-
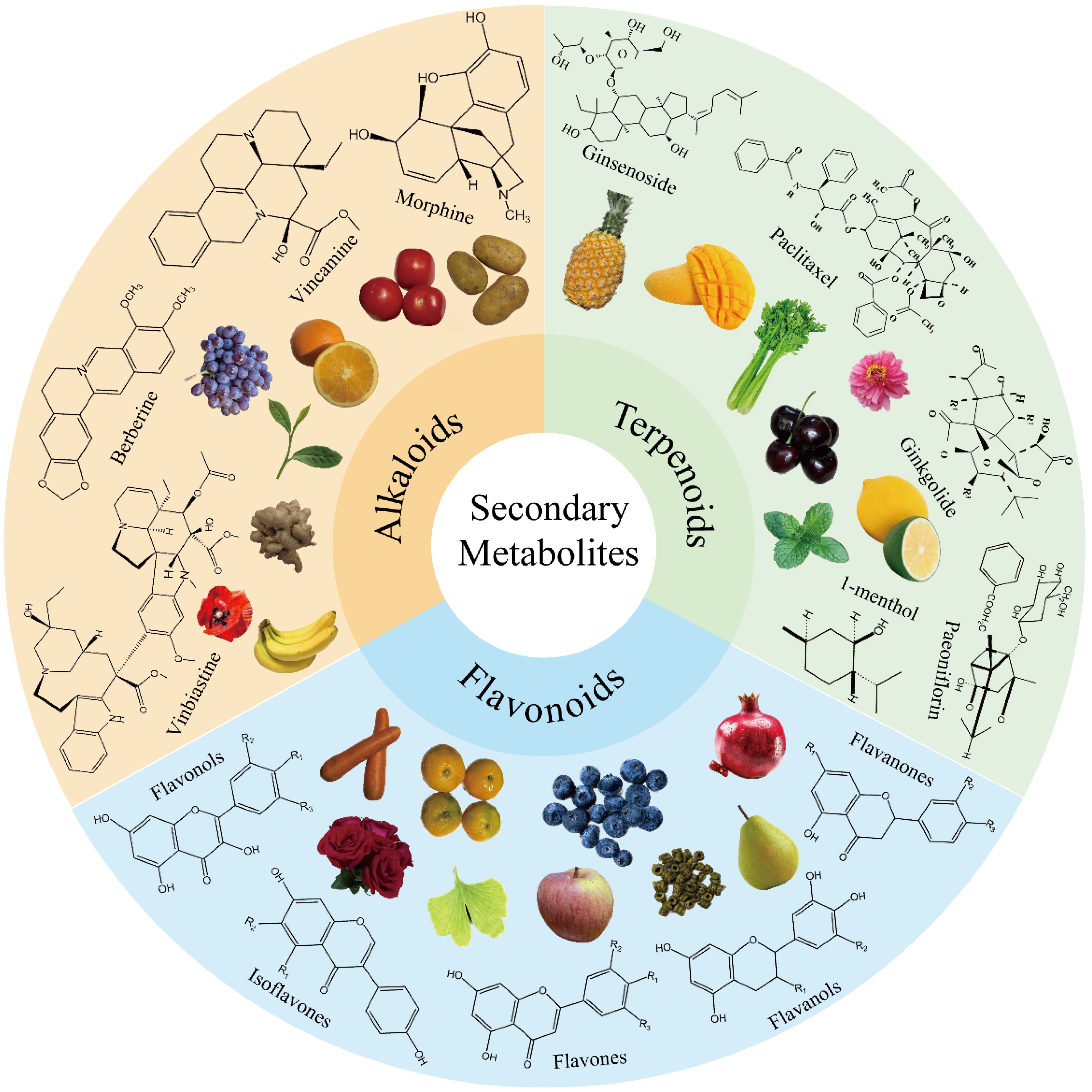
Secondary metabolites (SMs) are natural compounds that play a critical role in plants[1]. SMs fall into three categories based on their chemical structure and synthesis pathways: phenolic compounds (such as flavonoids, phenolic acids, coumarins, and tannins), terpenoids (such as monoterpenes, sesquiterpenes, diterpenes, and sesterterpenes), and nitrogen-containing compounds (such as alkaloids, cyanosides, glucosinolates, and non-protein amino acids). SMs are abundantly present in nearly all horticultural plants, which primarily include vegetable, fruit, ornamental, beverage-producing, and herbal medicinal plants (Fig. 1). These plants produce SMs that play a vital role in human life by providing food, nutrients, aesthetics, and medicinal treatments.
Flavonoids are the most abundant and widely distributed SMs in horticultural plants[2,3]. Terpenoids, on the other hand, are the largest class of plant metabolites, with over 40,000 distinct compounds which are widely distributed in medicinal and ornamental plants[4]. Alkaloids are primarily distributed in a wide range of horticultural plants, spanning various families and genera. These SMs are found in horticultural plants and play significant roles in contributing to the specific odor, color, taste, and nutrition of plant parts. Furthermore, they are essential for the survival of plants under various stresses. SMs actively participate in potential defense mechanisms, such as chemical warfare between plants and pathogens, as well as adaptation to cold/high temperatures, salinity, or drought environments. This review provides an overview of the classification, distribution, functions, and accumulation mechanisms of several important SMs (specifically flavonoids, terpenoids, and alkaloids) in enhancing horticultural plants' stress resistance and human health. The review also provides a direction for future research on utilizing SMs in breeding horticultural plants.
