-
Public safety is a basic need for human beings. Emergencies that threaten public safety are mainly divided into four categories: natural disaster emergency, industry emergency, urban emergency, and public health emergency[1]. Among them, public health emergencies are closely related to people's health, which causes casualties and social panic. During urbanization, densely populated places increase, providing convenience for public health emergencies, especially respiratory infectious disease outbreaks[2]. After natural disasters (e.g., earthquakes, floods) or accidents (e.g., fires, explosions), people's living conditions deteriorate, and the epidemic risk also increases. Respiratory infectious diseases are airbourne. Their high infectivity, widespread nature, and difficulty in control pose an increased threat to human safety. In the 20th century, influenza, spreading worldwide, claimed millions of lives, resulted in significant losses, and impeded social progress and development[3]. In the 21st century, respiratory infectious diseases have become increasingly prevalent, resulting in substantial economic, societal, and physical and mental health costs. In 2003, SARS was prevalent in more than 20 countries and regions, causing more than 8,000 infections and more than 800 deaths, with a high fatality rate. In 2009, the influenza A H1N1 virus epidemic broke out in America and spread globally, causing more than 200,000 deaths. In 2012, an outbreak of MERS spread to 27 countries, resulting in the closure of thousands of primary and secondary schools in South Korea. The COVID-19 outbreak at the end of 2019 still circulates worldwide and affects people's lives today. According to the World Health Organization (WHO), more than 700 million people worldwide have been infected with COVID-19, and more than 6.8 million have died[4].
The transmission mechanism of infectious diseases is the basis for risk assessment and the design of epidemic control measures. The pandemic of infectious diseases requires three elements: source of infection, transmission route, and susceptible population[5]. For respiratory infectious diseases, the source of infection mainly refers to the infected patients and the infectious substances produced by them. The transmission route refers to the route and medium through which infectious substances leave the source of infection and reach the susceptible person, mainly including three types of transmission: contact, droplet, and airborne transmission. The susceptible population refers to those who lack specific immunity to the infectious pathogens. People spend more than 80% of their time indoors[6]. Compared with the outdoors, indoor has a high incidence of public health events, and it is common for respiratory infectious diseases to spread between people indoors[7,8]. For the control of the epidemic of respiratory infectious diseases, attention should be paid to indoor environments.
In the COVID-19 pandemic, scholars studied the transmission law of respiratory infectious diseases[9−11], and evidence showed that airborne transmission is likely to be the main transmission route of respiratory infectious diseases. Compared with contact and droplet transmission, the airborne transmission mechanism is complex, and the requirements for epidemic control are also higher.[12] As shown in Fig. 1, relevant studies mainly focus on the characteristics of infection sources, airborne transmission routes, exposure of susceptible personnel, and risk assessment methods.
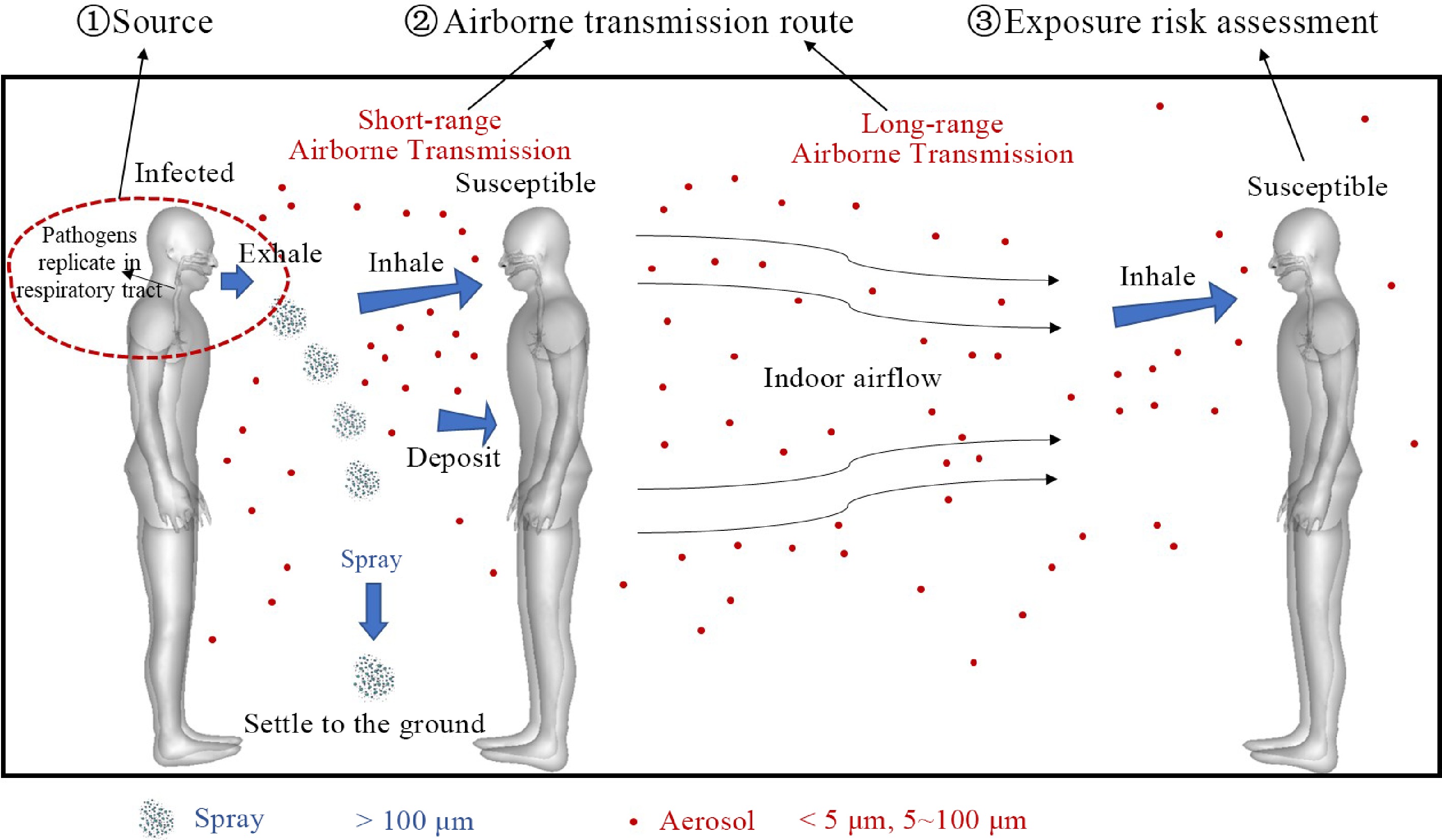
Figure 1.
Three main factors in indoor airborne transmission of respiratory infectious diseases: Source of infection, airborne transmission route, exposure risk assessment.
The airborne transmission process of respiratory infectious substances is usually dominated by indoor airflow. Human behavior could significantly affect the airflow field and change the diffusion law of exhaled infectious substances, which affects the regional risk[13]. During the SARS epidemic, a transmission case across seven rows of seats occurred on a flight, which was presumed to be related to the crew members' movement[14]. Among the reported cases of COVID-19 infection, some infections have also been caused by close interactive movement with patients. A risk assessment of respiratory infectious diseases in aircraft cabins showed that the risk assessment model considering personnel movement could better explain the transmission pattern of respiratory infectious diseases than the traditional static scenario model[15]. Hence, it is needed to explore the airflow characteristics of the human movement scene and its influence on the infectious substance diffusion. Respiratory protective gear can mitigate transmission of respiratory infectious disease, amongst them facemasks are commonly used against the COVID-19 pandemic, due to sound regulations, portability, and low costs. The effectiveness and mechanism of facemasks also gained great interest in research, which could help guide protective measures, and promote public safety.
