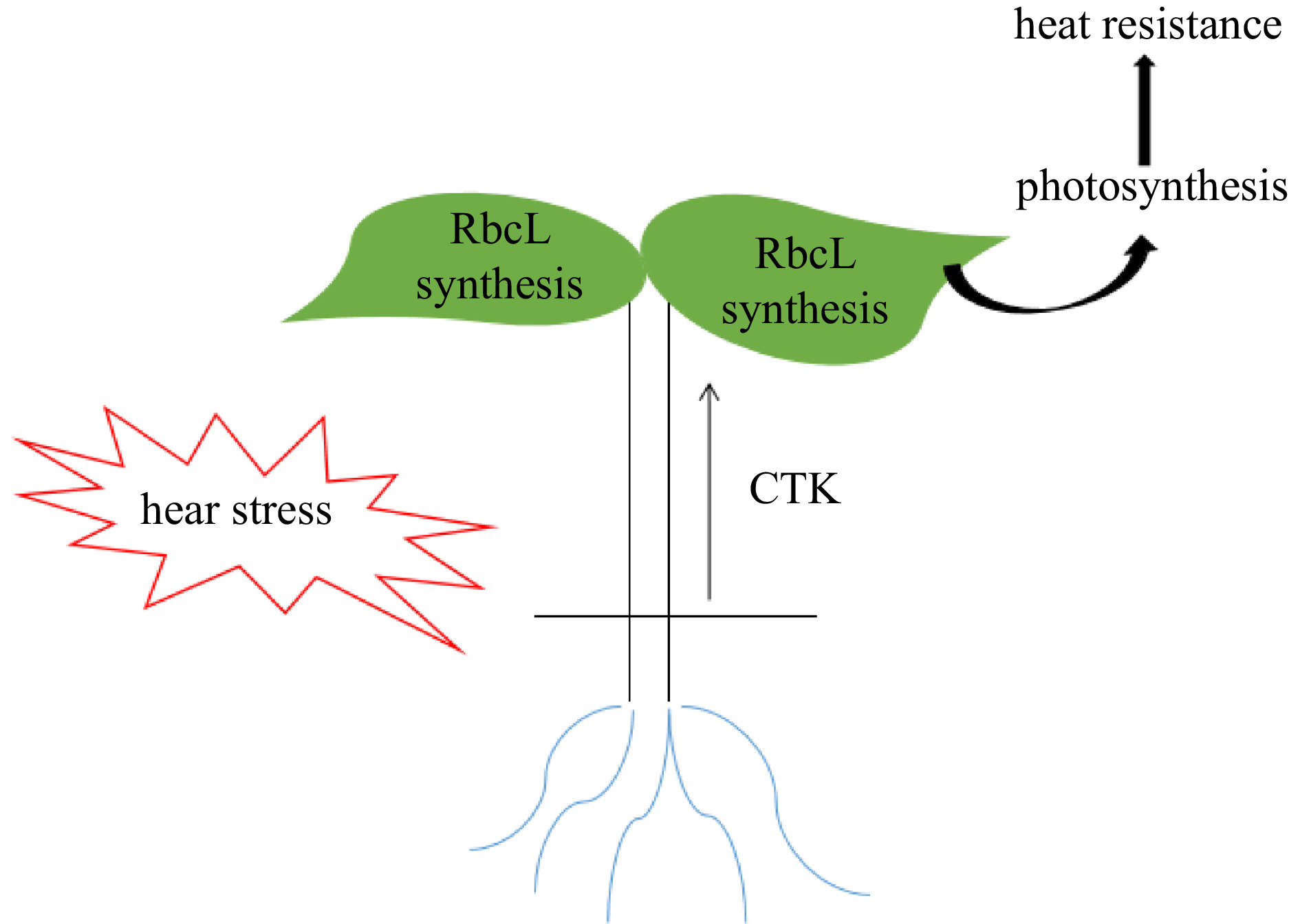
-
Being immobile, plants cope with various abiotic stresses throughout their life cycle in constantly changing environments, such as drought, salinity, and extreme temperatures[1, 2]. Among them, high temperature is becoming an increasingly devastating threat for crop growth and yield[3]. Extreme heat events have caused huge damage to crop production[4]. Generally, heat stress impairs photosynthetic activity, causing damage to the cell membrane, and even affecting enzymatic activities[5−7]. Rubisco and its activase (RCA) are very sensitive to heat stress, which severely impairs the efficiency of the photosynthetic carbon cycle. Thus, it is necessary to find ways and mechanisms to improve crop heat tolerance.
Grafting is an ancient horticultural technique that has been around for thousands of years[8]. It is widely used in plant cultivation programs to modify scions architecture, improve the vigor of the scions, and increase stress tolerance. What's more, grafting has become popular in recent years due to the fact that rootstocks can provide resistance to soil-borne diseases and abiotic stressors, especially in cucurbits and Solanaceous crops[9]. Grafting can improve the growth and resistance of potassium in tobacco, by improving K+ uptake, utilization, and loading capacity[10]. Previous study suggested that grafted plants can exchange of genetic information via either large DNA pieces or entire plastid genomes[11]. Grafting increases the salt tolerance of tomato by limiting the transport of sodium and chloride from the root to the shoot[12]. Cucumber grafted onto luffa and bitter gourd rootstock can improve heat stress tolerance[8, 13−15]. Compared with self-grafted plants, bitter gourd-grafted plants have stronger heat resistance. This is associated with the relatively high photosynthetic capacity of bitter gourd-grafted plants under heat stress[14,15]. While little is known about the molecular mechanisms in grafted plants and whether long-distance signaling from root to shoot involved it[16].
Cytokinins (CTKs) are a group of adenine derivatives with isoprenoid or aromatic chains connected to the N6 position of the adenine ring, which are important for regulating series of development processes and environmental responses[17−19]. In plants, isoprenoid CTKs, including N6-(Δ2-isopentenyl) adenine (iP), trans-zeatin (tZ), and cis-zeatin (cZ), are more abundant than aromatic CTKs[20]. CTKs play crucial roles in the response of the root to long-distance signals. CTKs are synthesized in roots, especially at high nitrate concentration, and translocated to the shoots, regulating the growth of shoots[21]. Exogenous application of CTK improved the plants to heat stress[22, 23]. Overexpression of the CTK biosynthetic gene isopentenyltransferase (IPT) enhanced the resistance to heat stress by elevating the endogenous CTK levels. Besides, the availability of macronutrients, such as nitrate (N) and phosphate (P), regulate the expression of IPT genes[24].