-
Flavonoids have a common diphenylpropane backbone (C6-C3-C6) in which two aromatic rings, namely the A ring and B ring, are connected by a three-carbon chain. The properties of flavonoids depend on the arrangement of hydroxyl, methoxy, and glycoside groups on C6-C3-C6. Flavonoids are generally classified into seven subcategories based on the degree of oxidation of the central heterocycle: flavonols, flavones, isoflavones, anthocyanins, flavanones, flavanols, and chalcones.
Flavonols, also known as 3-hydroxyflavonoids, are characterized by several specific substitutions in the A and B rings, which are connected by three-carbon chains. Positions 5 and 7 on the flavonol A ring are replaced by hydroxyl groups. Flavonols contain more 3-OH groups than other flavonoids. Vegetables and fruits such as cauliflower, onions, asparagus, and apples are rich in flavonols[5]. The chemical structure of flavones consists of 4H-amino-4-one, which has a phenyl substituent at position 2. Most flavones are 7-O-glycosides, found in celery, tea, red peppers, and oranges[6]. Isoflavones differ from other flavonoid classes in having a basic structural feature where a B-ring attaches to C-3 but not C-2[7]. Many fruits, vegetables, nuts, and grains contain small amounts of isoflavones[8,9]. The structural characteristic of anthocyanins is that the C ring of the basic parent nucleus is carbonyl-free, and one oxygen atom exists in the form of a salt. Anthocyanins are widely present in flowers, fruits, leaves, stems, and other parts of plants. They are pigments that give plants their blue, red, purple, etc. colors and are rich in fruits and vegetables such as blueberries, red cabbage, tomatoes, purple sweet potatoes, and eggplants[6,10]. Flavanones, also known as dihydroflavones, are obtained by cyclo-closure isomerization of 20-hydroxychalcone, creating stereogenic centers at carbon C-2[11]. Flavanones are found almost exclusively in citrus fruits, such as oranges and lemons[12]. Flavanols are characterized by hydroxyl groups bound to the 3rd position of the C ring. Flavanols are abundant in a variety of fruits, including apples, cherries, plums, apricots, and berries, especially in fruit skins[6,13]. Chalcones, are natural open-chain flavonoids that can carry up to three modified or unmodified C5-, C10-, and C15-isoprene moieties on the A and B rings. These bioactive products are widely distributed in the legume family, mulberry family, ginger family, and plantain family[14].
Terpenoids
-
Terpenoids are derived from the carbon isoprene unit isoprene diphosphate (IPP) and its allyl isomer dimethylallyl diphosphate (DMAPP). IPP and DMAPP can be synthesized by two different pathways: the mevalonic acid (MVA) pathway and the methylerythritol phosphate (MEP) pathway[15−17]. All terpenoids are classified according to the five-carbon isopentane units of the core structure. These include monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterterpenes (C25), triterpenes (C30) and polyterpenes (> C30)[18,19].
Monoterpenes consist of two units of isoprene and have the general molecular formula C10H16. Examples of monoterpenoids are terpineol (found in lilacs), limonene (found in citrus fruits), linalool (found in lavender), and myrcene (found in hops)[20]. They are odorous compounds that contribute partially to the fragrance of many flowers and fruits. Sesquiterpenes consist of three units of isoprene and have the general molecular formula C15H24. Examples of sesquiterpenes and sesquiterpenoids are farnesol, humulene, and farnesenes[21]. Diterpenes are nonvolatile C20 hydrocarbons derived from four isoprene units and have a diverse structure[22,23]. They can exist in linear, bicyclic, tetracyclic, pentacyclic, or macrocyclic forms. Diterpenes are characterized by ployoxygenated keto and hydroxyl groups[24]. Triterpenes are derivatives of the C30 precursor squalene, and there are more than 20,000 known members[25]. Tetraterpenes are composed of eight isoprene units and have the formulas C40 and C40H64[21]. The most extensively studied tetraterpenoids are carotenoids, which have more than 750 members[26, 27]. Terpenoids with more than 40 carbons are considered polyterpenes. Some plants produce a polyisoprene with trans double bonds, known as gutta-percha, while natural rubber consists of polyisoprene with cis double bonds[28].
Alkaloids
-
Alkaloids are typically synthesized from aliphatic amino acids and aromatic amino acids, and they follow two synthetic pathways: the pyruvate pathway and the shikimic acid pathway[1]. The precursors of alkaloids, mainly amino acids, are derived from metabolic pathways such as glycolysis[29]. Based on their biosynthetic precursors and heterocyclic ring systems, alkaloids are further classified into different types, including indole, tropane, piperidine, purine, imidazole, pyrrolizidine, pyrrolidine, quinolizidine, and isoquinoline alkaloids[30].
Indole alkaloids are characterized by the presence of serotonin, and there are approximately 2,000 compounds associated with this type of alkaloid[31]. Tropane alkaloids contain an 8azabicyclo [3.2.1] octane nucleus, which is derived from the amino acid ornithine. They can be found in angiosperms belonging to the Solanaceae, Brassicaceae, Erythroxylaceae, Convolvulaceae, and Euphorbiaceae families. Several important alkaloids, such as scopolamine, hyoscyamine, cocaine, and atropine, belong to this class and have significant medicinal uses[32,33]. Quinoline and isoquinoline alkaloids are another important group of heterocyclic aromatic alkaloids formed by the fusion of a benzene ring and a pyridine ring, commonly known as benzopyridine. They are found in various horticultural crops, such as poppies (Papaver somniferum) that produce morphine, cinchona trees (Cinchona spp.) that yield quinine, and bitter oranges (Citrus aurantium) that contain alkaloids like synephrine[34]. Purine alkaloids are derived from purines, specifically adenine and guanine, and are often referred to as xanthines. Caffeine, theobromine, theophylline, and aminophylline are the most important members of this group of alkaloids[35,36]. Piperidine alkaloids are widely distributed in the plant kingdom. These compounds are characterized by a saturated heterocycle, namely the piperidine nucleus, and are known for their toxicity[37]. Pyrrolizidine alkaloids consist of two five-membered rings known as necine bases, which share a nitrogen atom at position 4. They can be found in angiosperms belonging to the Boraginaceae, Compositae, Orchidaceae, Leguminosae, Convolvulaceae and Poaceae families[38]. Pyrrolidine alkaloids contain 5-membered rings consisting of the amino acids ornithine (or arginine in some cases) and lysine, along with acetate/malonic acid units[39]. Quinolizidine alkaloids consist of two fused 6-membered rings sharing a nitrogen atom and exhibit structural variations from simple to complex. Notable examples include quinine, obtained from the bark of the cinchona tree, and quinidine, found in the plant Remijia pedunculata[40].