Herein, we review studies on the respiratory infectious disease transmission mechanism in the past few decades. The following issues will be highlighted:
(1) Source characteristics of respiratory infectious diseases.
(2) Airborne transmission route of respiratory infectious diseases.
(3) Exposure and infection risk assessment for susceptible individuals.
(4) Effects of human movement on airborne transmission mechanisms.
(5) Effects of facemasks on source characteristics and infection risk.
-
Pathogens of respiratory infectious diseases (viruses, bacteria, etc.) usually replicate in a patient's respiratory tract and enter the environment through the patient's respiratory activities (breathing, speaking, coughing, sneezing, etc.)[5,16]. Most pathogen's particle sizes are in the nanometer to micron range, such as the COVID-19 virus at about 60~140 nm[17] and the influenza virus at about 80~500 nm[18]. These pathogens usually need to be attached to a carrier for airborne transmission. Droplets and aerosols produced by respiratory activity are typical carriers[19]. The carrying infectious substances are suspended, evaporated, and dispersed in the environment and may be deposited on objects or human surfaces or inhaled by humans, posing an infection risk[20].
The particle size and concentration characteristics of droplets generated by the respiratory activities of patients are key factors affecting their diffusion characteristics[21]. In the past few decades, scholars have studied the particle size distribution, quantity, concentration, and velocity of exhaled droplets. In terms of experimental research methods, early research mainly used high-speed cameras, solid impact, liquid impact, and other technologies. With the development of technology, optical particle counter, aerodynamic particle counter, laser particle size analyzer, electromigration particle size spectrometer, and other measurement methods have been frequently used. Individual differences exist in the particle size distribution of patients' exhaled droplets, but most are in 0.05~500 μm, especially 0.1~100 μm[22−24]. Considering the evaporation process, scholars combined experimental and computational fluid dynamics (CFD) methods to study the relationship between the initial particle size of the droplet, evaporation time, environmental relative humidity, and the final particle size and established a fitting curve. It showed that the final particle size of the droplet after evaporation is usually about 1/3 of the initial particle size[25,26]. In the COVID-19 pandemic, many scholars have collected and analyzed pathogens exhaled by patients. Most of these studies were conducted in indoor and healthcare environments, including air and environment sampling. Collection mechanisms include impact dust filters, cyclones, impactors, filters, water-based condensation, and passive sampling. The RT-PCR method was used to detect SARS-CoV-2 RNA in collected samples[27]. The particle size distribution of exhaled infectious substances varies from person to person. It is affected by the type of respiratory activity and the exercise state[28], and individualized analysis is required for specific case studies.
It is worth noting that the pathogens of different types of respiratory infectious diseases have different replication and shedding sites in the respiratory tract of patients. For example, the SARS virus usually replicates in a patient's lung, while the COVID-19 virus independently replicates and sheds in a patient's larynx[29]. It was found that a higher percentage of particles released from the lungs were deposited in the larynx during exhalation[30]. Moreover, the number of people infected with COVID-19 is much higher than SARS. Wu & Weng found that the difference in the replication site of infectious agents in the patient's respiratory tract will affect the exhaled proportion and indoor exposure[31]. The exhaled fraction of viruses that replicate independently in the larynx is higher than that which replicate only in the lungs, increasing the risk of exposure for susceptible persons. This finding provides a possible explanation for the high transmissibility of some respiratory infectious diseases such as COVID-19. Differences in where pathogens replicate in a patient's respiratory tract may affect their exhalation and the exposure risk of susceptible individuals. However, existing studies usually use the surface of the patient's mouth or nose as the release location of infectious substances, and the impact of the difference in the release site of pathogens in the respiratory tract is insufficient. Clarifying the exhalation process of pathogens in the respiratory tract of patients is the basis for assessing the indoor transmission rule and exposure risk of susceptible individuals. It has significant reference value for scientific protection measures.
-
Over a long historical period, contact and droplet transmission were considered to be the main transmission routes of respiratory infectious diseases, while airborne transmission routes were considered negligible. Until 1962, airborne transmission began to attract attention in the study of tuberculosis outbreaks[32]. However, the view that contact and droplet transmission were the main transmission routes remained mainstream for a period. After SARS in 2003, many scholars studied the transmission mechanism of respiratory infectious diseases. Morawska et al. studied indoor droplet diffusion law and personnel exposure assessment methods[33]. Qian & Li, and Li et al. studied the influence of indoor ventilation on the transmission mechanism of respiratory infectious diseases[34,35]. Melikov studied measures to reduce indoor risks, such as personalized ventilation and air purification[36]. Xie et al. and Wei & Li studied exhaled droplets' particle size distribution and transmission distance[37,38]. Liu et al. proposed the mechanism of short-distance airborne transmission of respiratory infectious diseases[39]. But until COVID-19, there was still controversy about the airborne transmission mechanism of respiratory infectious diseases, and only a few infectious diseases that caused cross-room infections were generally recognized as having airborne characteristics[40].
Since 2019, interdisciplinary studies under the COVID-19 pandemic have shown that airborne transmission is likely the main transmission route of respiratory infectious diseases[41−43]. Many scholars have conducted studies on the airborne transmission of respiratory infectious diseases based on actual cases[44,45]. Li summarized the main transmission routes of COVID-19 and proposed that short-range airborne transmission is the leading cause of cross-infection. Long-distance airborne transmission continues with short-range airborne transmission[46]. At present, scholars and official organizations such as the WHO have widely recognized the airborne transmission route of respiratory infectious diseases[47]. Airborne transmission, also known as aerosol transmission, refers to 'the spread of an infectious agent caused by the dissemination of droplet nuclei (aerosols) that remain infectious when suspended in air over long distances and time'[48]. Evidence of airborne transmission has been found for respiratory infectious diseases such as measles, influenza, and COVID-19. Richard et al. verified that SARS-CoV-2 could spread in the air through animal experiments[49]. Milton et al.[50] and Alsved et al.[51] detected viral RNA in the exhaled aerosol of influenza and COVID-19 patients through experimental sampling and found that aerosols smaller than 5 μm contained a high amount of virus. In addition, viruses have also been detected in the air of COVID-19 patients' wards or living environments[52].
Due to the similarity of transmission processes, airborne transmission is often confused with droplet transmission. The existing studies mainly distinguish them based on the difference in particle size, with large particles usually classified as 'droplets' and small particles as 'aerosols'. 'Droplets' are usually mainly affected by gravity and have a short diffusion distance, while 'aerosols' may be suspended for a long time, leading to long-distance transmission[53]. However, the demarcation of particle sizes remains a subject of controversy. The academic community has debated whether particles would settle to the ground within 1 to 2 m after being exhaled, and a commonly used threshold for distinguishing between droplet transmission and aerosol transmission was set at 5 μm. With further research, scholars found that this discrimination method may not be accurate: not only aerosols less than 5 μm have the possibility of long-distance airborne transmission, but also some droplets with medium particle size of 5~100 μm might also suspend in the air for a long time and be inhaled by susceptible people, leading to cross infection[54]. Considering that some droplets will evaporate into droplet nuclei before settling, and the final particle size is like that of aerosol, the actual particle size of droplet and aerosol is estimated to be 60~100 μm. It is generally believed that aerosols smaller than 30 μm can be suspended in the air for more than 1 min. Droplets between 30 and 100 μm are inhaled only near the patient and deposited only in the upper respiratory tract, and droplets larger than 100 μm are usually not inhaled directly. From an aerodynamic point of view, the probability of tiny aerosols entering the lungs after being inhaled is more significant, which may lead to infection of the alveolar tissue of the lower respiratory tract. Chen et al. showed that because the areas where droplets deposited and could infect susceptible persons (eye membranes, mouth, nose) accounted for only about 1.15% of the frontal area of the head[55], it is likely that the airborne route of small-size aerosols dominated the risk of infection even during close exposure[55]. The amount of virus in the exhaled aerosol of influenza patients was about 8.8-fold higher than that in large droplets[56], and similar findings have been observed in COVID-19 patients: more than 90% of the exhaled viral RNA of patients was present in aerosols, suggesting the role of viral aerosols in COVID-19 transmission. These studies suggest that airborne transmission is essential in transmitting respiratory infectious diseases.
