-
The 'Nam Hom' coconut (Cocos nucifera L.) is a tropical plant renowned as the 'Tree of Life' because every part of it may be used, including the coconut meat, coconut water, coconut shells, trunks, and coconut roots. Coconut, which is delicious, aromatic, and full of nutrients, is the world's most commonly cultivated palm plant[1]. Coconut water is a clear, colorless liquid with a pH of 7.0, 12%−15% sugar (w/w), 0.23% protein, and 0.02% fat. Its main sugars are glucose, fructose, maltose, and raffinose. It is also rich in sodium and potassium ions, while malic acid serves as the main acid[2]. The major metabolic compounds found in coconut water include chlorogenic acid, ferulic acid, lauric acid, oleic acid, palmitic acid, shikimic acid, succinic acid, and sucrose[3]. Coconut water, on the other hand, have a short shelf life and deteriorate quickly[4]. Cider production is one method for increasing the value of low-quality coconut[5]. Cider is a marvelously crafted low-alcohol beverage, fermented from fresh fruit juice with a deliciously clear and mellow taste. Abounding with vital nutrients like vitamins, minerals, and polyphenols, this drink boasts various health benefits, such as reducing the risk of cardiovascular diseases and combating inflammation[6]. Several factors influence the qualities of coconut cider over time. The temperature and duration of the fermentation process have a significant influence on the final product[7]. Temperature influences both the time and rate of yeast fermentation, as well as yeast metabolism, which influences the chemical composition and overall quality of the cider. It is not just the cider-making conditions that can affect the flavor of coconut cider, but also the choice of yeast strains used[8]. The yeast strains used in cider fermentation play a critical role in sugar metabolism, flavor development, and overall fermentation kinetics. Cider fermentation primarily involves two types of yeast, including Saccharomyces cerevisiae, the most commonly used yeast for alcoholic fermentation, especially in wines and ciders, efficiently ferments sugars like glucose, fructose, and sucrose, and is responsible for ethanol production; Non-Saccharomyces yeasts, such as Pichia, Hanseniaspora (Kloeckera), Candida, and Metschnikowia, often involved in the early stages of fermentation, contributing to the aroma and complexity of the cider, as producing volatile compounds, glycerol, and organic acids, but generally poor ethanol production[9]. The commercial K1V1116 yeast has been known for its ability to enhance the aroma of wines and ciders, producing higher levels of esters and thiols, contributing to fruity and floral characteristics, whereas EC-1118 is known for producing clean, neutral flavors, allowing the raw material character to shine through in the final cider[10]. The fermentation of cider involves a sequence of stages. Firstly, during yeast inoculation, when yeast (wild or selected) is introduced to the apple juice (must), it begins to metabolize sugars, primarily fructose and glucose. It is followed by ethanol production, the primary metabolic process of S. cerevisiae, in which sugars are converted into ethanol and carbon dioxide. This fermentation typically occurs at temperatures ranging from 10−25 °C. Finally, aroma compound production stage occurs, during which volatile compounds such as esters, higher alcohols, aldehydes, and organic acids are produced, contributing to the cider's aroma and flavor complexity[11]. Therefore, it is crucial to analyze the comprehensive chemical changes occurring in coconut cider throughout the fermentation process, considering various factors. Such analysis plays a vital role in the development of this product.
The omic method is a technique for analyzing the different types of coconut cider that are divided into two parts: Metabolomics refers to the systematic identification and analysis of all metabolites present in an organism or biological sample. Metabolites are divided into main metabolites, which are essential for the development of all living organisms. Secondary metabolites are produced in nature and are essential to the organisms that produce them[12]. Metabolomics application in food science and fermentation products allows researchers to identify and quantify these metabolites, providing insights into the metabolic pathways involved in fermentation and the impact on food quality[13]. There are some key applications of metabolomics in food fermentation: Quality control and process optimization by analysis can help monitor the fermentation process, identify key metabolites, and assess the quality and safety of fermented foods[14]. Characterization of fermentation products helps in understanding the chemical composition of fermented foods and determining their nutritional value and functional properties. Starter culture selection by analyzing the metabolic profiles of different strains, researchers can identify cultures that produce desired metabolites, improve fermentation efficiency, and enhance the sensory attributes of the final product[15]. Understanding fermentation pathways by studying the changes in metabolite levels over time, researchers can unravel the complex metabolic interactions between microorganisms and substrates, contributing to a deeper understanding of the fermentation process[16]. Authentication and adulteration detection can be used to detect and quantify specific metabolites that act as markers for the authenticity of fermented foods. This helps in identifying potential adulteration or the use of inferior raw materials in the fermentation process[17]. Overall, metabolomics in food science, specifically in the context of fermentation, offers a powerful tool for understanding the metabolic changes that occur during fermentation processes, optimizing product quality, and ensuring the safety and authenticity of fermented foods.
Flavoromics has become an effective alternative to targeted approaches for flavoring coconut cider. A non-targeted, data-driven approach was employed to identify as many metabolites as possible. The objective is to discover a correlation between food's chemical composition and its organoleptic properties[18]. Flavoromics, a branch of food science, plays a significant role in understanding the flavor profiles and aroma characteristics of fermented foods. It involves the analysis of volatile compounds and flavor-active molecules to gain insights into the sensory attributes and overall flavor development during fermentation processes[19]. There are some key aspects and applications of flavoromics in the context of food fermentation, including flavor characterization, where flavoromics enables the comprehensive analysis and identification of volatile compounds responsible for the unique flavors and aromas in fermented foods. By analyzing these compounds, researchers can better understand the flavor profiles and sensory characteristics of different fermented products, flavor optimization by studying the flavor-active compounds present in fermented foods, and flavoromics enables researchers to optimize fermentation processes and enhance the desired sensory attributes[20]. This can involve identifying key flavor compounds, understanding their formation pathways, and adjusting fermentation conditions to achieve the desired flavor outcomes, quality control and sensory evaluation in which flavoromics can be employed as a tool for quality control and sensory evaluation of fermented foods. Quantifying and monitoring specific flavor compounds help to assess the consistency, authenticity, and overall sensory quality of the final products, product development and innovation in which flavoromics provides valuable insights for product development and innovation in the fermentation industry[21]. By understanding the flavor profiles of different fermentation processes and their correlation with sensory preferences, researchers can create new and improved fermented food products that cater to consumer tastes and fermentation process monitoring by analyzing the volatile compounds produced during fermentation, flavoromics can aid in monitoring the progress and dynamics of the fermentation process. This helps in identifying key flavor development stages, optimizing fermentation time, and ensuring consistent flavor production[22]. In summary, flavoromics in food science, specifically within the realm of fermentation, offers a powerful tool to explore, understand, and manipulate the flavors and aromas of fermented foods. It assists in flavor optimization, quality control, product development, and process monitoring, ultimately leading to the creation of more enjoyable and appealing fermented food products.
