-
Among vegetables, bulb onion (Allium cepa L.) is the second most produced vegetable in the world after tomatoes[1]. However, onion production is hampered by several biotic and abiotic stresses. Among the biotic stresses, Iris yellow spot (IYS) is an impactful disease of onion that is caused by Iris yellow spot virus (IYSV) which belongs to the family Bunyaviridae and genus Tospovirus. This virus is spread by its principal vector, onion thrips (Thrips tabaci Lindeman) (Thysanoptera: Thripidae)[2,3] which acquire the virus during the larval stage and spread the virus during feeding on the leaves[4]. It can also be transmitted by another group of thrips known as tobacco thrips (Frankliniella fusca)[5]. Both thrips and the virus can overwinter on alternate hosts such as, winter annuals, to generate inoculum for the next growing season[6]. IYS was first reported in the Netherlands in 1992 on an ornamental plant, Iris hollandica which is used in the cut flower industry[7]. It was first noticed in the United States in an onion field in eastern Oregon and the Treasure Valley of southwest Idaho[8]. Later, it was also witnessed in other major onion-producing states of the United States such as Arizona, California, Utah, and Washington[4,9−17].
Symptoms of IYS on leaves appear as irregular to diamond-shaped, yellow to straw-colored lesions, that decrease the leaf photosynthetic area of the plant and eventually reduce bulb size and yield under severe disease conditions[9,13,18]. In addition to foliar symptoms, enlarged lesions along flower stalks (scapes) end up girdling them, causing scape collapse and lodging which decreases seed yield[13,18]. IYS can be controlled by managing the vector, using cultural practices such as sanitation, and host plant resistance[4]. Several registered insecticides such as carbamates, organophosphates, and pyrethroids have encountered the problem of induced thrips resistance in various onion production areas[19,20]. In addition, thrips eggs are protected in the tight axil of the leaves which makes insecticide spray coverage inadequate. Trap crops, such as, lacy phacelia (Phacelia tanacetifolia Benth.), buckwheat (Fagopyrum esculentum Moench.), and carrot (Daucus carota L.) to attract thrips and minimize thrips migration to onion plants, has been somewhat successful to reduce virus transmission[21]. Unfortunately, no chemical or biological control is available to eliminate IYSV once it is inside the plant. Therefore, this pathosystem can only be managed using an integrated pest management based multifaceted approach. Host plant resistance acts as a foundation for an effective integrated approach[22] and therefore, the most promising component to control IYS is the development of cultivars that are less impacted by thrips and IYSV.
No IYS-resistant onion cultivar has been found or developed after numerous screenings of onion cultivars yet[4,18,22−25]. It is necessary to develop or discover breeding lines that exhibit fewer thrips and less severe IYS symptoms to lessen bulb yield losses. Several studies have been conducted to evaluate onion germplasm for IYS susceptibility. In Washington state, US, IYS incidence ranged from 58% to 97% for 46 different long-day cultivars[23]. In a two-year evaluation of onion cultivars in Colorado, IYS incidence ranged from 16% to 100% in the first year and 13% to 61% in the second year[26]. For the last 14 years, the onion breeding program at New Mexico State University (NMSU) has been evaluating germplasm for reduced thrips number and IYS symptom expression[24,25,27−29]. In 2011, plants of the breeding line NMSU 05-33-1 exhibited a lower IYS severity than plants of 12 other NMSU breeding lines when they were grown in the winter season[24]. The breeding program has had success at reducing disease severity through selection for fewer IYS symptoms under conducive conditions for severe disease development. The field layout to conduct evaluation and screening studies at NMSU was designed in such a way that it ensures the presence of viruliferous thrips to spread IYSV throughout the field. This layout has been used to select for individual plants with reduced IYS symptoms and to further evaluate selection progress of those selections[30,31]. Plants of the selected onion breeding lines, NMSU 10-575-1, 10-577-1, and 10-582-1, exhibited less severe IYS symptoms than plants from the populations they were derived from[32]. In a recent study, plants of developed breeding lines, NMSU 12-236, 12-243, 12-335, 12-337, and 12-796 exhibited fewer thrips and fewer IYS symptoms than plants of a commercial check 'Rumba' and plants of their respective original populations demonstrating the selection progress[33].
This study was conducted to further characterize NMSU onion breeding lines selected for reduced IYS symptom expression. A similar field layout with some modifications was used to create an environment conducive for strong IYS disease pressure throughout the field. It was hypothesized that plants of the NMSU breeding lines that were less attractive to thrips early in the growing season would also exhibit a reduced/delayed IYS symptom expression as compared to plants of a commercial check cultivar. In addition, we hypothesized that a greater number of thrips per leaf early in the growing season would result in a greater disease severity than a lesser number of thrips per leaf early in the growing season. It was also hypothesized that plants of the NMSU breeding lines would produce larger-sized bulbs than plants of the commercial check cultivar due to reduction in IYS symptoms or a delay in symptom development of those breeding lines. The objectives of the study for two consecutive years were: a) to compare the NMSU onion breeding lines with the commercial check cultivar for the impact of thrips number early in the growing season on IYS severity; and b) to compare the NMSU onion breeding lines with the commercial check cultivar for the impact of IYS severity on bulb yield attributes.