Many factors affect the airborne transmission of infectious substances, such as respiratory airflow velocity, ventilation, walking, and other human activities, among which the key is indoor airflow[57]. Studies have shown that infections of respiratory infectious diseases mainly occur in indoor scenes with poor ventilation[58]. Temperature stratification in the displacement ventilation environment may lead to the concentration of infectious substances exhaled by patients at the height of the respiratory zone, increasing the risk of susceptible persons[59]. In general, airflow significantly impacted the spread range of infectious substances. When there is no wind, the concentration of virus droplets exhaled by patients in a static scene will decrease rapidly within 1~2 m[39]. However, some droplets can spread more than 6 m with wind[60]. In addition, the airflow velocity of infectious substances exhaled by patients can also affect the spread distance of infectious substances. For example, droplets released by sneeze may spread more than 8 m due to high initial velocity (> 15 m/s)[61]. Ai & Melikov studied the effects of factors such as air exchange rate, relative position, distance, and indoor airflow on individual transient exposure in the near-exposure scenario and found that individual exposure changes significantly in short-term interaction events, indicating that the research conclusions based on the steady-state scenario may not apply to the non-steady-state scenario[62].
Scholars have clarified the basic rules of airborne transmission of respiratory infectious diseases through experimental and numerical simulation studies. These studies provide a reference for understanding the transmission process of respiratory infectious diseases and designing prevention and control measures. From the perspective of the research trend, the early studies were mainly in steady-state scenarios. With the deepening of research, the studies that considered the transient changes of respiratory airflow were gradually enriched. The research on the transmission law of infectious substances in the dynamic scenario with human movement also attracts more attention.
-
To assess the spread and prevalence of infectious diseases, an essential basic work is to assess the infection risk of susceptible individuals in typical areas. For individual respiratory exposure and infection risk assessment methods of respiratory infectious diseases, scholars have carried out a series of studies on typical indoor scenarios such as classrooms[63], hospitals[64], conference rooms[65], and aircraft cabins[66]. Many methods have been established to analyze the susceptible individuals' exposure and risk of respiratory infectious diseases[67−70], including the Wells-Riley equation, Dose-Response model, Monte Carlo model, CFD-Eulerian method, CFD-Lagrangian method, and Experimental methods. These methods have been used to study human exposure and infection risk due to airborne transmission. It should be noted that the Wells-Riley equation and Dose-Response model are commonly used to assess the average infection risk. Only in combination with the Euler or Lagrangian methods can these two methods obtain the spatial distribution of pathogen concentration and infection risk[71].
There are differences in the specific principles of these models. The most critical parameters assess susceptible individuals' inhalation or exposure dose of infectious substances. Taking the Wells-Riley model as an example, Wells first proposed the 'quantum of infection' concept in 1955 to calculate the risk of airborne infection. The 'infective quantum' is the dose that directs infection to a susceptible individual and can be one or more pathogens that can attach to the droplet. In 1978, based on the 'infection quantum' concept, Riley et al. proposed and developed the Wells-Riley equation to predict the risk of airborne infection[72]. In the follow-up studies, scholars made various modifications considering the effects of filtration, particle sedimentation, respiration, non-steady state conditions, etc. However, for a long period, most studies using the Wells-Riley model are confined to a single enclosed space and assume that infectious substances are evenly distributed in space, which is different from the real situation. Qian et al. established a mathematical model to predict the spatial distribution of airborne infection risk of respiratory infectious diseases by combining the Wells-Riley model and CFD method, considering the inhomogeneity of spatial distribution of infectious substances, which can be used for indoor regional risk assessment. A large outbreak in Hong Kong during the SARS epidemic was used to verify the model's validity[73]. Guo et al. introduced the spatial flow impact factor (SFIF) on this basis. They combined with the Wells-Riley model to propose a method to obtain the spatial distribution of infection probability, which can be used to guide the optimization of the indoor layout of personnel and purifiers[74]. Zhang & Lin proposed a dilution-based infection risk assessment method. The applicable scope of the Wells-Riley model is further expanded[75]. In general, the risk assessment of airborne transmission of respiratory infectious diseases using the Wells-Riley model has the advantages of being simple and efficient. However, due to its assumption based on steady state, it needs to be modified in the risk assessment study of dynamic scenarios combined with the non-steady state calculation results to achieve a more accurate quantitative assessment of regional risk and individual exposure risk. In related studies, quantifying the proportion of inhaled air of susceptible individuals to the exhaled breath of patients combined with the Wells-Riley model to assess susceptible individuals' infection risk quickly has shown good application prospects[76−78].
Based on the transmission mechanism and exposure assessment study, scholars preliminarily defined the range of high-risk areas in typical environments. They proposed relevant control strategies, such as ventilation, disinfection, and maintaining a safe distance. They also have explored the impact of protective measures. To control the source, the influence of protective measures such as masks on the transmission range and safe distance of infectious substances was discussed[79,80]. Personalized ventilation, air curtains, and other means have shown good application prospects in terms of indoor ventilation and airflow control. Regarding reducing the activity of pathogens, scholars have carried out studies on ultraviolet, photocatalysis, high-efficiency filters, and other means. Yao et al. found that ozone and high temperatures may reduce viruses' activity in studying environmental factors[81]. However, most existing studies on the airborne transmission mechanism of respiratory infectious diseases are based on static scenarios, and the relevant conclusions (e.g., safe social distance) ignored the influence of human movement in dynamic scenarios[82,83].
-
Human activities can change indoor airflow and affect the infectious substances diffusion[84]. Since the 1990s, scholars have conducted studies on the effects of human behavior on flow field and pollutant diffusion. The involved behaviors include local movement (e.g., hand movement) and whole-body movement (e.g., walking), and opening the door or window, sitting down, and standing up. With the frequent occurrence of respiratory infectious diseases, studies on the influence of human movement on infectious substances and risk attract scholars' attention. Zhang et al. studied the impact of three passenger behaviors in cabins on air quality and human infection risk, showing that human behavior greatly impacted the flow field and pollutant distribution in the surrounding microenvironment[85]. Personnel walking induced airflow in the operating room would increase the concentration of bacteria on the operating table, leading to an increased risk of postoperative infection[86]. Recently, Liu et al. studied the interactions of exhaled buoyant jet flow and human motion-induced airflow in a water tank. An apparent stagnant layer in the wake region and might lead to long-time suspension for patient-exhaled droplets[87].