By employing a synergistic approach that combines flavoromic and metabolomic techniques, a highly efficient analysis was achieved to examine the flavor profiles and metabolite composition of 'Nam Hom' coconut cider.
-
High-purity chemicals purchased from Sigma Aldrich Co. (St. Louis, MO, USA) were used for extraction and derivatization. GC derivatization grade reagents and analytical grade were purchased from RCI Labscan Ltd. (Pathumwan, Bangkok, Thailand). All internal standards for metabolomics and reference standards for both metabolomics and flavoromics were purchased from Sigma Aldrich, while a C6−C30 n-alkane mixture from the same supplier was used to determine linear retention indices.
'Nam Hom' coconut
-
At the aromatic farm in Damnoen Saduak district, Ratchaburi, Thailand, the 'Nam Hom' coconut (Cocos nucifera L.) was sourced. To maintain its quality, the coconut water was filtrated and treated with 200 ppm potassium metabisulfite before being stored in high-density polyethylene (HDPE) plastic tanks, where it was kept at −18 °C to prevent any deterioration[23]. It was then thawed for 24 h at a temperature of 10 °C before use.
Coconut cider-making
Activate yeasts
-
To initiate the experiment, 5 g of dry commercial yeast were activated by adding them to 20 mL of tap water and then incubated at a temperature range of 35−40 °C for 15 min. The yeast strains used were Saccharomyces cerevisiae (EC1118) and Saccharomyces cerevisiae (K1-V1116) (Lallemand Inc., Montreal, Quebec, Canada). These two commonly used strains in cider production, K1-V1116 and EC-1118, both strains of Saccharomyces cerevisiae developed originally by the Institut Oenologique de Champagne (IOC).
Cider-making
-
The coconut water of 5 L was thawed and then adjusted to a desired level of sweetness (18 °Brix) by adding sucrose and a desired pH level of 4 by using a malic acid solution. The process starts by adding 4 mL of yeast solution (1 g/L) to the mixture, and fermentation takes place for 14 d at a temperature of 20 ± 2 °C, with 100 mL samples being collected every 2 d. Following fermentation, the coconut cider tanks were stabilized for 2 weeks at 0−4 °C temperature. The stabilized cider was then filtered through a cellulose membrane and packaged into 200-mL bottles with caps[24].
Physico-chemical analysis of coconut cider
-
The refractometer determined total soluble solids as Brix[25], the pH meter model 2-Star was used to measure pH[26], the vinometer was utilized to determine the alcohol content[27] and the reducing sugar was measured using the dinitrosalicylic (DNS) colorimetric method[28].
Metabolomics
Sample extraction and fractionation
-
The following extraction method was adapted from Limwiwattana et al.[29]. Separation of both polar and non-polar, using 10 mL of cider mixed with 10 mL of methanol : water solution (80:20, v/v) and 4 mL of dichloromethane. The mixture was separated into two phases, with the polar fraction present in the upper phase and the non-polar fraction in the lower phase. A sugar fraction was extracted from a 50 μL sample by silylation with N-trimethylsilyl imidazole (TMSIM) and pyridine. Next, hexane and water were added to the mixture to hydrolyze amino acids and fatty acids, resulting in the separation of the sugar and sugar alcohol fractions in the upper phase. The amino acid fractionation process began with an oximation of 200 μL of the sample, achieved by mixing 2 mg/mL hydroxylamine hydrochloride in pyridine. The mixture was then silylated using N-methyl-N-(trimethylsilyl) fluoroacetamide (MSTFA), and hydrolyzed amino acids were separated by the addition of hexane and water, which removed sugar. The resulting solution was subjected to a second round of silylation using MSTFA and acetonitrile.
The non-polar extract was used to separate the polar lipid and the fatty acid methyl ester (FAME). 1.5 mL of the dichloromethane phase sample was transesterified. After adding methyl tertiary-butyl ether, methanol, and 5.4-molar sodium-methylate in methanol, the sample was hydrolyzed in an aqueous solution. Dichloromethane and 0.35 M HCl solution were used to remove sugars and amino. Fatty acid methyl ester and polar lipids were fractionated and eluted with a solution using solid-state microphase extraction with C18-LP cartridges and MTBE (methyl tertiary butyl ether) : hexane (100:2 and 70:30, v/v, respectively). GC-FID is used for analyzing extracted samples.
GC/FID equipment and conditions
-
Metabolite compounds were analyzed using a gas chromatography instrument equipped with a flame ionization detector to perform gas chromatographic analysis. Separation was carried out using a DB-1 capillary column (60 m × 0.32 mm × 0.25 μm film thickness) with dimethylpolysiloxane as the stationary phase and hydrogen gas as the mobile phase. The oven temperature program was initiated at 100 °C, gradually increasing at a rate of 4 °C/min until it reached the final temperature of 320 °C, which was maintained for 25 min. The injection port temperature and detector temperature were set at 280 and 320 °C, respectively. A sample volume of 1 μL was analyzed.
Flavoromics
Sample extraction
-
The extraction method used in this study was adapted from Torrens et al.[30]. For the extraction of coconut water cider samples, an autosampler headspace solid-phase microextraction (HS-SPME) technique was employed. To enhance the extraction process, a 5 mL sample was spiked with 10 μL of a 0.1 mg/mL methyl nonanoate in methanol solution as the internal standard. Additionally, 1 g of NaCl was added to augment volatility within the headspace.
