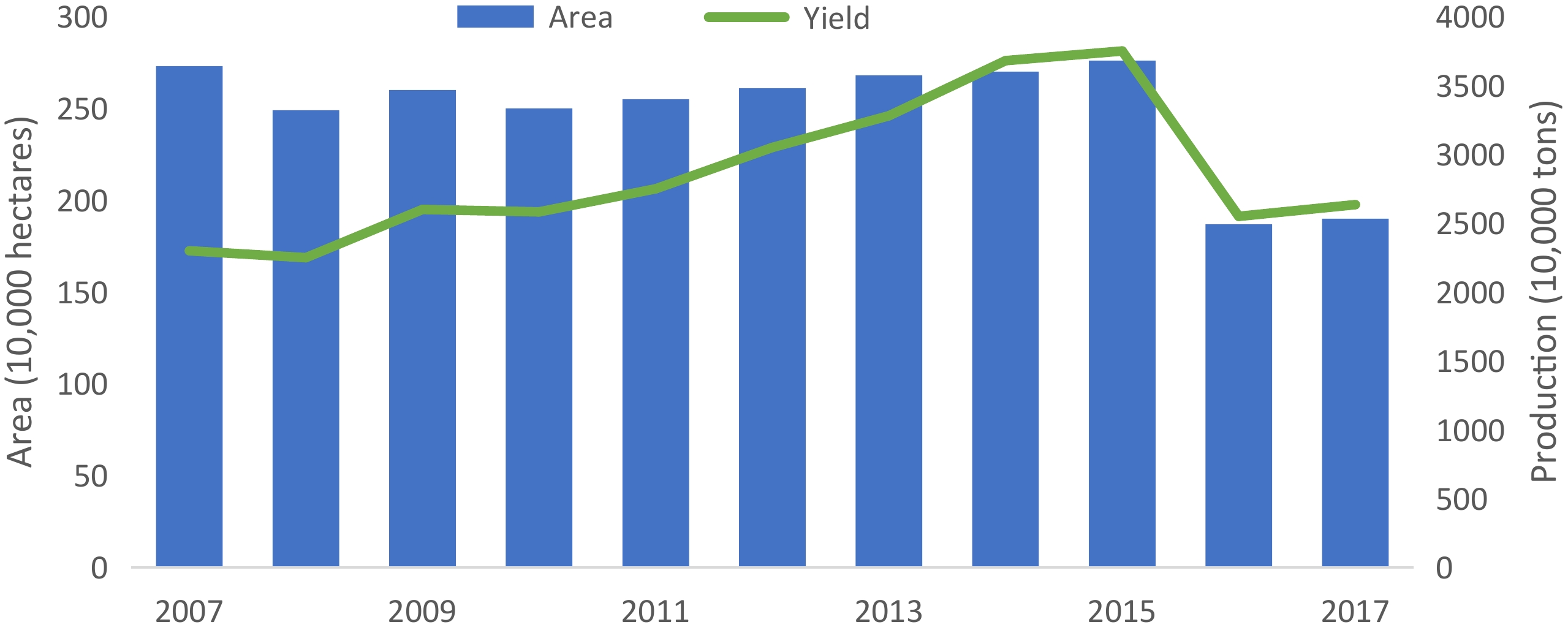
Figure 2.
Production area and quantity of tropical fruits in China between 2007−2017. From 2016, the statistics of tropical fruits in China excluded the data of citrus, lemon and other Rutaceae fruits; therefore, a downward trend was seen in 2016.
New fruit cultivars to supplement the traditional fruit industry in Hainan
-
Consumption of fruit continues to increase worldwide, with particular demand for organic, pollution-free and sustainably grown produce. Furthermore, there is a rapidly expanding market for functional foods which provide health benefits as well as nutritional properties. Consequently, the best possible breeding base of tropical and subtropical fruits is necessary to promote the transformation of the traditional agricultural industry into a modern commercial production and agricultural system[11,13,14]. The 14th 'Five-Year Plan' of Hainan Provincial Committee, released in 2021, aims to promote agricultural and rural modernization by proposing that rare, exotic and superior fruit cultivars – such as ice cream fruit and milk fruit – are grown in experimental and demonstration planting trials in Qionghai county. This will help to promote the construction of the Qionghai Open Agriculture Cooperation Experimental Zone[11]. At present, there are three main species of novel, high-quality fruits with large planting areas and high yield being grown in Qionghai: finger lime, yellow dragon cultivar and ice cream fruit.
