-
Milk proteins are the best protein source because of their essential amino acid score that helps in improving the protein digestibility corrected amino acids scores. The high quality protein content of both whey proteins (20%) and caseins (80%) are satisfying the requirements of human amino acid needs in addition to their digestibility and bioavailability[1].
Whey proteins are precious watery nutrients as they contain about half of the milk total solids that remain after the caseins are curdled during cheese making, it is a rich source of lactose, whey proteins, some milk salt and water-soluble vitamins[2]. Whey proteins are well-documented as valuable milk ingredients and are highly nutritious food[3]. Moreover, the nutraceutical properties that are derived from the metabolic hydrolysis of milk, its whey proteins, and their peptides include antimicrobial and antioxidant activities[4]. Additionally, the preventive and curative effects that the protein possess have practical applications in the treatment of anemia, liver complaints, and arthritis[5].
During cheese making, the valuable whey proteins that are lost while separating the whey from the milk[3], could be converted into attractive fermented or non-fermented products for human utilization and consumption[6]. For example, the use of advanced filtration techniques for valorization of whey cheese was found to recover these high value proteins[7,8].
Baobab (Adansonia digitata L.) is a grossly indigenous fruits[9]. Baobab fruit pulp obtained from the different regions of Sudan were found to contain high vitamin C (358.44 mg/100 g), calcium (393.55 mg/100 g) and phosphorus (91 mg/100 g) levels, in addition to their high protein content (5.2%)[10]. Baobab fruit pulp exhibits higher antioxidant properties[11]. Dry Baobab pulp in Sudan, is either eaten fresh, ground to prepare a refreshing drink, added to gruel during its cooling[10] or as flavor to ice cream[12,13].
Roselle (Hibiscus sabdariffa L.) belongs to the family Malvaceae, an annual shrub that is grown in many tropical or subtropical countries including Sudan. Moreover, the red calyces of Roselle are usually used for preparing a flavorful and tart beverage either cold or hot to utilize their rich content of the numerous beneficial bioactive compounds[14]. In Sudan, Roselle is grown extensively in Darfur and Kordofan states under rained conditions[15]. Roselle has antimicrobial antispasmodic and hypotensive effects as well as for uterine muscle relaxation[16]. The phytochemical content of Rosella that are reflected in the health of consumers enables its use in many functional food[17].
Doum palm (Hyphaene thebaica) is a palm tree adapted to the desert and it has edible oval fruit with a potent antioxidants activity due to its high content of polyphenols[18]. The fruits of Doum palm (Hyphaene thebaica) are rich in dietary fibers and carbohydrates and it contains anti-hypertension substances[19]. Moreover, the edible portion of Doum fruits showed good values for dietary fiber and vitamins B1, B3, and B6, besides the total phenolic and flavonoid contents and its antioxidant activity that encourage its use in the formula of some functional foods[20]. Therefore, the objectives of the present study are the utilization of whey proteins produced by the dairy industry to develop whey-based beverages using some of the Sudanese indigenous fruits (Roselle, Baobab, and Doum palm) and test the quality of the obtained products. The gross chemical composition and the levels of vitamin C, calcium and phosphorus were compared within different whey proteins based beverages. Also the microbial content and some sensory attributes were compared for the different produced whey proteins beverages
-
The liquid whey remaining after the processing of Mozzarella cheese were obtained from the Dairy Production Department, Faculty of Animal Production, University of Khartoum, Sudan.
Materials
-
Roselle (Hibiscus sabdariffa L.) powder, Baobab (Adansonia digitata L.) powder, Doum palm (Hyphaene thebaica) powder, sugar and Gum Arabic were bought from the markets of Khartoum, Sudan.
Methods
-
Three liters of whey proteins were obtained after making Mozzarella cheese and sieved before being subjected to heating for 15 min at 75 °C. They were left to cool at a temperature of 10 °C. After that, they were filtrated using a clean cheesecloth and stored at room temperature.
Preparation of indigenous fruit juices
-
Sixty grams of Roselle (Hibiscus sabdariffa L.) powder was socked into 200 mL distilled water for 24 h and then filtrated. Meanwhile, 15 g of each Baobab (Adansonia digitata L.) and Doum (Hyphaene thebaica) powder were separately dissolved in 200 mL of clean distilled water. Then the three prepared juices were added to make different whey proteins beverages.