GC/MS equipment and conditions
-
The following extraction method was adapted from Torrens et al.[30]. A gas chromatography instrument and a time-of-flight mass spectrometer was used, with a Stabilwax fused silica column (30 m × 0.25 mm × 0.25 μm film thickness) containing a stationary phase of polyethylene glycol. The oven temperature program began at 40 °C and increased at a rate of 5 °C/min until the final temperature of 150 °C was reached. The temperature was then rapidly increased at a rate of 40 °C/min until it reached 250 °C for identification. A sample volume of 1 μL was analyzed.
Statistical data analysis
-
Physicochemical measurements were obtained in triplicate, and the results were reported as the mean±standard deviation (SD). IBM's statistical package for the social sciences (SPSS) software version 21 (SPSS Inc., Chicago, IL, USA) was utilized for statistical analysis, employing one-way analysis of variance (ANOVA) and Duncan's multiple range test to determine significant differences between treatments at the 95% confidence level (p ≤ 0.05). Metabolites were identified by comparing them to reference standards, and their concentrations were expressed as mg/mL relative to the internal standard of each fraction. Flavor compounds were identified by comparing their mass spectra to standards in the National Institute of Standards Mass Spectral Database version 2.0. The retention indices (RI) were calculated by comparing the retention time of the n-alkanes series (C6–C30) with RI data from the literature that utilized the same GC column polarity[31,32]. XLSTAT-base version 2023 software (Addinsoft, New York, NY, USA) was employed for principal component analysis (PCA) and agglomerative hierarchical clustering analysis (AHC) on the relative concentrations of all metabolites and flavor compounds. Correlation network analysis was performed using non-parametric Spearman's rank correlation with the correlation/association tests mode in the XLSTAT base software, considering a significance level of p ≤ 0.05.
-
Table 1 shows the physical properties of coconut cider 'Nam Hom' during fermentation with K1-V1116 and EC-1118 yeasts: Brix, pH, reducing sugar, and alcohol. The Brix and reducing sugar contents of coconut cider were discovered to decrease during the fermentation process. Cider fermented with K1-V1116 yeast had less reducing sugar and Brix than cider fermented with EC-1118 yeast. The pH of cider fermented with K1-V1116 and EC-1118 yeasts slightly decreased during fermentation, resulting in higher acidity. On the other hand, the alcohol content of the cider fermented with both yeasts significantly increased (p ≤ 0.05) at every stage of fermentation. Alcohol production may result from yeast fermentation, according to the research by Han & Du[33]. The primary digestion of sucrose results in the production of glucose and fructose. Following that, the yeast uses glucose and fructose as precursors to produce alcohol and carbon dioxide in the following steps. As shown in Table 1, there were no significant differences in Brix, pH, reducing sugar, and alcohol content between cider fermented with K1-V1116 and EC-1118 yeasts. The obtained results indicated that the cider contained an alcohol content of approximately 7%−8%, aligning consistently with the typical range of alcohol content found in cider, which is 2%−8%[34].
Table 1. Physicochemical properties of 'Nam Hom' coconut cider produced by yeast K1-V1116 and EC-1118 during fermentation, including Brix, pH, reducing sugar, and alcohol.
Treatment 25% Ferment (0−2 d) 50% Ferment (4−6 d) 75% Ferment (8−10 d) 100% Ferment (12−14 d) K1-V1116 EC-1118 K1-V1116 EC-1118 K1-V1116 EC-1118 K1-V1116 EC-1118 Brix 16.83 ± 1.3a 16.83 ± 1.3a 14.00 ± 0.6b 14.33 ± 1.0bcf 13.13 ± 0.4bc 13.97 ± 0.6bcf 9.00 ± 1.1d 10.33 ± 1.2d pH 3.88 ± 0.15a 4.04 ± 0.06ae 3.66 ± 0.08ab 4.07 ± 0.05ae 3.44 ± 0.15bc 3.69 ± 0.43bcdf 3.39 ± 0.02bcd 3.43 ± 0.03bcdf Reducing sugar (mg/mL) 29 ± 2.61a 27.17 ± 1.47ab 25.5 ± 0.55b 24.17 ± 0.98bcd 24.5 ± 1.05cb 22.33 ± 0.52bcd 19.83 ± 0.75e 20.17 ± 0.17e Alcohol (% v/v) 0.83 ± 0.98a 0.67 ± 0.82a 4.0 ± 1.67b 2.50 ± 1.05ba 7.0 ± 0.63c 4.83 ± 0.75cb 8.17 ± 0.41dc 6.83 ± 0.41d * Values are means ± standard deviation (SD). Means in the same column indicated by different letters are significantly different at a 95% confidence level (p ≤ 0.05). Metabolomics and flavoromics of coconut cider
-
Omics methods refer to a collection of scientific techniques used to explore and analyze large-scale datasets in biology. These methods allow for the comprehensive study of specific 'omes' or complete sets of molecules, in biological systems. The suffix '-omics' signifies the broad analysis of particular biological components[12,13]. Metabolomics is a branch of omics methods, dealing with the comprehensive study of small molecules, or metabolites, within cells, tissues, or organisms. These metabolites are products of cellular processes, and their analysis provides insights into the biochemical activities occurring within a system. Metabolomics focuses on understanding the chemical fingerprints left behind by cellular processes and offers a snapshot of the physiological state of an organism or sample at any given time[13]. Flavoromics is a subfield of metabolomics that specifically focuses on the molecular compounds responsible for flavor. It involves the comprehensive analysis of volatile and non-volatile compounds that contribute to the sensory characteristics of food and beverages, such as taste and aroma[18]. Both metabolomics and flavoromics are powerful tools in the food industry, advancing product quality, safety, and innovation in food and beverage development. They are especially important in studying complex fermented foods and beverages, where a wide array of chemical transformations occur[19−21].
Figure 1 shows the PCA biplots of metabolites and flavor compounds of 'Nam Hom' coconut cider fermented with yeast K1-V1116 and EC-1118 representing 83.76% of the total variance, which classified the fermentation time into three major groups: Pre-Fermentation, In-Process, and Final-product. The classification aligns with the findings of Wattanakul et al.[35], the report depicted the division of mango wines using a PCA biplot into three stages: Pre-Fermentation, In-Process, and Post-Fermentation, or Final Product.