Cucumber (Cucumis sativus L.) belongs to the Cucurbitaceae family. It is a thermophilic species, and sensitive to extreme temperatures. Bitter gourd (Momordicacharantia L.), is a heat-resistant plant, widely distributed in tropical and subtropical regions of the world. As most of the members of Cucurbitaceae are highly compatible, grafting is widely used to improve stress resistance in production. Our previous research reported that grafting onto bitter gourd rootstock could improve the heat tolerance of cucumber through alleviating the decrease of photosynthetic-related protein expression under high temperature stress[14]. However, the mechanism underlying the tolerance to bitter gourd rootstock-induced systemic heat tolerance in cucumber shoots is largely unknown. In the current study, we found that bitter gourd rootstock enhanced the heat resistance of cucumber scions, is associated with protein accumulation related to photosynthesis, and the root-sourced CTK signaling involved in the bitter gourd rootstock-induced heat resistance. Our study suggested that cytokinin signaling is involved in the regulation of rootstocks on scion heat tolerance, which further lays a theoretical foundation for grafting to improve plant heat tolerance.
-
Fresh and dry weight, and growth of plants were significantly inhibited after heat treatment (42/32 °C) for 5 days (Table 1, Supplemental Fig. S1). The leaves of the self-grafted cucumber turned yellow and severely lost water after heat treatment (42/32 °C) for 5 days. The fresh and dry weight of the self-grafted plants decreased by 31.2 and 34.6%, but the plants grafted onto the bitter gourd rootstock under heat stress decreased by 18.2% and 18.4% (Table 1). In order to investigate the role of bitter gourd rootstock response to heat stress, we examined the physiological parameters changed after stressed, such as the net photosynthetic rate (Pn), chlorophyll content, and the maximum photochemical efficiency of PSII (Fv/Fm) of grafted plants after exposure to 42/32 °C for 2 days. There was no significant difference in weight, Pn, chlorophyll content, and Fv/Fm between self-grafted plants and bitter gourd-grafted plants under control temperature (Fig. 1). Heat stress decreased Pn, chlorophyll content, and Fv/Fm of self-grafted plants by 28.1%, 21.1% and 29.2%, after exposure to 42/32 °C, respectively (Fig. 1). However, the Pn, chlorophyll content and Fv/Fm value of plants grafted onto the bitter gourd rootstock only decreased by 6.8%, 18.6% and 12.2,% for the same treatments (Fig. 1), indicating that the bitter gourd rootstock had higher thermotolerance than the cucumber rootstock.
Table 1. Effects of high temperature on the growth of grafted cucumber seedlings.
Treatments Fresh weight (g·plant−1) Dry weight (g·plant−1) Cs-28 9.28 ± 0.11a 1.04 ± 0.07a Mc-28 8.99 ± 0.10a 0.98 ± 0.05a Cs-42 6.38 ± 0.20c 0.68 ± 0.02c Mc-42 7.35 ± 0.17b 0.80 ± 0.04b Cs-28: self-grafted plants subjected to 28/18 °C (day/night). Mc-28: bitter gourd-grafted plants subjected to 28/18 °C (day/night). Cs-42: self-grafted plants subjected to 42/32 °C (day/night). Mc-42: bitter gourd-grafted plants subjected to 42/32 °C (day/night). Data are means ± SE. The letters 'a', 'b', 'c', and 'd' indicate significant differences between treatments (p < 0.05). 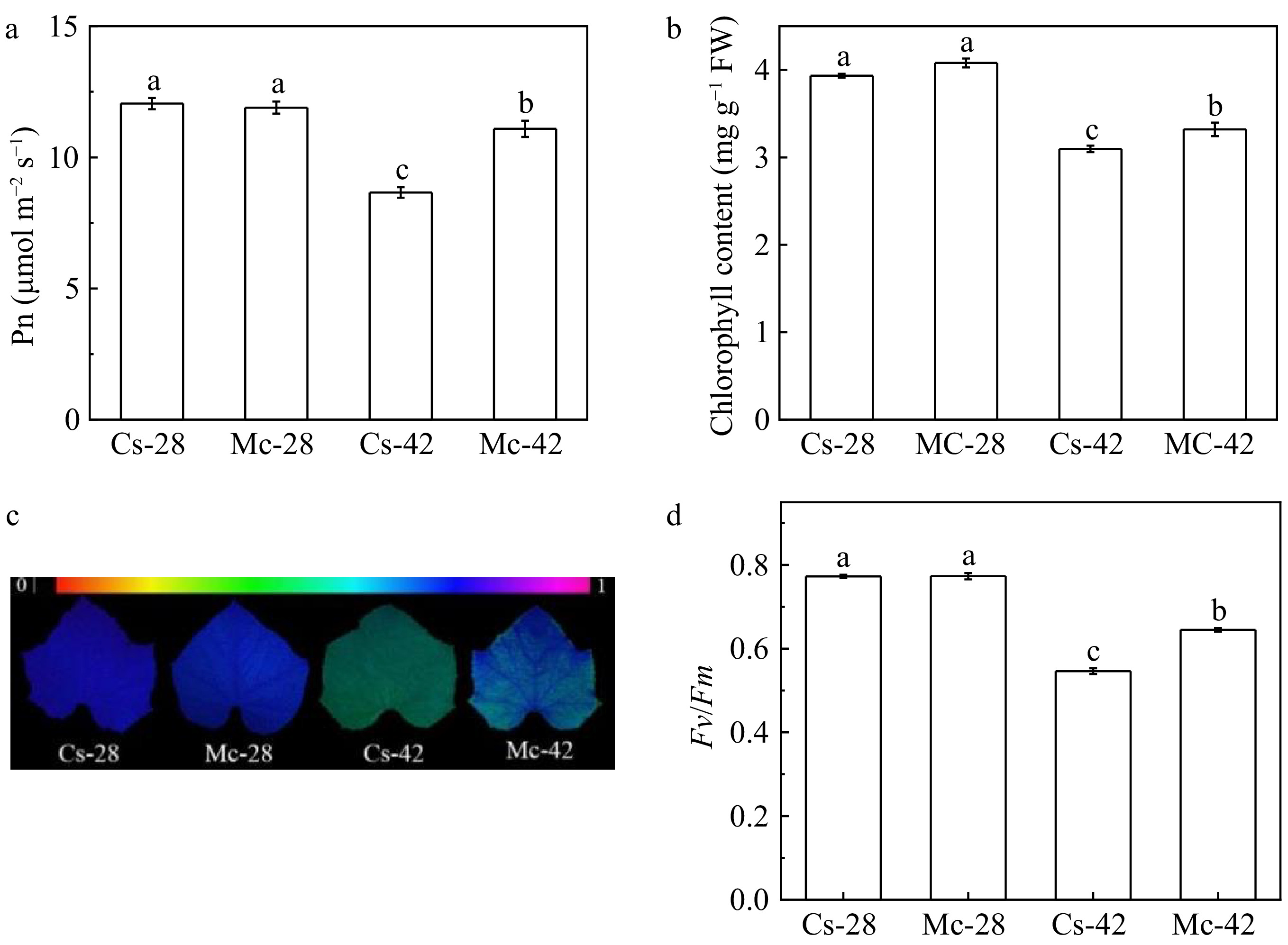
Figure 1.
