-
Ice cream fruit (Casimiroa edulis La Llave) is a tropical fruit tree from the Rutaceae family, also known by the common names white persimmon, white sapote, Mexican apple and fragrant meat fruit. It is a monoecious species, bearing male and female parts in the same flowers (existing simultaneously)[1]. It is called ice cream fruit in China because it tastes like vanilla ice cream after freezing. Ice cream fruit originated in Mexico and Central America, but is currently distributed across a range of tropical and subtropical regions[2]. The tree shows high levels of resistance against biotic and abiotic stresses[3]. The fruit is rich in flavor, with soft flesh, minimal fruit juice, a milk-like fragrance, and a sweet flavor reminiscent of ice cream[1]. This combination of characteristics makes them highly sought after by consumers.
At present, the commercial planting area of domestic ice cream fruit is not large, and the output is limited, resulting in a relatively high market price of around 50 Euros/kilogram (390 Chinese Yuan/kg). Similarly, it is not widely planted in China, only being introduced in Hainan, Yunnan and a few other places. However, as a tropical fruit with high economic value, there is considerable promise to expand its production as a high-end tropical fruit variety in the domestic market. Therefore, it is necessary to further study the cultivation technology of ice cream fruit - cross pollination.
Morphology and floral biological characteristics of ice cream fruit
Morphological characteristics
-
In the Hainan region, ice cream fruit trees can grow to 5−6 m, with gray-brown bark. The leaves are oval and lanceolate when young, with 3−5 leaflets typically comprising each leaf. They range between 6–12 cm long and 4–6 cm wide, with short-pointed or rounded leaf tips. The leaf margin has fine blunt teeth, with short hairs on the petiole, leaf shaft. The fruit begins green, turning more spherical and yellow and spherical as it ripens[3].
The flowers have five petals, with a yellow-green corolla borne at the leaf base, around 8−10 mm in size. The stamens are 2−3 mm long.
The medium-sized (5−10 cm diameter) fruit is shaped like a persimmon, with thin skin, white or yellowish flesh, and a smooth texture (Fig. 1). When refrigerated, it tastes like ice cream, with a sweetness of 17%−25%. A mature tree can produce 500 kg of fruit in one fruit period.
It is generally easy to cultivate, growing in a range of conditions (light, shaded, dry, wet, barren soil). It shows good cold resistance, withstanding temperatures as low as −2 to −4 °C.
Floral characteristics
-
Ice cream fruit is widely cultivated in the tropics and subtropics, and can also flower in temperate regions. Ice cream fruit can bear fruit more than once per year, with flowering occurring twice a year between January to February, and June to July[2]. The time from flower germination until flowering is around 15−20 d[1], but fertilisation rates and fruit survival vary widely among cultivars. Varieties producing little pollen or no pollen seem to have a lower fruit yield but a higher fruit survival rate; Varieties with more pollen have lower fruit survival rate.
Ice cream fruit nutritional composition
-
Ice cream fruit contains a lot of trace elements beneficial to the human body, such as potassium, calcium, iron and other minerals. It also contains high levels of several vitamins, including vitamin C (62 mg vitamin C/100 g); in some areas this fruit is colloquially known as the 'king of vitamin C'. Ice cream fruit is high in sugar, including fructose, glucose and other sugars, with a total sugar content as high as 16%[4].
Research status of ice cream fruit
-
Practical research into any crop is inseparable from the collection of germplasm resources, breeding techniques and the investigation of biotic and abiotic factors affecting it. At present, few research groups are conducting experiments on ice cream fruit in China, thus much basic experimental research and investigation is still required.
In China, ice cream fruit is currently cultivated on a large scale in Yunnan Xishuangbanna Botanical Garden and in the Hainan Shanda agricultural tropical fruit window. This survey uses ice-cream fruit grown by Hainan Qionghai Shanda agricultural company. The imported ice cream fruit came from the United States. This is one of the few only plantings areas in China.
Study purpose and significance
-
At the present time, commercial ice cream fruit plantings suffer from low to moderate fruit set rates following artificial pollination, as well as inconsistencies in fruit quality. In order to improve the yield and quality of ice cream fruit, we investigated different artificial pollination methods in this study. Different ice cream fruit varieties were crossed using the artificial hybrid method for paired pollination experiments, with pollen taken from one variety used to pollinate another variety. The resultant fruit yield and quality were used as metrics to determine the best-performing crosses.