For experiments, it is common to simulate human movement using rail cars and manikins. The development in mechanical technology, material science, ergonomics, and full-scale thermal manikin have progressed in the past few decades. They have been widely used in the field of human safety, human thermal injury, and the transmission mechanism of respiratory infectious diseases[88−90]. Han et al. measured the transient airflow around various parts of a manikin when it moved and its limbs swung, revealing that the flow field disturbance by body motion is much more significant than that of limb swing alone[88]. Therefore, many studies adopted the translational model to simplify human walking behavior. Kalliomäki et al. used a rail car with a full-scale manikin to study the behavior of opening doors and moving across rooms, indicating that human movement significantly promotes the diffusion of pollutants[91]. Halvoňová et al. studied the effect of human movement on other people's exposure, indicating that the interactive distance between the moving person and the stationary person may be the key parameter[92]. Bhattacharya et al. studied the induced airflow on both sides of real people walking at 1 m/s and found that the unilateral lateral influence range was about 0.6 m[93]. Based on the similarity criterion, some conducted experimental research on small-scale models. Poussou et al. studied the diffusion characteristics of pollutants with a small cabin model with a 1/10 scale in the water tank, indicating that human movement would carry pollutants to the passing area[94]. Tao et al. developed a smoke visualization method to show the wake phenomenon around small moving objects[95]. Using a 1/5 scale model, they found that human form significantly impacted the wake. Luo et al. used a 1/8 scaled manikin to study the characteristics of wake and found that there was obvious convection in the vertical direction around human movement[96]. Although the small-scale experiment has the advantages of saving space and cost, the effect is not ideal[97]. Therefore, full-scale experiments are needed to accurately study the real scale human movement airflow, especially the transient airflow characteristics of interactive human movement and its influence on the transmission mechanism of infectious diseases. The moving object's shape should be as close as possible to real humans. The commonly used methods for measuring motion-induced airflow include hot ball/hot wire anemometer, ultrasonic anemometer, laser Doppler anemometer, smoke visualization, particle image velocity measurement (PIV).
Over the past decades, the effects of human movement on airflow have been studied in detail. However, many studies did not consider the interaction between human movement and other people's respiratory airflow. In fact, interactive movement often occurs in the office, factory, train, aircraft cabin, and other indoor scenes. In this process, the airflow changes obviously and rapidly. Especially in the interactive movement between patients and susceptible persons, the interactive airflow may promote the long-distance transmission of exhaled infectious substances. Recently, Wu et al. conducted a series study on the transient airflow characteristics of this process and its influence on the transmission mechanism of respiratory infectious diseases[98,99]. The airflow characteristics of interactive human movement and its influence on respiratory infectious diseases' transmission were revealed. The mechanism and differences between interactive human movement's transient and continuous effects on individual respiratory exposure were quantitatively revealed. The 'enhancement effect' that interactive human movement leads to expanding the high-risk area of respiratory infectious diseases was proposed and verified[99] (Fig. 2).
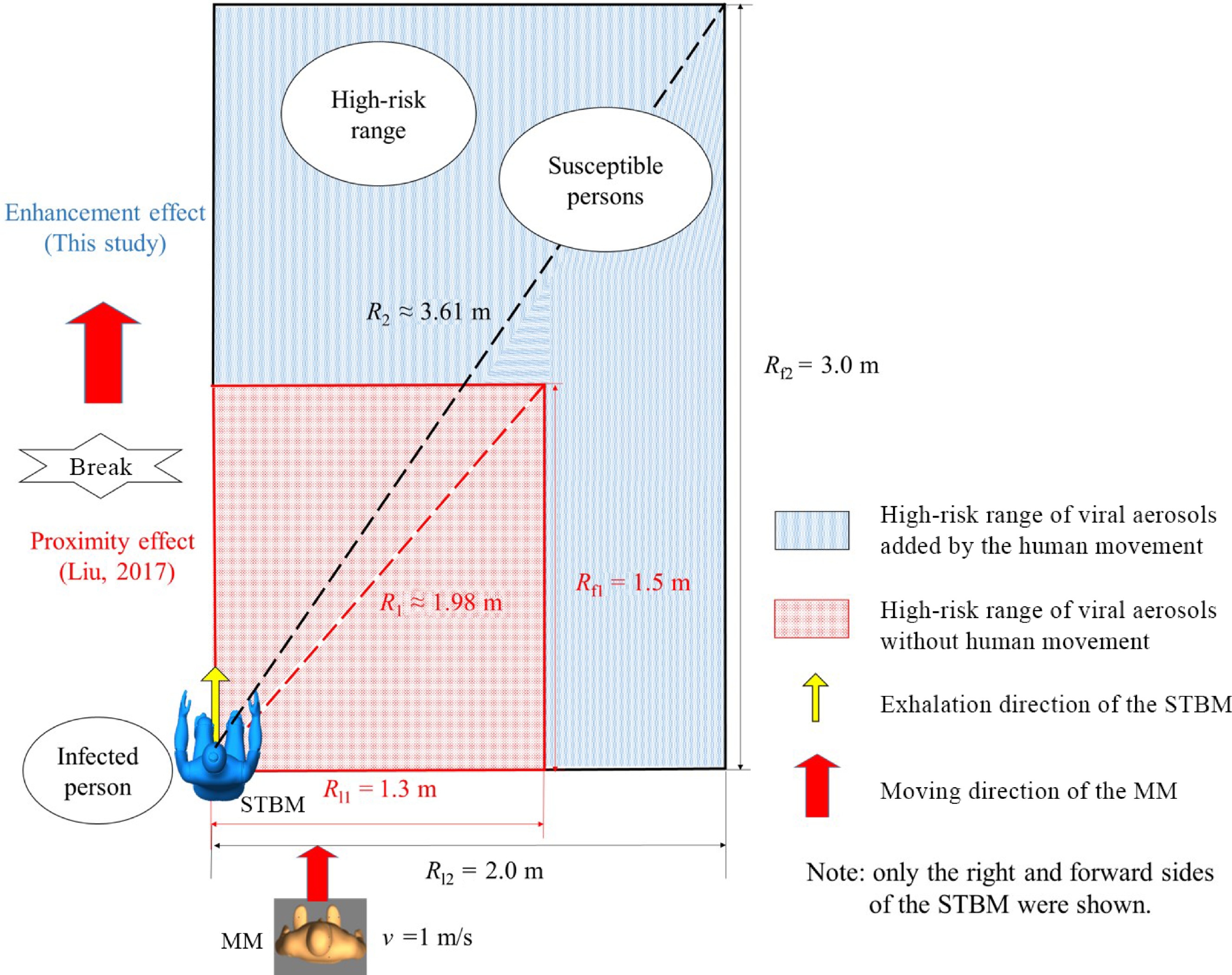
Figure 2.
The 'enhancement effect' of human movement on the high-risk range[99].
Due to limited experimental sites and expensive measuring instruments, studying all scenarios and variables is tricky. For invasive measurement methods, the presence of instruments will also interfere with the airflow field and pollutant diffusion process, affecting the accuracy of measurement results. Therefore, the CFD method was introduced into the study of indoor airflow in the 1970s[100] and was widely used in the following decades, and also used to study the transmission law of respiratory infectious diseases and individual respiratory exposure risk assessment[101−103]. With the improvement of computers, the CFD method has been used to study the impact of human movement on the airflow field and the respiratory infectious diseases spread. Compared with experiments, the CFD method can study the influence of parameters in detail. It has the advantages of being low cost, strong repeatability, and has rich visual information[104], which can provide reference for individual exposure and regional risk assessment. In CFD methods, numerical and turbulence models are the key factors affecting the accuracy of calculation[105,106].