-
Four NMSU intermediate-day onion breeding lines were evaluated for IYS symptom expression and its impact on onion bulb yield in two consecutive years, 2019 and 2020. These breeding lines have been selected for fewer thrips and lesser IYS symptom development[30,33]. Two thrips-attractive commercial cultivars were also included as checks: 'Rumba' in 2019, whereas 'Stockton Early Yellow' was planted in 2020 because the seeds of 'Rumba' were not available for the second year. NMSU entries are originated from different ancestors: NMSU 12-236 from NMSU 07-52-1, NMSU 12-238 and NMSU 12-243 from NMSU 07-53-1, and NMSU 12-337 from NMSU 07-54-1[27].
Location and experimental design
-
The seeds were sown in plastic flats filled with Metro-Mix 360 (Sun Gro Horticulture, Bellevue, WA, USA) and placed in a greenhouse during the month of January at the Fabian Garcia Science Center (FGSC) in Las Cruces, NM, USA (32.2799° N, 106.7725° W). For adequate seedling growth, Miracle-Gro (15-30-15; N-P-K; Scotts Miracle-Gro Company, Marysville, OH, USA) fertilizer was applied. The seedlings were transplanted into the field in March on the raised beds. Entries were arranged in a randomized complete block design with three blocks, each with four replications. The length of each plot was 3.3 m and two rows per plot were planted with a spacing of 7−8 cm between two plants. A drip irrigation system with control valves was used to deliver water and fertilizer to growing plants. Drip irrigation tape with the emitters (Irritec, Fresno, CA, USA) was buried in the center of the bed underneath the soil at a depth of 10 cm. Standard cultural practices were used for the production in southern New Mexico[34]. No pesticides were sprayed to control thrips which is not commercially recommended to follow for the crop management. To ensure an effective spread of thrips and to induce sufficient IYS disease pressure for evaluation, bulbs infected with the viruliferous thrips were planted in October of the previous year around the perimeter of the IYS field plot as border rows. The thrips-attractive cultivar, 'NuMex Freedom' was planted as spreader rows next to the border rows. Thrips were expected to move from the border to the spreader rows after flower stalk formation on the infected bulbs and eventually to the test entries in four replications per block when the plants in the spreader rows had matured (Fig. 1). This field layout is also not a commercially recommended practice for growers.

Figure 1.
Field layout to ensure an effective spread of thrips and to induce sufficient IYS disease pressure. Yellow borders represent the border rows planted with the onion bulbs infected with viruliferous thrips saved from the previous year and red borders refer to the spreader rows next to the border rows. Each block contains four replications of the test entries.
Data collection
-
Ten randomly selected plants from each replication (n = 120) were labelled using plastic labels. Total thrips per plant were counted by investigating the axil (the tight junction) of the basal portion of the visible leaves of these plants at various dates [8, 10, 12, and 14 weeks after transplanting (WAT)]. Further, the number of leaves of these same ten plants were counted at the same observation dates. The same plants were rated for IYS severity using a rating scale of 0 to 4; where 0 means no symptoms, 1 refers to 1−2 tiny lesions on a leaf, 2 means more than 2 medium-sized lesions, 3 refers to lesions merging on more than 25% of the leaf area, and 4 indicates that more than 50% of the leaf is dead[30]. IYS severity ratings were recorded at three different times (12, 14, and 16 WAT) for the first year and the rating was started at 10 WAT for the second year. Bulb diameter (in cm) and bulb weight (in grams) were also measured after harvesting (when 80% of the tops fell) from the same plants. Average number of days to reach maturity were estimated to gain insight into the onset of symptom manifestation and plant senescence for each entry. The number of thrips per leaf was estimated by dividing the total thrips per plant by the number of leaves for each observation date.
Statistical analysis
-
The means and standard errors for thrips count, IYS severity ratings, bulb diameter, and bulb weight were estimated using the PROC MEANS and PROC MIXED statements in SAS® Studio, a web-based environment known as SAS® OnDemand for Academics (SAS Institute Inc., Cary, NC, USA). For the mixed analysis, entries were considered fixed effects while blocks and replications were random effects. Differences between thrips-attractive check cultivars and the NMSU breeding lines for IYS severity were estimated using the PDIFF statement. Moreover, correlation coefficients among the studied traits on an individual plant basis were calculated using PROC CORR in SAS® Studio and correlation heatmaps were generated using the R package 'ggcorrplot'.
-
Straw-colored lesions of IYS were visible on flower stalks in the border rows and leaves of plants in the spreader rows by the end of April to early May (Fig. 2).

Figure 2.
Iris yellow spot (IYS) symptoms, (a) on leaves of plants in the spreader rows, and (b) on flower stalks in the border rows.
