-
The coconut palm (Cocos nucifera L.) is an important crop in tropical regions, serving as a source of food, income, and industrial products. However, coconut cultivation faces several challenges, including low productivity, weed management, soil erosion, and nutrient depletion[1−3]. Conventional weed control techniques, including herbicides and manual labor, come with inherent drawbacks related to costs, environmental consequences, and long-term viability. Consequently, there is a rising interest in investigating alternative and environmentally responsible approaches, like cover cropping, to confront these issues[4].
Cover crops are cultivated non-commercial crops that serve multiple purposes, including soil protection and modification, biodiversity enhancement, erosion control, and weed suppression. Intercropping coconut plantations with suitable cover crops can offer numerous agronomically and environmental benefits[5]. The Mucuna genus, consisting of approximately 100 species of climbing vines and shrubs in the legume family, has gained attention as a prominent candidate for cover crops due to its diverse qualities[6], including rapid growth, nitrogen fixation ability, weed-suppressive properties, and potential soil-improving attributes. This genus encompasses numerous species, with Mucuna pruriens var. utilis being the most extensively studied and widely recognized. However, other species, such as Mucuna cochinchinensis and Mucuna bracteata, also exhibit promising traits for cover cropping purposes[7,8]. Variations within the genus have been shown in Fig. 1.

Figure 1.
Variations of Inflorescences and fruits in Mucuna species. (a) M. pruriens var. pruriens (L.) DC.; (b) M. pruriens var. utilis (Wall. ex Wight) Baker ex Burck; (c) M. bracteata DC. ex Kurz; (d) M. gigantea (Willd.) DC.; (e) M. gracilipes Craib; (f) M. hainanensis Hayata; (g) M. revoluta Wilmot-Dear; (h) M. thailandica Niyomdham & Wilmot-Dear; (i) M. warburgii Lauterb. & K. Schum. Source: Authors' compilation based on information from Mucuna species[9]
The Mucuna genus displays a wide array of morphological traits that may differ among species[9]. Nevertheless, several common characteristics are shared by numerous species of Mucuna. An individual plant that is in a state of good health and optimal growth has the capacity to attain a length ranging from 3 to 18 m. Furthermore, this plant possesses a life cycle that is determined by both hereditary factors and environmental conditions, spanning a duration of 120 to 330 d[10]. Mucuna plants are typically climbing vines or shrubs with robust and sturdy stems. The stems may be herbaceous or woody, depending on the species. They tend to twine or rise, often utilizing nearby structures or plants for support. The branches of some species can reach considerable lengths, allowing them to ascend high into the canopy. Plants of this genus have a deep taproot system, and depending on the variety of soil conditions, some roots can grow up to 7−10 m in length[11]. Root hairs increase the surface area available for nutrient and water absorption while serving as a nodulation site[10]. Rhizobia bacteria live symbiotic with the legume root system for atmospheric nitrogen fixation[11].
The leaves of Mucuna spp. are typically characterized by their large size, alternate arrangement, and pinnately compound structure. Each leaf comprises three leaflets arranged along a central rachis, creating a trifoliate pattern. While the leaflets can exhibit variations in size, shape, and texture, they are generally ovate or elliptical with pointed tips. The leaf surfaces may range from smooth to covered with fine hairs, displaying shades of deep green to lighter green. These leaves are trifoliate in nature and may exhibit pubescence[12]. A mature leaf typically measures approximately 5−20 cm long and 3−15 cm wide.
Mucuna species are known for their striking and colorful bisexual flowers that form in clusters or racemes[10]. These flowers typically exhibit a papilionaceous structure, resembling the characteristic butterfly-like shape commonly found in legume family plants. These flowers are composed of five petals, which include a larger banner petal, two lateral wings, and two joined lower petals that create a keel. Depending on the specific species, the flower colors can vary significantly, encompassing shades of purple, blue, white, pink, or yellow. Following self-pollination, Mucuna flowers transform into elongated and fleshy seed pods[13]. These pods often have a distinctive curved or coiled appearance, contributing to their unique visual appeal. The size, shape, and coloration of the pods may exhibit variation across different species of Mucuna. The pods of Mucuna plants are known to yield seeds that are generally big and spherical, characterized by a compact covering of short hairs or bristles[12]. Depending on the specific species, these seeds can be highly abundant and vary in color, with options ranging from black and brown to mottled. Mucuna reproduction occurs through sexual means via seeds and vegetatively through stem cuttings with a high success rate. Innovative micro-propagation techniques have been developed to address challenges associated with low germination rates and poor seedling viability typically observed in Mucuna seeds[14].
According to Correia et al., Mucuna spp. can achieve optimal growth and development in environments with an annual rainfall ranging from 400 to 3,000 mm and temperatures between 19 and 27 °C[15]. It tends to enter the flowering stage earlier when the night-time temperature drops below 21 °C. Furthermore, Mucuna spp. is well-known for its ability to tolerate a wide range of pH levels (4.5−7.7), soil types, drought conditions, and high soil acidity levels[6,16]. Adaptability to diverse climates and soil conditions has influenced its preference as a cover crop[14,17]. However, as indicated by Blomme et al., it is essential to note that the growth and nodulation of Mucuna can be influenced by shading[11].
While Mucuna shows potential as a cover crop in coconut cultivation, there is a noticeable scarcity of comprehensive reviews that investigate its complete potential. Therefore, the objective of this review article is to undertake a thorough assessment of the existing scientific literature. With this review, we seek to derive valuable insights from both research findings and practical field experiences. The main objective of this study is to assess the effectiveness of Mucuna as a cover crop in coconut plantations. By conducting a comprehensive examination of the agronomic, environmental, and economic ramifications, our objective is to acquire a comprehensive comprehension of the potential benefits and obstacles associated with the integration of Mucuna into coconut-based agroecosystems.