Preparation of whey protein beverages
-
During this experiment, 10 different whey proteins beverages were developed based on 3 different treatments in addition to the control (Plain whey proteins). The three treatment utilized 30:70%, 50:50%, and 70:30% of the heated liquid whey proteins that were added to the selected fruit juices. After making all the mixtures of the whey proteins and the fruits juices, about 15 g of sugar and 2.5 g of Gum Arabic were added to each of the prepared fruit juices and each was blended separately.
Chemical analysis
-
The chemical composition of whey protein beverages was performed according to the methods of the AOAC[21].
The fat content was extracted by taking 10.94 mL of the whey proteins beverages sample and 10 mL sulfuric acid (density 1.815 gm/mL at 20 °C) into a clean dry Gerber. Then 1 mL of amyl alcohol (density 0.814–0.816 gm/mL at 20 °C) were added and the contents were thoroughly mixed till no white particles could be seen. After the centrifugation of the Gerber tubes (1,100 revolution per min) for 3 min, they were transferred into a water bath at 65 °C for 3 min and then the fat percent was taken directly from the fat column[21].
The total protein content of the whey protein beverages was determined using the Kjeldahl method[21]. In a clean and dry Kjeldahl flask, 10 mL of the whey proteins beverages sample was placed followed by the addition of catalyst powder (Na2SO4 and the equivalent of 0.1 mg Hg). Then 25 mL concentrated sulfuric acid (density 1.86 gm/mL at 20 °C) was added to the flask and the mixture was then digested on a digestion heater until a clear solution was obtained (3 h). The flasks were then removed and left to cool and the digested samples were poured into volumetric flasks (100 mL) and diluted to 100 mL with distilled water. About 5 mL were taken and neutralized using 10 mL of 40% NaOH. The distillate was received into a conical flask containing 25 mL of 2% boric acid and 3 drops of 0.1 of bromocresol green and methyl red) indicator. This distillation was continued until the volume in the flask was 75 mL. The flasks were then removed from the distillates and titrated against HCl (0.1N) until the endpoint was obtained (red color). The protein content was calculated as follows:
$ \rm {Nitrogen\;({\text{%}})=\dfrac{\rm T\,\times\, 0.1\,\times\, 20\,\times\, 0.014}{\rm Weight\;of\;sample}\,\times\, 100} $ $ \rm {Protein\;({\text{%}})=Nitrogen\;({\text{%}})\,\times \,6.38} $ Where, T: itration figure; 0.1: Normality of HCl; 0.014: Atomic weight of nitrogen; 20: Dilation factor.
The content of lactose was determined using the anthrone method[22]. The reagent was added to the clear filtrate from precipitation of a sodium bicarbonate solution of the whey protein beverages with 0·1 n-sulphuric acid. The reagent consists of 0.15% (w/v) anthrone in 70% (v/v) sulphuric acid. Ten mL of the ice-cold anthrone solution was added, with cooling, to 1 mL of the ice-cold solution containing 0–100 µg of lactose. The mixture was heated at 100 °C for 6 min and then cooled for 30 min. The optical density of the colored solution was then measured at 625 nn using spectrophotometer (UV mini 1240), Shimadzo, Japan.
The titratable acidity of whey proteins beverages was determined by taking 10 mL of the sample into a white porcelain dish and then 0.5 mL of phenolphthalein indicator was added. The titration was conducted using 0.1 N NaOH until a faint pink color that lasted for 30 s was obtained. The titration figures were then divided by 10 to get the percentage of the lactic acid[21].
The ash content was determined according to the AOAC method[21] by weighing 5 mL of whey protein beverages sample into a suitable clean dry crucible and evaporating to dryness on a steam bath. The crucibles were placed in a muffle furnace at 550 °C for 1.5–2 h. It was cooled in a desiccator and weighted. The ash content was then calculated as follows:
$ \rm {Ash\;({\text{%}})=\dfrac{\rm W1}{\rm W2}\,\times\, 100} $ Where, W1= Weight of ash; W2= Weight of sample.
The solid's non-fat content where then calculated mathematically by adding the sum of lactose, protein, and ash content.
Vitamin C content of the whey protein beverage samples (in duplicate) was determined by the method developed previously[23] using a UV/VIS Spectrophotometer (UNICAM, 8625).