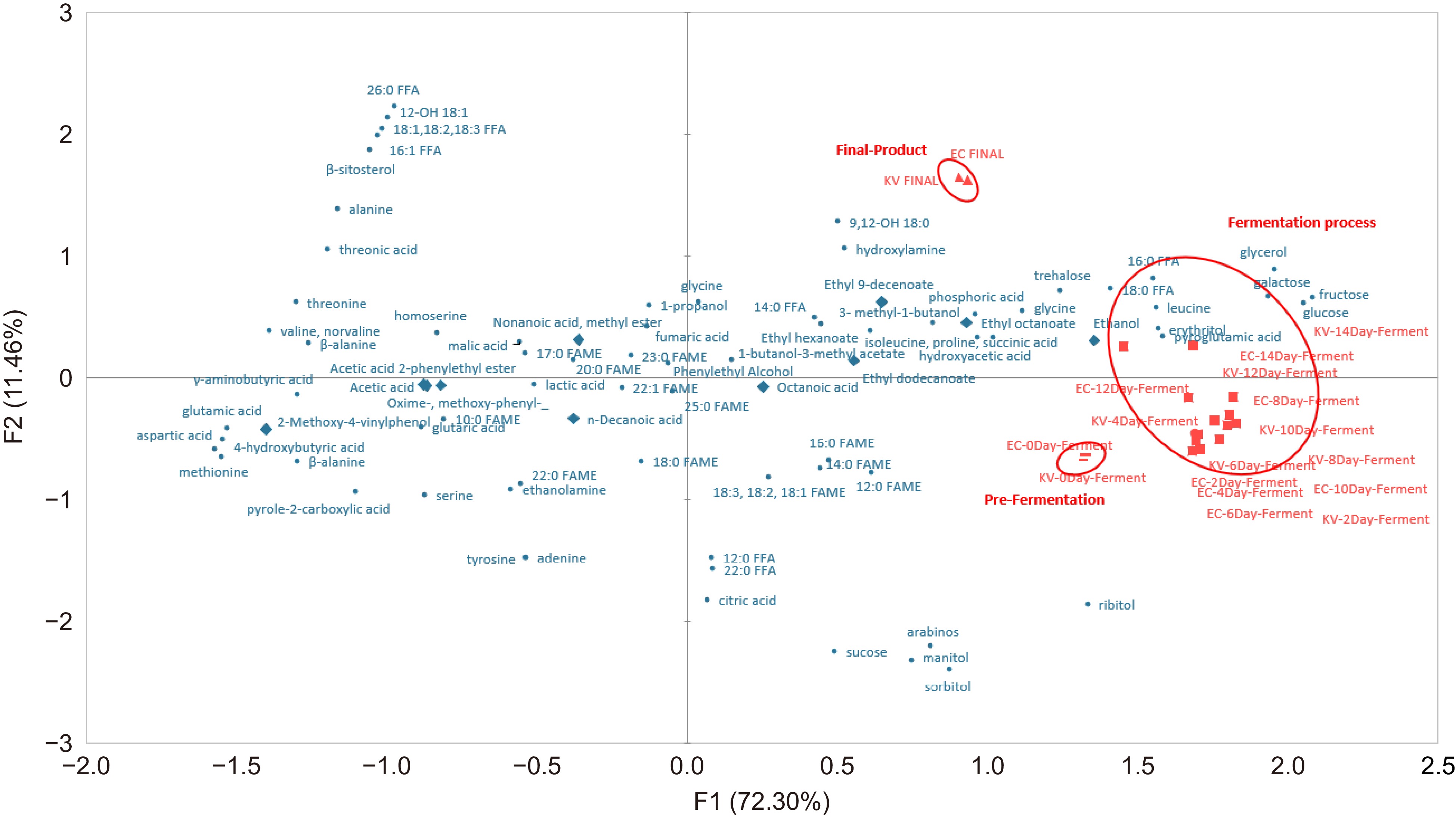
Figure 1.
Biplot (p ≤ 0.05) of principal component analysis (PCA) showing all identified metabolites (●) and flavor compounds (♦) in coconut cider at different stages of processing: Pre-Fermentation (—), In-Process (■), and Final Product (▲). FAME = fatty acid methyl ester, FFA = free fatty acid.
The first group was Pre-Fermentation, which was a zero-day fermentation that showed relationships with sugar groups such as sucrose, sorbitol, and arabinose, among others. This high concentration of sugars might be attributed to both the natural sugar content in coconut water and the addition of sugar for Brix adjustment at the beginning of fermentation. This finding was consistent with the report by Chen et al.[36] that discussed the presence of sugars during the initial fermentation of ginkgo rice wine. Sugars play a crucial role in wine fermentation by providing energy for microbial growth through carbohydrate metabolism throughout the fermentation process. Specifically, the sucrose level undergoes an initial increase followed by a subsequent decrease.
The group of fermentation at 2−14 d in the second group 'In-Process' showed a relationship with primary amino compounds, this association could be attributed to the yeast metabolizing certain amino acids and subsequently releasing them. It was worth noting that cider fermentation involves a significant presence of various amino acids, aligning with the findings reported by Mirás-Avalos et al.[37] regarding amino acids in grape wine. In grape wine, amino acids constituted approximately 40% of the total nitrogen content. During fermentation, yeast utilized certain amino acids while also producing some amino acids towards the end of the process. Final-Product was the third group. It was a group for cider at the end of the fermentation process. This group was closely related to a group of flavors such as ethyl 9-decanoate and ethyl octanoate, among others. Consequently, during the process of alcoholic fermentation, the yeast consumes the amino acids found in the coconut, potentially resulting in the production of alcohols, aldehydes, esters, and various volatile compounds. These findings align with the research conducted by Robinson et al.[38]. In their study, it was discovered that during the fermentation of grape wine, fatty acids could act as precursors for yeast to produce esters. Additionally, the amino acid was found to play a crucial role in the formation of volatile fatty acids, higher esters, and alcohols, leading to a significant increase in volatile content at the end of fermentation.
Figure 2 depicts the results of Agglomerative Hierarchical Clustering Analysis (AHC) that divided aromatic coconut cider into 3 groups: Pre-Fermentation, In-Process, and Final-Product. The analysis produced results consistent with those obtained from a PCA biplot, effectively categorizing the cider groups into three distinct categories based on fermentation time: Pre-Fermentation, In-Process, and Final Product.