Effects of heat stress on grafted cucumber plants on (a) net photosynthetic rate, (b) chlorophyll content, (c) images of the maximum photochemical efficiency of PSII, (d) the quantity of the maximum photochemical efficiency of PSII (Fv/Fm). The data of Pn was measured at 48 h after heat treatment, and the data of chlorophyll content and Fv/Fm were measured at 48 h and 24 h after heat treatment, respectively. All data are presented as means of three biological replicates (± SE). Means with different letters indicate significant differences at p < 0.05 according to Duncan's multiple range test. Three independent experiments were performed with similar results.
Effects of high temperature on the expression of photosynthesis-related genes and proteins in grafted cucumber seedlings
-
The results of the RT-PCR analysis showed that the expression of the RbcL and Lhcb2 genes reduced under heat stress in both of the grafted cucumber plants, but the bitter gourd rootstock efficiently alleviated the decreasing amplitude at high temperature (Fig. 2a, b). While the relative gene expression of RCA was up-regulated after exposure to high temperature, but compared to self-grafted plants, the bitter gourd rootstock suppressed its expression caused by high temperature (Fig. 2c). As shown in Fig. 2d, heat stress also decreased the level of RbcL and Lhcb2 protein in cucumber self-grafted plants. In addition, the RCA abundance was up-regulated by high temperature, especially in cucumber self-grafted plants.
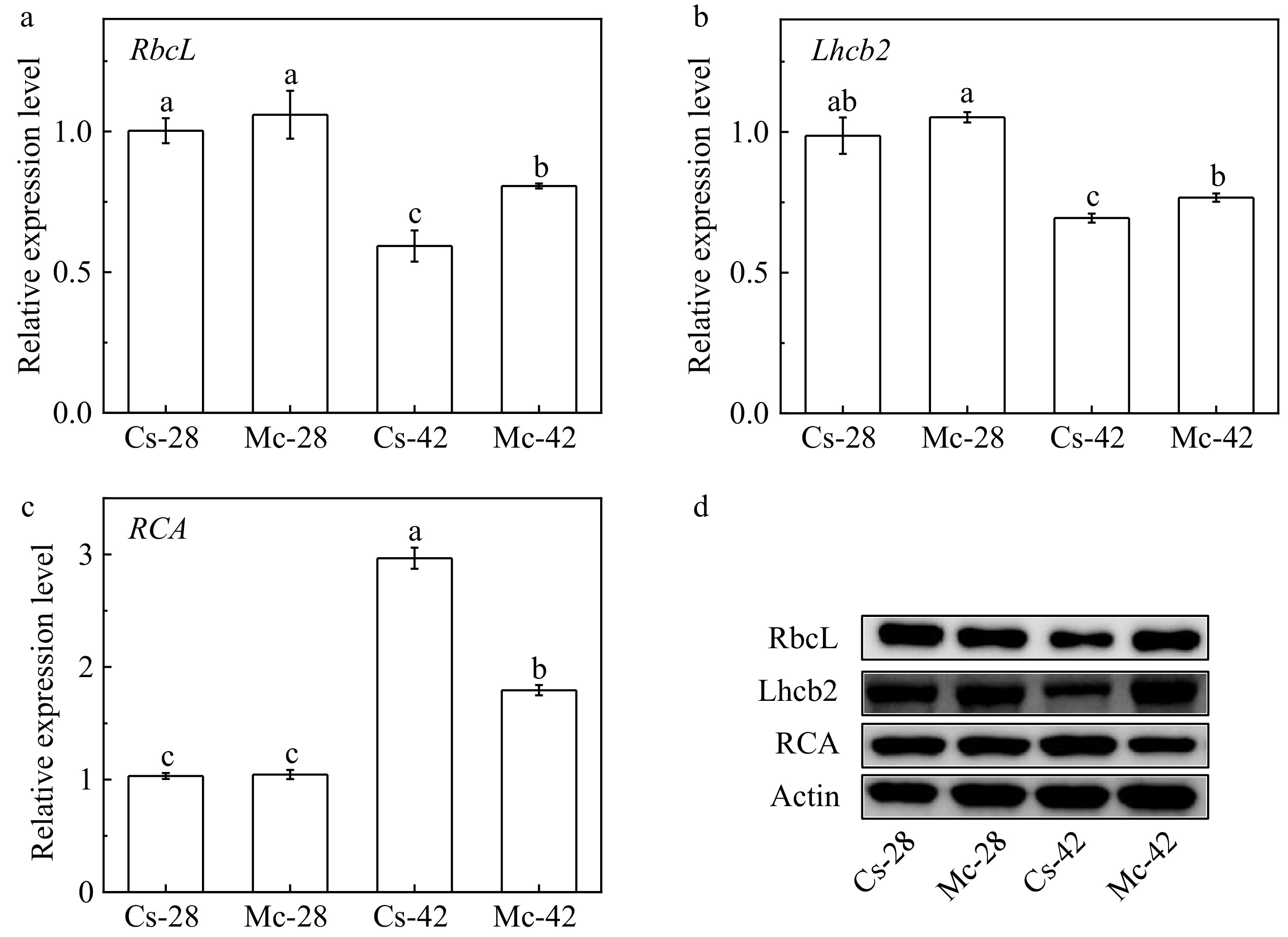
Figure 2.
Effects of bitter gourd rootstock on the expression of RbcL, Lhcb2 and RCA genes and their protein abundance in leaves of cucumber under heat stress. (a) The expression level of RbcL genes. (b) The expression level of Lhcb2 genes. (c) The expression level of RCA genes. (d) The expression level of RbcL, Lhcb2 and RCA proteins. Each bar represents a mean ± SE of three independent experiments. Means followed by different letters indicate significant differences between treatments (p < 0.05) according to Duncan's multiple range tests.
Effects of bitter gourd rootstock on CTKs contents in grafting cucumber shoots and roots
-
CTKs play a key role as long-distance root-to-shoot signals to regulate various growth and developmental processes in shoots[17−19]. Changes in CTK contents in both leaves and roots in response to heat stress were measured (Fig. 3). The content of trans-zeatin (tZ) in roots of self-grafted plants first increased and then decreased with the extension of the high-temperature duration (Fig. 3a). The highest tZ value was observed at 6 h, the content was approximately 2.5 fold higher than that of the control treatment, and the content of tZ was close to detection limit after heat stress for 48 h. The change trend of tZ content in the roots of bitter gourd rootstock grafted seedlings was similar to that of self-rooted cucumber grafted seedlings. However, after 48 h of heat stress treatment, the content of tZ only dropped to half of the normal temperature control (Fig. 3a). The content of trans-zeatin riboside (tZR) in the roots of the grafted plants was similar to that of tZ in the grafted seedlings. But, the peak value emerged at 3 h and 6 h after heat stress in self-grafted plants and bitter gourd grafted plants, respectively (Fig. 3c). The content of N6-(Δ2-isopentenyl) adenine (iP) in the roots of grafted cucumber seedlings did not change significantly with high-temperature treatment (Fig. 3e).
