-
Turfgrasses are divided into cool-season and warm-season turfgrasses according to the differences in climate types of origin. The optimum temperature range for cool-season turfgrasses aboveground parts is approximately 15−24 °C, and that for underground parts is approximately 10−18 °C[1]. Cool-season turfgrasses are mainly distributed in cool and humid, semi-humid, semi-arid and transitional zones. They have the characteristics of dark green leaves, and good adaptation to cold stress[2]. Commonly used cool-season turfgrass species include Kentucky bluegrass (Poa pratensis), tall fescue (Festuca arundinacea), perennial ryegrass (Lolium perenne) and creeping bentgrass (Agrostis stolonifera).
High temperature is one of the important environmental factors limiting the management and growth of cool-season turfgrasses. When the ambient temperature exceeds 30 °C, it hampers the growth of cool-season turfgrasses, leading to leaf wilting, yellowing, and even seedling mortality[3]. The cool-season turfgrasses in temperate and transitional zones are highly sensitive to extreme heat in the summer season. High temperature leads to the decrease of ornamental value and increase of maintenance cost of cool-season turfgrasses, which severely hinders the promotion and application of cool-season turfgrasses, posing an urgent challenge in turf production. The cultivation of heat-tolerant varieties is therefore the foremost priority in temperate and transitional zones. It is crucial to investigate the physiological and biochemical characteristics of cool-season turfgrasses in response to high temperatures, elucidate heat-tolerance-associated genes and proteins, and unravel the molecular mechanisms underlying the heat-tolerance response. In this review, the research progress of morphological and physiological changes induced by high temperature in cool-season turfgrass species were summarized, as well as the underlying molecular mechanisms governing the response to heat stress. Furthermore, potential strategies for enhancing the heat tolerance of cool-season turfgrasses were also explored.
-
Elevated ambient temperatures lead to suboptimal conditions for the growth of cool-season turfgrasses, resulting in reduced seed germination rates, decreased root vitality and tillering, wilting and yellowing leaves, and the occurrence of dead seedlings (Fig. 1). The seeds of three perennial ryegrass varieties, namely 'Yatsyn', 'Nui', and 'Mathilde', exhibited a significant decrease in germination rates when subjected to different temperatures. Specifically, the germination rates at 36 °C were only 3.3%, 29.7%, and 1.6% for three perennial ryegrass varieties, respectively, whereas that at 25 °C was 100%[4]. Previous studies have shown that the root growth and viability of tall fescue and creeping bentgrass decreased significantly under high temperature stress[5−7]. The tiller density (tiller number per unit area) of two varieties of creeping bentgrass ('L-93' and 'Penncross') experienced a significant reduction under high temperature treatment[8]. Tiller density is one of the important indexes to evaluate the turfgrass quality. The heat tolerance comparison among four representative cool-season turfgrasses species revealed that the turfgrasses initially exhibited leaf wilting and the emergence of dead spots, and ultimately leading to diminished grass cover and root growth under natural high temperature conditions[9]. The leaf senescence symptoms (such as chlorophyll decreases, leaf yellowing) of creeping bentgrass, perennial ryegrass, and bluegrass can also be induced by high temperatures[10−12]. The stay-green phenotype (leaves remain green phenotype for a long time) is one of the ornamental traits for evaluating the turfgrass quality. Heat sensitivity of cool-season turfgrass causes leaf senescence in summer season which increases turf management cost and limits the application of cool-season turfgrasses. Therefore, breeding of varieties with an improved stay-green trait in the summer season is highly required.