Main content of the study
-
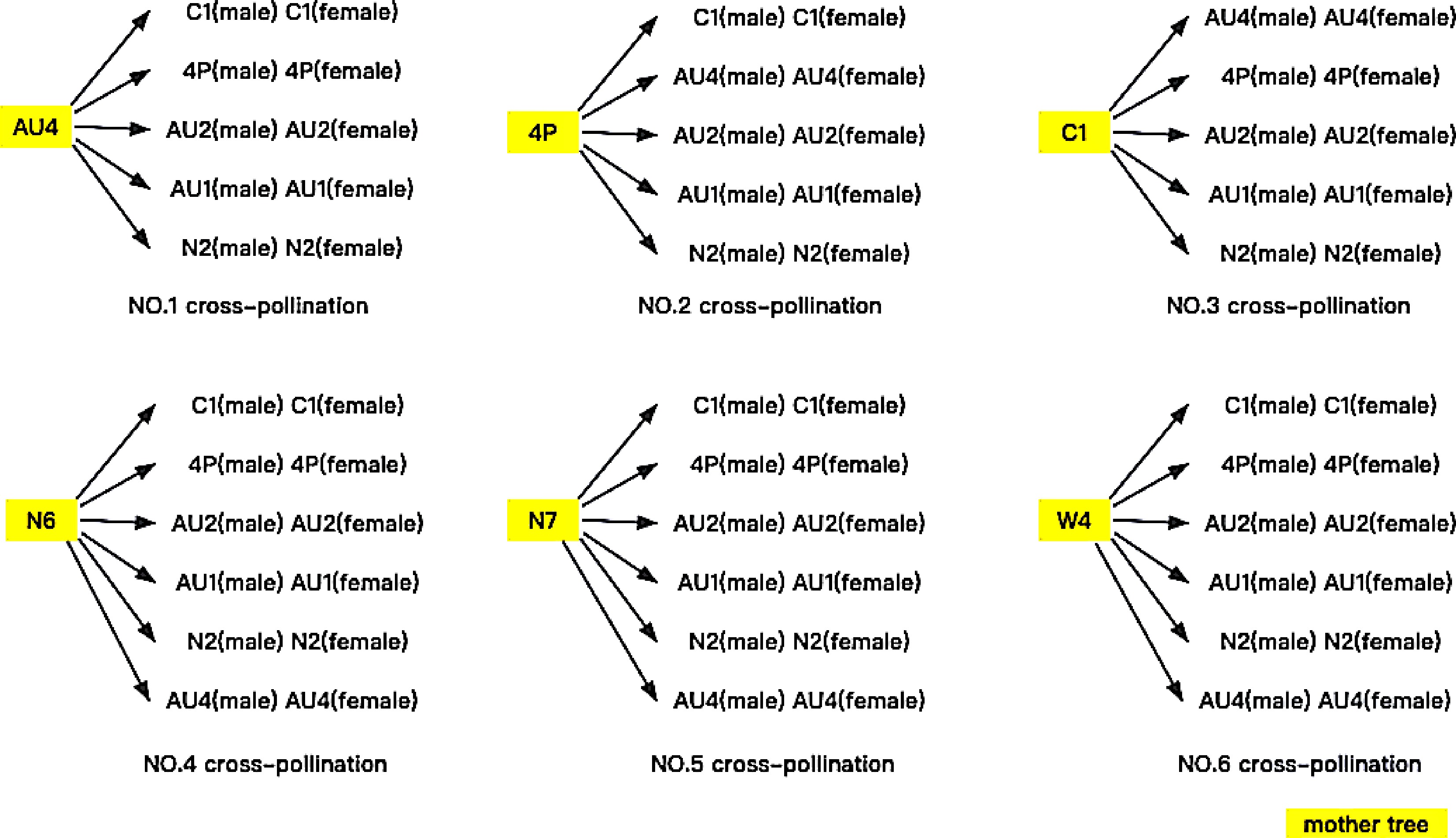
Ice cream fruit is a monoecious species, and pollen counts vary depending on the variety. A total of nine varieties were investigated in this work, being divided into three groups: little pollen (varieties AU4, 4P, C1), abundant pollen (N2, AU1, AU2) and no pollen (N6, N7, W4). The varieties with abundant pollen or little pollen were used as male parents, while the varieties with little pollen or no pollen were used as the female parents. In addition to cross-pollination, natural hanging fruit and artificial self-pollination were also investigated in each variety. After pollination, the flowers were bagged to avoid interference from external factors.
-
The different ice cream fruit varieties showed significant morphological differences in their stamens, as shown in Fig. 2.