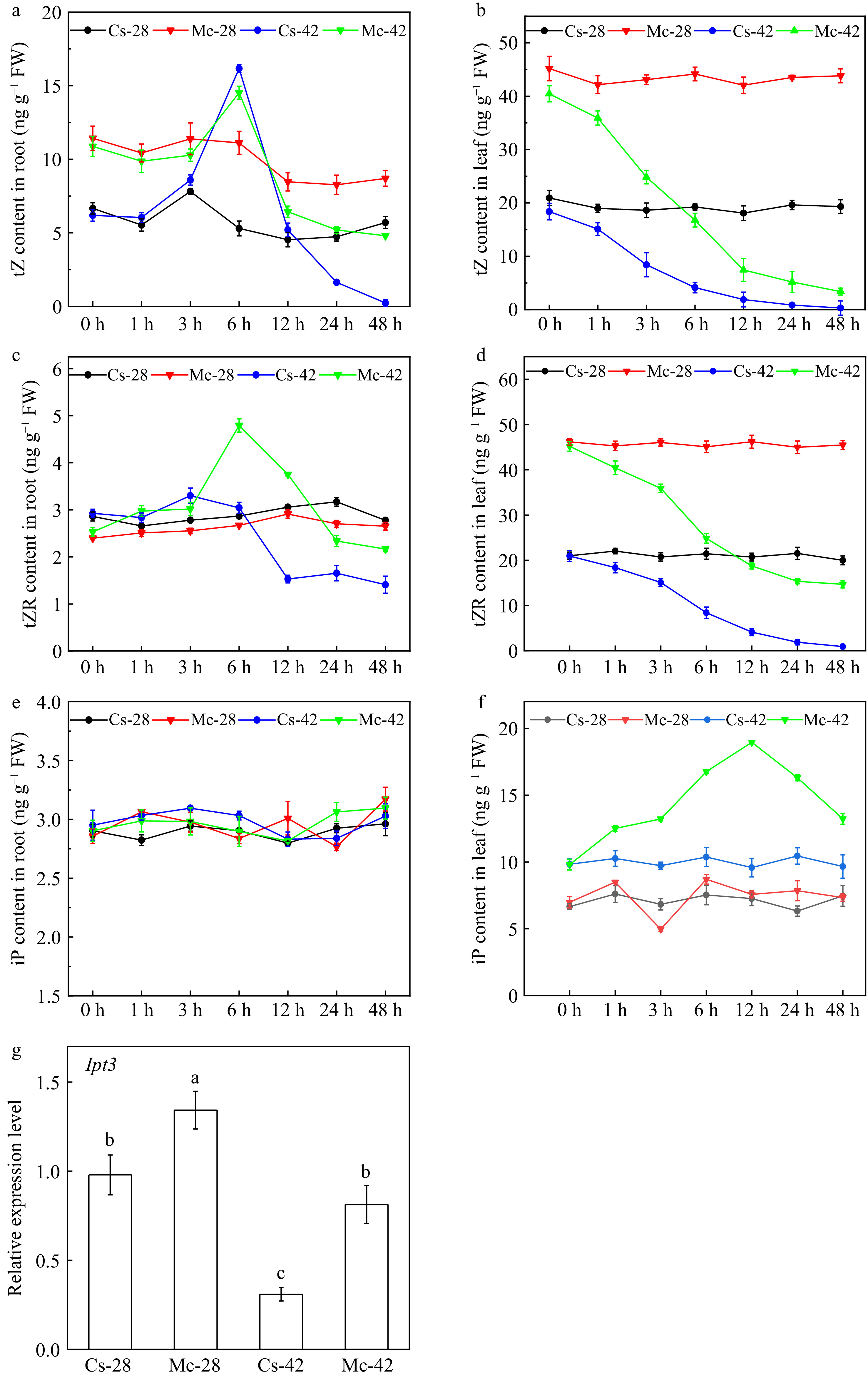
Figure 3.
Effects of bitter gourd rootstock on CTK content in cucumber leaves and roots, CTK biosynthesis in cucumber leaves. (a) The content of tZ in roots of grafted plants after exposure to heat stress for 0, 1, 3, 6, 12, 24, 48 h. (b) The content of tZ in leaves of grafted plants after exposure to heat stress for 0, 1, 3, 6, 12, 24, 48 h. (c) The content of tZR in roots of grafted plants after exposure to heat stress for 0, 1, 3, 6, 12, 24, 48 h. (d) The content of tZR in leaves of grafted plants after exposure to heat stress for 0, 1, 3, 6, 12, 24, 48 h. (e) The content of iP in roots of grafted plants after exposure to heat stress for 0, 1, 3, 6, 12, 24, 48 h. (f) The content of iP in leaves of grafted plants after exposure to heat stress for 0, 1, 3, 6, 12, 24, 48 h. (g) The expression level of IPT3 gene in leaves of grafted plants. Each bar represents the mean ± SE of three independent experiments. Means followed by different letters indicate significant differences between treatments (p < 0.05) according to Duncan's multiple range tests.
In contrast, the contents of tZ and tZR in the leaves of grafted seedlings decreased significantly with the extension of the treatment time. The content of tZ and tZR in self-grafted plants were close to the detection limit up to 48 h after high temperature treatment (Fig. 3b, d). But, with the extension of the treatment time, the contents of tZ and tZR in the leaves of bitter gourd rootstock grafted plants were always higher than that of the self-grafted plants (Fig. 3b, d). Interestingly, the iP content in the leaves of self-grafted plants did not change significantly with high-temperature treatment, but in bitter gourd rootstock-grafted plants the content of iP first increased and then decreased with the extension of the high temperature duration. After 12 h of heat stress, the iP content reached the peak position and was about 2.7 folds higher than that of the control treatment after heat stress for 48 h (Fig. 3f). Higher cytokinins in bitter gourd rootstock plants positively affected the expression of photosynthesis-related proteins and enhanced the heat tolerance of grafted seedlings.
In addition, we also measured the changes in IPT3, a key limiting gene for CTK biosynthesis, in grafting plants after exposure to heat stress. Under control conditions, IPT3 expression in bitter gourd grafted plants was 23% higher than that of self-grafted plants. After being exposed to heat stress treatment, the gene expression level of IPT3 in bitter gourd-grafted plants and self-grafted plants decreased by 33% and 72%, respectively (Fig. 3g).