Other SMs
-
In addition to alkaloids, flavonoids, and terpenoids, horticultural plants contain a wide range of other SMs. Some examples of these are glucosinolates, fatty acids, coumarins, and lignans, each possessing distinct structures and synthesis pathways. Glucosinolates, which are compounds containing sulfur, are mainly found in plants from the Brassicaceae family, such as cabbage, broccoli, radishes, and mustard greens. These metabolites contribute to the unique flavor and pungency of these crops. Glucosinolates are produced from amino acids and are synthesized through the elongation of the amino acid chain and the modification of the side chain[41]. Another group of SMs found in horticultural plants are fatty acids, which are essential components of cell membranes and are involved in energy storage and signaling. Fatty acids are synthesized through biosynthesis pathways, such as the fatty acid synthase complex. Common sources of fatty acids include olive oil, palm oil, avocado, and nuts/seeds. Coumarins are aromatic compounds that consist of a benzene ring fused with a lactone ring. They are synthesized through the phenylpropanoid pathway and can be found in various horticultural plants, including citrus fruits, cherries, and grasses. Coumarins contribute to the defense of plants against pathogens and pests[42]. Lignans, on the other hand, are complex phenolic compounds that contain multiple benzene rings connected by linkages. They are derived from the phenylpropanoid pathway and can be found in plants such as flaxseed, sesame seeds, and berries. Lignans have been associated with various health benefits, including antioxidant and anticancer properties.
-
Horticultural plants are susceptible to one or more abiotic stressors such as drought, low temperatures, flooding, and high salinity. The impact of these stressors on fruit and ornamental trees is becoming increasingly severe due to escalating global urbanization, industrialization, and frequent extreme weather events. Additionally, the production of vegetables and ornamental herbaceous plants not only faces adverse environmental conditions but also pests and diseases induced by facility cultivation, leading to a decrease in both yield and quality. Certain SMs can be induced to help horticultural plants adapt and resist adverse environmental pressures, which are crucial for their growth, development, and yield formation (Fig. 2).
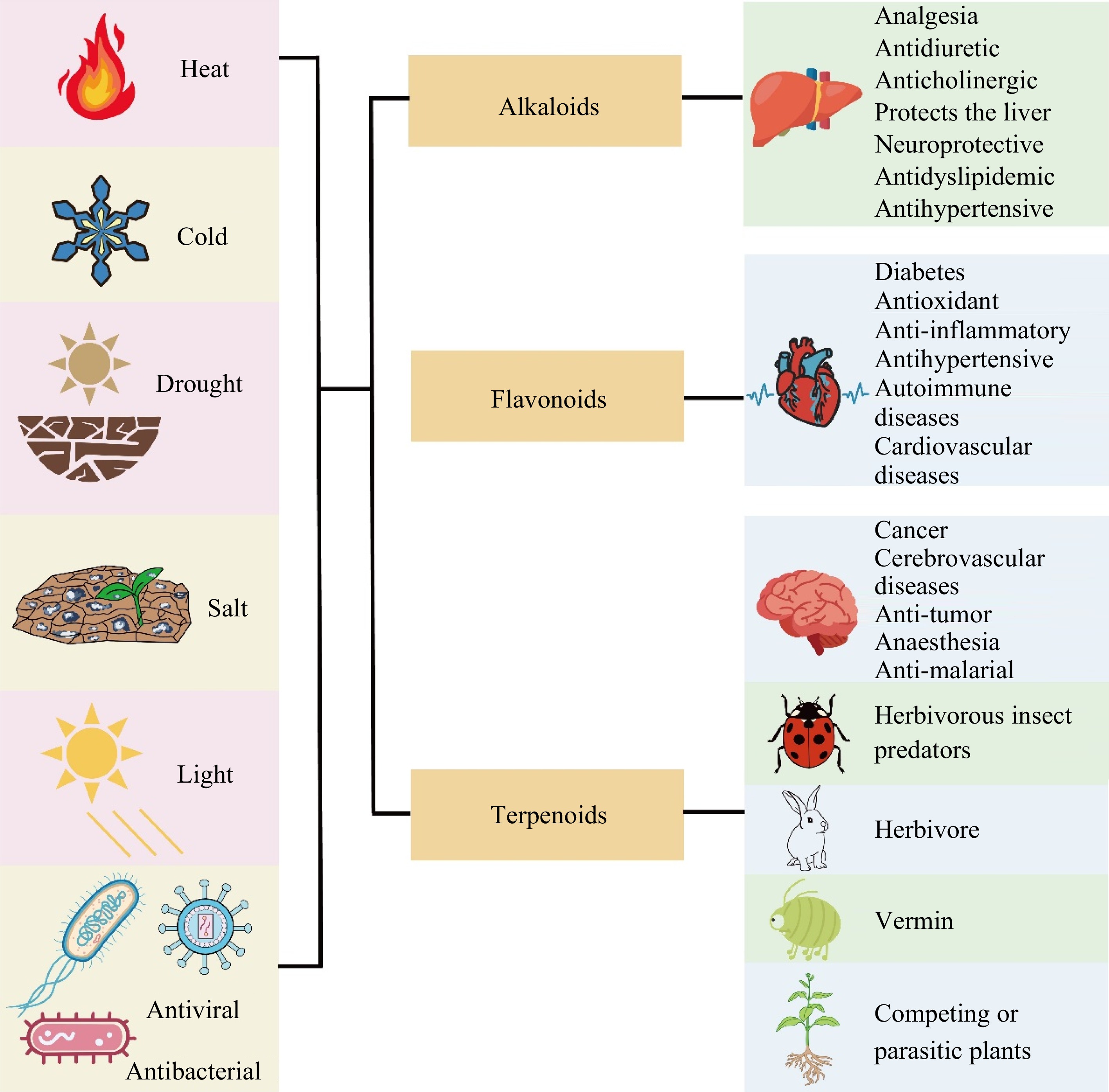
Figure 2.