-
Climbing nature and quick growth characteristics contribute significantly to their exceptional competitive abilities against weed populations in agricultural fields[4]. The allelopathic potential of the Mucuna genus is particularly noteworthy when compared to that of typical cover crops[18]. The primary allelochemical present in Mucuna species is L-DOPA, with leaves containing notably higher concentrations, accounting for approximately 1% of their dry weight. The presence of these allelopathic chemicals results in the manifestation of inhibitory effects on the growth of adjacent plants, as reported by Fujii[19]. The root exudates of Mucuna primarily affect nearby plants, thereby contributing to its localized weed suppression effect. Therefore, the efficacy of weed management under field conditions is influenced by both Mucuna biomass production rate and planting distances[20]. Research conducted to evaluate weed density in different pre-rice cassava cropping systems over three consecutive years has proved that Mucuna was the most effective cover crop for suppressing weeds, followed by cowpea (Vigna unguiculata), soybean (Glycine max), and lablab (Lablab purpureus) (Table 1)[21]. Furthermore, Fig. 2 provides evidence that coconut planted with Mucuna bracteata in a three-row intercropped system optimizes ground cover, making it the most suitable planting technique out of those analyzed for achieving extensive ground cover[22].
Table 1. Comparison of weed density in different pre-rice cassava/legume cover cropping practices in rice at 9 weeks after transplanting.
Cassava/cover
cropping systemsWeed density (No. of weeds/m2) 2011 2012 2013 Cassava/Mucuna 71f 139d 129f Cassava/Cowpea 102.5e 171c 156e Cassava/Soybean 123.3d 188c 169cd Cassava/Lablab 137.4c 200b 178c Cassava mono-cropping 165b 224b 218b Natural fallow 199.8a 325a 377a Means followed by the same letter(s) within the same column are not significantly different at a 5% significance level. Source: Authors' compilation based on information from intercrops and weed management practices[21]. 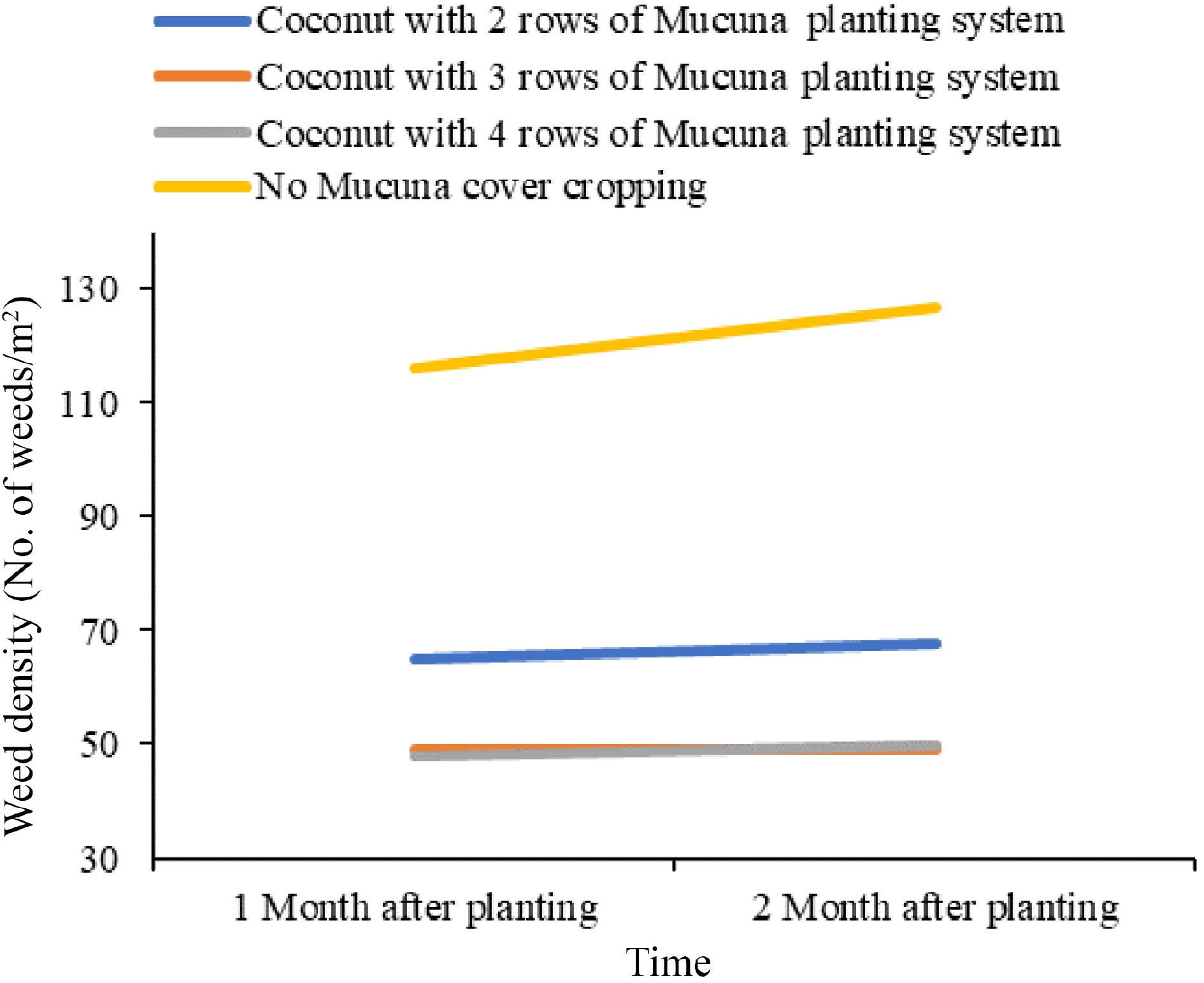
Figure 2.