Plants of the thrips-attractive check cultivars had a greater number of thrips per leaf at the first two observations in both years than the NMSU breeding lines as hypothesized (Figs 3 & 4). A fewer number of thrips had also been observed in the past for the initial observation dates for the NMSU lines[30,33,35]. The NMSU lines could be less attractive to thrips due to their leaf morphological traits. Thrips attractiveness to onion leaves depends on the leaf color and chemistry of epicuticular wax composition[33,36]. It has been observed that plants with yellow-green leaves and lesser amounts of epicuticular wax exhibiting 'semi-glossy' texture attract fewer onion thrips. Conversely, plants with bluish-green leaves exhibiting 'waxy' texture possess higher levels of wax which make them more attractive to thrips[22,24,37]. Later, it was determined that a particular type of epicuticular wax, hentriacontanone-16 (H16) was present in higher amounts on bluish-green leaves (waxy). However, cultivars with yellow-green leaves (semi-glossy) exhibited lower levels of H16[36]. Plants of NMSU 12-236, 12-238, and 12-243 lines have been classified as semi-glossy and plants of NMSU 12-337 were categorized as glossy. However, 'Rumba' and 'Stockton Early Yellow' cultivars were classified as waxy type phenotypes[27]. Therefore, adult thrips were first attracted to the plants of the check cultivars. Since adult thrips are going to those plants first, they can either lay eggs or infect the plants with IYSV. With more adult thrips attracted to the plants of the checks, there will be more eggs being laid and more feeding sites in which IYSV could be transmitted. This higher adult thrips number can result in an increased number of juvenile thrips and IYS lesions later. The juvenile thrips tend to be noticed first because they develop quicker than the IYS symptoms.

Figure 3.
Thrips in the leaf axils of plant (left) and IYS symptoms at 14 weeks after transplanting (WAT) (right) on (a) the thrips-attractive cultivar ‘Stockton Early Yellow’, and (b) an NMSU breeding line.
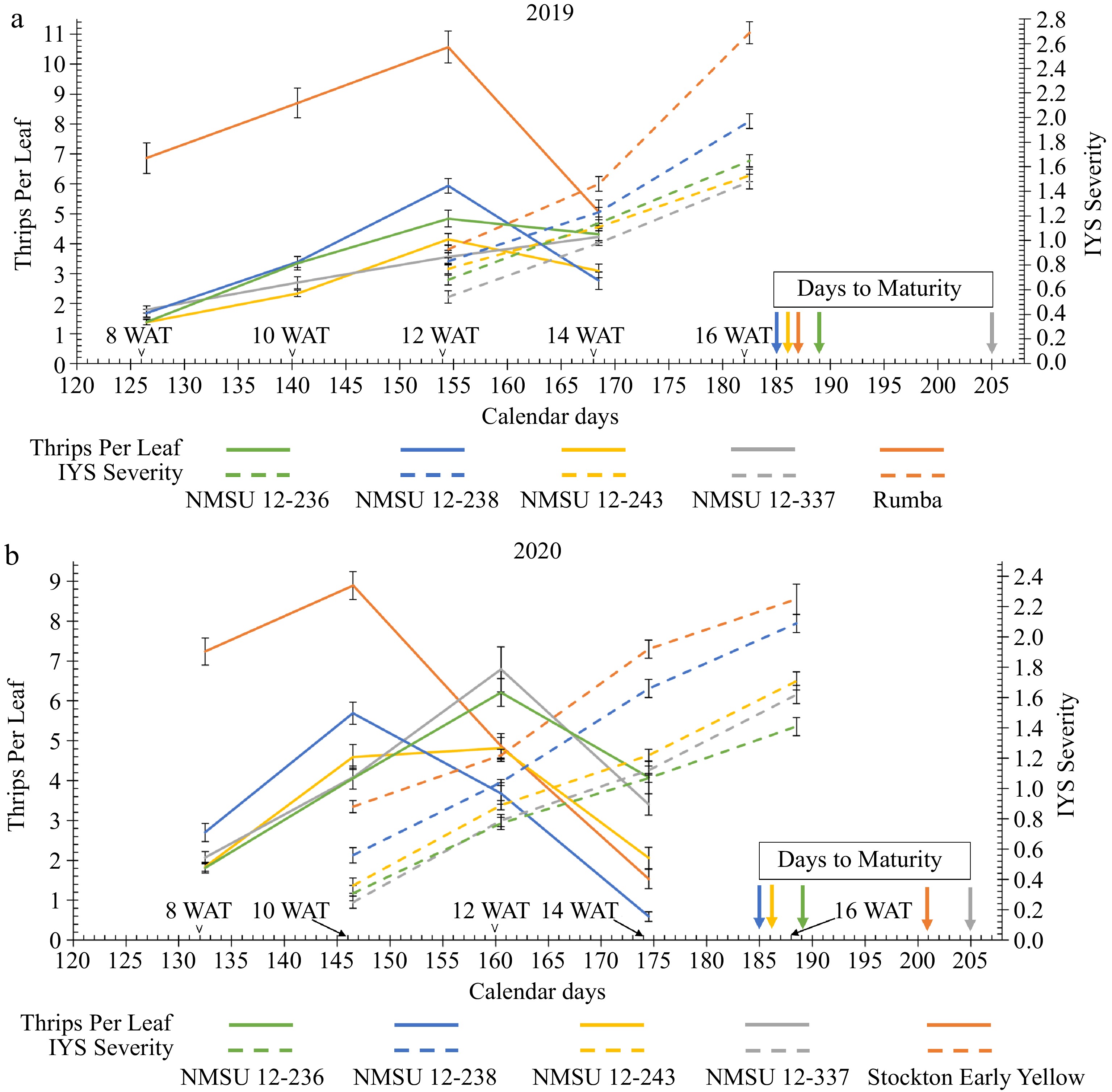
Figure 4.