Effect of exogenous tZ on photosynthesis of grafted cucumber seedlings
-
To understand more insight of CTK contribution on photosynthetic capacity in grafted plants, we applied exogenous tZ treatment to grafted plants. As expected, exogenous tZ enhanced the heat resistance of the grafted cucumber by increasing photosynthesis. The results demonstrated that 10 μM tZ was the most effective in alleviating heat stress. As shown in Fig. 4a, the Fv/Fm increased with an increase in tZ concentration up to 10 μM. And further increase in tZ concentration up to 20 μM did not significantly increase the value of Fv/Fm. Furthermore, the heat-induced decline in Pn was attenuated by the spraying of tZ, and the greatest induction was observed in plants that at the concentration of 10 μM. These results indicate that exogenous spraying of tZ can effectively improve the photosynthetic parameters under high-temperature stress.
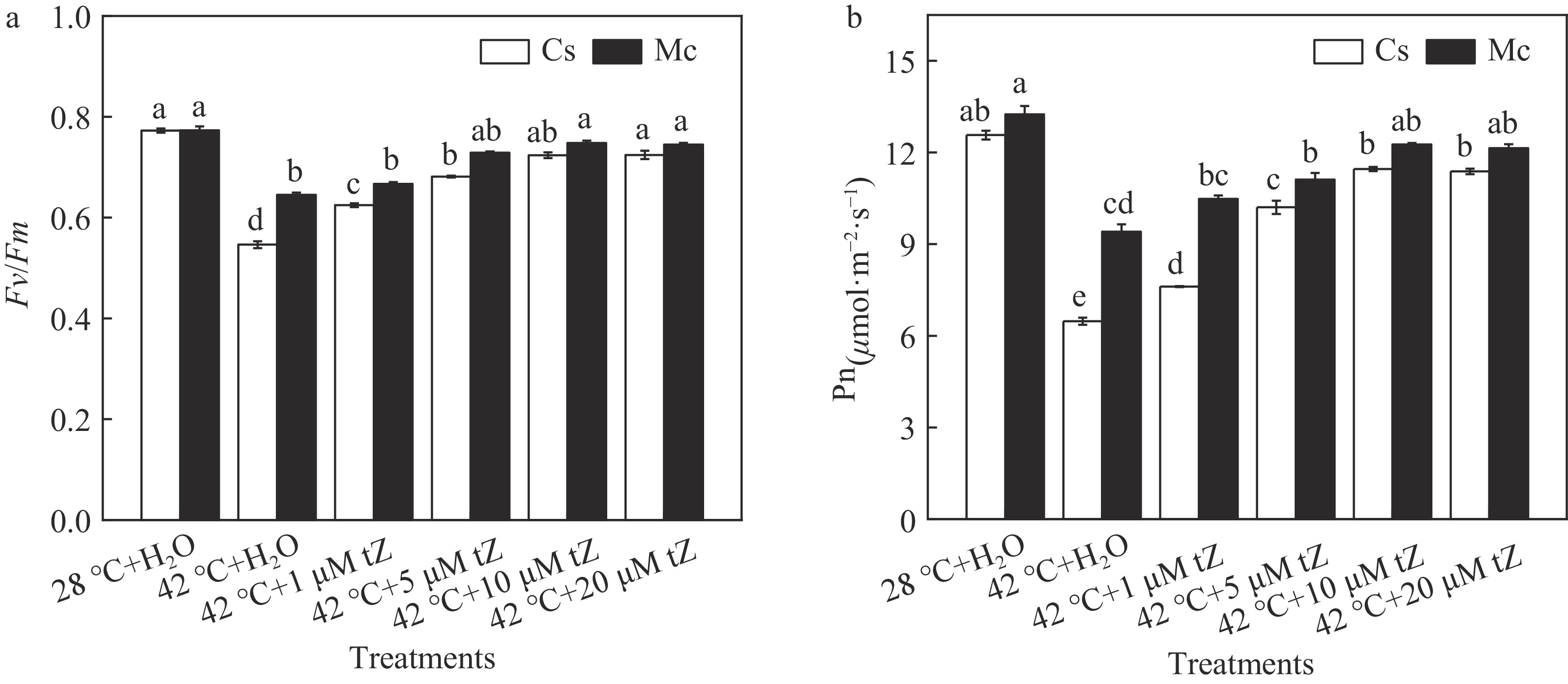
Figure 4.
Effects of exogenous trans-zeatin (tZ) on Fv/Fm and Pn of grafted plants under heat stress. (a) Changes in the maximum photochemical efficiency of PSII (Fv/Fm). Plants were pretreated with 0, 1, 5, 10, 20 μM tZ for 12 h and then plants were exposed to heat stress at 42 °C for 48 h. (b) Changes in the net CO2 assimilation (Pn). Plants were pretreated with 0, 1, 5, 10, 20 μM tZ for 12 h and then plants were exposed to heat stress at 42 °C for 48 h. Each bar represents the mean ± SE of three independent experiments. Means followed by different letters indicate significant differences between treatments (p < 0.05) according to Duncan's multiple range tests.
In addition, we also measured the gene expression level of RbcL and the activity of Rubisco. Grafting plants pretreated with 10 μM tZ for 12 h significantly improved the expression level of RbcL gene under heat stress (Supplemental Fig. S2). Moreover, exogenous tZ could effectively alleviate the decrease in Rubisco enzyme activity caused by high temperature stress (Supplemental Fig. S2).
Effect of root-sourced CTK on Rubisco large subunit accumulation and Carboxylation activity under heat tolerance
-
To confirm the role of root-sourced CTK in photosynthesis-related proteins, we examined the effect of lovastatin (Lov, an inhibitor of the synthesis of isoprenoids and especially of CTKs) on bitter gourd rootstock-induced RbcL expression and subsequent heat tolerance after exposure to 42 °C for 48 h. As shown in Fig. 5, bitter gourd rootstock-induced RbcL protein accumulation under heat stress, but the protective effect was completely prevented by pretreatment with Lov. Furthermore, 10 μM tZ can effectively promote the accumulation of RbcL protein. These results strongly indicate that CTK was involved in the bitter gourd rootstock-induced accumulation of RbcL protein and subsequent heat tolerance.