Comparison of weed growth in different Mucuna planting systems. Source: Authors' compilation based on information from potential use of Mucuna bracteate as a cover crop[22].
The smothering effect exerted by Mucuna leads to reduced germination percentages, slower germination speed indices, and the development of weak, unhealthy seedlings in other plant species[23]. Additionally, Mucuna has emerged as a promising bio-herbicide[24], providing an environmentally friendly alternative to herbicide usage in organic farming systems. Notably, the cultivation of Mucuna pruriens var. utilis has demonstrated effective control of cogon grass (Imperata cylindrica), an invasive weed in many regions of Sri Lanka[25,26]. Mucuna has also displayed efficient weed management capabilities against Bermuda grass (Cynodon dactylon) and nutgrass (Cyperus rotundus)[27]. These findings highlight the potential of Mucuna as an effective tool for weed control in agricultural systems, especially for managing problematic and invasive weed species.
Improving soil properties
-
Mucuna exhibits distinct characteristics in nodule formation, nitrogen release, litter production, and soil improvement compared to other legumes. While Mucuna produces relatively larger nodules, the number of nodules per plant and unit area is generally lower than other legume species[28]. However, regarding nitrogen cycling, Mucuna has shown a higher potential for releasing net nitrogen from its plant biomass during decomposition than other species like Pueraria phaseoloides and Stylosanthes hamata[29]. Moreover, Mucuna species have been observed to yield significantly higher quantities of organic matter in comparison to P. phaseoloides and other ground cover plants, rendering them a suitable choice for mulch cover and a valuable contributor to soil carbon and plant nutrients[30]. According to Sakiah & Hasibaun, lands with Mucuna bracteata cover on flat and sloping terrain contained 2.58% and 2.22% organic matter content, respectively[31]. In contrast, if these lands lacked this plant cover, they would register only 1.98% and 1.44% of organic matter respectively. Additionally, one year after establishment well-grown Mucuna cultivation can generate approximately 8−10 t ha−1, whereas other cover crops typically produce about 4.4 t ha−1. The actual yield of Mucuna is influenced by factors such as the duration of the growing season and the overall health of the soil[12]. The aforementioned characteristics definitively identify the Mucuna species as a highly efficient provider of green manure.
The deep-rooted characteristic of Mucuna confers a significant advantage by effectively minimizing soil compaction. This is accomplished by establishing channels for water entry and promoting root penetration. Consequently, this unique characteristic helps prevent nutrient leaching and improves soil structure[31,32]. Studies have shown that soil integrated with Mucuna exhibits a greater capacity for forming larger and more stable soil aggregates than other cover crops[33]. The persistent generation of binding chemicals, such as organic acids and humic substances, by the flourishing soil microorganisms facilitated by the biomass of Mucuna can be ascribed to this phenomenon[34]. These diverse soil organisms are one of the key indicators of healthy soil[35]. In addition, the root system of Mucuna has been seen to significantly improve certain hydro-physical characteristics of the soil. These improvements include a reduction in bulk density, an increase in total porosity, and the promotion of aeration porosity. This, in turn, creates an optimal soil environment for enhanced plant growth[36]. It is imperative to consider that the impact of cover crop intensity may vary depending on certain management strategies, as illustrated in Fig. 3. It illustrates the suitability of Mucuna cover cropping and its proper management for flat lands as well as slopy lands in terms of soil physical properties like bulk density, total pore spaces, infiltration capacity, and permeability. This reduces risks of waterlogging, runoff, and erosion while promoting factors beneficial to plant production like water availability, drainage, and root development.