Trend of number of thrips per leaf and Iris yellow spot (IYS) severity of the NMSU breeding lines and the thrips-attractive check cultivars, (a) 'Rumba' in 2019 and, (b) 'Stockton Early Yellow' in 2020 at various calendar days [8, 10, 12, 14, and 16 WAT (weeks after transplanting)] in the growing season.
Later in the season, thrips number started to drop after a certain point when plants started to senesce. In 2019, thrips number started to fall near the calendar day 155 [12 weeks after transplanting (WAT)] (Fig. 4a). However, in 2020, thrips number dropped around day 145 (10 WAT) for two entries, 'Stockton Early Yellow' and NMSU 12-238, and day 160 (12 WAT) for others (Fig. 4b). Earlier decline in the second year for these two entries might be attributed to the early advent of the Southwest US monsoon and heavy rainfall, which is unfavorable to thrips due to their dislike for water[38]. A greater number of thrips during the early stage of plant growth leads to the earlier development of IYS symptoms[39]. For plants of the NMSU breeding lines, a lower number of thrips early in the growing season led to a postponement in IYS symptom development in both years (Fig. 4). This pattern of development suggests that selecting for IYS tolerance or resistance would be more successful by looking for entries that manifest symptoms gradually as opposed to those that manifest symptoms quickly. Additionally, leaf senescence may intensify IYS symptom manifestation as plants get closer to maturity or normal leaf senescence may be mistaken for IYS symptoms[25].
Plants of the NMSU breeding lines were rated for IYS severity at 12, 14, and 16 WAT with initial symptoms at 10 WAT for the second year as symptoms were visible early in the season on the check cultivar 'Stockton Early Yellow'. IYS severity increased from 12 to 16 weeks and 10 to 16 weeks in 2019 and 2020, respectively (Fig. 5) which has also been observed to increase over time in a previous study[40]. In 2019, plants of the NMSU lines exhibited a reduced IYS severity in comparison to 'Rumba' for all observation dates except for NMSU 12-238 at 12 WAT (Fig. 5a). Similarly, in 2020, a reduced IYS severity was recorded for plants of the NMSU lines in comparison to 'Stockton Early Yellow' for all the observation dates except for NMSU 12-238 at 12 and 16 WAT (Fig. 5b).
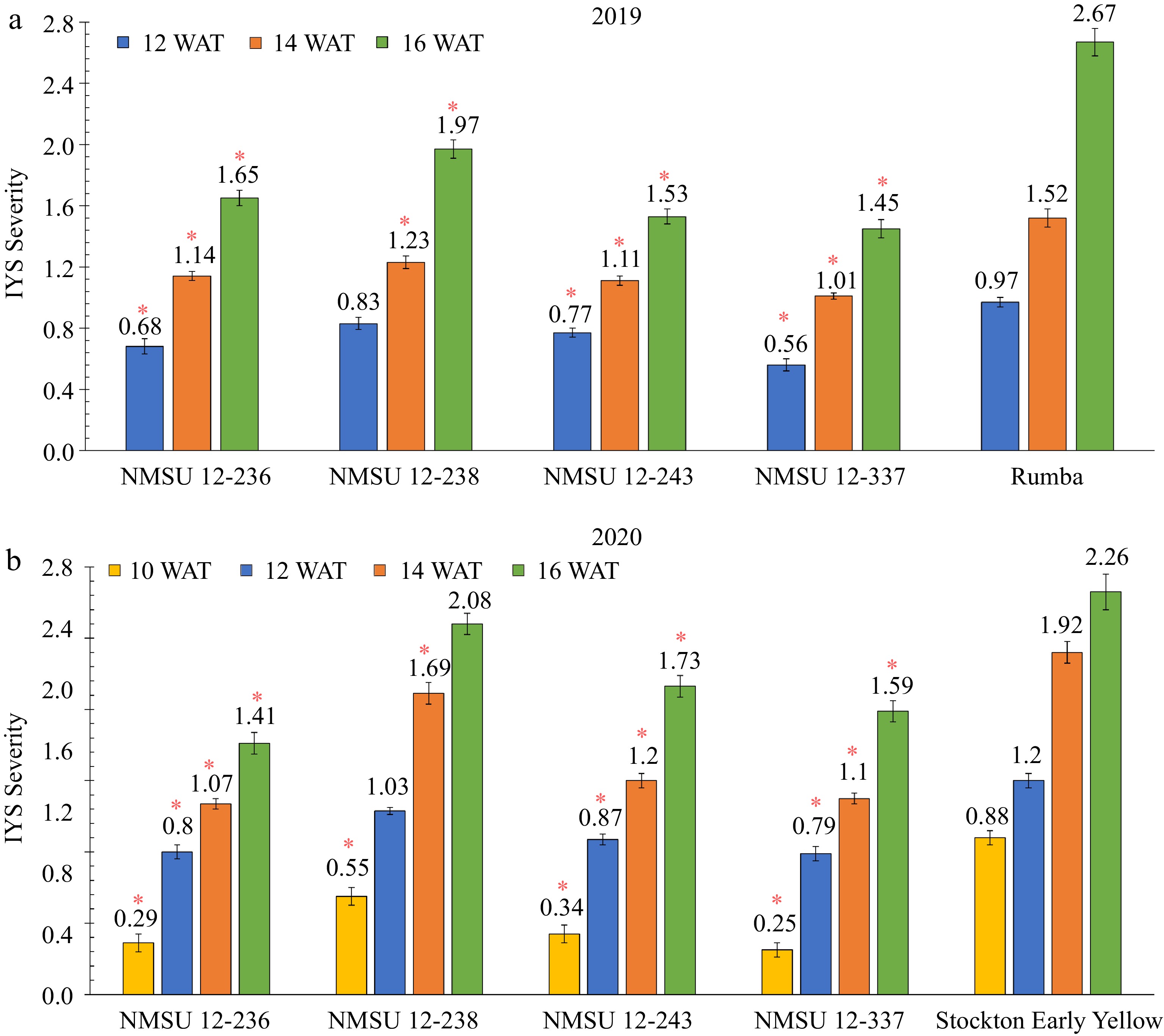
Figure 5.