Finger lime
-
Finger lime (Citrus australasica), also known as Australian finger lime or caviar lime, is a native Australian fruit, with the flesh comprising small, caviar-like vesicles (Fig. 3). Its caviar-like texture makes it one of the most unique citrus species, in addition to its unique aroma and crisp taste. Australian finger lime is sought after by boutique restaurants around the world. Furthermore, due to the wide genetic diversity that naturally exists in this species, cultivars of finger lime are available with yellow, red, pink, purple, black, blue and green flesh. At present, Australia remains the largest producer of finger limes. Furthermore, most variety development and technical research is concentrated in Australia. One of the major contemporary breeding challenges is developing thornless varieties, as existing varieties bear thorns up to an inch (25 mm) long.

Figure 3.
Finger lime (Citrus australasica), also known as Australian finger lime or caviar lime, cultivated in Hainan.
In 2011, the Hainan Grand Modern Agriculture Development Co. Ltd. began to import Australian finger lime cultivars for experimental cultivation and selective breeding purposes. After a decade of this work, more than 10 high-quality commercial cultivars suitable for domestic cultivation have been identified. Furthermore, a 20 hectare block has been established as the first domestic plantation of finger lime, forming the current basis for most of Asia's finger lime production. The typical current domestic and international market price of this crop is US
${\$} $ Yellow dragon cultivar
-
Yellow dragon fruit (Selenicereus undatus), also known as white-fleshed pitahaya (pitaya), bird's nest fruit and Kirin fruit, is a spineless dragon fruit originally from Ecuador. The fruit has a golden appearance, while the flesh has a silk-like, bird's nest texture. In addition to its unique organoleptic properties, the yellow dragon cultivar is rich in dietary fiber and vitamin C. Bird's nest fruit is one of the highest quality and sweetest pitaya cultivars (Fig. 4).
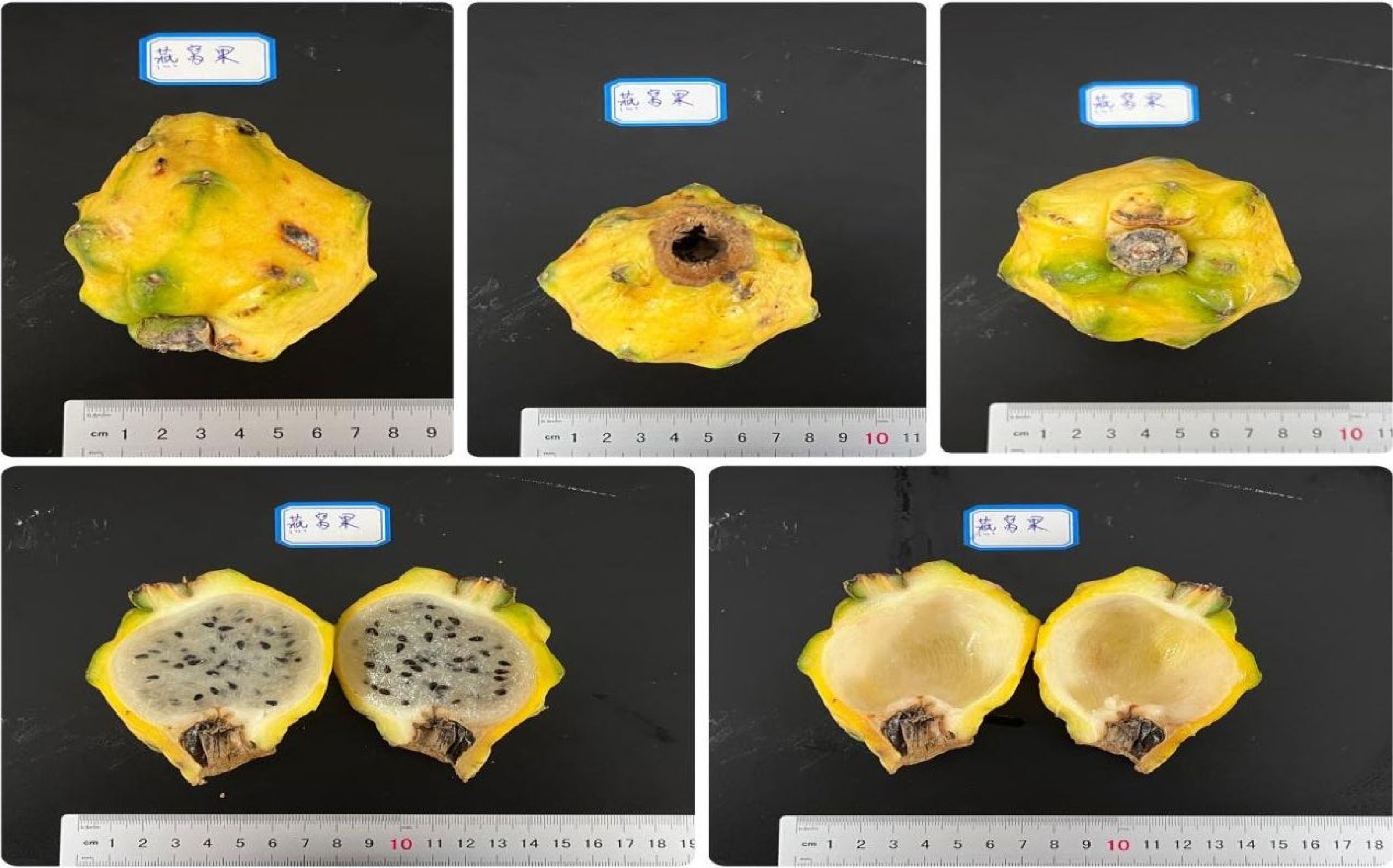
Figure 4.
Different views of the bird's nest fruit, a yellow Pitaya cultivar known as Yanwo in Hainan (Selenicereus undatus).