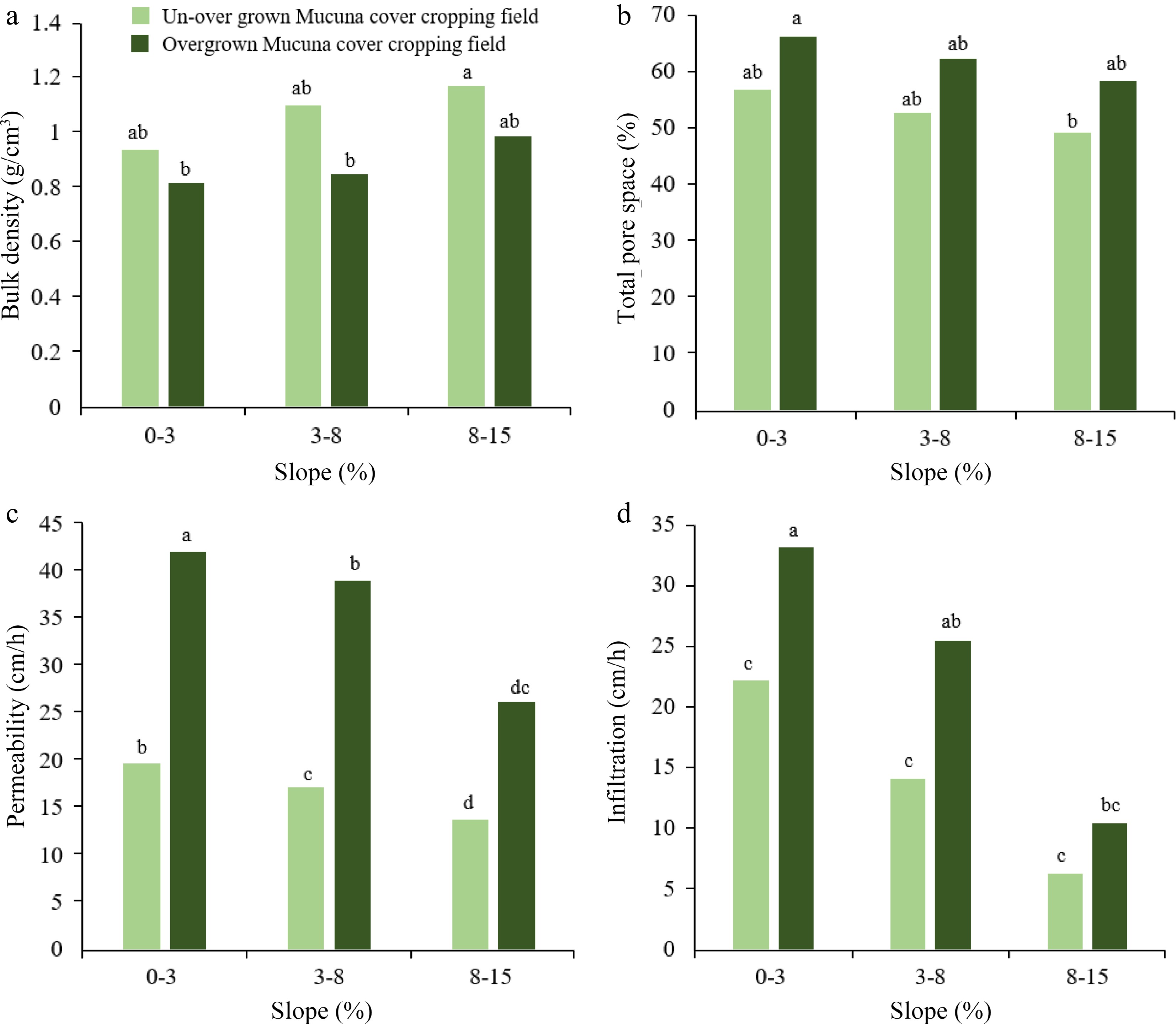
Figure 3.
Variation of soil physical properties in two Mucuna cover crop management systems in different topography levels. (a) changes in bulk density with land slope; (b) changes in total pore space with land slope; (c) changes in permeability with land slope; (d) changes in infiltration with land slope. Source: Authors' compilation based on information from effect of Mucuna bracteata on soil physical properties, runoff and erosion[34]. Note: Letters followed by the same lowercase letter in the same parameter show not significantly difference on a significant level at 5%.
Mucuna pruriens var. utilis has gained significant recognition as a preferred ground cover in West Africa for mitigating soil degradation caused by traditional agricultural methods[37]. It can be employed as a monoculture short fallow cover crop for severely degraded soils, while a maize-mucuna relay cropping system proves suitable for moderately degraded soils[37]. These approaches underscore the versatility and efficacy of Mucuna in combatting soil degradation and advancing sustainable agricultural practices in the region.
Pest and disease control
-
The Mucuna genus is well-known for its remarkable ability to combat pests and diseases, rendering it a valuable crop for safeguarding other plants[38]. Mucuna species employ a variety of mechanisms to regulate the intrusion of plant-parasitic organisms. The decomposition of deceased Mucuna plant material enriches soil health, fostering a conducive environment for both micro and macro-organisms and serving as a carbon and energy source for all soil inhabitants[34]. Studies have demonstrated that cultivating Mucuna promotes the proliferation and growth of beneficial organisms, including nematodes, earthworms, millipedes, centipedes, coleoptera, diptera, and isopoda, while simultaneously suppressing populations of phytophagous nematodes and ants[39].
Mucuna aterrima, as an example, emits both non-volatile and volatile organic compounds from its above-ground portions, which hold promise in combating root-knot nematodes (Meloidogyne incognita)[40,41]. The levels of these allelochemicals differ across various plant components, with the vine, leaves, petioles, fine roots, and primary roots displaying distinct lethal concentration (LC50) values (Fig. 4)[42]. Furthermore, the seeds of Mucuna pruriens contain a peptidase inhibitor that impedes the population growth of the Melon fruit fly (Zeugodacus cucurbitae) by increasing larval mortality, reducing pupal weight, and inhibiting adult emergence[43]. These discoveries underscore the potential of Mucuna species to offer natural resistance against pests and diseases. The emission of allelochemicals and the facilitation of symbiotic organisms collectively augment the pest control and plant defense capabilities of Mucuna crops. The inclusion of Mucuna within integrated pest management (IPM) techniques has the potential to diminish dependence on synthetic pesticides and foster the adoption of agricultural practices that are more sustainable and ecologically conscious.