Functions of alkaloids, flavonoids and terpenoids in biotic and abiotic stresses and human health.
Light
-
Plants rely on solar energy to grow through a process called oxygenic photosynthesis. However, when the intensity of light becomes excessive, it can lead to photoinhibition and oxidative stress. These adverse effects can lower photosynthetic efficiency, impair carbon assimilation, and ultimately hinder both plant growth and yield. Conversely, insufficient light intensity can prompt shade avoidance responses in horticultural plants, which can unfavorably affect their overall development, leading to reduced biomass accumulation and yield. Specialized molecules, such as flavonoids, have a crucial role in protecting horticultural plants from the harmful effects of excessive light and ultraviolet radiation[43].
Under high light intensity, plants may produce higher levels of SMs as a protective response to absorb excess light and prevent damage caused by reactive oxygen species (ROS). Anthocyanins act as antioxidants, scavenging ROS generated under UV stress. In response to UV radiation, the fruits of berries, grapes, and tomatoes usually accumulate more anthocyanins[44]. Apples, particularly their peels, contain proanthocyanidins that aid in protecting the fruit from abiotic stresses such as UV radiation. These compounds act as natural sunscreens, absorbing harmful UV light and reducing potential damage to apple cells[45]. Carotenoids act as photoprotective agents by dissipating excess energy absorbed by chlorophyll, thus protecting plants from photoinhibition. Horticultural crops like tomatoes (Solanum lycopersicum) and carrots (Daucus carota) accumulate carotenoids under high-light conditions. Anthocyanins act as antioxidants, scavenging ROS generated under UV stress[46]. They also help maintain the quality and nutritional value of the fruit by reducing oxidative damage. Many other horticultural plants, such as roses (Rosa spp.)[47], petunias (Petunia spp.)[48], geraniums (Pelargonium spp.), coleus (Solenostemon spp.), ornamental cabbage, and kale (Brassica oleracea), produce vibrant flower colors due to the accumulation of anthocyanins. These pigments also act as photoprotective compounds by absorbing excessive light energy and preventing photoinhibition in chloroplasts. Moreover, flavonoids, including flavones, flavonols, and flavan-3-ols, act as natural sunscreens in horticultural plants. For instance, tea (Camellia sinensis) leaves contain flavonoids like catechins, which protect against photodamage. These compounds absorb UV radiation, shield the photosynthetic apparatus, and prevent the generation of ROS. Flavonoids also contribute to the regulation of plant growth and development under light-stress conditions. Plant flavonoids can function as signaling molecules, UV filters, and ROS.
Terpenoids assist plants in reducing the impact of light stress by removing ROS and protecting cellular components. Limonene, serveing as a photoprotective compound, participates in the dissipation of excess light energy and reduces the risk of oxidative damage caused by ROS[49]. Under light stress conditions, citrus plants such as oranges and lemons increase the production and accumulation of limonene as a protective response. Geraniol, a monoterpene alcohol and signaling compound found in the essential oils of several rose species, helps the plant adapt to high light intensities[20]. Certain diterpenes are known to possess antioxidant and photoprotective properties, making them crucial for light stress resistance. For example, the herb Sage (Salvia officinalis) accumulates abietane diterpenes, including rosmarinic acid, which function as antioxidants and photoprotective compounds, counteracting the negative impacts of excessive light[50]. Furthermore, certain sesquiterpenes exhibit antioxidant and anti-inflammatory properties, making them potential candidates for safeguarding plants against light stress. Chamomile (Matricaria chamomilla) accumulates sesquiterpenes, such as α-bisabolol and chamazulene, which enhance the plant's resistance to light stress by providing antioxidant and anti-inflammatory activities[51].
Alkaloids are thought to be involved in antioxidant defense mechanisms, contributing to the maintenance of cellular homeostasis and protection against photodamage. Solanine acts as a protective compound against light stress-induced damage through its antioxidant and anti-aging properties. In potatoes, exposure to excessive light triggers the accumulation of solanine, which helps regulate membrane stability, maintain photosynthetic efficiency, and mitigate oxidative damage induced by intense light[52]. Moreover, catharanthine and vindoline, along with other bioactive compounds in Madagascar periwinkle (Catharanthus roseus), can scavenge ROS produced under high light conditions and reduce oxidative stress[53]. Caffeine is a well-known alkaloid that naturally occurs in coffee plants. Under conditions of increased light exposure, caffeine can accumulate in coffee leaves[35,54]. Morphine accumulation in opium poppy plants can be stimulated by various environmental stresses, including light stress[55].