Comparison of Iris yellow spot (IYS) severity of four NMSU onion breeding lines, NMSU 12-236, NMSU 12-238, NMSU 12-243, NMSU 12-337, with the thrips-attractive check cultivars, (a) 'Rumba' in 2019, and (b) 'Stockton Early Yellow' in 2020. Entries were arranged in a randomized complete block design with three blocks, each with four replications. Bars with '*' indicates that selected breeding lines are different from the thrips-attractive entries at p ≤ 0.05. Ten randomly selected plants per plot or replication were rated for IYS severity on a scale of 0 to 4, where 0 = no symptoms, 1 = 1 to 2 small lesions per leaf, 2 ≥ 2 mediumsized lesions per leaf, 3 = lesions coalescing on more than 25% of the leaf, and 4 ≥ 50 % leaf death[30]. The observation dates were 10, 12, 14, and 16 weeks after transplanting (WAT).
Relationship among thrips infestation, Iris yellow spot (IYS) severity, and bulb yield
-
If an onion cultivar is attractive to thrips carrying IYSV, then there is a greater chance of a positive correlation between thrips number per leaf and IYS severity in the early stage of the growing season. This, in turn, is expected to result in a negative correlation between IYS severity and bulb yield traits. Assuming that cultivars not attractive to thrips are planted, it is expected that there might be a weak to no correlation between thrips and IYS severity, as well as, between IYS severity and bulb yield traits. Thus, the correlation among thrips per leaf (Tpleaf), IYS severity ratings, bulb weight, and bulb diameter were determined for the plants of the NMSU breeding lines in comparison to the thrips-attractive check cultivars. The correlation between the number of thrips per leaf at 8 WAT and the severity rating of IYS at 12 WAT was of particular interest in determining whether a high thrips count early on leads to more severe IYS symptoms at the initial observations based on the performance of the check cultivars. In addition, we wanted to determine the correlation between the final IYS severity rating at 16 WAT and bulb yield attributes to assess the impact of the disease.
Both thrips-attractive cultivars exhibited the same trend for the correlations among the desired traits. Plants of 'Rumba' in 2019 exhibited a positive correlation (r = 0.53) between Tpleaf at 8 WAT and IYS severity at 12 WAT. Moreover, a negative correlation was observed for IYS severity at 16 WAT with bulb diameter (r = −0.24) and bulb weight (r = −0.23) (Fig. 6a). Similarly, 'Stockton Early Yellow' plants in 2020 exhibited a positive correlation between Tpleaf at 8 WAT and IYS severity at 12 WAT (r = 0.47). The lack of correlation between the number of thrips observed at 8 WAT and IYS severity at 10 WAT indicates that the symptoms of IYS had not developed enough to have a correlation with the number of thrips. In addition, a negative correlation was observed for IYS severity at 16 WAT with bulb diameter (r = −0.32) and bulb weight (r = −0.34) (Fig. 6b).