To reduce computational costs, numerical models are often simplified. Early studies used simplified cylinder or prism to represent humans[107,108]. However, simplifying the human model (e.g., ignoring the gap features) will affect the results. Therefore, with the development of laser 3-D and CT scanning technologies, numerical human body models with real human shape for CFD research has gradually become the mainstream[86,96,109]. It can better simulate and restore the influence of real human movement. The induced airflow caused by moving objects increases with movement intensity[110,111], and the peak wake velocity can reach about 1.8 times the movement velocity. Han et al. quantitatively studied the influence of personnel movement in the cabin on droplet propagation and regional deposition distribution[112], indicating that personnel exercising in the cabin usually face a higher infection risk. Choi & Edwards studied the behavior of five people walking through a door in turn[113]. Luo et al. found that people's movement will promote the diffusion and mixing of indoor pollutants at different heights, and increase the individual's respiratory exposure[114]. Researchers have different views on the impact of human movement on indoor particle concentration and diffusion. Wang & Chow believe that walking may reduce the suspended droplets[115]. In contrast, Cao et al. showed that higher walking speed may prolong suspended aerosol concentration decay time and increase the respiratory exposure risk[116]. These differences may be related to human movement patterns, scene layout, and ventilation differences. So, building a numerical model closer to the real human body shape is necessary. Hence, there may be significant spatial differences in the impact of human movement on the diffusion process of pollutants, and the impact on specific areas needs to be further studied.
Selecting a suitable turbulence model is crucial to the accuracy of numerical simulations. Reynolds mean (RANS) methods such as renormalization group RNG k-ε turbulence model, and large eddy simulation (LES) methods are widely used. Poussou et al. found that the RANS methods can simulate wake changes caused by human motion[94] and has high computational efficiency. Therefore, although the method is less effective than the LES method in restoring the details of transient turbulence[108], the RANS method is still widely used in the numerical simulation of human movement. Scholars designed specific scenarios and provided references for selecting turbulence models by comparing experimental and numerical simulation results. Chen verified the effectiveness of calculating indoor airflow by turbulence model through experimental results[117]. Blocken analyzed the RANS and LES methods in simulating indoor airflow and pollutant transmission[118]. Luo et al. studied a moving manikin's wake by PIV experiment and numerical simulations, found that the LES method could better restore experimental results[119]. Overall, the LES method performs well in transient simulation and restoring turbulence details, while the RNG k-ε turbulence model has a good balance of comprehensive performance. The choice depends on the actual scenario and the calculation needs.
Due to the COVID-19 pandemic, the effects of human movement on the transmission of respiratory infectious substances have received more attention. Li et al. simulated the diffusion of droplets from coughing in walking patients. The droplets tended to be distributed below the waist of patients, meaning that children may have a higher risk of infection than adults[120]. Chen et al. studied the impact of head movement on the transient exposure risk of people in close dialogue scenes. They found that when the heads of patients remained motionless, the exposure level of susceptible people was relatively high[121]. Furthermore, some scholars conducted simulations on multi-person interactive motion. Zhao et al. studied the impact of multi-person, side-by-side directional walking in a terminal on airflow and pollutant diffusion[122]. Liu et al. simulated the impact of people taking a van passenger escalator on the diffusion of exhaled droplets of patients. Infectious substances significantly followed the wake[123], and results also suggested that the multi-person interactive movement may promote long-distance airborne transmission[124] (Fig. 3).
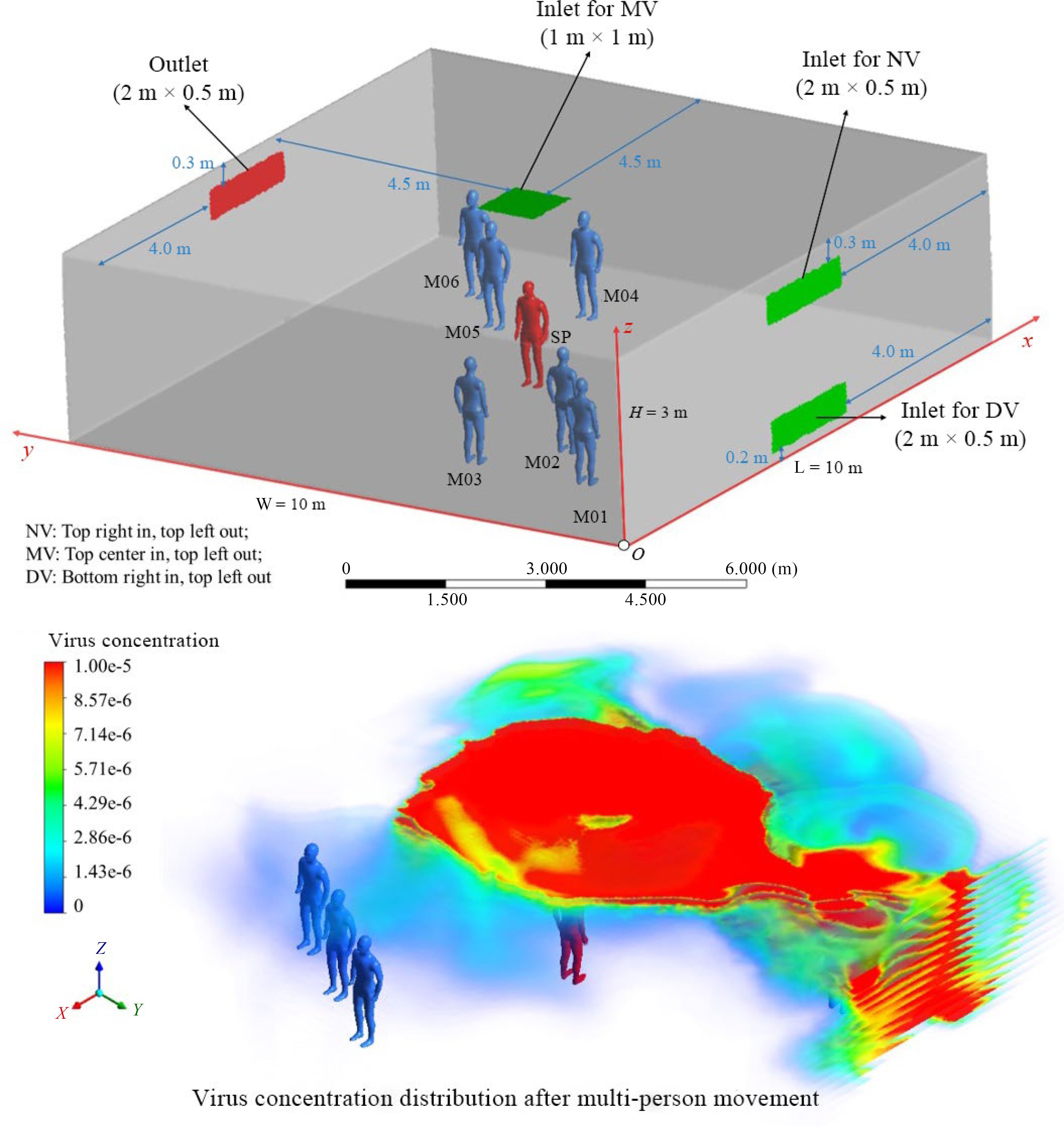
Figure 3.
Multi-person movement's effects on airborne transmission[124].
Overall, human movement will promote indoor air mixing, enhance the diffusion of droplets, and might reduce particle removal speed. The whole-body movement's influence is the most significant. It is necessary to consider the influence of human movement on airflow and infectious material diffusion for epidemic control.