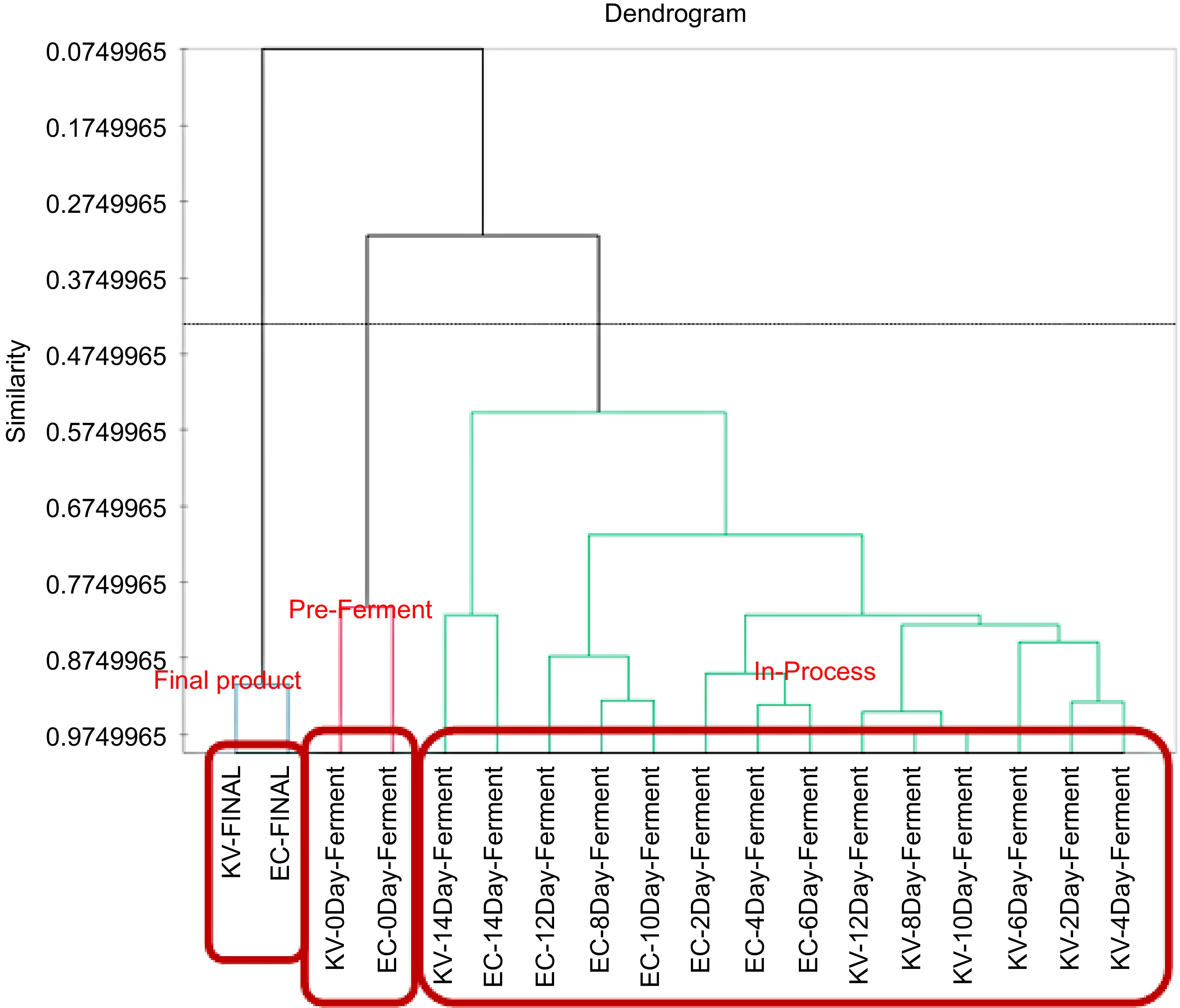
Figure 2.
Dendrogram (similarity mode of agglomerative hierarchical clustering) of fermentation coconut cider at different fermented times (p ≤ 0.05).
The relative content of metabolite and flavor compounds
-
The metabolite analysis of coconut cider fermented with the yeast strains K1-V1116 and EC-1118 detected a total of 152 peaks, of which 64 were identified. These identified peaks were divided into four major groups, consisting of 11 sugar groups, 30 amino acid groups, 12 FAME groups, and 11 polar lipid groups. Both yeast strains exhibited similar metabolite conversion propensity, with high levels of sugars such as glucose, sucrose, and fructose being observed during the early stages of fermentation. The majority of the carbohydrates in the cider were converted to alcohol during the fermentation process. The study demonstrated that sugar was an efficient precursor for the synthesis of phenols and aromatic amino acids, resulting in a significant decrease in the sugar content of cider fermented with both yeasts. The decrease in mannitol during the fermentation process indicated that lactic acid bacteria were not present in the cider, as mannitol is a marker of good cider quality. Lactic acid bacteria utilize fructose to produce mannitol; therefore, high levels of mannitol could imply bacterial contamination[39]. On the other hand, the glycerol content significantly increased during fermentation because glycerol could be the final product of glycerol pyruvic fermentation[40], which was similar to the yeast-fermented cider 2 strains.
Figure 3 demonstrated a slight increase in the major acid, malic acid, which is found in coconut water. Furthermore, no changes were observed in the citric and lactic acid concentrations, indicating the absence of lactic bacteria contamination. Leucine and isoleucine were significantly increased because they are metabolic byproducts of pyruvic acid via leucine and isoleucine biosynthesis and can synthesize acetyl-CoA by the action of pyruvate dehydrogenase, which enters the TCA cycle (citric acid cycle). L-isoleucine is essential for the formation of proteins, breaking down food, and producing energy. L-leucine is necessary for protein synthesis, muscle repair, and the production of growth hormones[41]. Furthermore, the amounts of leucine and isoleucine in cider fermented with both yeasts were comparable.