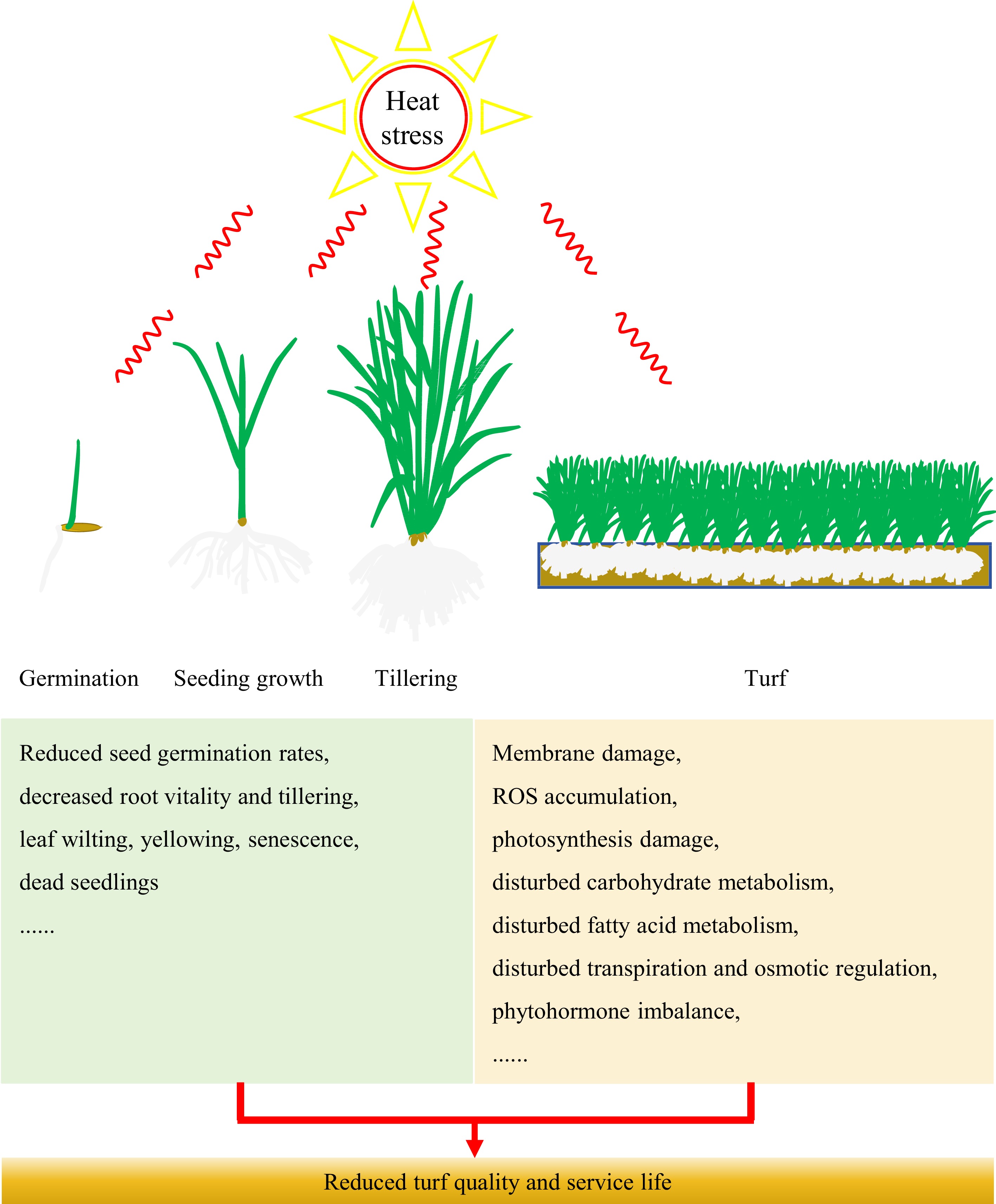
Figure 1.
Morphological and physiological characteristics of cool-season turfgrasses under heat stress.
Oxidative damage and antioxidant defense
-
Reactive oxygen species (ROS) are an array of highly active molecular oxygen derivatives[13]. The generation of ROS primarily occurs in chloroplasts photosystem system I and photosystem system II, and when photorespiration is activated, peroxisomes also produce ROS[14]. High temperature can induce a substantial accumulation of ROS in plants, which changes the properties of membrane proteins and membrane lipids, inducing lipid peroxidation and enzyme inactivation, thus increasing membrane permeability, causing damage to plants[13,14]. Simultaneously, plant cells possess a repertoire of enzymatic defense mechanisms, such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX), etc[3,14]. Therefore, the changes of ROS levels and antioxidant enzyme activities are employed as crucial criteria for assessing the thermotolerance of cool-season turfgrasses. For example, the heat-tolerant varieties of creeping bentgrass, perennial ryegrass, and tall fescue exhibit decreased ROS levels, reduced lipid peroxidation, increased membrane stability as well as antioxidant enzyme (SOD, POD, and CAT) activity when compared with heat-sensitive ones under high temperature stress[15−17]. Similarly, heat-tolerant warm-season turfgrasses showed higher ROS removal capacity due to higher antioxidant enzymes (SOD, POD, and CAT) activity when compared with heat-sensitive cool-season turfgrasses under high temperature stress[18,19]. After high temperature treatment, the activities of CAT, SOD and POD in perennial ryegrass initially increased before subsequently decreasing[17]. SOD, POD, and CAT function as effective ROS scavengers to detoxify overproduced ROS and maintain oxidative balance in plant cells, which helps to improve the heat tolerance and ornamental performance of cool-season turfgrasses[20].