-
Facemasks can slow or stop the transmission process of respiratory infectious diseases, and they are classified into source control and respiratory protection[125]. Source control is placing facemasks on the infection source to reduce emission, and respiratory protection is placing facemasks on the susceptible individual, i.e., the receiver. Source control modifies exhalation flow and respiratory protection modifies inhalation flow. Figure 4 shows the facemask protection model.
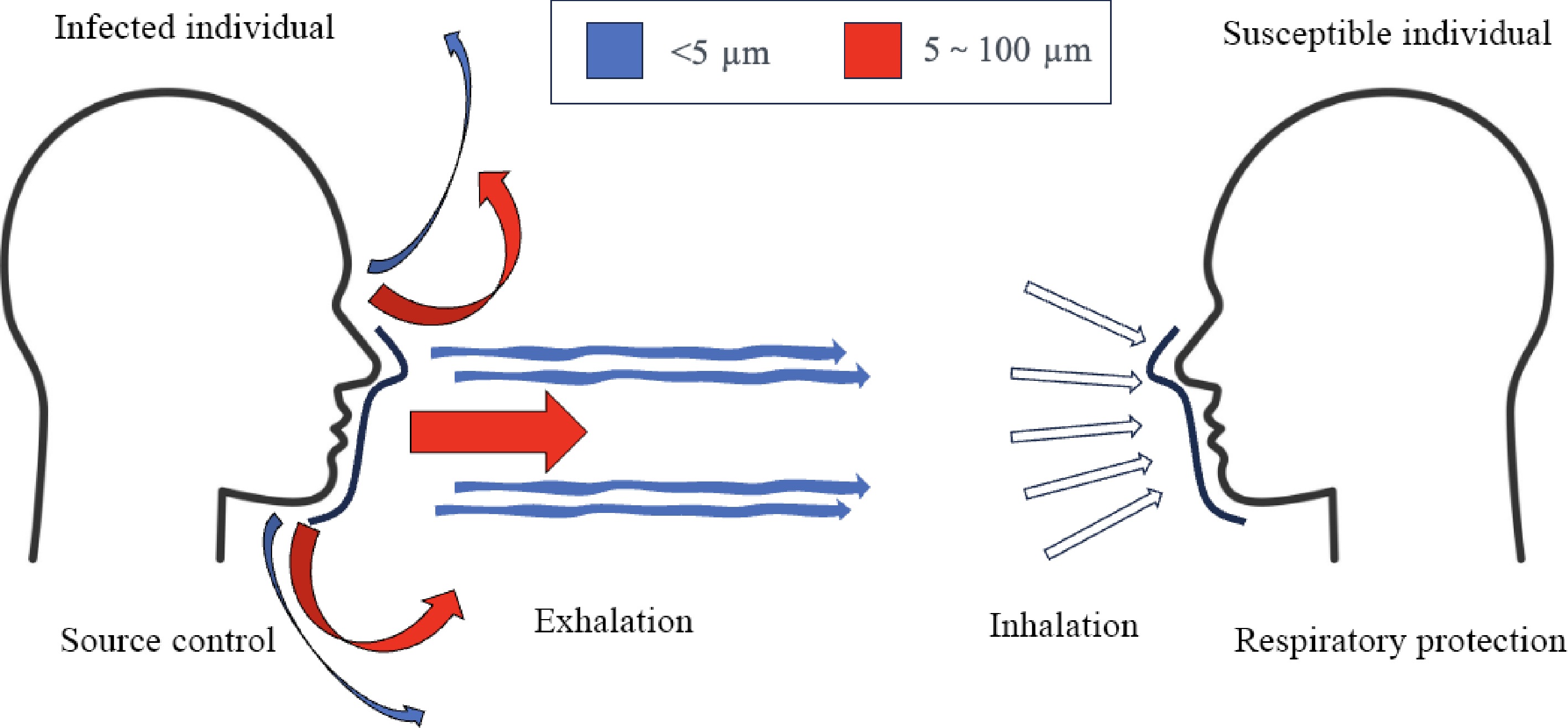
Figure 4.
Model of facemask protection. Source control aims to reduce virus-laden droplets exhaled from infected individuals, and respiratory protection aims to protect susceptible individual from inhaling droplets emitted by infection sources.
Effect of facemasks on source control
-
Scholars quantitatively studied the effect of wearing masks on significantly reducing the exhaled droplets number and limiting the diffusion range of aerosols[126,127]. Chu et al.[128] carried out a systematic analysis on countermeasures of COVID-19 including physical distancing, facemasks and eye protection. The result shows that facemasks could reduce infection risk significantly, while N95 facemasks provide better protection than surgical masks. However, Bartoszko et al.[129] compared surgical and N95 masks and found that both masks offer similar protection against viral respiratory infectious diseases. Adjodah et al.[130] found a statistical association between mask mandates and decreased newly infected cases, deaths, and hospital admissions. Meta-analysis conducted by Liang et al.[131] shows similar results that facemasks had a protective effect against viruses.
Based on an analysis of facemask protection mechanism, Schmitt & Wang[125] identified three types of facemasks: high-performance respirators, surgical/medical and community masks. High-performance respirators are designed to offer both source control and respiratory protection. Surgical/medical masks and community masks can be used for source control, but are inadequate for respiratory protection due to looser fit and lower protection efficiencies. High performance respirators including N95, KN95, and FFP2 respirators have played an important role against SARS-CoV-2 Omicron variant. Baker et al.[132] reported that instituting of N95 respirators and daily testing rapidly abated infection clusters in hospitals. KN95, N95, and FFP2 are equivalent in protection effectiveness. KN95 follow the standard GB2626-2019 issued by China in December 31, 2019. There are also facemasks like FFP2 and N95 that follow different standards offer protection which is equivalent to that of KN95. FFP2 (filtering face piece 2) follow the European standard EN 149-2001+A1: 2009, and N95 follow American standard NIOSH 42 CFR Part 842019. All three kinds of facemasks are designed for respiratory protection, Details of these regulations are shown in Table 1.
Table 1. High-performance respirator regulations.
Region Regulation Name Particle Filtration
Effciency (PFE, %)FE test flowrate Particle diameter (µm) China GB2626-2019 KN90 ≥ 90 30~100 L/min, continuous flow 0.075 ± 0.020 KN95 ≥ 95 KN100 ≥ 99.97 Europe EN 149-2001+A1: 2009 FFP1 ≥ 80 95 L/min, continuous flow 0.02~2 FFP2 ≥ 94 FFP3 ≥ 99 America NIOSH 42 CFR Part 84-2019 N95 ≥ 95 20.0~65.0 L/min, breathing 0.075 ± 0.020 Source control (exhalation protection) offered by facemasks is expected to change dynamics of exhaled air jets, and thus reduce expulsion of aerosols of respiratory fluids into the environment. Lindsley et al.[133] assessed source control performance of cloth masks (community masks), medical masks and high performance respirators, and found that filtration efficiency ranged from 17% to 71% for coughing and 35% to 66% for exhalation. Filtration efficiency is affected by multiple factors such as aerosol particle size, respiratory flowrate, and mask-face fit factors. Ni et al.[134] defined outward fitted filtration efficiency (oFFE) that takes particle diameter, respiratory flowrate and mask fit factor into consideration in order to represent realistic source control effectiveness. Qualitative visualizations have been conducted on breathing, speaking and laughing using a Schlieren imaging setup[135,136], Bourrianne et al.[137] used infrared imaging and particle image velocimetry to visualize exhalation flow with and without facemasks. Experiments showed that facemasks confined exhaled flows within tens of centimeters in front of a person, and turned jetlike exhaled flows into quasivertical buoyancy-driven flows.