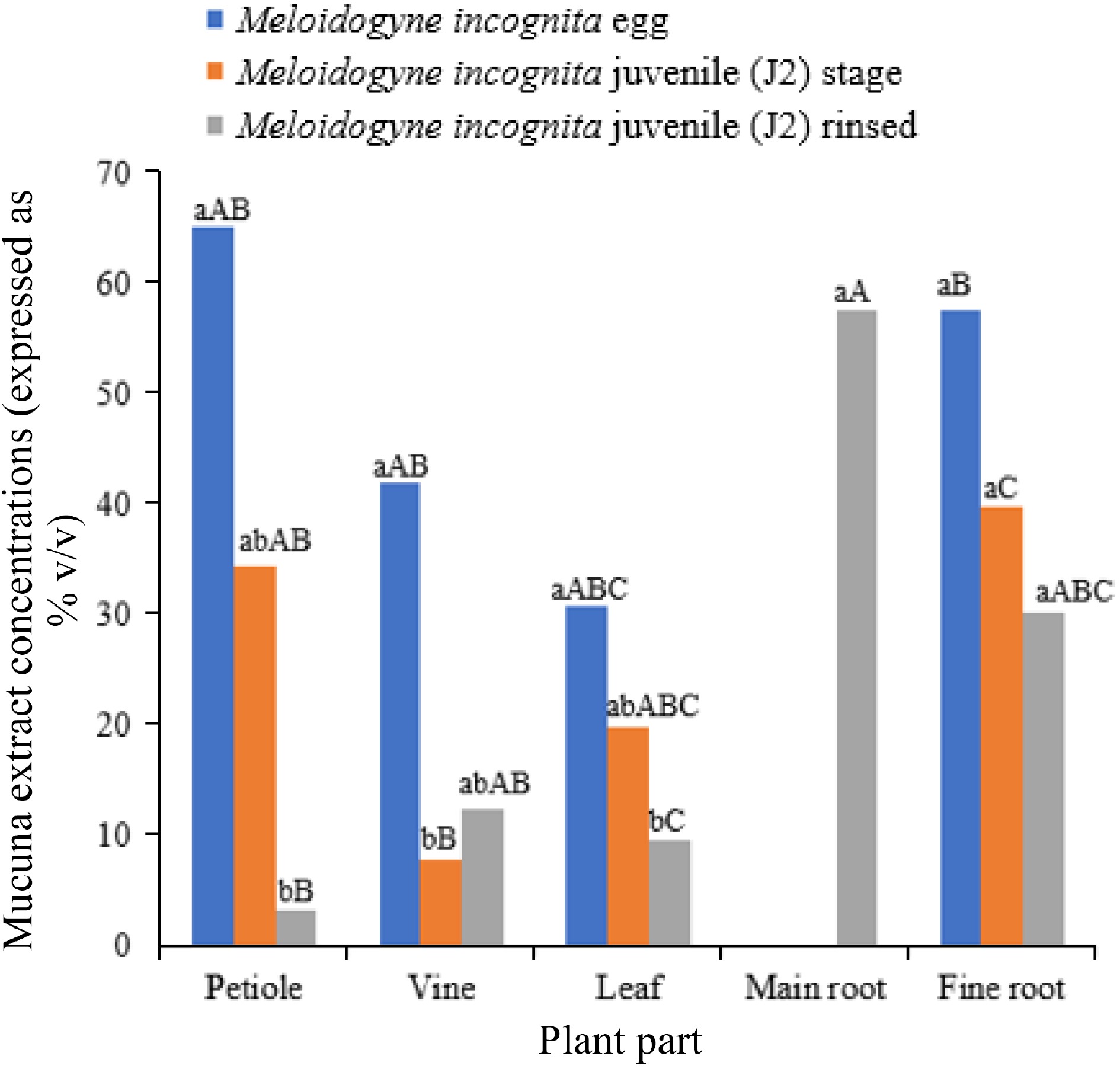
Figure 4.
Impact of Mucuna pruriens extractions for 50% reduction Meloidogyne incognita. Source: Authors' compilation based on information from germination and growth study[42]. Note: Values followed by the same lowercase letters and same uppercase letters are not different (p < 0.05) separately. Values are taken by Bon p-value adjustments.
Feeding material and human diet
-
Mucuna species have been extensively studied as potential fodder or supplementary feed for various livestock in animal husbandry. Different researchers have documented their potential in the diets of dairy cows, pigs, goats, guinea pigs, cane rats, and poultry[44−47]. However, certain monogastric animals may exhibit sensitivity to the anti-nutritional compounds found in Mucuna, rendering it toxic for them, as highlighted by Carew & Gernat[45]. On the other hand, for sheep, both M. pruriens seeds and pods have demonstrated potential as raw materials in diet formulation, contingent upon their metabolizable energy content, as indicated by Garcia-Galvan et al.[48].
Moreover, the seeds of M. pruriens have been subject to investigation as a dietary supplement in preparing fish meal for common carp (Cyprinus carpio L.), thus aiding in the fulfillment of the protein needs of fish[49]. To ensure safe use, the seeds should be harvested at the boosting stage and dried properly to 75%−80% dry matter basis within 3−5 d[50]. Moreover, it has been observed that mature Mucuna seeds are consumed as a cereal in human diets, particularly in South Asian countries like Sri Lanka, due to their nutritional value[51]. The seeds have been found to possess various biological activities, such as cytotoxicity, phytochemical, anti-parasitic, and antioxidant properties[52]. However, Mucuna seeds contain anti-nutritional compounds, including tannins, cyanides, hemagglutinins, anticoagulants, and trypsin inhibitors[53]. Seed yield varies with the environmental conditions, management practices, and soil conditions.
Fortunately, these anti-nutrients can be removed during household preparations using simple steps[6]. The process involves isolating and cleaning mature, high-quality seeds and soaking them in water for 24 h, with water changes at intervals. The soaked seeds are then boiled for 4−5 h, with water changes, before de-husking and drying. Autoclaving can be employed for industrial use to eliminate heat-labile anti-nutritional factors[49]. Ultimately, Mucuna seeds can be incorporated into the human diet in various forms, including whole seeds, flour, or as part of beverages when combined with other foods[6].