In 2016, the Hainan Grand Modern Agriculture Development Co. Ltd. in Hainan, first introduced the yellow dragon cultivar to Hainan. After establishing suitable cultivation techniques and promoting this crop to farmers, an area of around 200 hectares was planted, with the fresh fruits winning the bronze award at the 2019 Beijing World Horticultural Expo.
At present, the domestic market price of bird's nest fruit is around US
${\$} $ Ice cream fruits
-
Ice cream fruit (Casimiroa edulis) is a fruit tree native to Mexico and Central America (Fig. 5); it is also known by the names white sapote and Mexican apple. The fruit is rich in potassium, vitamin C and various trace elements. Its pulp is soft and melts in the mouth, with a flavor described as sweet banana. Some consumers freeze the ripe fruit and eat the frozen flesh as a healthy and nutritional replacement for ice cream. Shanda Agriculture in Hainan has taken the lead in introducing and cultivating various cultivars of ice cream fruit in China. In addition to cultivating or developing more than 40 cultivars – including three-leaf, W4, caramel gold, caramel, vanilla, papaya and honey – Shanda Agriculture also established the first successful experimental planting in this country. Currently, it has a commercial plantation of approximately 50 hectares (around 2,400 plants) in Hainan Province and a further 400 hectares of demonstration crops.
The domestic market retail price of fresh ice cream fruit has reached US
${\$} $ Summary
-
In conclusion, the yellow heart dragon fruit, finger lime and ice cream fruit have a growing history as popular tropical fruits, on both the international and domestic Chinese markets. However, the local agricultural sector in Hainan faces some challenges in expanding its production. These include identifying and implementing optimum planting techniques, breeding cultivars with increased heat tolerance, shorter branches and increased compactness, and higher yield. In addition, more research is needed to clarify their nutritional and health properties, as well as how these properties are influenced by different agronomic conditions. Answers to these questions will be greatly beneficial for expanding the local, large-scale cultivation of these fruits.