For the determination of calcium and phosphorus of whey proteins beverages samples, the residual of the ash extract was used. The phosphorus was determined by spectrophotometer (UV mini 1240), Shimadzo, Jaban. The estimation of phosphorous content was conducted using the vanadate-molybdate yellow calorimetric method[24]. Also the the calcium content was performed as was described previously[24]. The calcium content was determined by taking 5 mL of the ash extract solution into a 50 mL conical flask and the volume was completed to 25 mL by distilled water. Then 50 mg of meroxide indicator, 3−5 drops of sodium hydroxide were added, and the mixture was titrated against 0.01 N (EDTA). After calibration, the solution changed from pink to purple.
$\rm Ca + Mg \;mmol/L = (V \times\, N\, \times 1000)/5\; mL$ $ \rm {Ca \;mmol/L=(V\,\times\, N\,\times\, 1000)/5\;mL} $ Where, N: normality of EDTA; V: volume.
Microbiological examination
-
Plate count agar (Biomark, B 298) medium was used to determine the total bacterial count at 32 °C for 48 h. It was obtained in a dehydrated form and each rehydrated liter of the medium was composed of casein enzymic hydrolysate (5.0 g), yeast extract (2.5 g) dextrose (1.0 g), and agar (15.0 g). According to the manufacturers' instructions, 23.5 g were suspended in 1,000 mL distilled water; it was boiled until dissolved completely and sterilized
The M17 broth media (HIMEDIA, M1029-500G) medium was obtained in dehydrated form and it was used for the enumeration of the lactic acid bacterial count after solidifying with the agar. This medium consists of casein enzymic hydrolysis 2.50 g/L, peptic digest of animal tissue 2.50 g/L, peptic digest of soyabean meal 5.00 g/L, yeast extract 2.50 g/L, beef extract 5.00 g/L, ascorbic acid 0.50 g/L, magnesium sulphate 0.25 g/L, lactose 5.00 g/L and disodium-B-glycerophosphate 19.00 mg/L. The final pH was adjusted to 7.1 ± 0.1 at 25 °C. About 42.25 g were suspended in 1,000 mL distilled water. The medium was dissolved completely and 13 g of the agar was added before the sterilization.
The manufacturer' instructions were followed carefully for the preparation of both media, they were sterilized using the autoclave (15-pound pressure for 15 min)[25]. Similarly, the mixer, tips, and distilled water were sterilized using the autoclave (15 min at 121 °C)[25]. However, sterilization of glassware; Petri- dishes, test tubes, pipettes, flasks, and bottles; were done using dry heat (hot oven) at 160 °C for 1 h.
The plate count agar medium was incubated at 32 °C for 48 h[26] and the lactic acid bacterial count was determined at 37 °C anaerobically for 24 h on M17 agar[27]. A colony counter was used to count the different types of colonies and the results were present as cfu/mL.
Sensory evaluation
-
The sensory evaluation was conducted according to a previously described method[28]. All the obtained data were evaluated using the penal test sheets.
Statistical analysis
-
The obtained data were subjected to Statistical Analysis Systems (SAS). A comparison of means was performed using Duncan Multiple Range test (p ≤ 0.05)[29]. A Microsoft Excel sheet was used to plot the figures.
-
The data obtained for the chemical composition of whey proteins beverages (Table 1) showed that the fat content in the different juices used was less than that obtained for the plain whey proteins. However, significantly (p ≤ 0.05) higher fat content was found in the whey enriched with Doum juice (0.93%) compared to other juices. This might be because the fruits of Doum palm have a slightly high content of fat (2.57%)[30]. Moreover, the functional properties of the essential nutrients that are possessed and provided by Doum fruits have an important role in addressing many problems related to food in patients with diabetic and hypertensive conditions[31]. The low values of the fat in the Baobab fruit pulp is the reason for the lowest mean obtained for fat content in whey proteins enriched with Baobab[13].
Table 1. Effect of fruits type on the chemical composition of whey proteins beverages.