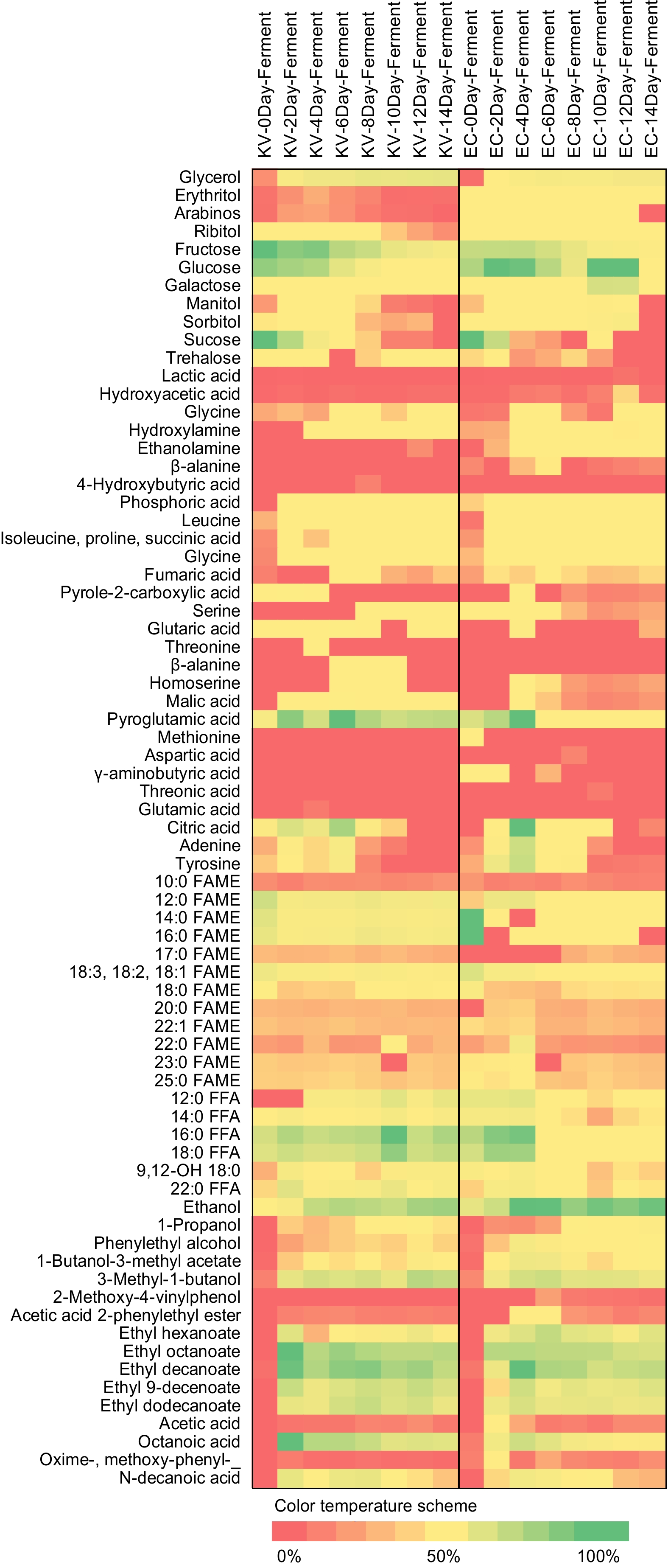
Figure 3.
Heat plots of metabolites and flavor compounds in coconut cider fermented with yeast K1-V1116 (left) and coconut cider fermented with Yeast EC-1118 (right) at different fermentation times. FAME = fatty acid methy ester, FFA = free fatty acid.
During the fermentation process, there was a reduction in the amount of fatty acid methyl esters or FAMEs (Fig. 3). Free fatty acids or FFAs, on the other hand, are synthesized from acetyl-CoA during yeast fermentation, increasing its concentration. The primary FFAs found in coconut are lauric acid (12:0) and palmitic acid (16:0), while FAMEs such as myristic acid (14:0) and oleic acid (18:1) are also present[42]. Oleic acid and linoleic acid are examples of unsaturated long-chain fatty acids that can be converted to ester compounds through anaerobic fermentation. This reaction is called esterification and it occurs when these fatty acids react with alcohol. Esterification continues to occur throughout the fermentation process, resulting in the production of large amounts of acid and ethanol in the resulting cider[43].
Both types of cider were discovered to contain elevated levels of stearic acid and lauric acid (Fig. 3). Stearic acid contributes to heart health by reducing cholesterol levels and lowering the risk of heart disease. Furthermore, stearic acid possesses anti-inflammatory properties that can potentially alleviate inflammation and provide health benefits for chronic conditions such as cardiovascular disease, diabetes, and certain types of cancer[44]. Lauric acid exhibits potent antimicrobial properties, targeting bacteria, viruses, and fungi. Extensive research has shown its effectiveness in inhibiting the growth of diverse pathogens, including those responsible for skin infections, respiratory infections, and foodborne illnesses. Additionally, lauric acid demonstrates notable antiviral activity. These antimicrobial properties of lauric acid contribute to its potential immune system support by inhibiting the growth of harmful pathogens. This can enhance the body's natural defense mechanisms against infections and promote a healthy immune response[45]. In this study, it was found that cider fermented with K1-V1116 yeast exhibited higher levels of stearic acid and lauric acid compared to cider fermented with EC-1118 yeast.
This study found that the FAME and FFA content in cider fermented with K1-V1116 and EC-1118 yeasts followed similar trends (Fig. 3).
Flavoromics analysis of coconut cider identified 16 peaks, accounting for 23% of the total 63 peaks detected in the metabolite analysis of cider fermented with the yeast strains K1-V1116 and EC-1118. These identified peaks were classified into eight categories: four alcohols, four volatile acids, one volatile phenol, and seven esters. Among these, esters, including acetate and ethyl esters, were the primary volatile compounds present in the cider. Coconut cider contains complex components such as sugars, proteins, and organic acids, which can interfere with the detection and quantification of flavor compounds by masking signals or altering compound extraction efficiency. Variations in the fermentation process or the specific yeast strains used (K1-V1116 and EC-1118) could be also result in lower production of certain flavor compounds compared to other cider types.
The most common acetate esters found in the cider fermented with both yeasts were 1-butanol-3-methyl acetate (isoamyl acetate) and acetic acid 2-phenyl ethyl ester (2-phenyl acetate), as reported by Hazelwood et al.[46]. These acetate esters are produced through a reaction between alcohol and acetyl-CoA, with the alcohol being generated by amino acid metabolism during fermentation.