Temperature
-
Temperature stress, both high and low temperatures, induces physiological, biochemical, and molecular changes in the metabolic processes of plants. This in turn leads to the production and alteration of SMs. Horticultural plants are particularly affected by temperature stress, as it causes a decrease in growth rates and delays in development. As a result, these plants become more susceptible to specific pathogens and diseases.
The impact of SMs in horticultural plants is therefore significant in the context of temperature stress. The accumulation of specific flavonoids in horticultural crops under the influence of high and low-temperature stress demonstrates their crucial roles in plant defense and stress response. These flavonoids serve as protective compounds by aiding in antioxidant defense, photoprotection, and reducing oxidative damage caused by extreme temperatures. Flavonoids have been observed to respond to high-temperature stress. For instance, the accumulation of quercetin in response to heat stress helps onions resist heat-induced oxidative damage and contributes to quality preservation[56]. Similarly, in strawberries, flavonoids like anthocyanins accumulate under high temperatures, thereby reducing oxidative stress and maintaining fruit quality[57]. In alfalfa, flavonoids can alleviate the detrimental effects of heat stress during fertilization and early seed maturation[58]. Conversely, cold temperatures stimulate the accumulation of anthocyanins, resulting in the characteristic deep purple or red coloration. Catechins are another type of flavonoid that accumulate in the leaves of tea plants under low-temperature stress. The production and accumulation of catechins are, therefore, induced as an adaptive response to cold temperatures. During cold adaptation and freezing, flavonoids effectively remove ROS and act as potent antioxidants. In freezing conditions, when water is transferred from cells to intercellular ice crystals, flavonoids are expected to be more strongly distributed into the lipid phase of the cell membrane, therefore stabilizing it[59]. Reed, on the other hand, synthesizes a substantial amount of flavonoids, such as daidzin, daidzein, and mglycitin, which mitigate the negative effects of heat stress[60].
Terpenoids are beneficial for horticultural plants in managing temperature stress, as they act as protective compounds that regulate temperature, reduce oxidative damage, and enhance resilience. Specifically, terpenoids like carotenoids function as antioxidants and protect the photosynthetic machinery from heat and light damage. They help dissipate excess energy as heat, reducing the risk of photoinhibition. In tomatoes, the accumulation of carotenoids, such as lycopene, safeguards the plant against heat stress[61]. Another terpenoid, menthol, provides a cooling sensation when applied or consumed. Menthol activates specific receptors and acts as a cooling agent. Its accumulation in peppermint leaves helps regulate leaf temperature and minimize oxidative damage caused by high temperatures. This alleviates the negative effects of heat stress on the plant. Furthermore, linalool, which is present in basil, serves as a cryoprotectant. It helps regulate membrane fluidity and prevents cellular damage caused by cold temperatures. When linalool accumulates within basil leaves, it improves the plant's ability to withstand and survive cold stress conditions[62].
Alkaloids also have a significant role in the resistance of horticultural plants to high and low temperature stresses. Horticultural plants, like Mentha piperita and Catharanthus roseus, display an increase in alkaloid levels under heat stress[63]. Similarly, varieties of narrow-leaved lupine experience a rise in alkaloid content with increasing temperatures. Heat stress can induce the biosynthesis and accumulation of solanine in potato plants as a protective response. Solanine acts as a defense compound, assisting in protecting against heat-induced oxidative damage, maintaining membrane stability, and scavenging ROS. However, it is important to note that solanine can be toxic if consumed in large quantities, so the higher levels present during heat stress may not be desirable for consumption[64]. Capsaicin, a well-known alkaloid responsible for the spicy heat sensation in chili peppers, has been observed to accumulate more in response to high-temperature stress in chili pepper fruits. This accumulation of capsaicin is thought to be an adaptive response to heat stress, serving as a defense compound against mammalian herbivores. Although the precise role of capsaicin in protecting against high-temperature stress is not well understood, it may contribute to the overall stress tolerance of chili pepper plants[65].
Osmotic stress
-
Osmotic stress, which includes drought and salt stress, has a significant impact on plant growth and productivity. Plants respond to osmotic stress by regulating gene expression, ultimately restoring cellular homeostasis, detoxifying toxins, and promoting growth[66]. Furthermore, the accumulation of SMs often occurs when plants are exposed to such stresses. SMs play a crucial role in helping plants adapt to their environment and withstand osmotic stress conditions. One specific example is the buildup of flavonoids, which grant drought resistance to plants[67]. Quercetin, in particular, assists in scavenging ROS generated during drought stress, thus alleviating oxidative damage. The accumulation of quercetin in tomatoes contributes to drought tolerance and helps maintain plant health[68,69]. Pomegranates contain proanthocyanidins in the fruit peel and arils. These compounds contribute to the fruit's resilience under abiotic stresses like drought. Proanthocyanidins in pomegranates have been associated with improved plant water relations, helping to mitigate the effects of drought stress[70]. Additionally, anthocyanins may accumulate in grapevine berries under salt stress conditions, resulting in berry pigmentation. Moreover, in apples, the accumulation and enhancement of anthocyanin production aid in drought stress resistance[71,72].