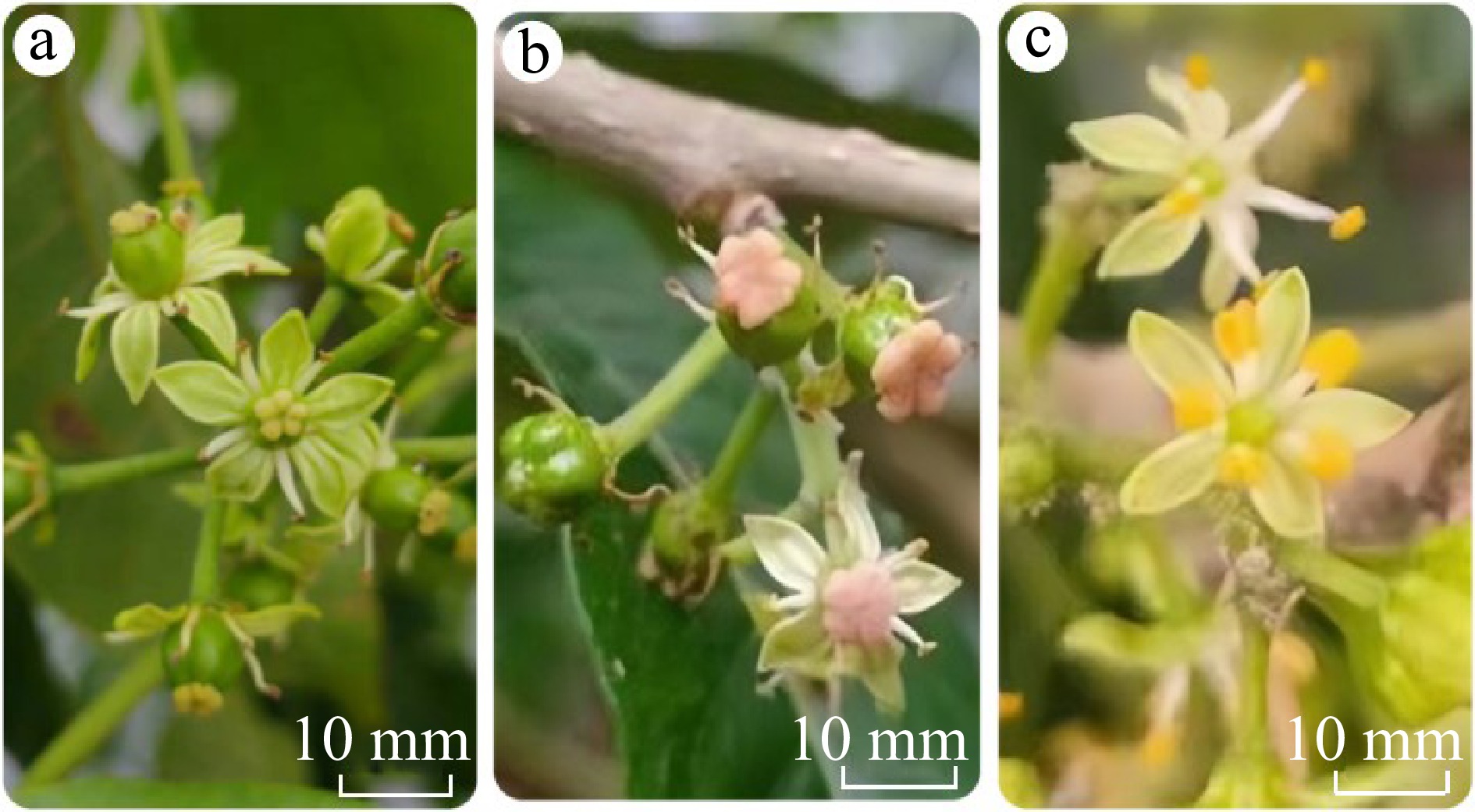
Figure 2.
(a) W4 variety – no pollen. (b) 4P variety – little pollen. (c) AU2 variety – abundant pollen.
The varieties with no pollen showed large ovaries, with a visible, yellow pistil stigma. The stamens and yellow and black, with dry and infertile anthers.
The varieties with little pollen stigma is pink or yellow-pink, and the ovary and stigma are abnormally large. Five stamens are very small, the anthers are yellow-white or yellow-black, and the pollen content is very little.
The varieties with abundant pollen pistil is very small, and the stigma is not obviously light green, the capital part is also very small, but the five stamens are very thick, especially the anther flower sac is also very large, showing bright yellow, the pollen content is very high.
In Fig. 3, the development process of naturally self-pollinated fruit is recorded, with the corresponding data for this experimental group shown in Table 1.
Table 1. Statistics of the naturally self-pollinated fruit.
Breed Rate of fruit set
(%)Width
(cm)Length
(cm)Sugar
(%)Abundant pollen AU1 0% − − − AU2 25% 4.1 ± 0 4 ± 0 10.7 ± 0 N2 50% 4.7 ± 0.2 3.95 ± 0.25 9.1 ± 0.1 Little pollen 4P 66% 7.5 ± 0.2 6.9 ± 0.1 11.7 ± 0.5 AU4 50% 8.3 ± 0.2 7.6 ± 0.3 10.5 ± 05 C1 100% 6.6 ± 0.2 5 ± 0.1 10.9 ± 0.1 Comparing the two groups (abundant pollen and little pollen) shows that the fruit set rate, fruit size and sweetness were all higher in varieties with little pollen, compared to those with more abundant pollen. Within each group, variety N2 showed the best performance for the abundant pollen varieties, while variety 4 showed the highest sweetness and acceptable size and fruit set amongst the varieties with little pollen. However, variety C1 did show the highest rate of fruit set.