Medicinal properties
-
Mucuna pruriens, in addition to serving as a beneficial ground cover, possesses remarkable medicinal, pharmaceutical, and nutritional properties, as highlighted in the study by Lampariello et al.[54]. The plant exhibits a diverse range of therapeutic activities, making it a subject of interest in various fields of medicine. Research has revealed that Mucuna pruriens exhibits a wide range of beneficial properties, including anti-venom, anti-ischemic, aphrodisiac, anti-microbial, anti-neoplastic, anti-inflammatory, anti-epileptic, anti-protozoal, and anti-diabetic effects, along with neuroprotective properties[55]. In Ayurvedic medicine, M. pruriens is highly valued for its medicinal attributes. The seeds, in particular, have been found to enhance antioxidant defenses, improve the quality of semen in infertile men, and reduce lipid peroxide levels[56,57]. Additionally, the seeds contain a significant amount of 'Levodopa,' making them a potential treatment for Parkinson's disease[55,58]. Furthermore, various parts of the M. pruriens plant have therapeutic applications. The roots of Mucuna are recognized for their potential to alleviate constipation, address ulcers, and reduce fever. In contrast, the leaves are traditionally considered beneficial for addressing debility, relieving headaches, and reducing inflammation[59]. The bioactive compounds present in Mucuna pruriens seeds have shown anti-microbial effects against pathogenic bacteria such as E. coli, Bacillus subtilis, and Salmonella typhi[60]. Furthermore, Mucuna also contains therapeutic peptides, such as proteinase and glycosidase inhibitors, which have been identified for their potential in treating liver cancers and hepatitis C virus (HCV) infections. Additionally, they can influence the pharmacokinetics of co-administered drugs[61].
Phytoremediation properties
-
Certain Mucuna species exhibit phytoremediation properties, enabling them to thrive in soils contaminated with crude oil[17,62]. This capability holds significant promise as a solution to combat pollution-related land degradation. Additionally, the edible seeds of Mucuna species stand out as a cost-effective and nutrient-rich source of carbohydrates, protein, lipids, amino acids, fibre, and minerals when compared to other widely consumed legumes like Glycine max, as highlighted by Mang et al.[63].
Economic benefits
-
Introducing the Mucuna genus into agricultural fields offers an environmentally friendly approach that can substantially reduce labor demands and dependence on external synthetic chemicals, including herbicides, pesticides, fertilizers, and particularly synthetic nitrogen fertilizers, as Buckles emphasized[64]. Through the use of this approach, agricultural practitioners have the potential to reduce production costs, preserve energy resources, and concurrently advance the sustainability of both the land and the environment. As a result, this methodology possesses the capacity to augment the financial gains of farmers.
-
When cultivating Mucuna as a cash crop, it is recommended to use a seed rate of 50 kg ha–1, with planting spacings set at 0.6 m × 0.6 m[12]. For use as a cover crop, Mucuna can be grown either as a sole cover crop or in a mixed cover cropping system, with a spacing of 0.8 m × 0.4 m and two seeds per hole at a seed rate of 30 kg ha–1[4,37].
Mucuna can be directly seeded in non-prepared fields or fields prepared using herbicides or ploughing, slashing, burning, or cleaning[65]. In order to enhance the rates of germination, it is recommended to administer seed treatments (see Table 2). Additionally, slashing can be practiced for more efficient seeding to stimulate germination[37]. In cases where seeds are limited and costly, stem cuttings can be utilized as a planting material. Hanum found that seedlings established from stem cuttings exhibited a higher growth rate than seed-sown seedlings (Fig. 5)[66].
Table 2. The impact of various seed treatments on Mucuna pruriens seed germination. Source: Authors' compilation based on information from study on seed treatment effects[67].
Treatments Germination (%) Control (no seed treatment was practised) 53 Cold water soaking for 24 h 58 Hot water soaking at 80 °C for 5 min 79 Acid treatment (commercial H2SO4) for 3 min 83 Scarification (seeds were rubbed against the hard surface for 5 min) 93 Cow-dung pelleting 63 Panchakavya soaking (3% concentration for 6 h)