Parameters Plain whey proteins Whey enriched with Baobab juice Whey enriched with Roselle juice Whey enriched with Doum juice Fat (%) 1.03a 0.71b 0.36c 0.93a Protein (%) 4.45b 1.41c 5.79a 5.33ab SNF (%) 8.59c 17.98a 15.40b 16.39ab Lactose (%) 4.78d 16.36a 8.30c 9.99b Acidity (%) 0.48b 0.38c 0.30d 0.58a Means with the same superscripts letters in the same column are not significantly different (p > 0.05). Significantly (p ≤ 0.05) higher protein content was obtained in whey proteins enriched with Roselle juice (5.79) followed by that enriched by Doum juice (5.33%) compared to the plain whey proteins (Table 1). Moreover, the proportion of protein content (6.46% and 6.29%) was found to increase with increasing of Doum juice (70% and 50%, respectively) as shown in Table 2 and this might be due to the protein content of Doum palm, which revealed 7.05%[30]. Also, the plain whey proteins revealed 4.45% proteins (Tables 1 & 2). Whey proteins contain highly nutritious food ingredients[3].
Table 2. Comparison of the chemical composition of whey proteins beverages using different type and concentration of fruits.
Parameters Concentrations (%) Fat (%) Protein (%) SNF (%) Lactose (%) Acidity (%) Plain whey proteins − 1.03 4.45 8.59 4.48 0.48 Whey enriched with Baobab juice 30:70 − 0.17 18.48 17.93 0.50 50:50 2.13 0.36 18.00 17.16 0.37 70:30 − 3.69 17.14 13.98 0.28 Whey enriched with Roselle juice 30:70 0.23 5.81 8.31 8.43 0.19 50:50 0.17 5.81 12.31 8.31 0.33 70:30 0.14 5.74 15.38 8.25 0.34 Whey enriched with Doum juice 30:70 0.26 6.46 17.09 9.24 0.28 50:50 0.25 6.29 16.26 8.42 0.17 70:30 0.08 3.24 15.81 8.25 0.10 The solid non-fat content of whey protein beverages revealed significantly (p ≤ 0.05) higher values for the whey protein beverages enriched with Baobab pulp (17.98%), followed by that enriched with Doum juice (16.39%) and Roselle juice (15.40%) compared to the plain whey proteins (8.59%). This could be because the Baobab fruit has a high content of carbohydrates (21.09%)[32]. Moreover, the carbohydrate content of dry Baobab fruit pulp is relatively high[33]. The reason could be because the total solids content of Baobab pulp are high[13].
The significant (p ≤ 0.05) high lactose content (16.36%) was reported for the whey proteins enriched using Baobab juice (Table 1). This could be justified by the fact that the dry fruit pulp of Baobab is a rich source of carbohydrates[33].
The titratable acidity in the whey enriched with Baobab revealed the highest significant (p ≤ 0.05) value (0.58%) followed by that obtained for the plain whey proteins (0.48%). The obtained higher value justified the use of Baobab in the fermentation of some foods. The obtained titratable acidity (0.38%−0.42%) in yogurt was found to comply with the required minimum standard (0.6%) stated for commercial yogurt[34].
Table 3 showed significant (p ≤ 0.05) variations in the values of vitamin C in the different whey protein beverages. The significant (p ≤ 0.05) high content of vitamin C was found for Baobab enriched whey proteins (140.93 mg/100 mL). The obtained higher levels of vitamin C demonstrated the antioxidant properties of the three used fruit juices (Tables 3 & 4). Moreover, vitamin C content was found to increase with the increasing proportion of the Baobab juice extracts (167.22 and 166.39 mg/100 mL for 70% and 50%, respectively). The high vitamin C content of the raw Baobab fruit pulp justified these findings[10,11,33,34]. The value of vitamin C was found to increase with the increasing fortification level of Baobab pulp into yogurt[34]. Moreover, the high natural content of vitamin C in the pulp of Baobab fruit, enables its good antioxidant activity[35]. Vitamin C and A in Baobab fruit pulp were estimated as 236 and 80 mg/100 mL, respectively[36].
Table 3. Effect of fruit types on vitamin C and some minerals contents of whey proteins beverages.
Parameters Whey enriched with Baobab juice Whey enriched with Roselle juice Whey enriched with Doum juice Vitamin C (mg/100 mL) 140.93a 91.28c 129.28b Phosphorus (mg/100 mL) 0.90b 1.29a 0.94b Calcium (mg/100 mL) 1.23b 8.50a 0.93c Means bearing similar superscripts letters in the same column are not significantly different (p > 0.05). Table 4. Effect of fruits type and concentration on the vitamin C and some minerals contents of whey proteins beverages.