To compare the effects of natural and artificial pollination, another experiment was carried out with artificial self-pollination conducted at different times every day. The bag surrounding each flower was gently shaken each time to ensure that the pollen was transferred from the stamens to the stigma; this process was repeated until the stamens and stigma withered, and the fruit began to grow, indicating that pollination was successfully completed.
As shown in Table 2, the fruit set rate of ice cream fruit varieties with abundant pollen increased in the case of artificial pollination. However, there was no significant change in the size or sweetness of the fruit quality. It is known that artificial pollination can slightly improve the fruit set rate of abundant pollen varieties, but it has no significant effect on fruit quality.
Table 2. Statistics of experimental data of artificial self pollination.
Breed Fruit set
(%)Width
(cm)Length
(cm)Sugar
(%)Abundant pollen AU1 33% 5.9 ± 0 5.5 ± 0 10.0 ± 0 AU2 50% 4.4 ± 0.5 4.0 ± 0.1 9.5 ± 0.5 N2 40% 6.0 ± 0 4.5 ± 0 8.6 ± 0 Little pollen 4P 66% 7.7 ± 0.1 6.4 ± 0.8 11.0 ± 1.0 AU4 50% 8.9 ± 0.1 7.5 ± 0.1 10.2 ± 0.2 C1 100% 7.8 ± 0.1 6.9 ± 0.1 9.5 ± 0.1 Therefore, artificial cross-pollination and hybridization studies were conducted to find possible combinations which could provide improved fruit quality compared to self-pollination.
Artificial cross-pollination (male/female)
-
Tables 3−8 show the experimental results from the artificial cross-pollinations, broken down by each female parent. Additionally, each table shows the results with and without emasculation.
Table 3. Experimental data for the cross-pollination with AU4 as the maternal parent, with and without emasculation.
Group Mother tree Father tree Fruit set (%) Length (cm) Width (cm) Sugar (%) Female AU4 (little pollen) Little pollen C1 33% 7.5 ± 0.1 7.4 ± 0.1 8.9 ± 0.1 4P 50% 8.8 ± 0.2 7.9 ± 0.2 10.4 ± 0.1 Abundant pollen AU1 40% 8.15 ± 0.1 7.75 ± 0.1 10.4 ± 0.2 AU2 50% 9.2 ± 0.1 7.95 ± 0.1 9.5 ± 0.1 N2 75% 9.47 ± 0.1 8.47 ± 0.1 10.5 ± 0.5 Male Little pollen C1 0% − − − 4P 50% 8.05 ± 0.1 7.95 ± 0.2 8.5 ± 1 Abundant pollen AU1 33% 8.7 ± 0.2 8.5 ± 0.1 8.0 ± 2 AU2 33% 8.5 ± 0.3 7.9 ± 0.1 8.1 ± 0.2 N2 50% 9.2 ± 0.3 8.04 ± 1 8.5 ± 1 Table 4. Experimental data for the cross-pollination with 4P as the maternal parent, with and without emasculation.
Group Mother tree Father tree Fruit set (%) Length (cm) Width (cm) Sugar (%) Female 4P (little pollen) Little pollen C1 50% 7.8 ± 0.6 7.0 ± 0.2 9.8 ± 1.2 AU4 66% 6.9 ± 0.1 6.3 ± 0.2 8.78 ± 1.0 Abundant pollen AU1 50% 8.7 ± 0.6 6.57 ± 1.0 9.8 ± 0.5 AU2 50% 7.35 ± 0.2 6.45 ± 2.0 8.5 ± 1.5 N2 80% 7.7 ± 1.0 7.075 ± 1.0 11.0 ± 1.0 Male Little pollen C1 42% 8.5 ± 0.3 6.9 ± 0.2 10.5 ± 0.5 AU4 50% 8.0 ± 2.0 7.95 ± 2.0 10.0 ± 0.8 Abundant pollen AU1 50% 7.15 ± 2.0 6.55 ± 3.0 9.2 ± 0.6 AU2 42% 6.8 ± 0.4 6.1 ± 0.5 8.87 ± 0.4 N2 62% 7.5 ± 0.3 7.4 ± 0.6 9.84 ± 0.3 Table 5. Experimental data for the cross-pollination with C1 as the maternal parent, with and without emasculation.