(a mixture of 2.5 kg of cow dung, 1.5 L of cow urine,
1 L of cow milk, 1 L of cow curd, 500 g of cow
ghee and 1.5 L of sugar cane juice)67 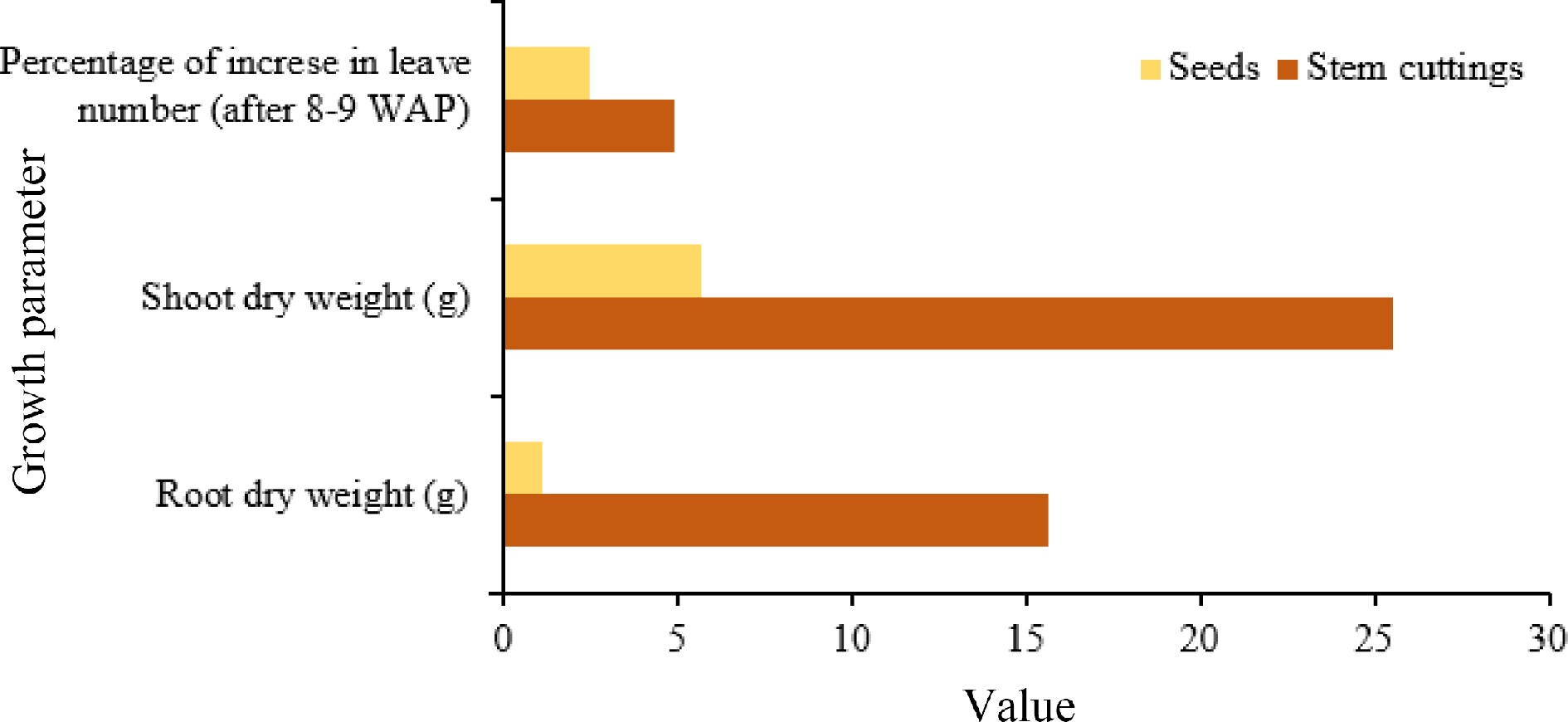
Figure 5.
Comparison of growth of Mucuna from seeds and cuttings. Source: Authors‘ compilation based on information from growth pattern study[66].
Following the second year of establishment, Mucuna forms a dense vegetation cover, covering the entire ground at a rate of 2−3 square meters per month[31]. Unlike some other cover crops, plants in the Genus Mucuna display rapid vegetative growth right from emergence, as noted by Hartkamp et al.[68]. Incorporating rhizobial inocula at the planting stage is beneficial to enhance plant establishment and development[69].
Mucuna responds better to natural fertilizers than synthetic fertilizers, especially goat urine, which increases the number of leaves, tendril length, and dry weight of the plants. The best result for tendril lengths (69.95 cm) was observed when 300 ml of goat urine and 60 min of soaking time were applied[70]. Despite being known for its pest and disease resistance, Mucuna shows susceptibility to various fungi such as Cercospora stizolohii, Mycosphaerella cruenta, Phyllosticta mucunae, Phymatotrichum omnivorum, Phytophthora drechsleri, Rhizoctonia solani, Sclerotium rolfsii, Uromyces mucunae, bacteria including Bacterial leaf-spot, Xanthomonas stizolobiicola, Pseudomonas stizolobii, and Pseudomonas syringae, and parasitic plants such as Striga gesnerioides[27]. This vulnerability may add to the costs for farmers. Proper maintenance and pruning are also essential due to its significant and aggressive vegetative growth, producing larger leaves, thick stems, and climbing habits[29]. This growth habit can also make it a competitor for young seedlings of the main crop, necessitating careful management.
When Mucuna has completed approximately 50% to 70% of its growth cycle, which typically occurs at or after the pod-filling stage, it is appropriate to incorporate it into the soil. During this stage, the maximum amount of plant dry weight and nitrogen can be effectively assimilated into the soil. Integration can be achieved by cutting and burying the plant material in the ground, cutting and spreading it on the field, utilizing ex-situ plant biomass, or creating secondary products like compost from plant waste[10]. Several studies have proposed that Mucuna could be a suitable alternative for cover cropping in lieu of alley cropping, owing to its numerous advantages[71]. However, recommendations for these types of cover crops may differ based on practicability, the nature of the main crop, the composition of weeds, environmental factors, and local realities[72].
-
To comprehend the economic significance of Mucuna species, it is imperative to comprehensively characterize the bioactive compounds found in different plant parts and elucidate their mechanisms of action in disease suppression[55]. Proper pre-breeding procedures should be conducted to boost crop diversification and identify and evaluate pivotal genes with advantageous agronomic traits[13,73]. Given the global concern regarding zinc deficiency in tropical agricultural soils, assessing the impact of Mucuna cover cropping on soil zinc levels offers valuable prospects for future research[72].
Furthermore, it is essential to explore the effects of Mucuna cover crops on biodiversity, encompassing beneficial insects, pollinators, and soil organisms. A growing amount of attention is being paid to assessing how the crop contributes to the provision of ecosystem services like habitat and pollination support, as well as how it might strengthen agroecosystems' resilience to climate change by reducing soil erosion, improving water and nutrient management, and increasing carbon sequestration.
Examining the allelopathic effects of Mucuna cover crops on primary crops and other plant species is crucial to interpreting their potential for beneficial and detrimental interactions. These avenues of research will play a significant role in shaping our understanding of the multifaceted contributions of Mucuna species in sustainable agriculture and ecosystem management.