Parameters Concentrations
(%)Vitamin C
(mg/
100 mL)Phosphorus
(mg/
100 mL)Calcium
(mg/
100 mL)Whey enriched with Baobab juice 30:70 167.22 0.87 0.70 50:50 166.39 0.59 1.40 70:30 89.19 0.55 1.60 Whey enriched with Roselle juice 30:70 56.24 1.23 2.30 50:50 101.23 1.85 2.30 70:30 116.25 2.11 0.90 Whey enriched with Doum juice 30:70 156.17 0.73 1.30 50:50 139.44 0.98 0.80 70:30 92.22 1.13 0.70 High level of vitamin C in Doum juice extract (129.28 mg/100 g) was also found during this study (Table 3). However, a lower value (31.74 mg/100 g) was reported for Doum fruit nectar samples[37]. This supported the fact that Doum fruit contains potent antioxidants[18]. Moreover, in a different product, it was reported that adding various percentages of powdered Doum fruit resulted in a proportional increase in the content of the total phenol as well as the antioxidant of the low-fat frozen yogurt compared with the control[38]. Also, the fermented milk of camel that was flavored using concentrated extracts of Doum was found to cause a significant increase in the content of phenolic components that showed correlation with the concentrations of the added levels of the juices[39]. On the other hand, the red pigment of Roselle showed a higher antioxidant activity when the fruit is consumed as beverages and the added Roselle extract resulted in more increase of the total phenolic content of yogurt fortified with different levels of Roselle extract and carrot juice[40].
The calcium content that was obtained in the whey proteins enriched with the Roselle juice (8.50 mg/100 mL) revealed significantly (p ≤ 0.05) higher values (Tables 3 & 4). This might be because the Roselle extract is slightly higher in calcium content as was reported previously[41]. Similarly, high calcium content was obtained for the whey proteins enriched with the Baobab fruit juice extract supported the report, which stated that the fruit pulp of dry Baobab has a high content of calcium and vitamin C[33]. The Baobab fruit pulp contains about 295 mg/100 g of calcium[10]. The range of calcium in the Baobab was 4.10−4.30 mg/100 g[36]. The calcium requirements during growth, pregnancy, and lactation are increased[42]. Therefore, yogurt enriched the Baobab as a drink would be beneficial in maintaining the high calcium requirements for pregnant women, lactating mothers, children, and the elderly[34]. On the other hand, powdered Doum fruit contains adequate K, Ca, Na, and Mg[20,31,38]. Utilization of Doum palm (Hyphaene thebaica) powdered fruit showed useful application in food products because of its fiber and mineral content and its potential in health[43].
The content of phosphorus reported for the whey proteins enriched with Roselle juice (1.29 mg/100 mL) showed significant (p ≤ 0.05) high values (Table 3). The phosphorus content was found to increase with increasing the used proportion of Roselle juice (Table 4). This reflected the richness of Roselle juice in its phosphorus content (2.78 mg/100 mL)[44]. However, the Baobab fruit content for phosphorus was in a range of 1.70−1.90 mg/100 g[36]. Among humans, a high content of phosphorus is desirable for increasing bone health[41].
Microbiological findings of whey proteins beverages
-
The microbial analysis of whey beverages (Table 5; Fig. 1) showed a high count for the total viable bacteria in the whey protein before heat treatment (log 5.10 vs 2.58), which matches very well with the objectives of heat treatment as a preservation method. The whey proteins enriched with Doum juice showed the lowest result for the total viable bacteria count (Table 5; Fig. 1). It was reported that Doum nectar did not show any viable bacteria during the storage period at the ambient and refrigeration temperatures[37]. Also, the antimicrobial activity of Hibiscus extracts against many bacteria was reported. Hence the extracts of H. sabdariffa have the potential to be used as antimicrobials in a food beverage system[14]. Information on microflora associated with the dried calyx of H. sabdariffa and its Zobo juice will help in designing appropriate techniques for the preservation of the juice[45]. The Hibiscus extracts have a potential use in the prevention of pathogenic growth in foods and beverages[14].
Table 5. Effect of fruits type and concentration on the microbiological loads of whey proteins beverages.