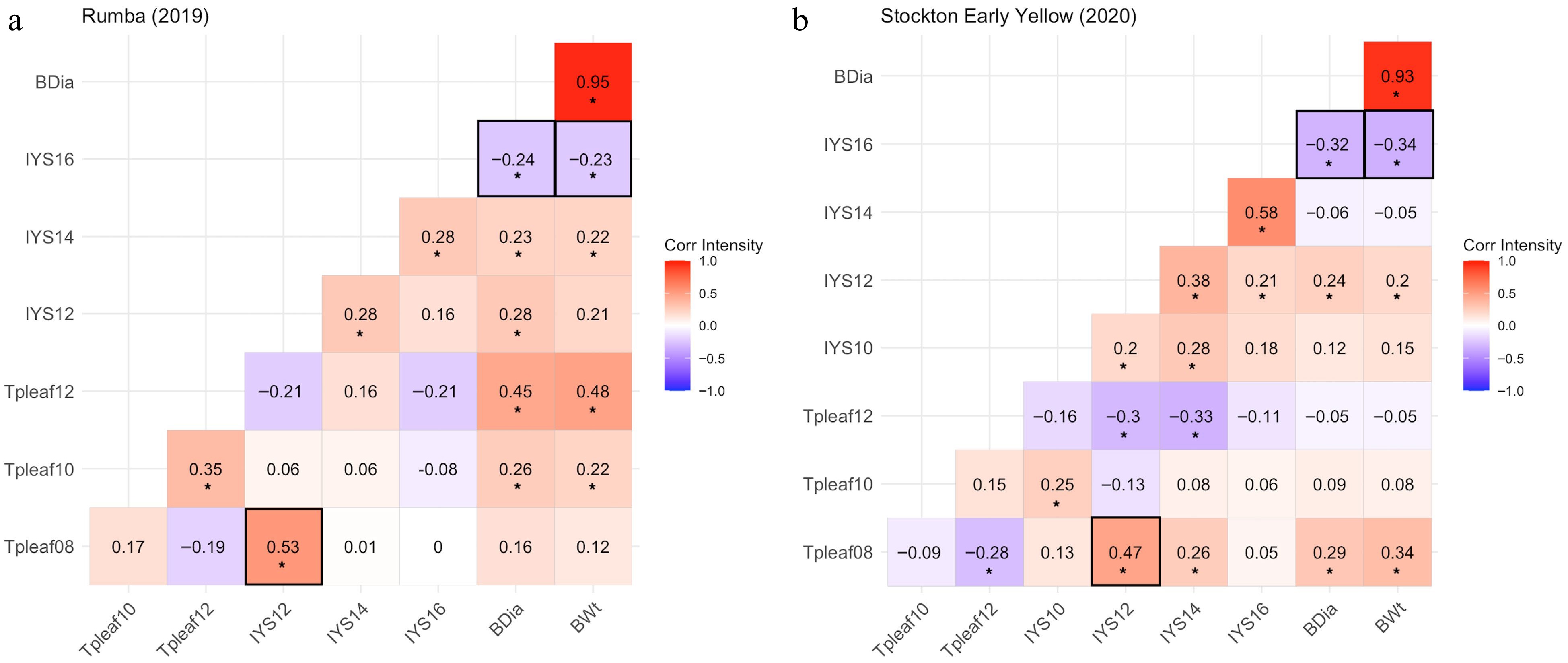
Figure 6.
Correlation heatmaps for thrips-attractive check cultivars, (a) 'Rumba' (2019) and (b) 'Stockton Early Yellow' (2020), demonstrating correlation coefficients among thrips per leaf (Tpleaf), Iris yellow spot (IYS) severity, and bulb yield parameters (BDia = bulb diameter and BWt = bulb weight) at various observation dates. '*' Indicates that correlation coefficients are significant at p ≤ 0.05. Data were collected for thrips per leaf at 8 (Tpleaf08), 10 (Tpleaf10), and 12 (Tpleaf12) weeks after transplanting (WAT), and IYS severity ratings at 10 (IYS10), 12 (IYS12), 14 (IYS14), and 16 (IYS16) WAT.
There was no relationship between thrips and IYS severity for three of the NMSU entries. However, one entry had a positive correlation, but it was not as strong as the check cultivars. Plants of the NMSU 12-236 line demonstrated a positive correlation (r = 0.27) of Tpleaf at 8 WAT with IYS severity at 12 WAT in the first year. Similarly, in the second year, a positive correlation (r = 0.35) was observed for Tpleaf at 8 WAT with IYS severity at 12 WAT (Fig. 7a). Plants of the NMSU 12-238 had no correlation between Tpleaf at 8 WAT and IYS severity at 12 WAT in 2019 and 2020 (Fig. 7b). Also, plants of the NMSU 12-243 exhibited no correlation between Tpleaf at 8 WAT and IYS severity at 12 WAT (Fig. 7c). Furthermore, plants of the NMSU 12-337 did not show any correlation between Tpleaf at 8 WAT and IYS severity at 12 WAT in both years (Fig. 7d). Thrips abundance and IYS incidence was found to be positively correlated for the susceptible cultivars in previous research also[2,40−42].