In both yeast-fermented ciders, ethyl ester groups such as ethyl hexanoate, ethyl octanoate, ethyl decanoate, ethyl 9-decanoate, and ethyl dodecanoate were present (Fig. 3). Due to the higher abundance of EC-1118 yeast compared to K1-V1116 yeast, cider fermented with EC-1118 yeast had a more pronounced fruity aroma than K1-V1116 yeast-fermented cider[47]. Ethyl esters are a by-product of the hydrolysis of acyl-CoA that produces medium and long-chain fatty acids. Acetyl-CoA is produced by yeast metabolism from pyruvate, which interacts with the hydroxyl group of alcohol[48] and participated in the synthesis of these ethyl esters[49].
During fermentation, amino acids undergo transamination to α-keto acids in the Ehrlich pathway, which are then decarboxylated to aldehydes. These aldehydes are further reduced to higher alcohols, including ethanol, 1-propanol, 3-methyl-1-butanol (isoamyl alcohol), and phenyl ethyl alcohol, using NADH-dependent chemical reactions[50]. Both yeast-fermented ciders had approximately the same total alcohol content.
Volatile acids were detected in both EC-1118 and K1-V1116 fermented ciders, with EC-1118 having a higher concentration (Fig. 3). Tanoic is utilized as a substrate in the esterification process. Additionally, methoxy-phenyl-oxide levels increased during fermentation.
Considering that both yeast strains showed similar tendencies in metabolic conversion, with comparable changes in Brix, reducing sugar content, pH, and alcohol content, K1-V1116 and EC-1118 were both highly regarded for coconut cider production, each with its own strengths. K1-V1116 could be used for aromatic preservation and ester production, while EC-1118 was favored for robust, high-alcohol fermentations. K1-V1116 is particularly appreciated for enhancing aromas, whereas EC-1118 is prized for producing clean, high-alcohol fermentations[10]. Both strains could be commonly used depending on the desired characteristics of the coconut cider, whether sparkling, dry, or fruity.
Pairwise correlation analysis between metabolites and flavor compounds during the fermentation of coconut cider
-
Figure 4 illustrates the correlation between metabolites and flavoring agents in aromatic coconut cider. Sugars, namely sucrose, glucose, and fructose, exhibited a strong affinity for esters and ethanol, which may elucidate the connection between ethanol synthesis from carbohydrates and ester production. Moreover, the cider's sensory attributes related to the mouthfeel, including sweetness, texture, and softness, could be attributed to ester formation[51].
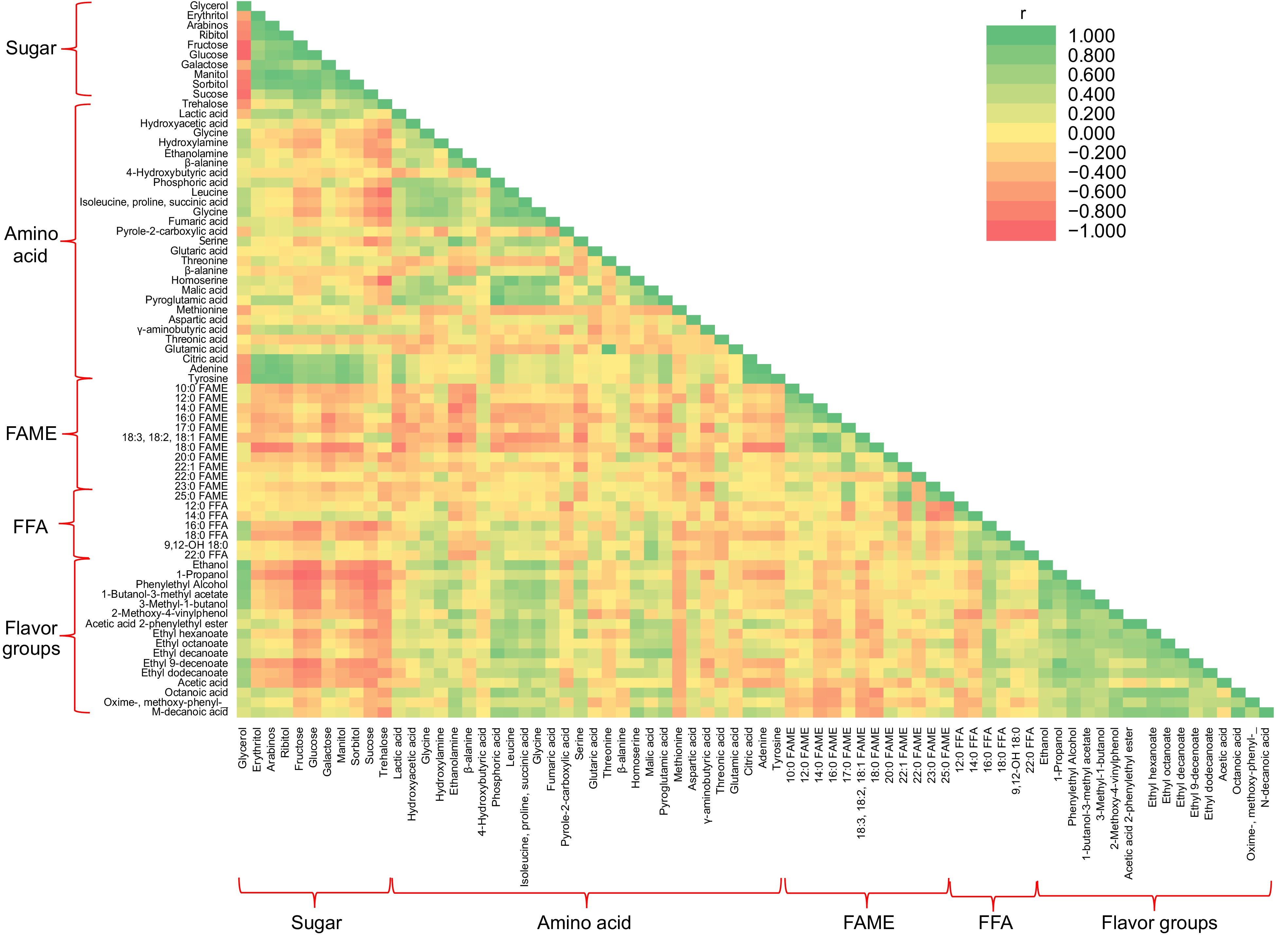
Figure 4.