Parameters Concentrations (%) Total violet bacterial count Lactic acid bacterial count Whey proteins
before heat
treatment
(Control A)5.10 3.72 Whey proteins
after heat
treatment
(Control B)2.58 2.49 Whey enriched with Baobab juice 30:70 3.69 2.99 50:50 4.24 3.06 70:30 3.26 3.00 Whey enriched with Roselle juice 30:70 3.28 3.17 50:50 4.65 3.12 70:30 3.27 3.07 Whey enriched with Doum juice 30:70 2.67 2,53 50:50 3.09 3.48 70:30 2.67 2.53 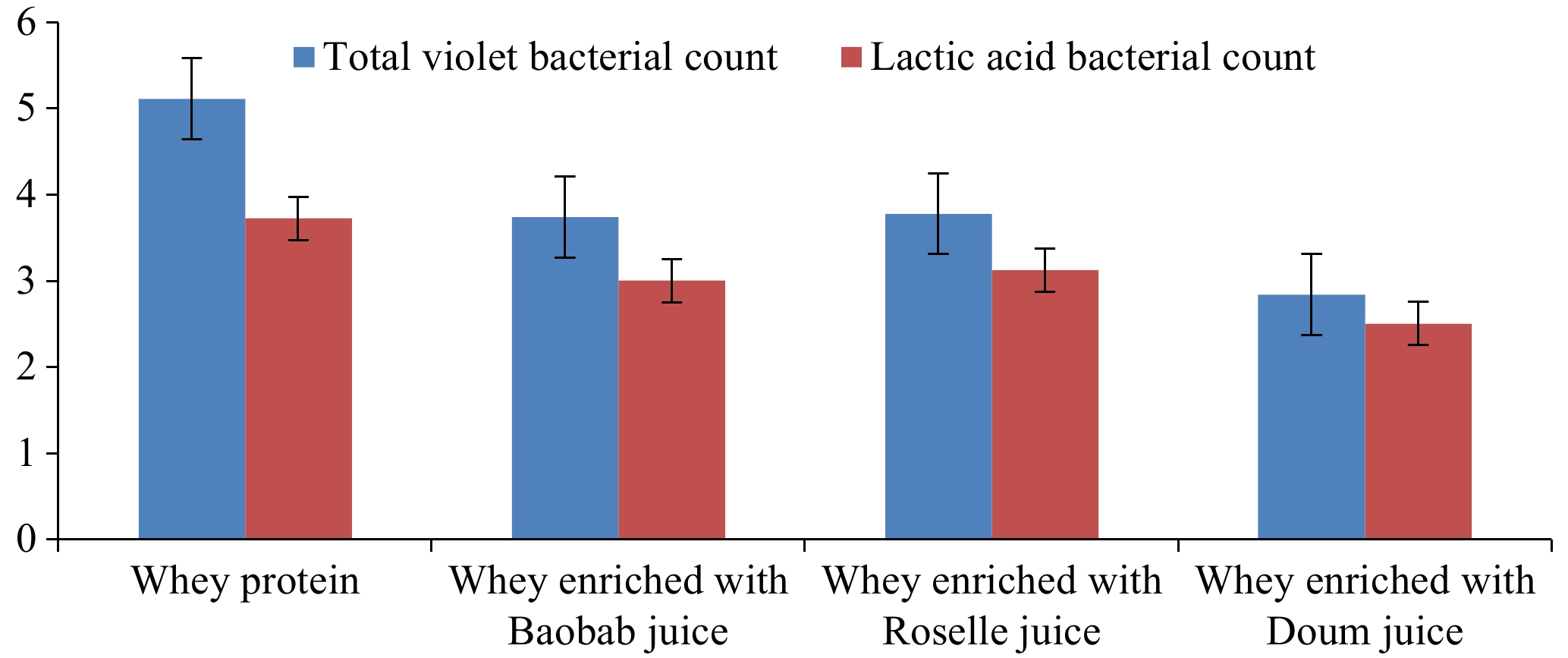
Figure 1.
Variations of the microbiological loads of whey proteins beverages using different types and concentrations of fruits.
The total viable account was intermediate in whey proteins enriched with Baobab (Table 5; Fig. 1). Similarly, the 5% Baobab ice cream revealed a low total bacterial count compared to that made using 3% Baobab, which is indictive of its antimicrobial action[12]. This might be because its fruit pulp has higher values of vitamin C in addition to its antioxidant activity that play a positive role in reducing the microbial loads and hence extending the shelf-life of foods and beverages[32,35]. The microbial levels in formulated fruit juices should be 102 to 103 cfu/mL) according to the standards of the Sudanese Standards and Meteorology Organization[46].