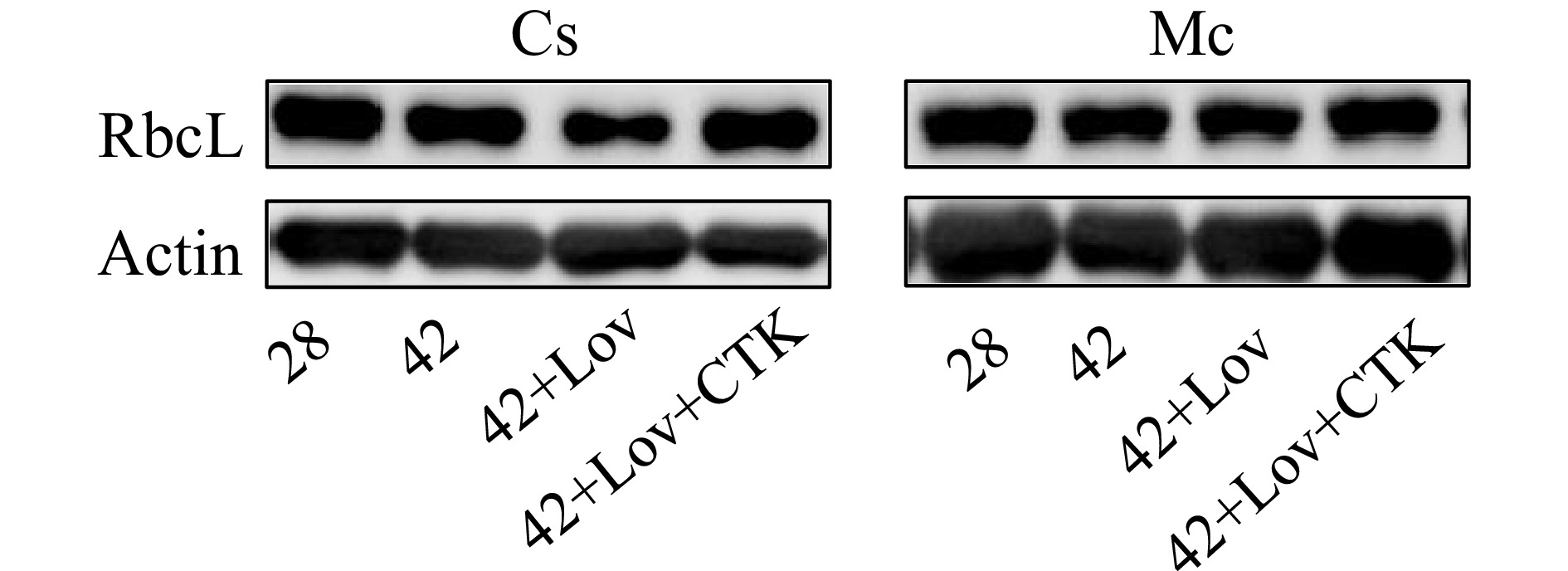
Figure 5.
Effects of CTK signal on RbcL accumulation. Plants grafted onto cucumber and bitter gourd were treated with 42 °C for 48 h; after 10 μM Lov pretreatment for 12 h, then plants were exposed to 42 °C for 12 h; 10 μM Lov pretreatment for 12 h, then plants were exposed to heat stress for 12 h, and then replenishment group were sprayed with 10 μM tZ. The control groups were treated with 28 °C.
-
Grafting is a widely used viable technique that enhances stress resistance, and improves the yield and quality of crops in agriculture. However, grafting mainly focuses on the application of rootstocks to improve resistance of scions[8, 16]. Grafting onto rootstocks with longer roots can improve the stomatal conductance of grapes and enhance the resistance under low and moderate water deficient conditions[25]. In the present study, we found that heat resistant bitter gourd-rootstock significantly improved the accumulation of biomass and photosynthetic capacity of cucumbers under high-temperatures. The higher heat tolerance of bitter gourd rootstock grafting was associated with a lower decline of Pn, Fv/Fm, and chlorophyll content and the values of these components was higher in bitter gourd grafted cumber seedlings as compared with cucumber self-grafting plants under heat stress (Fig. 1). Therefore, grafting is an effective agricultural technique to prevent yield reduction and protect the photosynthetic apparatus.
Rubisco, the key enzyme of the Calvin cycle, and is the most abundant protein in plants[26, 27]. However, Rubisco-mediated CO2 fixation in chloroplasts is catalytically slow, competitively inhibited by oxygen, and makes inefficient use of water[28, 29]. Various strategies have been used to increase plants photosynthetic performance, such as the engineering of Rubisco, optimizing the Calvin Cycle, and changing the expression level of enzymes within the Calvin Cycle. By increasing the thermo-stability of RCA in Arabidopsis, improved photosynthesis and growth rate under a moderate heat stress[30, 31]. In the present study, we found that increased heat tolerance associated with increasing in transcript abundance and protein expression of RbcL and Lhcb2, suggests that the use of bitter gourd as rootstock might promote the accumulation of RbcL and Lhcb2 to improve plant heat tolerance (Fig. 2a, c & d). However, in our study, RCA was up-regulated by heat, and the abundance and protein expression of RCA were lower in bitter gourd rootstock grafting plants than in self-grafted plants under heat stress (Fig. 2b, d). It might account for the number of Rubisco protein lost activity in self-grafted plants is more than that in bitter gourd grafted plants under heat stress. The catalytic chaperone RCA can remove sugar phosphate inhibitors from an inactive uncarbamylated enzyme or an inhibited carbamylated Rubisco[32]. High temperature causes chlorophyll degradation, and chlorophyll degradation causes LHCII damage[7, 33]. The highest accumulation of Lhcb2 protein and chlorophyll content was found in bitter gourd-grafted plants under heat stress (Fig. 1b, Fig. 2d). These results suggest that bitter gourd rootstock-induced heat tolerance was associated with a accumulation of photosynthetic proteins.
CTKs involved in rootstock-induced RbcL accumulation and subsequent heat tolerance
-
Cytokinins are key regulators of a wide range of plant growth processes, with highly flexible and adaptable properties[34]. Under heat stress, endogenous CTKs are involved in regulation of stomatal aperture and subsequently transpiration[35]. Exogenous application of CTK alleviates the inhibition of heat stress on photosynthetic activity and delays chlorophyll decrease[36, 37]. In this study, the contents of tZ and tZR in root, and iP in bitter gourd grafted-plants leaves were significantly increased after short term exposued to heat stress. On the other hand, long-term heat stress profoundly decreased the three kinds of CTKs in both roots and leaves (Fig. 3a−f). Interestingly, the IPT3 gene did not increase in leaves (Fig. 3g). In the case of short-term heat stress, the endogenous CTK was increased. Under heat stress, the effect of CTK on stomata opening followed by stimulation of leaf transpiration is crucial in the early phase of the stress response[38].The opening of the stomata is beneficial to increase the leaf transpiration rate and reduce the leaf surface temperature. CTK levels are maintained mainly by CTK biosynthesis and degradation genes in plants[39]. The IPT3 gene in grafted plant leaves was decreased after exposure to high temperature, indicating that compared with self-grafted cucumber bitter gourd-grafted cucumber alleviated the decrease of CTK transported from roots to shoots, and these findings suggest that CTK acts as signal molecule involved in heat stress responses.