Lower triangular heat map represents pairwise correlation analysis between metabolites and flavor compounds during the fermentation of coconut cider. Each square represents Spearman's rank correlation coefficient at a significance level of p ≤ 0.05. Orange-red, strong negative correlation (r < −0.7); green, strong positive correlation (r > 0.7). FAME = fatty acid methyl ester, FFA = free fatty acid.
The amino acids exhibited a strong positive correlation with the FFA group and flavoring agents, but a negative correlation with FAME. This is because amino acids serve as precursors for alcohol synthesis and flavoring agents, while FAME is negatively associated with them. Higher alcohol production during fermentation is a byproduct of yeast amino acid metabolism through the Ehrlich pathway and yeast sugar metabolism[46].
FAME, including methyl dodecanoate (12:0), hexadecanoic acid (16:0), octadecenoate (18:0), and linoleic acid (18:2), exhibited a positive correlation with sugars and esters due to the role of esters in fatty acid biosynthesis. The role of ester production in yeast metabolism is unclear, as it may either generate esters to remove toxic fatty acids from yeast cells or be an unintended byproduct of yeast sugar metabolism during fermentation, which may not benefit yeast cells[52]. On the other hand, FAME showed a significant negative correlation with several amino acids and flavor compounds, such as alcohol and acetic acid groups. Additionally, no significant relationship between FAME and FFA was observed.
The results of this study could be used to control various factors and conditions during cider production to create coconut cider with a unique aroma and high levels of bioactive components.
-
The study investigated the fermentation process of coconut cider using two yeast strains and examined its physicochemical properties, metabolomics, and flavoromics. During fermentation, it was found that the Brix and reducing sugar contents decreased, with K1-V1116 yeast resulting in lower levels compared to EC-1118 yeast. The pH slightly decreased, indicating increased acidity, while the alcohol content significantly increased.
Metabolomics analysis revealed the conversion of sugars to alcohol, a decrease in mannitol (indicating the absence of lactic acid bacteria), and an increase in glycerol as the final product of glycerol pyruvic fermentation. During the fermentation of cider using both types of yeast, there was a modest increase in malic acid concentrations, whereas the concentrations of citric acid and lactic acid remained constant, suggesting the absence of lactic bacteria contamination. The levels of leucine and isoleucine increased, indicating their role as metabolic byproducts and their involvement in protein synthesis and energy production. Cider fermented with K1-V1116 and EC-1118 yeast demonstrated an elevation in beneficial fatty acids, specifically stearic acid and lauric acid. Flavoromics analysis identified various volatile compounds, including esters, alcohols, volatile acids, and phenols. Acetate esters and ethyl esters were found to contribute to the fruity aroma of the cider.
Correlation analysis revealed strong associations between sugars and esters/ethanol, amino acids and free fatty acids (FFAs)/flavor compounds, and fatty acid methyl esters (FAMEs) with sugars/esters.
Overall, this study provided important insights into the fermentation process of coconut cider and its impact on physicochemical properties, metabolite conversion, and aroma formation. The findings might be used to guide the control of fermentation conditions and factors to produce coconut cider with unique aromas and higher levels of bioactive components.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. This research was conducted under the project AG-BIO/60-005-012, titled 'Utilization of health in driving value addition along the food industry chain from rice, palm oil, and coconut', at the Center for Agricultural Biotechnology, Kasetsart University. The English proofreading service and native grammar checking support provided by the Kasetsart University Research and Development Institute (KURDI) are acknowledged.
-
The authors confirm contribution to the paper as follows: study conception and design: Songprasert P, Na Jom K; data collection: Songprasert P; analysis and interpretation of results: Songprasert P, Na Jom K; methodology: Songprasert P, Ruangchaisirawet Y; resources: Lorjaroenphon Y, Morakul S; funding acquisition: Na Jom K, Lorjaroenphon Y, Morakul S; supervision: Na Jom K; visualization: Lorjaroenphon Y, Morakul S; draft manuscript preparation: Songprasert P, Ruangchaisirawet Y; writing-review & editing: Na Jom K, Lorjaroenphon Y, Morakul S. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Songprasert P, Ruangchaisirawet Y, Lorjaroenphon Y, Morakul S, Na Jom K. 2025. Effect of two yeast strains and fermentation time on metabolomics and flavoromics of Nam Hom (aromatic) coconut (Cocos nucifera L.) cider. Beverage Plant Research 5: e007 doi: 10.48130/bpr-0024-0039
Effect of two yeast strains and fermentation time on metabolomics and flavoromics of Nam Hom (aromatic) coconut (Cocos nucifera L.) cider
- Received: 20 August 2024
- Revised: 18 November 2024
- Accepted: 09 December 2024
- Published online: 17 March 2025
Abstract: The fermentation process of 'Nam Hom' coconut cider was investigated using two yeast strains: K1-V1116 and EC-1118. During the fermentation period, the Brix and reducing sugar levels exhibited a persistent decline, while the alcohol content continued to escalate. Additionally, a slight decrease in pH indicated a progressive increase in acidity. The fermentation time was divided into three main stages: Pre-Fermentation, In-Process, and Final-Product. Both yeast strains exhibited similar tendencies in metabolite conversion, with elevated levels of sugars such as glucose, sucrose, and fructose observed during the early stages of fermentation. Flavoromics analysis identified a range of volatile compounds, including esters, alcohols, volatile acids, and phenols. Notably, acetate esters and ethyl esters were identified as contributors to the fruity aroma of the cider. Correlation analysis revealed strong associations between sugars and esters/ethanol, amino acids, and free fatty acids (FFAs)/flavor compounds, and fatty acid methyl esters (FAME), with sugars/esters. These findings provide valuable insights for controlling fermentation conditions and factors to produce coconut cider with distinctive aromas and increased levels of bioactive components.
-
Key words:
- Coconut cider /
- Metabolomics /
- Flavoromics /
- Nam Hom /
- Cocos nucifera L.