The lactic acid bacteria were higher in whey proteins enriched with Roselle fruit juice (Table 5; Fig. 1). This might be because the Roselle fruit juice extract is slightly higher in acidity content than other fruits used (Tables 1 & 2), which supported a previous findings[44]. Also, the present findings supported the previous report, which stated that the use of Doum palm extracts in the aqueous form will lead to the increase of both viability and activity of probiotics dairy starter cultures that are used in the manufacture of some special dairy products[47]. Moreover, the whey proteins drawn from cheese and buttermilk provide a suitable matrix for enhancing the growth and viability of probiotic microorganisms for the potential development of probiotic dairy-based beverages[48].
In this study the base of the whey protein beverages is the Mozzarella cheese, hence some of the estimated useful lactic acid bacteria in the different whey proteins enriched juices are rich. This also supported the functional properties of the prepared juices as the health benefits of the starter cultures as a probiotic is well documented. The dairy-based whey proteins beverages display many health benefits because of their high contents of the antioxidant activity, bioactive peptides and the essential amino acids present in the whey[48,49]. In addition to their role in the mitigation of blood glucose and decreased appetite[50]. Thus, consuming dairy products enriched with probiotics provide anti-hyperglycemic effect and many other health benefits that depend upon the type of probiotic culture used and the consumed dairy products[51].
Sensory attributes of whey protein beverages
-
The sensory organoleptic evaluation of whey proteins beverages (Table 6; Fig. 2) showed that the whey proteins enriched with Roselle juice showed significantly (p ≤ 0.05) high acceptable score for color and this is because of its red color, which is due to the anthocyanins present in Roselle fruit[52]. When the extracts of Hibiscus are made into beverages or juice, its red color gained the desirability of the consumers[14]. The addition of Roselle extract (0.2%–0.4%) to carrot juice was found to improve the functional properties of yogurt and increased its sensory scores for up to 21 d[40]. Moreover, the US Food and Drug Administration has accepted H. sabdariffa as a natural flavoring substance because their calyces are extracted in water[14]. However, the whey enriched with Doum juice was significantly (p ≤ 0.05) high acceptable for its taste followed by Baobab. Similarly, higher scores were estimated for the sensory attributes with the addition of up to 33% powdered Doum fruit[38].
Table 6. Comparison of the sensory characteristics of whey proteins beverages using different types and concentrations of fruits.
Fruit juice Concentrations (%) Color Flavor Taste Whey proteins enriched with Baobab juice 30:70 1.70 2.60 2.30 50:50 1.50 2.20 2.50 70:30 2.50 2.60 1.60 Whey proteins enriched with Roselle juice 30:70 2.10 1.80 2.25 50:50 1.80 2.45 2.45 70:30 1.95 2.25 2.25 Whey proteins enriched with Doum juice 30:70 1.20 2.25 2.65 50:50 1.45 2.25 3.00 70:30 2.30 2.10 2.50 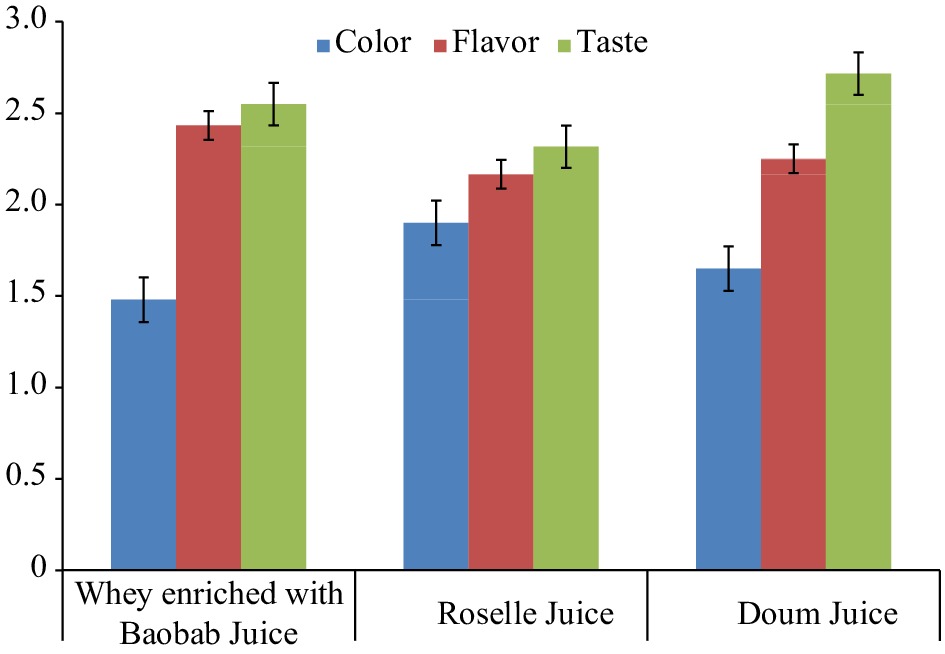
Figure 2.