Challenges in germplasm procurement and variety development in Hainan
Lack of wild resource collection and foreign germplasm introduction
-
Even with the establishment of germplasm in various nurseries, much of the germplasm diversity remains in the botanical (wildtype) 'species', which is in many cases not sufficiently investigated[15−18]. This stands in contrast to many developed countries around the world, where great importance is attached to the collection, documentation, protection and utilization of fruit tree germplasm resources. For example, the American National Plant Germplasm System (NPGS) has established a number of GenBank branches with different specialties, such as apple, pear, citrus and small fruit trees. To date, the NPGS has preserved more than 600,000 accessions and 16,300 species, 80% of which are from abroad[19−22]. Similarly, more than 60% of the germplasm resources preserved in Japan, Russia and Italy have been introduced from foreign countries. In contrast, foreign germplasm resources form just 15% of China's current GenBank, which mainly comprises cultivated cultivars at present[23−25].
Lack of germplasm evaluation and utilization
-
Globally, only around 2% of existing plant resources have been characterized in-depth, including identification and evaluation as food crops. To date, there has not been sufficient accurate phenotype identification of germplasm resources and exploration of genotypes at a genome-wide level, making it difficult to meet the needs of various breeding programs. In a similar vein, more gene excavation and identification of new genes in existing and new germplasm resources are required to transform existing breeding efforts into economic opportunities.
For example, the development of new cultivars of finger lime, yellow heart dragon fruit and ice cream fruit in China is mainly hindered by limited laboratory and equipment capabilities, the shortage of highly trained researchers in this field, and particularly the lack of professional, targeted and standardized facilities focused on the identification and evaluation of germplasm resources[12, 13, 26]. In terms of identification and evaluation techniques, there is limited evaluation of major phenotypic traits, relatively poor facilities for identifying and evaluating stress resistance traits, and a low importance given to the genotype-level characterization of important traits[27].
Rapid urbanization, modernization and industrialization are threatening valuable germplasm resources and leading to the extinction of novel breeds from the face of the earth. Alongside the challenges of climate change, environmental pollution and foreign species invasion, the need for a thorough, contemporary germplasm resources survey in Hainan – as well as other areas of the world – is becoming apparent. Further steps to preserve these genetic resources are also required, such as designating protected habitats and collecting specimens for preservation in GenBank. There is also a need to improve the state of current GenBank, which can suffer from a short-term focus, leading to the scattering or loss of collected germplasm samples over time.
Lack of genomic data integration
-
Rapid advances in genome sequencing technologies have driven breakthroughs in crop genomic research. China is a world leader in crop genomics research, having completed the genome sequencing of rice, wheat, corn, canola, and cucumber, as well as identifying the functional properties of many genes in these crops. Furthermore, Chinese researchers are pioneering the way in genotype identification based on high-throughput genome sequencing, elucidating genomic associations with the functional properties of rice and maize.
The combination of High-Seq and Pac-Bio genome sequencing technology with the latest optical physics mapping technology has allowed the assembly of any species' genome to the chromosomal level[14, 28−30]. Read lengths of up to 15 kb became possible, further reducing sequencing costs and improving throughput. More recently, Bio-Nanotechnology has announced a second-generation optical physical map that will allow ultra-long read lengths and provide a scaffold for large genome assembly[31−33]. Through these new technologies, the genomes of many species will soon be updated with fine-map level sequencing. This further strengthens the role of the genome for breeding and genetic research, allowing a series of new application technologies such as genome editing tools including Meganucleases (MNs), Zinc Finger Nucleases (ZFNs). Transcription Activator Like Effector Nucleases (TALENs) and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) with associated protein[34, 35].
In terms of tropical fruit species, these recent genomics advances are being used to develop new cultivars of Pitaya and Mango[36−40]. At present, the genomes of only a few economically significant species (such as cassava, banana, rubber tree, coconut, mango, papaya, pineapple, lychee and Dendrobium officinale) have been sequenced. However, in-depth genomic research work and comparative genome analysis is rarely reported[41].
Biotechnological tools to improve tropical fruits
-
Based on the latest modern genomic technology, it is possible to get a species' chromosome fine-level reference map, with the aid of simplified resequencing technology. This can be used to analyze the genetic diversity of core populations, investigate species origin and evolution, identify the genetic basis of key economic agronomic traits, and support original research into new variety development.