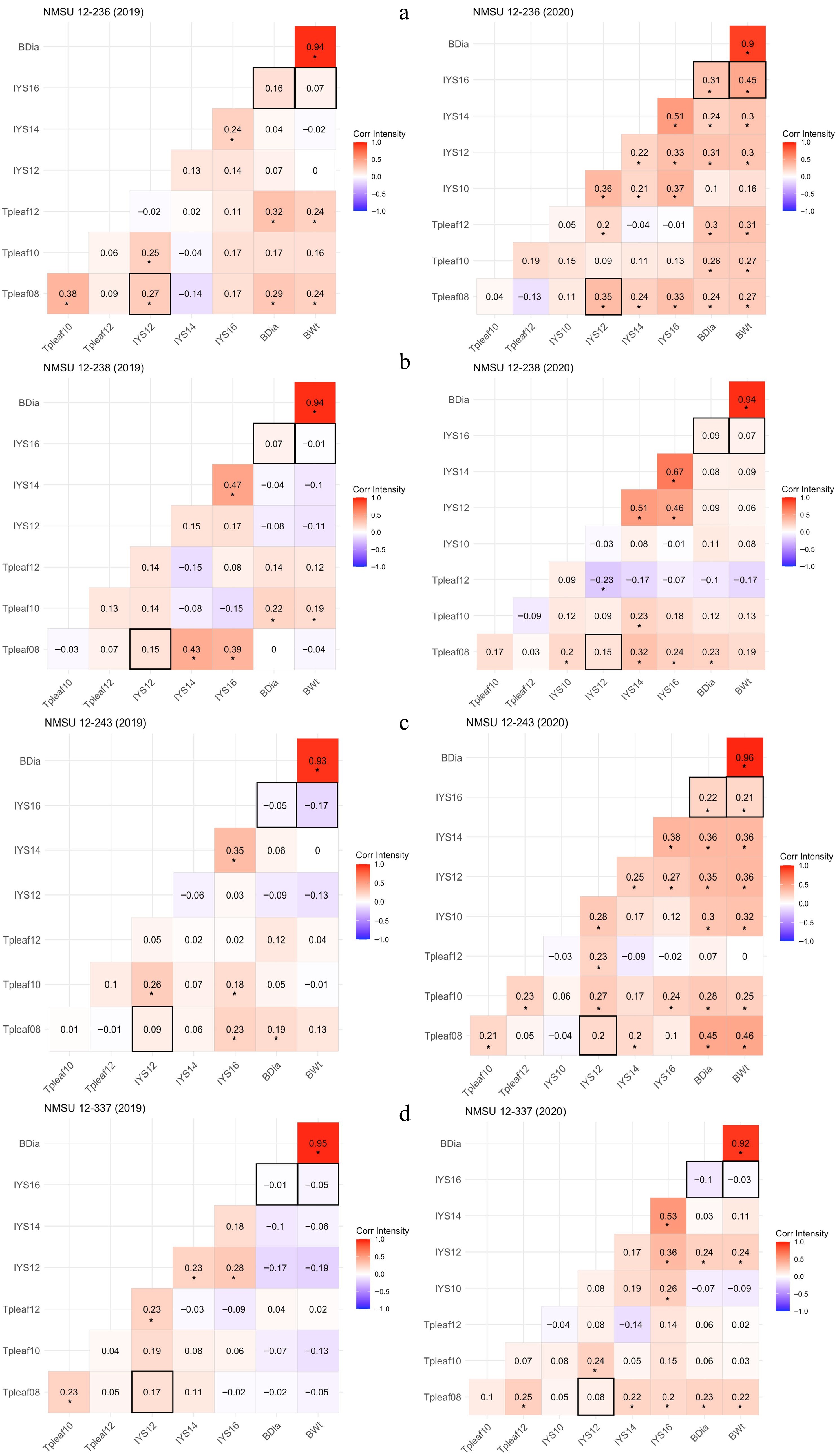
Figure 7.
Correlation heatmaps for NMSU onion breeding lines, (a) NMSU 12-236, (b) NMSU 12-238, (c) NMSU 12-243, and (d) NMSU 12-337, demonstrating correlation coefficients among thrips per leaf (Tpleaf), Iris yellow spot (IYS) severity, and bulb yield parameters (BDia = bulb diameter and BWt = bulb weight) at various observation dates in 2019 and 2020. '*' Indicates that correlation coefficients are significant at p ≤ 0.05. Data were collected for thrips per leaf at 8 (Tpleaf08), 10 (Tpleaf10), and 12 (Tpleaf12) weeks after transplanting (WAT), and IYS severity ratings at 10 (IYS10), 12 (IYS12), 14 (IYS14), and 16 (IYS16) WAT.
All NMSU entries exhibited no relationship between IYS severity at the last observation date (16 WAT) and traits related to bulb yield in 2019. However, two of the NMSU entries presented a positive correlation between IYS severity and bulb yield traits in 2020. There was no correlation between IYS severity at 16 WAT and bulb yield traits in the first year for plants of the NMSU 12-236. Though, in the second year, IYS severity displayed a positive correlation with bulb diameter (r = 0.31) and weight (r = 0.45) (Fig. 7a). IYS severity at 16 WAT was not correlated with bulb yield attributes in both years for plants of the NMSU 12-238 (Fig. 7b). Further, IYS severity at 16 WAT did not reveal any correlation with bulb yield traits for plants of NMSU 12-243 in 2019. However, severity presented a positive correlation with bulb diameter (r = 0.22) and bulb weight (r = 0.21) in 2020 (Fig. 7c). No correlation was also revealed between IYS severity at 16 WAT and bulb yield traits in both years for plants of NMSU 12-337 (Fig. 7d). IYSV incidence and bulb yield was negatively correlated for the jumbo (207-314 g) and colossal (315−458 g) bulb size classes of the susceptible cultivar 'Teton' and moderately resistant 'Sterling', respectively[18,43,44]. Moreover, a negative correlation had also been detected between IYS severity and bulb yield in other results[45,46]. It had been observed that the moderately resistant cultivars are less impacted by thrips and IYSV and produce higher bulb yield[40].