Group Mother tree Father tree Fruit set (%) Length (cm) Width (cm) Sugar (%) Female C1 (little pollen) Little pollen AU4 60% 7.27 ± 1.0 6.76 ± 1.0 10.3 ± 1.0 4P 33% 6.9 ± 0.4 5.9 ± 0.3 10.0 ± 0.5 Abundant pollen AU1 25% 6.8 ± 0.5 6.2 ± 0.6 8.0 ± 0.4 AU2 33% 5.9 ± 0.4 5.9 ± 0.3 10.2 ± 1.0 N2 50% 6.8 ± 0.6 6.8 ± 0.3 11.0 ± 0.5 Male Little pollen AU4 50% 6.9 ± 0.2 6.8 ± 0.4 10.3 ± 1.0 4P 0% − − − Abundant pollen AU1 0% − − − AU2 33% 6.8 ± 0.4 5.9 ± 0.1 10.0 ± 0.5 N2 50% 7.1 ± 0.5 5.9 ± 0.2 9.16 ± 1.0 Table 6. Experimental data for the cross-pollination with N6 as the maternal parent, with and without emasculation.
Group Mother tree Father tree Fruit set (%) Length (cm) Width (cm) Sugar (%) Female N6 (No pollen) Little pollen C1 0% − − − 4P 40% 7.0 ± 0.5 6.5 ± 0.2 8.75 ± 1.0 AU4 50% 6.8 ± 0.2 6.3 ± 0.2 10.0 ± 0.4 Abundant pollen AU1 50% 7.2 ± 0.2 5.9 ± 0.3 8.2 ± 0.2 AU2 33% 5.8 ± 0.4 6.2 ± 0.4 8.0 ± 1.0 N2 50% 6.9 ± 0.2 6.7 ± 0.3 10.0 ± 0.6 Male Little pollen C1 50% 6.6 ± 0 6.5 ± 0 8.0 ± 0 4P 33% 7.4 ± 0 6.5 ± 0 9.5 ± 0 AU4 33% 6.5 ± 0.15 6.4 ± 0.4 8.0 ± 1.0 Abundant pollen AU1 66% 6.6 ± 0.5 5.95 ± 0.1 9.5 ± 0.5 AU2 50% 6.6 ± 0.1 6.45 ± 0.1 0.59 ± 0.5 N2 66% 6.6 ± 0.6 5.95 ± 0.1 10.5 ± 1 Table 7. Experimental data for the cross-pollination with N7 as the maternal parent, with and without emasculation.
Group Mother tree Father tree Fruit set (%) Length (cm) Width (cm) Sugar (%) Female N7 (No pollen) Little pollen C1 50% 6.8 ± 0.2 6.4 ± 0.2 10 ± 0.4 4P 33% 7.2 ± 0.2 6.8 ± 0.2 9.4 ± 0.4 AU4 33% 6.4 ± 0.2 6.2 ± 0.4 9.4 ± 0.4 Abundant pollen AU1 33% 6.7 ± 0.3 6.6 ± 0.6 10.0 ± 0.2 AU2 33% 7.8 ± 0.4 6.8 ± 0.5 9.0 ± 1.0 N2 50% 8.8 ± 0.4 7.4 ± 0.1 9.5 ± 1.0 Male Little pollen C1 28% 5.8 ± 0.1 5.6 ± 0 8.5 ± 0.5 4P 50% 7.2 ± 0 6.8 ± 0 10.0 ± 0 AU4 25% 6.4 ± 0 6.3 ± 0 8.4 ± 0 Abundant pollen AU1 0% − − − AU2 40% 6.8 ± 0 6.1 ± 0 9.0 ± 0 N2 50% 6.8 ± 0.4 6.0 ± 0.2 10.0 ± 1.0 Table 8. Experimental data for the cross-pollination with W4 as the maternal parent, with and without emasculation.
Group Mother tree Father tree Fruit set (%) Length (cm) Width (cm) Sugar (%) Female W4 (No pollen) Little pollen C1 60% 7.2 ± 0 6.5 ± 0 16.0 ± 0 4P 50% 6.3 ± 0.3 5.8 ± 0.1 13.5 ± 0.5 AU4 0% − − − Abundant pollen AU1 50% 5.6 ± 0 5.4 ± 0.2 10.7 ± 0.3 AU2 50% 7.1 ± 0 6.2 ± 0 13.0 ± 0 N2 60% 6.8 ± 0.2 6.0 ± 0.1 14.2 Male Little pollen C1 25% 7.1 ± 0.3 6.4 ± 0.2 13.7 ± 1.0 4P 50% 7.0 ± 0.1 6.4 ± 0 11.7 ± 0.6 AU4 0% − − − Abundant pollen AU1 50% 6.9 ± 0 6.8 ± 0 12.8 ± 0 AU2 50% 5.8 ± 0 4.9 ± 0 13.6 ± 0 N2 60% 7.1 ± 0.6 6.0 ± 0.3 12.8 ± 0.2 When the AU4 variety was used as the maternal parent (with emasculation), the best performance was found using N2 as the pollen source, which yielded a 75% rate of fruit set and the largest fruit size (9.5 cm × 8.5 cm), as shown in Table 3. It also had a high sugar content (10.5%).