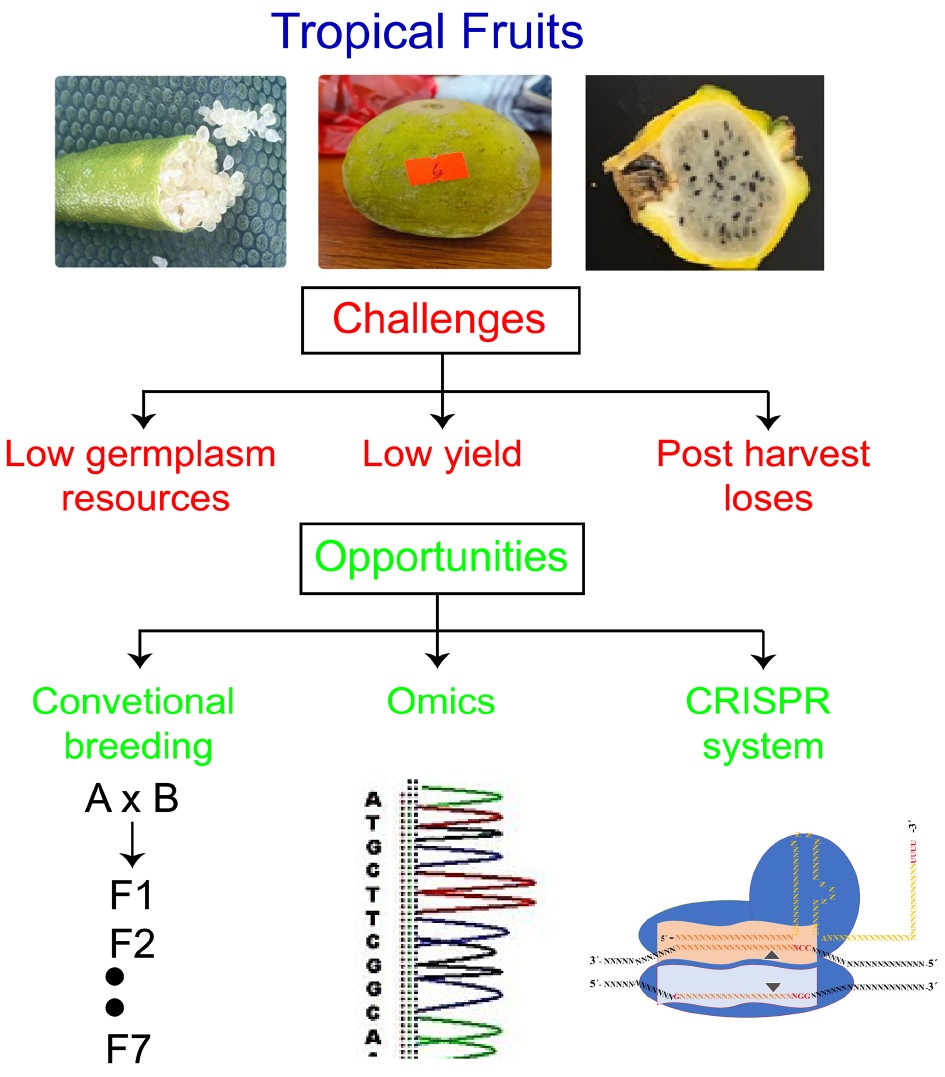
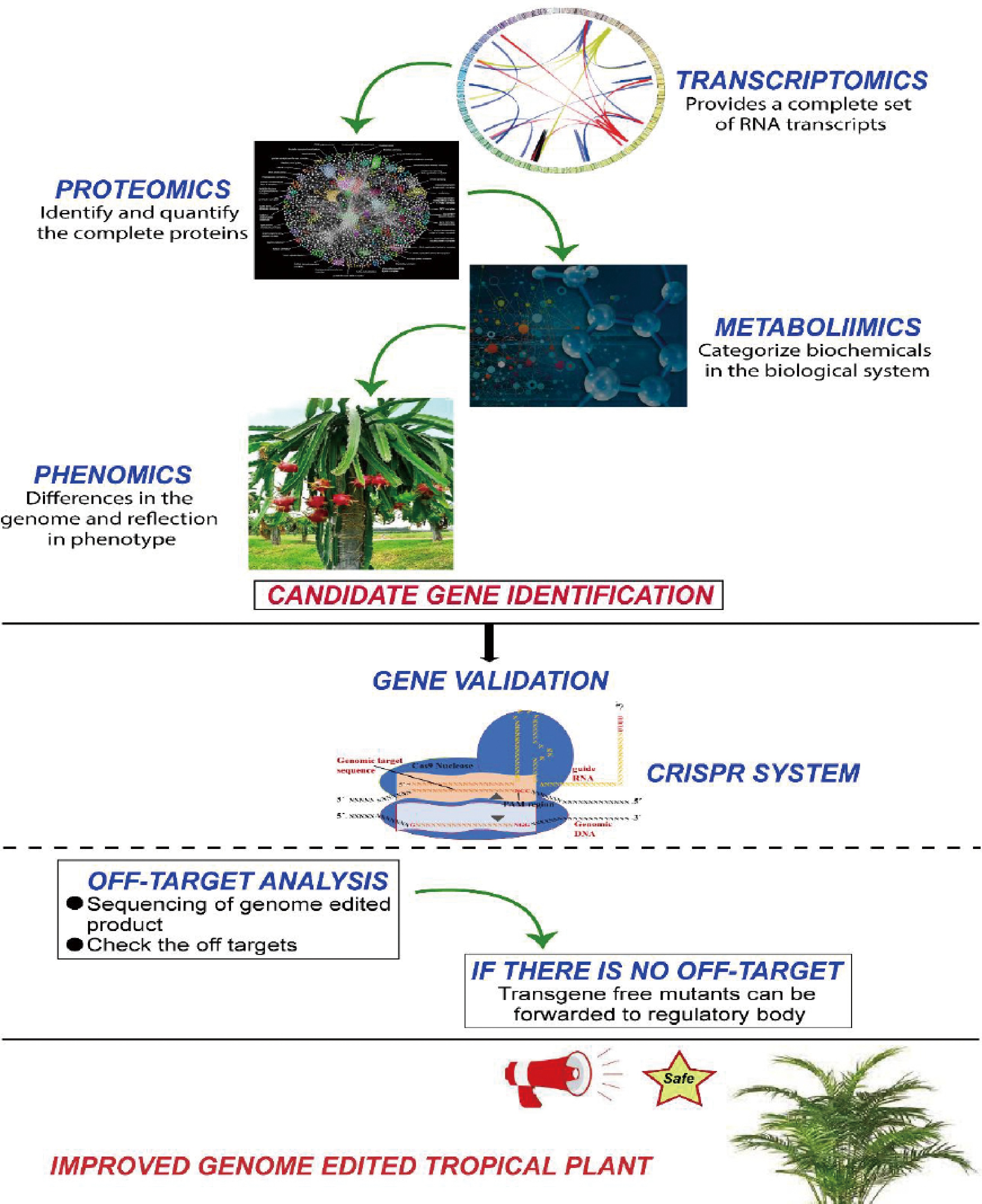
In addition to all emerging biotechnological tools, omics and genome editing can open a new era for the improvement of tropical fruits including yellow heart dragon fruit, finger lime, ice-cream fruit and others. Under changing climate scenarios, these tropical fruits can face severe challenges from biotic and abiotic stresses. However, omics-based tools provide an opportunity to identify the novel loci that control genetic regulation of specific traits such as drought or disease resistance. In tropical fruit crops, transcriptome, proteome, metabolome and phenome have revolutionized the research areas by linking the physical traits to the genome[42] in crops including mango[43−45], pitaya[46−49], durian[50−52], finger lime[53, 54], avocado[55−58], guava[59], peach[60, 61], jackfruit[62], pineapple[63] and papaya[64, 65].