Respiratory protection (inhalation protection) changes dynamics of inhaled air flows and stop virus-laden droplets from entering the respiratory tract. van der Sande et al.[138] investigated respiratory protection efficiency of multiple facemasks by short and long term inward protection experiment. Results showed that reductions in viral exposure and risk of infection were seen in experiments with all types of masks, and high performance respirators provided most protection despite imperfect fit (Fig. 5) . Hu[139] reviewed advances in in vivo sampling facemask devices. Filtration residuals collected by facemasks can be used for investigation of inhaled and exhaled pathogens, thus contributing to personal viral exposure and regional infection risk assessment.
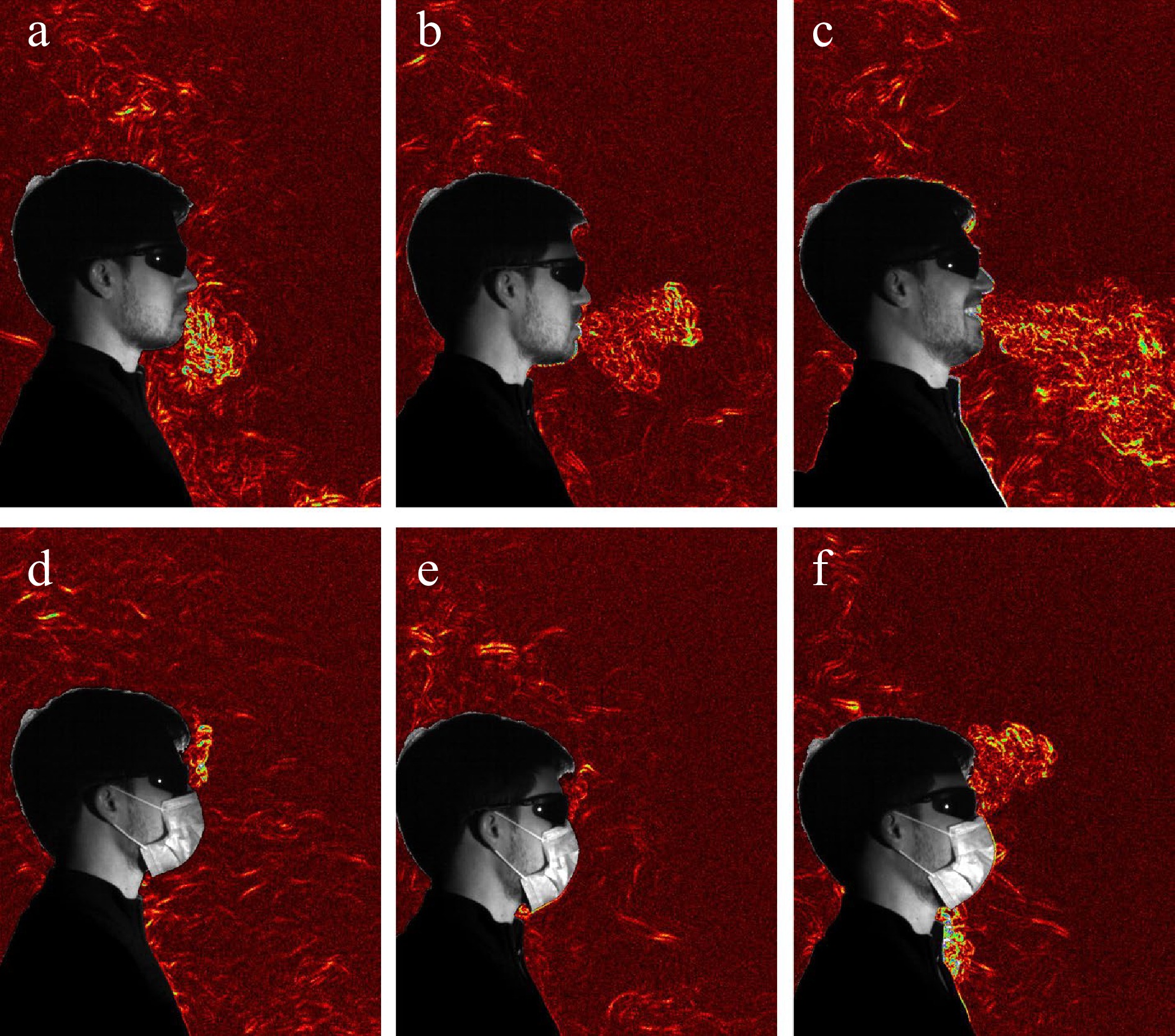
Figure 5.
Synthetic schlieren images of exhaled flows changes with and without a facemasks under different scenarios: (a), (d) breathing quietly; (b), (e) saying 'also'; (c), (f) laughing[136].
Infection risk assessment method considering facemask application
-
Application of facemasks can alter personal and regional infection risk. On the macroscopic level, epidemiology methods are applied to determine effectiveness of facemasks from real world epidemic data, while simulation methods are used to understand facemask-altered epidemic dynamics, and forecast epidemic spread. Zeng et al. performed an epidemiology investigation on effect of facemasks on COVID-19. Results showed that facemasks showed statistical correlation to reduction of daily infection cases. Cheng et al.[140] performed epidemiological analysis on confirmed cases of COVID-19, and compared masking populations with non-mask-wearing populations. They found that COVID-19 infection case number was significantly lower in the masked regions. Mniszewski et al.[141] developed epidemic simulation model of large social networks, and experimented impact of facemask usage on the spread of epidemic. Results showed that facemasks alone have limited effect on epidemic spread, but could become more effective when combined with other interventions such as hand sanitizer. The SEIR model is the most used model for epidemic dynamics simulation. Maged et al.[142] found that usage of facemasks reduced reproduction number of SARS-CoV-2 by 49%, when 60% of 1 million simulation population wore facemasks. Kai et al.[143] used stochastic dynamic network based compartmental SEIR model and agent-based Monte Carlo simulation to investigate effect of facemask usage and latency of mask mandates. A modified SEIAQR model was adopted by Yadav & Singh[144] for analysis of efficacy of vaccine and facemasks.
On the microscopic level, effectiveness and regional risk assessment are carried out by experimental and simulation methods. Experimental methods include techniques such as light scattering[145,146], thermal imaging[147] and particle tracing[148]. These methods qualitatively determine infection risk by visualizing respiratory flow, and quantitively assess infection risk by measuring properties of respiratory flow field. Simulation methods mainly refers to CFD simulations. CFD has been used to quantify the infection risk of inhalation[127]. Properties of respiratory flow and infection risk can be determined using Eulerian-Lagrangian multiphase model. The transmission process including viral droplet evaporation, breakup and turbulent dispersion can be well simulated[149]. Another CFD simulation focused on variance of protection of facemasks with respiratory particles diameters[150]. Smaller particles (diameters smaller than 10 µm) tended to escape through leaking flow, while most larger particles were caught by facemasks. Besides CFD models, other methods were adopted. Ni et al.[134] proposed a reduced order model, using electric circuit to represent respiratory flow, so that fraction and distribution of leaking flow can be determined. Liu et al.[151] simulated close contact behavior on a subway system, and found that virus exposure could be reduced by 82% if all passengers wore surgical masks.