Similarly, for 4P as the maternal parent, 80% fruit set was found with emasculation and with N2 as the pollen source (Table 4). This cross also showed moderate size and high sugar content. However, the largest fruit were found using AU1 as the pollen source.
The final little-pollen variety used as a maternal parent was the C1 variety, which generally showed poorer performance than the other two maternal parents previously discussed.
The best performance was seen using AU4 pollen on emasculated flowers, which gave a 60% rate of fruit set, the largest fruit size and a high sugar content (Table 5). N2 performed acceptably as a pollen source, and was also the best cross on non-emasculated flowers.
For the maternal parent varieties which did not produce pollen, N7 generally had poorer performance, while N6 and W4 had acceptable performance. Again, N2 was the best pollen source for most maternal varieties.
With N6 as the maternal parent, N2 and AU1 tied for the highest fruit set rate (66%) under non-emasculated conditions; however, the fruit was only moderate in size (Table 6). N2 also gave the sweetest fruit.
For N7 as the maternal parent, several combinations gave a 50% fruit set rate, while most other pollen sources gave quite poor rates of fruit set (Table 7). The better-performing pollen sources included C1 and N2 (for emasculated flowers) and 4P and N2 (for non-emasculated flowers). N2 also gave the largest fruit under emasculated conditions (8.8 cm × 7.5 cm).
Finally, with the W4 maternal parent, N2 again performed well under both emasculated and non-emasculated conditions (60% fruit set rate), with moderately sized fruit (Table 8). C1 also gave 60% fruit set when applied to emasculated flowers and good-sized fruit, as well as an exceptionally high sugar content (16%).
In general, most crosses showed higher fruit set rates and better fruit quality when the stamen is emasculated. The index of fruit quality as the best paired pollination group is a general method for pollination variety research[5], so we calculated the formula of the best paired parent variety of each parent to obtain the index.
Therefore, the best matching parent and the fruit quality of each parent in the case of male elimination can be shown as in Table 9. Overall, the best pollination combination between different ice cream fruit varieties was found using variety 4P and N2 as the parents (Table 9).
Table 9. Male parent cultivars and index of the best cultivar pairing for each female parent cultivar with emasculation.
Cross Fruit set (%) Length (cm) Width (cm) Sugar
(%)Index of
fruit qualityMother tree Father tree C1 AU4 60% 7.2 ± 1.0 6.7 ± 1.0 10.3 ± 1.0 10.2 4P N2 80% 7.7 ± 1.0 7.1 ± 1.0 11.0 ± 1.0 14.4 AU4 N2 75% 9.4 ± 0.1 8.4 ± 0.1 10.5 ± 0.5 14.2 N6 N2 50% 6.6 ± 0.6 5.9 ± 0.1 10.5 ± 1.0 8.3 N7 N2 50% 8.8 ± 0.4 7.4 ± 0.1 9.5 ± 1.0 8.5 W4 C1 60% 7.2 ± 0 6.5 ± 0 16.0 ± 0 13.5 Hybrid fruit of 4P and N2
-
For the best-performing artificially pollinated hybrid between varieties 4P and N2, the fruit weight was 280 g, with a longitudinal width of 8.1 cm and a transverse diameter of 8.5 cm. This combination showed a 88.9% fruit set rate, with a 62.5% fruit survival rate. The skin of the fruit was green, with golden-yellow flesh, and a sugar content of 12%. This new variety has obvious advantages in both fruit quality and yield, and it shows great potential in the future agricultural production process (Fig 4).
-
To gain further insight in the reproductive biology of ice cream fruit, a number of comparative observations on the flower morphology and growth are reported here.
Ice cream fruit varieties with abundant pollen showed a large number of flowers, although with very small ovaries and stigmas. However, the anthers were large. Each branch can bear up to 12−20 flowers.
Varieties with little pollen had significantly reduced numbers of flowers, although the ovaries were larger. Each branch bore 8–12 flowers.
Finally, the ice cream fruit varieties with no pollen had an unusually large ovary, around 2−4 times larger than that of the other varieties flowering at the same period. The anthers are infertile and do not produce pollen. The stigmata on the pistil is also very large, after successful pollination, the stigma withers gradually.
Pollination of different varieties of ice cream fruit
-
In natural self-pollination experiment, the following conclusions could be drawn:
(1) Ice cream fruit varieties with abundant pollen generally show lower fruit sizes, average to low sweetness, and low to moderate rates of fruit set.
(2) The ice cream fruit of little pollen varieties has good fruit quality and the fruit set rate is relatively high.
Furthermore, we observed that in the absence of artificial intervention, ice cream fruit setting rate, fruit quality and sweetness were worse. This indicates that the ice cream fruit benefits from manual pollination intervention.