In order to further justify the CTK role in signaling transmission in the heat shock response of bitter gourd rootstock grafted plants, we applied Lovastatin (an inhibitor of CTK biosynthesis) which inhibited CTK production. In a previous study, Lovastatin treatments inhibited endogenous CTK production in the seminal root and also reduced rice root growth[40]. Study proposed that proteome-level interactions maybe involved in some cytokinin-temperature signaling cross-talk[41]. In the expression level of present study, the RbcL protein decreased in grafted plants after Lovastatin treatment, and increased by spraying tZ (Fig. 5). All of these findings indicate that endogenous CTK induced Rubisco expression in grafted plants in response to heat stress.
-
In conclusion, we have demonstrated that grafting to the heat resistant rootstock can improve the cucumber plants heat tolerance by alleviating photosynthesis inhibition. This enhancement of photosynthetic capacity was associated with the accumulation of chlorophyll content, the lower decrease of RbcL transcription and Rubisco activity, and the higher content of CTKs. The higher accumulation of RbcL protein in the bitter gourd rootstock plants was attributed to an increase in the content of CTKs transported from roots (Fig. 6). These results support the hypothesis that a systemic response involving root−shoot communication during heat stress is mediated by CTK. Taken together, root-sourced CTK signaling is involved in the bitter gourd rootstock-induced heat resistance and plays a critical role in root−shoot communication in response to heat stress.
-
Two different species, cucumber (Cucumis sativus L., cv. Jinyou No.35, Cs) and bitter gourd (Momordica charantia L., cv. Changlv, Mc) were used as the scions and rootstock, respectively. Plant materials were made by applying cleft grafting, and self-grafted plants were denoted as controls. Seeds of rootstock were sown in 15-cell trays, and seeds of scions were sown in 108-cell trays filled with commercial organic substrate.
When the second true leaves of the rootstock seedlings fully developed and the cotyledons of the scions expanded completely, cleft grafting was performed. After grafting, plants were transferred to the graft healing chamber and maintained at a temperature of 25−30 °C and a relative humidity of 85%−100% for 7 d until the graft union completely healed. The grafted seedlings were then moved to growth chambers with the following environmental conditions, 12 h photoperiod, temperature of 28/18 °C (day/night) and photosynthetic photon flux density (PPFD) of 300 μmol m−2·s−1, relative humidity of 70%−75%.
Experimental design
-
When the third leaves of grafted seedlings fully expanded, they were exposed to a variety of treatments: 1) self-grafted plants subjected to 28/18 °C (day/night), Cs-28; 2) bitter gourd-grafted plants subjected to 28/18 °C (day/night), Mc-28; 3) self-grafted plants subjected to 42/32 °C (day/night), Cs-42; 4) bitter gourd-grafted plants subjected to 42/32 °C (day/night), Mc-42. After being exposed to high temperatures, plant leaf samples (the third leaf from the bottom) were harvested at different time points for various purposes, such as follows the gene relative expression level of IPT3 at 6 h for quantify the chlorophyll fluorescence and the gene expression level of photosynthetic proteins (RbcL, RCA, Lhcb2) at 24 h, to determination the content of chlorophyll, photosynthetic protein (RbcL, RCA, Lhcb2) expression levels and the net photosynthetic rate (Pn) at 48 h; the cytokinin content (tZ, tZR, iP); and for the measurement of fresh and dry weight. All the leaf samples were immediately flash-frozen in liquid nitrogen and stored at −80 °C for subsequent gene expression and protein analysis.
To investigate the effects of exogenous tZ on grafted cucumber plants tolerance to heat stress, cucumber self-grafted and bitter gourd-grafted seedlings were sprayed with tZ (Sigma-Aldrich) at 1, 5, 10, and 20 μM at the four-leaf stage, where distilled water spray was used as the control. The tZ solutions were prepared by dissolving the solute in dimethyl sulfoxide (DMSO) followed by dilution with distilled water. The plants were exposed to a heat stress of 42/32 °C after 12 h of spraying of tZ at the above mentioned-concentrations. The maximum photochemical efficiency of PSII (Fv/Fm) at 24 h, and the net CO2 assimilation (Pn) at 48 h were measured after exposure to heat stress. A 10 μM concentration of tZ was used to investigate the effects of tZ on the gene expression of RbcL, the protein expression level of RbcL, and the enzyme activity of Rubisco.
The tZ, tZR and iP contents in both leaves and roots were measured at 0, 1, 3, 6, 12, 24, 48 h after exposure to heat stress. To examine the role of CTKs in rootstock to heat resistance, the leaves of self-grafted seedlings were pretreated with 20 μM Lovastatin (Lov, a synthetic inhibitor of cytokinin), solutions were prepared by dissolving the solute in DMSO, and after 12 h of treatment, and plants were transferred to a growth chamber at 42/32 °C for heat stress. The protein expression level of RbcL and Rubisco activity were measured after 48 h of heat stress.
Plant growth, fresh and dry weights, chlorophyll content, and net photosynthetic rate
-
After 5 d of treatment, the plants were washed with sterile distilled water, dried with bibulous paper, and then measured fresh (FW) and dry (DW) weights. For the measurement of dry weight, plants were incubated at 105 °C for 15 min, followed by 70 °C for 72 h following which the measurements were made. After 48 h of treatment, the leaves of grafted plants were soaked in absolute ethanol followed by centrifuge at 5,000 ×g for 2 min and the supernatant was collected. The absorbance of the supernatant was detected at 649 nm and 665 nm. The total chlorophyll, chlorophyll a, and chlorophyll b content were calculated according to relative equations, respectively[42].
Chla = 13.95 × OD665 − 6.88 × OD649
Chlb = 24.96 × OD649 − 7.32 × OD665
After 48 h of treatment, the Pn was measured with a portable photosynthesis system (LI-6400, LI-COR Inc., Lincoln, USA), at a temperature of 25 °C, 85% relative humidity, a cuvette air flow rate of 500 ml min−1, and an ambient CO2 concentration of 380 μmol mol−1. A PPFD of 600 μmol m−2·s−1was provided by a mixture of red blue light-emitting diodes.
Chlorophyll fluorescence measurements
-
Chlorophyll fluorescence was determined with an imaging-PAM (pulse-amplitude-modulated) chlorophyll fluorometer (Heinz Walz, GmbH, Effeltrich, Germany) of the third leaf from the bottom after exposure to heat treatment for 24 h. The plants were dark-adapted for 30 min to measure the maximum photochemical efficiency of PSII (Fv/Fm). The values of Fv/Fm and the quantum efficiency of PSII (ΦPSII) were determined using the entire area of the third leaf from the bottom[43].