Terpenoids also play a significant role in the resistance to osmotic stress. They aid in alleviating the adverse effects of drought and salt stress by enhancing antioxidant defense mechanisms, regulating stomatal closure, maintaining membrane stability, and mitigating oxidative damage. In drought conditions, the increased production of diterpenoid antioxidants, such as α-tocopherol and isoconnamol, protects Rosmarinus officinalis and Salvia officinalis from oxidative damage, thus enhancing their drought tolerance[73,74]. The high salvianolic acid and tanshinone content improves photosynthesis in S. miltiorrhiza under drought stress and safeguards cells against oxidative damage, thereby enhancing salt stress resistance[75]. Sesquiterpene β-caryophyllene can accumulate in tomato plants under salt stress. It acts as a stress-signaling compound and contributes to the plant's tolerance against salt stress. Its accumulation helps regulate ion transport, maintain osmotic balance, and reduce oxidative damage caused by salt-induced ROS[76].
Alkaloids play a crucial role in osmotic stress by contributing to several important functions. They help maintain osmotic balance, regulate ion transport, and safeguard cellular structures from damage caused by salt. Some alkaloids, including betaine and glycine betaine, accumulate in response to high salt concentrations in the soil. For example, betaine accumulation in sugar beets is essential for maintaining cellular homeostasis and enhancing salt tolerance. Betalains, another group of alkaloids, act as antioxidants by scavenging ROS generated during salt stress. This antioxidant activity protects against oxidative damage and sustains physiological functions in beetroot[77]. In mandala plants, salt stress induces the accumulation of alkaloids in young leaves. Additionally, alkaloids are known to play a role in the resistance of horticultural plants to drought stress. Proline, for instance, acts as an osmoprotectant and assists in maintaining cellular osmotic balance. In grapes, proline accumulation helps improve water use efficiency and enhance drought tolerance[66]. Plantlets of Papaver somniferum, when exposed to drought stress, produce elevated levels of alkaloids. However, it is crucial to note that research on the specific roles of alkaloids in drought and salt stress resistance in horticultural plants is relatively limited compared to other compounds such as terpenoids and flavonoids. Further studies are necessary to explore additional alkaloids and uncover their functions in enhancing stress tolerance in horticultural crops under drought and salt stress conditions.
Other SMs resist abiotic stresses
-
In addition to alkaloids, flavonoids, and terpenoids, phenolic acids and tannins also play a crucial role as SMs in the resistance of horticultural crops to adversity. Phenolics, specifically, are particularly important in protecting fruits and vegetables from low-temperature stress. These phenolic acids possess antioxidant and antimicrobial properties, effectively safeguarding crops from oxidative stress and pathogenic bacteria. By reducing peroxidation in fruit and vegetable cells, phenolics maintain their healthy structure and function. Moreover, the application of exogenous SA treatment can promote kiwifruit phenolic metabolic processes and enhance its cold resistance[78]. On the other hand, tannins are thought to contribute to defense mechanisms that deter pests and prevent herbivory. By utilizing tannin biostimulants under salt stress, changes in the root architecture of tomato plants can be induced and their salt tolerance can be improved[79]. Overall, the presence of these SMs in horticultural crops enhances their resilience to adversity by providing enhanced defense mechanisms, allowing them to thrive.
-
SMs in horticultural plants play a critical role in resisting biological stresses. These compounds serve as defense mechanisms against various pathogens and pests, enhancing the plant's ability to withstand and combat living stresses. SMs like alkaloids, flavonoids, and terpenoids can possess antimicrobial, insecticidal, antifungal, or nematicidal properties. They function as chemical deterrents that hinder the growth and development of pathogens, pests, and herbivores. Moreover, SMs can boost the plant's immune response by activating defense signaling pathways and fortifying cell walls. By effectively resisting biological stresses, SMs contribute to the overall health and resilience of horticultural plants, ensuring successful growth, development, and productivity[80].
The defense against biological attacks may be considered the most important and extensively studied aspect of SMs. Flavonoids are capable of protecting plants against damage caused by viruses, fungi, bacteria, and herbivores. They can disrupt lipid bilayers, leading to bacterial membrane rupture. They are also able to impede multiple vital processes, including biofilm formation, cell envelope synthesis, nucleic acid synthesis, electron transport chain, and ATP synthesis, to effectively combat bacterial infections[81]. Citrus fruits, such as oranges and lemons, are rich in flavanone glycosides like hesperidin and naringin. These compounds contribute to the fruit's resistance against microbial pathogens[82]. In various horticultural crops, such as tomatoes, onions, and peppers, flavonoids like quercetin and kaempferol have demonstrated insecticidal activity against pests like aphids, thrips, and caterpillars[83]. Flavonoids in grapes contribute to the plant's defense against herbivores like insects and mammals. These compounds exhibit repellent or toxic effects, deterring feeding and providing protection against damage[84].
Terpenoids can function as elicitors, triggering defense mechanisms in plants against pests. Marigold (Tagetes spp.) for example, produce terpenes, including limonene and linalool, which have insect-repellent properties. These compounds help deter pests like aphids, nematodes, and whiteflies. Planting marigolds among vegetable crops can help reduce pest damage and the need for synthetic insecticides. The terpenoid compound called farnesene, found in apple fruits and their leaves, possesses nematicidal activity against plant-parasitic nematodes[85]. Certain terpenoids also act as chemoattractants for herbivores, with the hoterpenoid derivative of nerolidol, known as 4,8-dimethyl-1,3(E),7-nontriene, attracting insect herbivore predators and further reinforcing plant defenses[86]. Some terpenoids in crops like tomatoes and peppers contribute to their resistance against herbivorous insects by acting as antifeedants, reducing feeding activity and restricting insect population growth[84].