-
While the genus Mucuna has proven successful in Sri Lankan rubber plantations, its utilization in coconut plantations remains relatively uncommon. Nonetheless, Mucuna exhibits the potential to emerge as an ideal cover crop within the Sri Lankan agricultural sector due to its adaptability and multifaceted advantages. In the role of a cover crop, Mucuna stands poised to significantly contribute to sustainable agriculture by mitigating soil degradation and enhancing hydro-physical properties. It demonstrates the capacity to effectively suppress weeds, pests, and diseases in agricultural fields, thus reducing the reliance on chemical inputs. Additionally, the capacity of Mucuna to augment the soil's carbon content and supply vital nutrients has the potential to improve both crop yield and land productivity. Beyond these primary benefits, Mucuna offers a spectrum of secondary advantages. It serves as a nutritious food source for humans and livestock, providing valuable animal husbandry feeding materials. Additionally, the plant is a valuable source of raw materials for medicinal and pharmaceutical production, further amplifying its economic significance. The efficacy of Mucuna cover cropping is contingent upon meticulous site selection, which necessitates careful consideration of various elements including the inherent qualities of the primary crop, prevailing weed populations, environmental conditions, and local circumstances. Tailored recommendations for implementing Mucuna cover cropping can be formulated based on these variables to ensure its effective adoption. For widespread acceptance and adoption, it is imperative to conduct thorough research into the agronomic practices of Mucuna cover cropping, particularly in the context of coconut cultivation across diverse agroecological zones within the country. Maximizing the secondary benefits derived from Mucuna plants can serve as a compelling incentive for farmers to embrace this sustainable agricultural practice more readily. In conclusion, the implementation of Mucuna cover cropping in Sri Lanka's agriculture sector has the capacity to yield a wide range of environmental and economic benefits, hence promoting sustainability and enhancing prosperity.
-
The authors confirm contribution to the paper as follows: study conception and design: Atapattu AJ, Dissanayaka DMNS; data collection: Udumann SS, Dissanayaka DMNS; data analysis: Atapattu AJ, Dissanayaka DMNS, Udumann SS; draft manuscript preparation: Atapattu AJ, Dissanayaka DMNS, Nuwarapaksha TD; tables, and figures preparation: Dissanayaka DMNS, Nuwarapaksha TD. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in the manuscript.
-
We want to express our appreciation to the technical staff of the Agronomy Division of the Coconut Research Institute. Mrs. Asanki Jayamali, Mrs. Madhuwanka P. Gayadari, and Mr. Namal K. Gunarathna deserve special recognition for their enormous contribution. We would like to express our deep gratitude to the editor and two anonymous reviewers for their valuable comments and critical evaluation.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Dissanayaka DMNS, Udumann SS, Nuwarapaksha TD, Atapattu AJ. 2024. Harnessing the potential of Mucuna cover cropping: a comprehensive review of its agronomic and environmental benefits. Circular Agricultural Systems 4: e003 doi: 10.48130/cas-0024-0001
Harnessing the potential of Mucuna cover cropping: a comprehensive review of its agronomic and environmental benefits
- Received: 20 September 2023
- Revised: 30 November 2023
- Accepted: 11 December 2023
- Published online: 05 February 2024
Abstract: The coconut plant (Cocos nucifera L.), an essential tropical agricultural commodity, encounters a range of obstacles including the proliferation of unwanted weed vegetation, deterioration of soil quality, and depletion of essential nutrients. Conventional methods such as herbicide application and manual labor possess constraints, prompting a growing interest in environmentally sustainable alternatives such as cover cropping to address these challenges. Mucuna, a diverse genus of climbing vines and shrubs in the legume family, has drawn attention for its potential as a beneficial cover crop, offering various agricultural and environmental benefits. Mucuna species are known for their rapid growth, ability to fix nitrogen, and weed-controlling properties, making them well-suited for enhancing soil health and fertility. Moreover, their deep taproot systems contribute to soil aeration and compaction alleviation. The allelopathic potential of Mucuna offers an eco-friendly approach to weed control, reducing reliance on synthetic herbicides. In addition, the inclusion of Mucuna in the soil has the potential to enhance the population of beneficial organisms and support greater biodiversity. Therefore, this can potentially lead to beneficial effects on the implementation of sustainable agricultural methods. Mucuna provides various secondary benefits in addition to its primary agronomic advantages. The seeds and biomass of this plant function as a valuable source of nourishing fodder and feed for a diverse range of livestock, hence enabling the implementation of animal husbandry techniques. Additionally, Mucuna seeds exhibit potential as a nutrient-dense food source for human consumption, boasting demonstrated medicinal properties such as neuroprotective effects and potential in managing diabetes. Incorporating Mucuna cover cropping within coconut plantations can yield several benefits, including improvements in soil hydro-physical properties, enhanced pest and disease control, increased land productivity, and a reduced environmental footprint compared to conventional agricultural methods. The ability of Mucuna to adapt to varied climatic and soil conditions further increases its potential as a long-term and environmentally beneficial option. This review highlights the importance of Mucuna cover cropping and suggests customized recommendations. Furthermore, it proposes future research avenues, such as exploring its role in bolstering climate change resilience and its phytoremediation capabilities, to broaden our comprehension of this versatile cover crop. In conclusion, utilizing the potential of Mucuna inside coconut plantations is a possible path toward sustainable agriculture and environmental protection.
-
Key words:
- Allelopathic effect /
- Coconut /
- Conventional agriculture /
- Leguminous plant /
- Soil health