Hormone extraction and fractionation for UPLC analysis
-
The extraction and determination of cytokinin (iP, tZ, and tZR) was performed according to Bieleski with little modification[44]. Briefly, frozen leaves (about 0.5 g) were crushed to a fine powder using a Tissue Lyser, with a zirconia bead in a 10 ml centrifuge tube, and then soaked in 5 ml of extraction solvent (methanol: formic acid: water = 15:1:4, v/v/v). The homogenate was kept at −20 °C for at least 16 h. After centrifugation at 10,000 ×g for 15 min, the supernatant was transferred to a new centrifuge tube, and the pellet was re-extracted with 2 ml of extraction solvent, and combined with the first supernatant. The crude extract supernatants were filtered through the Sep-Pak C18 cartridge and dried under a stream of N2. The dried samples was resuspended with 50% methanol. Then, ultra-performance liquid chromatography (UPLC) was used to determine the three common forms of cytokinin iP, tZ, tZR.[45−48].
Total RNA isolation and gene expression analysis
-
Total RNA was extracted from cucumber leaves according to the manufacturer's instructions using an RNA simple Total RNA Kit (Tiangen, China). One microgram of total RNA was used to reverse transcribe to a cDNA template using the ReverTra Ace qPCR RT Kit (Takara, Japan). Quantitative real-time polymerase chain reaction (qRT-PCR) assays were performed using SYBR Green PCR Master Mix (Takara, Japan) in a StepOnePlusTM real-Time PCR System (Applied Biosystems, USA). The PCR conditions consisted of denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 58 °C for 15 s and extension at 72 °C for 30 s. The cucumber actin gene was used as an internal control. Gene-specific primers were designed according to the cDNA sequences as described in Supplemental Table S1. Relative gene expression was calculated by the 2−ΔΔCᴛ method[49].
Western blot analysis
-
The cucumber leaves were ground in liquid nitrogen and homogenized in extraction buffer (30 mM Tris-HCl (pH8.7), 1 mM MgCl2, 0.7 M sucrose, 1 mM ethylenediami-netetraacetic acid (EDTA), 1 mM DTT, 1 mM phenylmethanesulfonyl fluoride (PMSF) and 1 mM ascorbic acid) to extract proteins[50]. Protein concentrations were measured using a Bio-Rad protein assay kit (USA), denatured at 95 °C for 5 min and stored at −20 °C for further analysis. The denatured protein extracts (10 μg) were separated using a 12% SDS-PAGE for Western blotting, and the proteins on the SDS–PAGE gel were transferred to a 0.45μm poly vinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% non-fat dry milk for 1 h, washed with TBST buffer (including Tris-HCl, NaCl, and tween 20) three times, and incubated with a mouse anti-Rubisco large subunit monoclonal antibody, a rabbit anti-Rubisco small subunit monoclonal antibody, a rabbit anti-Lhcb2 monoclonal antibody, a rabbit anti-Rubisco activase monoclonal antibody, and a rabbit anti-actin antibody for 2 h. The membrane was washed with TBST buffer and incubated at room temperature for 1 h with Goat Anti-Rat IgG HRP-conjugate antibody or Goat Anti-rabbit IgG HRP-conjugate antibody. Finally, the membrane was washed with TBST three times and developed using ultra-sensitive ECL chemiluminescence reagent.
Statistical analysis
-
At least three independent biological replicates were used for each determination. All data were statistically analyzed using SPSS 23.0 software (SPSS Inc., Chicago, IL, USA), and the mean differences among the treatments were calculated with Duncan's multiple range test at p < 0.05.
This work was supported by the China Agriculture Research System (CARS-23-B12).
-
The authors declare that they have no conflict of interest.
- Supplemental Fig. S1 Effects of Momordca rootstock on root and shoot morphology of grafted cucumber plants under high temperature stress (a) shoot part (b) root part. Plants were exposed to heat stress at 42 °C for 48 h. Cs-28: self-grafted plants subjected to 28/18 °C (day/night). Mc-28: Bitter gourd-grafted plants subjected to 28/18 °C (day/night). Cs-42: self-grafted plants subjected to 42/32 °C (day/night). Mc-42: Bitter gourd-grafted plants subjected to 42/32 °C (day/night).
- Supplemental Fig. S2 Effects of exogenous trans-zeatin (tZ) on RbcL gene expression level and Rubisco activity of grafted plants under heat stress. (a) Changes in the gene relative expression level of RbcL Plants were pretreated with 10 μM tZ for 12 h and then plants were exposed to heat stress at 42 °C for 48 h. (b) Changes in the Rubisco activity. Plants were pretreated with 10 μM tZ for 12 h and then plants were exposed to heat stress at 42 °C for 48 h.
- Supplemental Table S1 Primers used to quantify the expression levels of genes using in real-time PCR analysis.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Han S, Shu S, Wang Y, Jahan MS, Sun J, et al. 2022. Cytokinin plays a critical role in bitter gourd rootstock-induced thermotolerance of cucumber. Vegetable Research 2:4 doi: 10.48130/VR-2022-0004
Cytokinin plays a critical role in bitter gourd rootstock-induced thermotolerance of cucumber
- Received: 10 October 2021
- Accepted: 03 March 2022
- Published online: 28 March 2022
Abstract: Plants, as sessile in nature, are constantly confronted with diverse biotic and abiotic stresses throughout their life cycle in the changing environment. As a result, plants evolved root-shoot communications to optimize plant growth and development, and regulate responses to environmental stresses. Here, we examined the roles of root-sourced cytokinin (CTK) response to heat stress in grafted cucumber seedlings. Cucumber plants grafted onto cucumber roots and bitter gourd (Momordica charantia) roots were exposed to heat to examine their heat tolerance by assessing the levels of photosynthetic capacity, CTK contents, chlorophyll-a/b-binding protein (Lhcb2), ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and its activating enzyme (RCA) content, and the enzyme activity of Rubisco. Bitter gourd rootstock enhanced cucumber scions heat stress tolerance. This enhancement was positively correlated with a higher content of CTK in both leaf and root parts, chlorophyll contents, and Rubisco abundance and activity. In addition, the higher level of CTK and Rubisco content in bitter gourd grafted plants shoots than in cucumber self-gafted plants shoots were attributed to an increase in CTK transport from roots in grafted plants under high-temperature conditions. These results indicated that CTK transfer from bitter gourd rootstock to scion and triggered the accumulation of Rubisco in leaf, thus improving the heat resistance of bitter gourd-grafted plants.
-
Key words:
- Cucumber /
- Grafting /
- Heat stress /
- Cytokinin /
- Root-shoot communication.