Alkaloids serve as natural insecticides, deterring herbivorous insects or acting as toxic agents. For example, nicotine, an alkaloid present in plants, acts as an insecticidal compound by affecting the nervous system of insects and resulting in their mortality[83]. Pyrethrins, derived from plants like chrysanthemums, are alkaloids that possess insecticidal properties and are commonly used in natural insecticide formulations[87]. Alkaloids can act as deterrents, reducing feeding activity of herbivorous insects and protecting plant tissues. For instance, Harmine is an alkaloid found in plants such as passionflower (Passiflora spp.) and certain vegetables. It can protect plant tissues by discouraging feeding activity and reducing insect damage[88]. Alkaloids can also provide protection against herbivores by acting as toxic substances. Plants like nightshades (Solanaceae) produce alkaloids such as solanine and tomatine, which are toxic to many insect pests and herbivores[83].
-
SMs in horticultural plants play a significant role in human health. These compounds, such as flavonoids, terpenoids, and alkaloids, exhibit a wide range of bioactive properties. They have been associated with antioxidant, anti-inflammatory, antimicrobial, anticancer, and cardiovascular benefits, among others. Including horticultural plants rich in SMs in our diet can contribute to promoting overall well-being and reducing the risk of chronic diseases. Additionally, these plants are often used in traditional medicine and natural remedies due to their therapeutic properties, further highlighting their role in human health (Fig. 2)[89, 90].
Health benefits of flavonoids
-
Numerous studies have indicated that flavonoids possess health-promoting properties. Flavonoids are capable of hindering the entry and binding of viruses into cells, impeding viral replication or translation, and preventing virus release[90]. Additionally, they exhibit various antifungal mechanisms such as interfering with plasma membranes, inducing multiple mitochondrial dysfunctions, and inhibiting the formation of cell walls, cell division, RNA and protein synthesis, which are significant for human health[91]. Cranberries (Vaccinium macrocarpon) are recognized for their high flavonoid content, especially flavonols and proanthocyanidins, which have antibacterial properties[92]. These flavonoids can hinder bacterial adhesion to cellular. Similarly, green tea (Camellia sinensis) similarly contains flavonoids, specifically catechins, with epigallocatechin gallate (EGCG) being the most plentiful. EGCG has shown antiviral activity against various viruses including influenza A virus, human immunodeficiency virus (HIV), and hepatitis C virus (HCV)[93].
Numerous in vitro and in vivo studies have demonstrated that flavonoids possess antioxidant properties, providing therapeutic and preventive benefits for various degenerative diseases and disorders such as cardiovascular diseases, neurodegenerative diseases, diabetes, inflammation, autoimmune diseases, and cancer. For instance, grapes are a rich source of flavonoids, including resveratrol, quercetin, and catechins. Resveratrol, in particular, exhibits therapeutic potential due to its antioxidant, anti-inflammatory, and cardioprotective effects[94]. Several studies have shown its potential as a cancer-preventative agent. Similarly, apples, with their diverse range of flavonoids such as quercetin and kaempferol, serve similar functions. Numerous studies have investigated the anti-inflammatory and anti-allergic effects of quercetin. Flavonoids can decrease the activity of xanthine oxidase, NADPH oxidase, and lipoxygenase in the human body. They can also reduce the activity of 15-lipoxygenase (15-LOX) and cyclooxygenase (COX), thus reducing inflammation and platelet aggregation. Inhibiting these enzymes can also protect LDL against oxidation and regulate capillary pressure to restore normal function[95]. Additionally, flavonoids may induce cell apoptosis, inhibit angiogenesis, exhibit antioxidant activity, and have cytotoxic or inhibitory effects on cancer cells[96]. It's important to note that while flavonoids in horticultural crops have shown potential antibacterial, antiviral, and human health-promoting effects, their specific effectiveness may vary depending on factors such as concentration, bioavailability, and interactions with other compounds. Further research is still needed to fully understand and harness the potential of flavonoids in these areas.
Benefits of terpenoids in human health
-
Terpenoids have various applications in human society. Hemiterpenes, for example, are being researched as potential biofuel sources[97]. Monoterpenes are found in essential oil compounds and contribute to the aroma and flavor of plants, serving as important components for several agricultural, pharmaceutical, cosmetic, and food-related uses. Terpenes such as linalool and linalyl acetate are present in lavender (Lavandula spp.) essential oil. Peppermint (Mentha × piperita) essential oil contains terpenes such as pinene, carvacrol, camphor, menthol, and limonene, which have been widely used in industrial applications[98]. Its calming and relaxation properties make it a popular choice for aromatherapy aimed at stress relief. Menthol, in particular, provides a cooling sensation that is refreshing and soothing, making it a common ingredient in toothpaste and chest rubs. Tea tree essential oil, derived from Melaleuca alternifolia, contains terpenes, including terpinen-4-ol, with demonstrated antimicrobial properties. It is widely used in skincare products and as a natural remedy for skin issues[99]. Moreover, glycosylated triterpenes, such as saponins, serve to protect plants from pathogenic microorganisms and pests, while select triterpenoids function as signaling molecules and have substantial implications in the food, health, and biotechnology industries[100].
Additionally, terpenoids possess pharmacological properties that promote human health. For instance, pinene found in pine trees exhibits anti-inflammatory characteristics and has the potential to improve lung airflow, thus potentially aiding respiratory conditions such as asthma. Furthermore, it has been shown to possess antibacterial and antifungal effects[101]. Ginkgolides are natural platelet activation antagonists that possess neuroprotective and reparative effects, making them crucial in ischaemic stroke treatment[5]. Cannabinoids have been utilized for thousands of years due to their anxiolytic and anesthetic properties[6]. Artemisinin is a widely used anti-malarial medication that protects millions of people from malaria annually[102].