Plants of the NMSU breeding lines showed less severe IYS symptoms as compared to plants of the thrips-attractive cultivars. Therefore, their correlation coefficients among studied traits also differed when compared to the thrips-attractive check cultivars for their respective year. These correlation studies did not exhibit any negative correlation between IYS severity and weight and diameter of bulbs for the NMSU lines suggesting less to no impact of IYS on bulb yield due to delayed symptom development. This result implies that observed IYS symptom expression does not impede the photosynthetic activity of leaves (source) to adversely influence the onion bulb size (sink) because plants reach near senescence by that time. It has already been confirmed that these NMSU breeding lines' plants generated comparable or greater leaf numbers and bulb weights when compared to the check cultivars[47]. It has also been established that these NMSU lines were more tolerant to thrips and IYSV and exhibited a higher photosynthetic rate resulting in larger and heavier bulbs in comparison to the check cultivars[48].
-
NMSU breeding lines were compared with commercially available thrips-attractive onion cultivars, and results showed that the NMSU lines selected for lower IYS severity were more tolerant to IYS disease. Therefore, the selected onion breeding lines are less likely to experience bulb yield losses. To strengthen the correlation studies in this experiment, observations on the number of thrips and IYS severity were taken four times at the interval of 2 weeks for two consecutive years. This approach minimizes observation errors and ensures a representative sample of thrips population. In the results, the bulbs produced from the commercial check cultivars were much smaller than the standard size which weighs 123 g in the US market classes. In contrast, the NMSU lines had jumbo (207−314 g) and medium (123−206 g) class bulb weights on an average[43,44]. This suggests that the NMSU lines can produce larger bulbs and maintain their market value even under high IYS pressure. The results reported were obtained without the use of insecticides to control thrips, which is not a common commercial practice. However, it should be noted that plant performance could potentially be improved by incorporating some form of chemical control. Furthermore, the field layout in this study is not recommended for commercial onion production. In that system, the incidence of IYS would be expected to be much lower. Therefore, it is possible that under low disease pressure, the breeding lines may produce a colossal (315−458 g) market class of yellow onion bulbs. Moreover, the potential yield losses to the onion industry of the United States due to IYS and onion thrips was 10%−15% of the farm gate value which is estimated at 7.5−12 million dollars[49]. The development of thrips and IYS-tolerant cultivars can play an important role in minimizing yield losses without the utilization of pesticides. This approach will harness ecological advantages such as biodiversity conservation, lower environmental impact, better soil health, and reduced greenhouse gas emissions; hence contributing towards sustainable agriculture. Ultimately, using cultivars less impacted by thrips and IYSV can provide an economical and environment-friendly approach to manage IYS disease.
Authors thank Ray Muhyi, undergraduate and graduate students for their help in managing the crop, data collection and harvesting. This research was funded by the USDA-NIFA Specialty Crop Research Initiative, grant number: 2018-03407, and the New Mexico Agricultural Experiment Station.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Sharma S, Cramer CS. 2023. Reduced Iris yellow spot symptom expression in the selected onion germplasm. Vegetable Research 3:26 doi: 10.48130/VR-2023-0026
Reduced Iris yellow spot symptom expression in the selected onion germplasm
- Received: 11 May 2023
- Accepted: 07 August 2023
- Published online: 21 August 2023
Abstract: Iris yellow spot virus (IYSV) is responsible for causing Iris yellow spot (IYS), which is a devastating disease for onion plants. Onion thrips is another major threat to onion, which not only harm the leaves by feeding on them but also spread IYSV throughout the plant, leading to reduced bulb size. Managing this disease requires an integrated approach that involves using germplasm lines that are less affected by both thrips and IYSV. In this study, we evaluated four breeding lines selected by the onion breeding program at New Mexico State University (NMSU) and two commercially available thrips-attractive check cultivars. The study was conducted using a randomized complete block design with three blocks, each including four replications. The field layout was designed to ensure the presence of viruliferous thrips to spread IYSV throughout the field. Data on thrips number per plant, leaf numbers, IYS severity, and bulb yield attributes were collected multiple times during the growing season from ten randomly selected plants in each replication. Plants of the NMSU lines possessed fewer thrips early on which led to a postponement in IYS symptom development when compared to plants of the check cultivars. Plants of the NMSU lines produced larger bulbs which suggests that delayed IYS symptoms reduced the impact of disease on bulb yield. The use of IYS-tolerant NMSU cultivars can provide growers with an eco-friendly and economical approach to manage IYS.
-
Key words:
- Viruliferous thrips /
- Bulb yield /
- Iris yellow spot virus /
- Correlation studies