In the experiments with artificial cross-pollination, we observed an increase in fruit set rate but no change in fruit sweetness or quality. This indicates that the ice cream fruit may have the characteristics of self-incompatibility, which limits the development of ice cream fruit related industries. In order to increase the yield, we must cross-pollinate and cross to find the best pair of pollinators. It is suggested that mixed pollen should be used for artificial pollination when growing fruits in the future[6]. The experimental results are consistent with this view.
The experimentation results from artificial cross-pollination (Tables 4−9) showed that the fruit set rate, fruit size and sweetness were all improved. Based on the cross-pollination results from different varieties, we can conclude:
Firstly, the best-performing variety as the male parent (i.e., the pollen source) is the variety N2, one of the abundant pollen varieties. In general, varieties producing more high pollen showed better performance as the pollen source, and can be used as the pollen source tree in agricultural production.
Second, the best-performing variety for the female parent was variety 4P, which was one of the varieties bearing little pollen. It showed high bud quality and good fruit characteristics, recommending its use as the mother fruit tree in agricultural production and planting.
Third, the combination of the pollen-free variety W4 as the mother tree and the little-pollen variety C1 as the pollen source tree showed a higher sweetness in their hybrid fruits than that of any other hybrid. This combination could be used as a special, high-value hybrid to enrich the agricultural production of ice cream fruit.
Finally, male group and female group contrast. The fruit set rate, fruit quality and fruit sweetness were better in the female group. In the process of agricultural production, artificial cross pollination needs to emasculation. Other research has shown that the average yield of the same maize variety was increased significantly by emasculation than by no emasculation[7]. The experimental results are consistent with this view.
Selecting appropriate varieties for cross-pollination is one of the key technologies to obtain high quality and high yield[8]. Hybrid technology has played an important role in plant genetics and breeding[9]. In particular, the abundant pollen variety N2 significantly improved fruit quality when used as a pollen source in this study, particularly when combined with variety 4P as the mother fruit tree.
-
The experimental field work was conducted at the Tuhao field station and Houpo field station, both owned by Hainan Shengda Modern Agricultural Development Co, Ltd, (Hainan, China).
The ice cream fruit varieties used in this experiment were three varieties producing abundant pollen (N2, AU1, AU2) and three varieties producing little pollen (AU4, 4P, C1) and three varieties producing no pollen (N6, N7, W4). The varieties used in this experiment were randomly selected in the experimental field. All the experimental varieties were planted at appropriate temperature, humidity, sufficient light, with optimum growth conditions and no signs of abnormal flowers and fruits[10,11]. The tree were kept free of diseases and insect outbreaks during the trial.
Instrument, equipment and appliances
-
The handheld glucose meter I used was the Nohawk HP-TD1.
Test method
Observation of natural self-pollination
-
No artificial interventions were made for the natural self-pollination experiment[12,13]; only the selected ice cream fruit trees and the sample flowers in the flower germination period were marked. Observations were made throughout the natural flowering and fruit set process. The fruit set rate and fruit quality data were also collected. In this experimental group, nine flowers were documented from each variety, for three abundant pollen varieties (N2, AU1 and AU2), three little-pollen varieties (AU4, 4P and C1). This gave a total of six experimental groups and 54 data points.
Artificial self-pollination and cross-pollination (male/female)
-
For the artificial self-pollination work, the selected ice cream fruit trees and the sample flowers were marked, bagged and isolated during the flower germination period. The flowers were then artificially self-pollinated (i.e., between the flowers of the same fruit tree). For this experiment, nine flowers were recorded from each variety, for the three abundant pollen varieties (N2, AU1 and AU2) and the three little-pollen varieties (AU4, 4P and C1). Again, this gave six experimental groups and 54 data points.
For the artificial cross-pollination work, one fruit tree of either an abundant pollen variety (N2, AU1, AU2) or a little-pollen variety (AU4, 4P and C1) was selected as the 'father' to collect pollen from. The varieties producing no pollen (N6, N7, W4) or little pollen (AU4, 4P and C1) were selected as mother trees (i.e., the flower source).
For each of the six female trees used as the mother, either five or six other varieties were used as the pollen source to cross-pollinate the parent. Five cross-pollinations were performed where the mother was a little-pollen variety, while six cross-pollinations were performed where the mother was a no-pollen variety. Consequently, there were a total of 15 crosses for the little-pollen mother varieties, and 18 crosses for the no-pollen mother varieties, as shown graphically in Fig. 5. Again, nine flowers were tracked for each cross-pollination experiment.
As a further investigation, this experiment was divided into two groups: male and female. This gave a total of 66 experimental treatments, yielding data on 594 flowers and fruit (Fig. 5).