Benefits of alkaloids in human health
-
Alkaloids play an important role in humans as they can increase the activity of the prefrontal cortex, thalamus, and visual system. They act as competitive inhibitors of muscarinic acetylcholine receptors, leading to anticholinergic effects. Alkaloids are also used as agents for sympathetic stimulation by directly targeting alpha and beta receptors. They have antipsychotic and antihypertensive activities, as well as presynaptic alpha-2 adrenergic blocking, mild antidiuretic effects, and antineoplastic properties. Additionally, alkaloids exhibit various other activities, including anti-inflammatory, analgesic, ganglion-blocking, insecticidal, and hepatoprotective effects, as described by Debnath et al.[103]. For example, berberine, an alkaloid found in plants such as goldenseal (Hydrastis canadensis) and Oregon grape (Berberis spp.), exhibits these properties. It has been shown to have antiviral effects against several viruses, including herpes simplex virus (HSV), respiratory syncytial virus (RSV), and influenza viruses[104]. Pyrrolizidine alkaloids, found in plants like comfrey (Symphytum spp.) and coltsfoot (Tussilago farfara), demonstrate antibacterial properties against bacteria such as Staphylococcus aureus and Escherichia coli[105].
Alkaloids are also used to enhance immune function, nutrition, and physical performance, and can be found in common foods, drinks, and supplements. For instance, caffeine in coffee offers antioxidant, anti-inflammatory, and stimulatory benefits. Theobromine and paraxanthines in cocoa act as antioxidants. Gingerol and gingerol in ginger possess antioxidant, anti-inflammatory, antibacterial, and antitumor capabilities[106, 107]. Pilocarpine, an alkaloid present in jaborandi leaves (Pilocarpus spp.), has medicinal properties and is well-known for its effect in inducing saliva production to treat xerostomia (dry mouth)[108].
-
Plants communicate via volatile SMs in response to biotic stress, with terpenoids being the most widely studied among them. Terpenoids serve a variety of functions, including acting as alarm substances, defense emissions, tracking markers, and deterrent food repellents[66]. Terpenoids as alarm signals that trigger or induce defense responses in neighboring plants or tissues of the same plant that have not yet been attacked[109]. These alarm signals are not only effective within species, but also between species. The emission of volatile terpenoids, specifically glucosinolates, is observed when wild radish (Raphanus raphanistrum) experiences herbivory or pathogen attack. The volatile terpenoids act as signaling molecules that instruct nearby mustard plants to produce defense-related compounds and enhance their resistance against herbivores or pathogens. Consequently, this type of plant-to-plant communication through volatile terpenoids allows mustard plants to orchestrate their defenses and increase their overall resilience to biological stress[110]. Some terpenoids also act as chemoattractants for herbivores. The compound 4,8-dimethyl-1,3(E),7-nontriene, a hotter terpenoid derivative of nerolidol, acts as an attractant for insect herbivore. This, in turn, helps strengthen the plant's defenses. In addition, the shrub known as Artemisia tridentata, or sagebrush, emits volatile terpenoids, such as camphor and limonene, into the atmosphere. Sagebrush plants of the same species can recognize conspecifics by terpenoids profile and adjust their growth and defense strategies accordingly. When sagebrush plants are exposed to their own volatile cues, they allocate additional resources to defense against herbivores and exhibit slower growth, suggesting a form of kin recognition and kin-directed defense[111].
-
Horticultural plants have a wide variety of SMs such as flavonoids, terpenoids, and alkaloids that play an important role in resisting adversity. These compounds help plants withstand heat, drought, salt, UV radiation, and attacks by pests and pathogens. In addition, these SMs play an important role in improving human health and treating human diseases. In the future, there is a growing interest in exploiting the potential of SMs in horticultural plants. Our goal is to better understand the function of these compounds under stress and in human health. In addition, exploring the potential of these SMs for industrial applications such as biopesticides and pharmaceuticals is an exciting avenue for future research. Overall, understanding and harnessing the functions of SMs in horticultural plants holds great promise for the development of more resilient and productive crop varieties to meet the demands of climate change and global population growth.
-
The authors confirm contribution to the paper as follows: study conception and design: Li W, Tang H; draft manuscript preparation: Wang Q, Xie H; manuscript revision: Li W, Tang H; All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This work was supported by the National Natural Science Foundation of China (Grant Nos 31971408 and 32101558), the Natural Science Foundation of Jiangsu Province (Grant No. BK20210801).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Tang H, Wang Q, Xie H, Li W. 2024. The function of secondary metabolites in resisting stresses in horticultural plants. Fruit Research 4: e021 doi: 10.48130/frures-0024-0014
The function of secondary metabolites in resisting stresses in horticultural plants
- Received: 22 December 2023
- Revised: 21 February 2024
- Accepted: 12 March 2024
- Published online: 03 June 2024
Abstract: Horticultural plants contain various secondary metabolites (SMs), including flavonoids, terpenoids, and alkaloids. These compounds play a critical role in plant growth, helping the plants adapt to different types of stress and providing numerous health benefits to humans. Advances in detection methods, such as liquid phase mass spectrometry and spatial metabolomics, have enabled the identification and characterization of a growing variety of SMs and their unique functions in horticultural plants. Recent studies have investigated the effects of SMs on horticultural plants under different biotic and abiotic stresses. This article offers a detailed review of the functions of key representative SMs in horticultural plants, focusing on their responses to biotic and abiotic stresses and their impact on human health. Furthermore, we explore the potential of using these compounds to improve the resistance of horticultural plants through future breeding efforts.
-
Key words:
- Horticultural plants /
- Secondary metabolites /
- Flavonoids /
- Terpenoids /
- Alkaloids /
- Stresses /
- Human health