Variations of the sensory characteristics of whey proteins beverages using different fruits.
Significantly (p ≤ 0.05) higher scores were reported in the flavor of whey proteins enriched with Baobab (Table 6; Fig. 2). This is in line with the conclusion stating that adding 3% Baobab to camel milk ice cream resulted in an improvement of its flavor[12]. The significantly (p ≤ 0.05) high acceptability of Doum juice could be because using Doum palm fruit gave the product a sweet taste, special flavour, and colour[53]. Hence, there is a possibility of producing high quality fermented milk from camel with good appearance, colour, flavour, body and texture when adding Doum extract[39].
-
Variations reported for some of the compositional contents of the obtained whey protein beverages might be due to the different chemical composition of fruits used. The sensory evaluation showed the best result of the whey proteins enriched with the fruits of Doum palm (Hyphaene thebaica). The possibility of using some of the valuable Sudanese local fruits should be promoted and utilized for enriching the whey protein. This will help in improving the nutritional content and acceptability of the selected fruit juices and the whey proteins and thus contribute globally to obtain functional foods.
-
The contribution of the authors regarding this paper was as follows: study conception and design: Saied MNAM, El Zubeir IEM; collection of data: Saied MNAM; analysis and interpretation of the results: Saied MNAM; draft manuscript preparation: Saied MNAM, El Zubeir IEM. Both authors reviewed and approved the final version of the manuscript.
-
The data generated and analyzed during the current study are available from the corresponding author (Ibtisam E. M. El Zubeir) on reasonable request.
-
The authors would like to extend their acknowledgments to the staff members in the Department of Dairy Production, Faculty of Animal Production, U. of K. for their technical help during the laboratory work.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Saied MNAM, El Zubeir IEM. 2024. Utilization of whey proteins in beverages using Baobab (Adansonia digitata L.), Roselle (Adansonia digitata L.) and Doum (Hyphaene thebaica) fruits. Food Materials Research 4: e016 doi: 10.48130/fmr-0024-0007
Utilization of whey proteins in beverages using Baobab (Adansonia digitata L.), Roselle (Adansonia digitata L.) and Doum (Hyphaene thebaica) fruits
- Received: 14 October 2023
- Revised: 06 March 2024
- Accepted: 07 April 2024
- Published online: 12 June 2024
Abstract: The loss of valuable nutrient content of the whey proteins obtained from the dairy industry in general is disposed of causing sewage pollution. Therefore, this study aims to utilize the nutrient content of whey proteins to beverages using some Sudanese indigenous fruits: Baobab (Adansonia digitata L.), Roselle (Hibiscus sabdariffa L.) and Doum (Hyphaene thebaica) juices at a rate of 30:70, 50:50 and 70:30% for preparation of beverages. The processed beverages were evaluated for some chemicals, microbial, and sensory properties. Whey proteins enriched with Doum juice revealed significantly (p ≤ 0.05) high-fat level (0.93%), while that enriched with Roselle was significantly (p ≤ 0.05) higher in the protein value (5.97%). Whey proteins enriched with Baobab juice significantly (p ≤ 0.05) revealed a higher value for vitamin C (140.93 mg/100 mL). The values obtained for calcium and phosphorus were significantly (p ≤ 0.05) high in the whey enriched with Roselle juice (8.50 and 1.29 mg/100 mL, respectively). The heat treatment of whey proteins showed significant (p ≤ 0.05) reduction in the total viable bacterial counts. Moreover, the lactic acid bacteria were higher in plain whey proteins followed by those enriched Roselle and Baobab juices. Also, the whey proteins enriched with Roselle juice showed significantly (p ≤ 0.05) the best color. However, the whey proteins enriched with Doum juice significantly (p ≤ 0.05) revealed the best scores for flavor and taste. Hence, the present study concluded that it is possible to use some indigenous Sudanese fruits to utilize the whey proteins as functional food instead of its negative effect on the environment.