Experimental operation procedures
Selected varieties (fruit trees)
-
For the biological and morphological observations, a total of 24 fruit trees were observed for different purposes (Table 10). The nine ice cream fruit varieties are planted in a large area in Hainan Shengda Agricultural Company, which belongs to the current commercial varieties[14]. The paired pollination completion experiments and experimental results are of great significance to their commercial production[15,16].
Table 10. Fruit tree different applications.
Different varieties Different applications Abundant pollen varieties (N2, AU1 and AU2) Observation of natural self-pollination 3 Artificial self-pollination 3 Artificial cross-pollination parent tree 3 Little-pollen varieties (AU4,
4P and C1)Artificial cross-pollination mother tree (male) 3 Artificial cross-pollination mother tree (female) 3 Artificial cross-pollination parent tree 3 No pollen varieties (N6, N7 and W4) Artificial cross-pollination mother tree (male) 3 Artificial cross-pollination mother tree (female) 3 Sum 24 Selection of the flowers
-
From each tree, three flowers were randomly selected and marked for observations[17]. The flowers were bagged in the artificial self-pollination and cross-pollination experiments to avoid external factors[18,19].
Pollen collection and preservation
-
Ice cream fruit pollen from the required parent was collected at the morning of the crossing, to avoid little pollen quality and quantity[20,21]. Using tweezers, the pollen was gently collected into separate bags for preservation[22]. No pollen was collected on rainy days, as moisture can affect pollen quality[23].
Artificial pollination
-
Artificial self-pollination was carried out using bagged flowers[24]. Pollen from the required variety was gently shaken onto the stigma; this was repeated at different times every day until the stamens withered or fell off[25−27].
Artificial cross-pollination was also carried out on bagged flowers; however, the stamens were removed (where required) at the germination stage[28,29]. As with the artificial self-pollination work, either cotton swabs or tweezers were used to transfer pollen from the required variety to the stigma of the mother flower at different times each day. This was repeated until the stamens fell off and the stigmata withered[30−32].
Emasculation
-
For experiments where emasculation was required, the stamens of the flower were gently cut off at the germination stage. Care was taken to ensure the rest of the pistil and stigma were not harmed during this process[33,34].
Measurement statistics
-
When the fruit ripened, it was picked and the length and width measured. The sugar content was also measured[35]. Finally, the relevant data was transferred into Microsoft Excel for subsequent processing and analysis[36].
This study was funded by Hainan Province Science and Technology Special Fund (ZDYF2022XDNY190), the Project of Sanya Yazhou Bay Science and Technology City (SCKJ-JYRC-2022-83) , University level scientific research project of Hainan University (XTCX2022NYB09), and Hainan Provincial Natural Science Foundation of China (421RC486).
-
The authors declare that they have no conflict of interest.
-
Received 19 September 2022; Accepted 19 July 2023; Published online 10 August 2023
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Lv W, Johnson JB, Zaman QU, Zhu M, Liu H, et al. 2023. Artificial pollination can improve fruit set and quality in the ice cream tree (Casimiroa edulis). Tropical Plants 2:12 doi: 10.48130/TP-2023-0012
Artificial pollination can improve fruit set and quality in the ice cream tree (Casimiroa edulis)
- Received: 19 September 2022
- Accepted: 19 July 2023
- Published online: 10 August 2023
Abstract: Ice cream fruit (Casimiroa edulis La Llave) is a member of the Rutaceae family native to South America, which has recently been introduced to China. In order to improve the yield of ice cream fruit, we carried out a hybridization experiment with paired pollination. Pollen was collected in the morning and carefully stored, before it was applied to the stigmata of sprouted flowers, using a cotton swab or tweezers. This artificial pollination is repeated at different times of the day. This was continued until the stamens fall off the flowers, the stigmata withered, and the fruit began to develop. Fruit yield and quality were recorded as the principal metrics of the success of each hybrid. At the Hainan Tropical Fruit Window Agricultural Company, nine different ice cream fruit varieties were selected and divided into three groups (abundant pollen, little pollen and no pollen) according to the amount of pollen that each variety naturally produced. Four experimental conditions were investigated: 1. No artificial pollination (natural self-pollination); 2. Artificial self-pollination; 3. Cross-pollination (no emasculation); 4. Cross-pollination (with emasculation). The results indicated that the fruit from cultivars producing abundant pollen generally had poor quality, smaller size, average sweetness, and a medium rate of natural fruit set. In contrast, cultivars producing little pollen showed better fruit quality, higher fruit fertility and increased success rates for artificial pollination. The N2 ice cream fruit cultivar showed the best characteristics as the paternal line (pollen source), while the 4P cultivar showed the best characteristics as the maternal line (flower source). The New varieties (N2 × 4P) performs well in fruit quality, fruit size, fruit sweetness and fruit setting rate. Consequently, this cross-pollinated cultivar is recommended for agricultural production and planting.
-
Key words:
- Ice cream fruit /
- Flowers /
- Fruit set /
- Pollination