Overall, scholars care about the effectiveness of facemasks in curtailing the transmission of respiratory infectious diseases, with a particular emphasis on source control. Facemasks serve a dual function: source control and respiratory protection. High-performance respirators, including N95, KN95, and FFP2 masks, are pivotal in providing both source control and respiratory protection. They have proven instrumental in mitigating infections. Source control involves placing facemasks on infected individuals to reduce the emission of virus-laden droplets. Filtration efficiency, aerosol particle size, respiratory flow rate, and mask fit are influential factors. Visualizations and experiments demonstrate how facemasks alter exhaled airflows, confining them near the wearer. Respiratory protection, on the other hand, prevents the inhalation of virus-laden droplets. High-performance respirators have been found to offer effective protection, even with less than perfect fits. A range of methods, including CFD simulations and experimental techniques, have been employed to assess facemask efficacy at the microscopic level. Epidemiological studies confirm the substantial positive effects of facemasks when used in conjunction with other interventions.
-
Public health emergencies are more frequent and have become a massive threat to public safety nowdays. Although the WHO declared the end of the global health emergency for COVID-19, there remains a possibility of large-scale respiratory epidemics in the future. The COVID-19 pandemic shows that many infections occur during interactive movement between susceptible and patients. Therefore, further elucidating the transmission mechanism of respiratory infectious diseases, especially in dynamic scenarios, is significant for guiding the formulation of epidemic control measures. Further research can be carried out in the following aspects.
Patients' pathological characteristics and personnel protection characteristics
-
The difference in respiratory infectious substances in patients' respiratory tract replication sites significantly impacted exhalation characteristics and indoor individual exposure. For different types of respiratory infectious diseases, it is necessary to combine the pathological characteristics of patients, consider various respiratory activities (such as sneezing and coughing), and comprehensively consider factors such as lung environmental humidity, aerosol condensation, respiratory rate, and phase. Wearing facemasks in indoor spaces with many people has become the norm. The impact of facemasks on the protective effect, such as the filtration efficiency of pathogens and the gaps generated during the breathing process, deserves further study to improve the accuracy of risk assessment and guide the scientific setting of prevention and control measures.
Multi-pose human database for personalized and refined evaluation
-
The body size difference of personnel will significantly affect the airflow characteristics of interactive motion, and most existing studies have chosen medium body size manikins to carry out research. Volunteers with different body characteristics should be recruited. The personalized, multi-pose numerical human model database should be entirely constructed in combination with CT imaging and laser 3D scanning technologies, and quantitative research should be carried out in-depth using the human movement experiment platform and numerical simulation methods to provide more comprehensive information and rules of human movement airflow. A refined risk assessment model should be established, considering factors such as individual immunity differences and vaccination status.
Interdisciplinary cooperation and consider people's activity patterns' effects
-
The types of indoor human activities are diverse, the multi-person movement scene flow field is complex, and the risk of infectious disease transmission is high. In the future, environments with high human activity, such as railway stations, transportation hubs, industrial production workshops, and other densely populated areas, should leverage video data, image recognition, and other tools to analyze typical behavior patterns. Additionally, combining these findings with CFD methods can help study airflow characteristics and explore effective intervention methods. The transmission mechanism of respiratory infectious diseases is multidisciplinary and complex, involving virology, respiratory physiology, aerosol dynamics, fluid mechanics, building ventilation systems, human behavior, risk assessment, and other fields involving experimental, numerical simulation, theoretical analysis, and other technical means. In the next step, we should continue to promote interdisciplinary cooperation research to deeply explore the influence of personnel interaction on the transmission mechanism of infectious diseases. Quantifying individual exposure and regional risk changes in dynamic scenarios provides a scientific basis for formulating prevention and control strategies for respiratory infectious diseases. On this basis, to explore efficient infectious disease prevention and control measures to protect public safety better and promote sustainable economic and social development.
-
Respiratory infectious diseases could trigger public health emergencies. In the past decades, scholars have carried out many studies on the transmission mechanism of respiratory infectious diseases and have clarified the characteristics of infection sources, airborne transmission mechanisms, and exposure risk assessment methods for susceptible populations. The effects of human movement on the airborne transmission mechanism were summarized. The fundamental issues are the airflow characteristics induced by human movement, the influence of human movement on the indoor flow field and pollutant concentration changes, and established experimental and CFD methods suitable for studying unsteady airflow. A regional dynamic risk assessment method was established. These research results can be applied to guide indoor ventilation design and infectious disease control measures. These findings provide scientific reference for the prevention strategies of respiratory infectious diseases.
The research has shown the following trends. First, more research focuses on the non-steady state and local interactive environment. During the current time scale, the steady-state research has changed to more complex non-steady-state and transient research, and more attention has been paid to the accurate assessment of key elements such as airflow characteristics, particle concentration, and individual respiratory exposure in transient processes like human movement in the environment. At the spatial scale, it has changed from focusing on the characteristics of the whole-space airflow field to the microenvironment airflow characteristics around the human body (e.g., the breathing zone) and the local space of human interaction. Second, quantitative research tends to be personalized and refined. With CT, laser scanning, and computer performance development, constructing numerical human models with real human shapes and respiratory boundaries has become a common research method with CFD simulations. The assessment of individual respiratory exposure and infection risk has also changed from the overall regional risk assessment to the precise quantitative assessment of individual exposure and risk. Third, facemasks play a vital role in mitigating the transmission of respiratory infectious diseases. High-performance respirators, with their dual source control and respiratory protection mechanisms, are crucial in reducing disease spread. The research findings contribute to understanding facemask dynamics, offering valuable insights for public health measures and interventions.
-
The authors confirm contribution to the paper as follows: study conception and design: Wu J, He F, Xie Z, Pan Y, Weng W; data collection: Wu J, He F, Fu M, Li Y, Wang J; analysis and interpretation of results: Wu J, He F, Pan Y, Weng W; draft manuscript preparation: Wu J, He F, Fu M, Pan Y, Weng W. All authors reviewed the results and approved the final version of the manuscript.
-
The data that support the findings of this study are available on request from the corresponding author.
This study was supported by National Natural Science Foundation of China (Grant No. 52074163, 72034004), Research Funds of Center for Big Data and Population Health of IHM (Grant No. JKS2022006).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Tech University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wu J, He F, Xie Z, Fu M, Li Y, et al. 2024. Review on respiratory infectious disease transmission mechanism: effects of human movement and facemask use. Emergency Management Science and Technology 4: e004 doi: 10.48130/emst-0024-0006
Review on respiratory infectious disease transmission mechanism: effects of human movement and facemask use
- Received: 18 January 2024
- Revised: 24 February 2024
- Accepted: 04 March 2024
- Published online: 29 March 2024
Abstract: Respiratory infectious diseases can cause public health emergencies, threatening human well-being, social operation, and economic development. Clarifying the transmission mechanism of respiratory infectious diseases is essential for control measures. We review the main research findings on the transmission mechanism of respiratory infectious diseases in recent decades. The source characteristics of respiratory infectious diseases, the airborne transmission mechanism, the exposure of susceptible persons, and the infection risk assessment methods are discussed. Given that the dynamic scenario of respiratory infectious disease transmission has attracted wide attention in recent years, we summarize the effects of human movement on indoor airflow, pathogen diffusion, and human exposure. Considering the everyday use of facemasks, the effects of facemasks on source characteristics and infection risk are also discussed. Finally, future research prospects are proposed. The transmission mechanism of infectious diseases can be comprehensively explored by delving into patients' pathological characteristics and personnel protection measures. This exploration can be facilitated by establishing a multi-pose manikin database, enabling personalized and refined evaluations. Interdisciplinary cooperation will play a pivotal role in fostering a holistic understanding. Furthermore, it is crucial to account for the impact of individuals' activity patterns on disease transmission dynamics. This review is expected to reference public health emergency management.