At the same time, genome editing tools – such as clustered regularly interspaced short palindromic repeats (CRISPR) with its associated proteins – are disrupting the plant breeding landscape by allowing direct editing of genetic targets. The main objective of genetic improvement of crops is to enhance the genetic diversity and breeding potential for higher yield and tolerance against biotic and abiotic stresses. The combination of emerging biotechnological tools such as omics and genome editing can enhance the genetic diversity in tropical crops, as shown in Fig. 5. Omics helps to find the genetic loci and genome editing techniques provide opportunity to validate those loci by insertion, deletion and replacement of any nucleotide or fragment. To knockout the function of negative regulator or overexpression of positive regulator can be carried using CRISPR system. CRISPR system can pave a way to validate the candidate genes and improve the fruit crops against biotic and abiotic stresses[34, 35].
Furthermore, these new genetic tools can be utilized in tandem. Omics approaches help to identify the target loci for specific phenotypic traits, while the CRISPR system can target the locus to validate the predicted loci. (Fig. 6). Negative regulators can be mutated to enhance any phenotype of the tropical fruit including yield, biotic and abiotic tolerance. Transgenes-free mutants can be forwarded to government institutions to release as a variety for human consumption.
-
This brief review has highlighted the opportunities and challenges in the Hainan tropical fruit industry, with a particular focus on the promising species of finger lime, yellow dragon cultivar and ice cream fruit. Work is ongoing to use plant morphological and physiological traits to develop and establish standard tropical fruit cultivars with high levels of adaptability, biotic and abiotic resistance, enhanced quality and yield. The main areas of future focus are expected to be group genome sequencing of core germplasm resources, deep mining of fruit genomes for sources of heat resistance and improved storage properties, using genomic edition technologies and artificial mutagenesis to develop improved cultivars. Genome editing and omics tools can help researchers to enhance the socio-economic status of the farmers and can tackle the climate change scenario, if the government approves the genome-edited crops in the future. Concerted efforts in these areas will help define the genetic foundations of Hainan's tropical fruits, accelerate the development and cultivation of new cultivars, promote the full use of China's abundant tropical resources, and improve the competitiveness of the global tropical fruit market.
This study was funded by Hainan Province Science and Technology Special Fund (ZDYF2022XDNY190), the Project of Sanya Yazhou Bay Science and Technology City (Grant number: SCKJ-JYRC-2022-83), and Hainan Provincial Natural Science Foundation of China (421RC486).
-
The authors declare that they have no conflict of interest.
-
Received 8 October 2022; Accepted 2 December 2022; Published online 26 December 2022
-
Collection of germplasm resources from a wide range of domestic and exotic sources
Evaluation of the genetic yield potential of tropical fruits
Proposed solution to use conventional and modern techniques to achieve the goal
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhu Z, Johnson J, Zaman QU, Wang H. 2022. Challenges and opportunities to improve tropical fruits in Hainan, China. Tropical Plants 1:13 doi: 10.48130/TP-2022-0013
Challenges and opportunities to improve tropical fruits in Hainan, China
- Received: 08 October 2022
- Accepted: 02 December 2022
- Published online: 26 December 2022
Abstract: Tropical fruits play a major role in the economic and social development of Hainan (China). Despite favorable climatic conditions, the yield of tropical fruits in Hainan remains low, in part due to the low genetic potential of currently grown tropical fruit varieties. Consequently, there is a need to improve yield potential by exploiting the genome and germplasm resources of tropical fruit species, minimizing post-harvest losses, and improving transportation standards. In this study, we intend to collect germplasm resources from a wide range of domestic and exotic sources to evaluate the genetic yield potential and nutritional quality of fruit using plant morphology, taxonomy and physiological parameters. In this review, we aim to identify current bottlenecks in the Hainan tropical fruit industry and propose solutions through the use of conventional breeding and new biotechnological tools, including the use of omics and CRISPR to enhance yield and tackle biotic and abiotic stresses of tropical fruit species. Producing new fruit cultivars in Hainan, either through conventional strategies or the use of genome editing technology such as CRISPR, could help improve the socioeconomic status of this region. Furthermore, increasing the genetic potential and production of new cultivars can help in meeting the demands of new trade agreements with various nations under the 'One Belt, One Road' initiative, Boao Forum for Asia, ASEAN agreements, and the Shanghai Cooperation Organization.
-
Key words:
- Challenges /
- Opportunities /
- Improve /
- Tropical /
- Fruits /
- Omics