Cell membrane thermal stability and fatty acid metabolism
-
The primary impact of high temperature stress on plants is the impairment of cell membrane integrity and the elevation of plasma membrane permeability. Cell membranes, composed of lipids and proteins, are highly organized structures and considered to be the most temperature-sensitive components in plant cells[21,22]. High temperature stress causes damage to the membrane structure, resulting in the disintegration of membrane lipids and an increase in fluidity, permeability, and loss of cell electrolytes[22]. Malondialdehyde (MDA), a byproduct of peroxidation in unsaturated fatty acids, serves as a crucial indicator for assessing the extent of lipid membrane peroxidation[23]. Under high temperature stress, a substantial quantity of superoxide free radicals, hydroxyl free radicals, and MDA accumulate in plant cells, which leads to alterations in membrane proteins and membrane lipids, resulting in increased membrane permeability and damage to plants[24]. Previous studies have shown that the heat-tolerant varieties of tall fescue and creeping bentgrass stocks exhibited lower MDA content, lower lipid peroxidation level and higher membrane stability under high temperature stress than those in heat sensitive ones[25−27].
Fatty acids play crucial roles as integral components within cellular membranes, the endoplasmic reticulum, the Golgi apparatus, and chloroplast[28,29]. Under high temperature stress, a large amount of ROS accumulate in plants, which leads to the oxidation of fatty acids, causes the change of the composition and saturation of fatty acids, and disrupts the structure of phospholipid bimolecular layer, thus increasing fluidity and permeability, destroying the integrity of the membrane, increasing the leakage of organic and inorganic ions in the cells, thereby affecting plant heat tolerance[29,30]. The fatty acid content of tall fescue heat-tolerant varieties was significantly lower than that of heat-sensitive varieties under high temperature stress[25]. The content of saturated fatty acids in the leaves of creeping bentgrass increased proportionally with the duration of high temperature treatment[27]. Previous studies have further shown that high levels of saturated fatty acids is helpful to reduce the damage of high temperature to plant cell membrane[25,31]. Therefore, regulation of fatty acid metabolism provides new approaches to maintain cell membrane stability under high temperature condition for cool-season turfgrasses.
Photosynthesis and carbohydrate metabolism
-
Photosynthesis is one of the most heat-sensitive physiological processes in plants[32]. Under high temperature stress, the thylakoid membrane structure of plant chloroplasts and the thermal stability of each component within the photosynthetic system are changed, the photochemical reactions in the thylakoid and carbon metabolism in the chloroplast matrix are impaired[24,33,34]. Moreover, the chlorophyll and photosynthetic pigment content, the maximum quantum efficiency of photosystem II, transpiration rate, photosynthetic rate and activities of membrane-associated enzymes are inhibited, thus reducing the photosynthetic rate of plants under heat stress condition[24,33−36]. Zhang et al. found that knockdown of perennial ryegrass STAY-GREEN (SGR) gene resulted in heat sensitivity as evidenced by degradation of photosystem protein (including Lhca3, Lhcb1/2/3/5, PsaA, PsbA (D1), PsbD (D2) and RbcL), decrease of the photosystem II quantum yield, and increase of energy dissipation level when compared to wild type (WT)[37]. Bi et al. observed that the potential to protect the photosystem II of heat-tolerant tall fescue varieties was higher than that of heat-sensitive varieties due to increased gene expression of PsbA (encoding protein subunits of photosystem II core reaction center complex) under high temperature stress[16]. Overexpression of creeping bentgrass SMALL HEAT SHOCK PROTEIN 17 (HSP17) reduced heat tolerance in Arabidopsis by reducing chlorophyll and inhibiting plant photosynthesis[38]. In the above studies, a positive correlation between heat tolerance and photosynthesis efficiency of cool-season turfgrasses was observed, which indicated that maintaining high photosynthesis efficiency was very important to improve heat tolerance of plants.
Carbohydrates serve as a crucial intermediary storage product bridging the gap between photosynthesis and growth utilization, not only providing energy for plant growth and development, but also playing a pivotal role in regulating plant stress tolerance[39]. High temperature will destroy the carbon assimilation process in photosynthesis and the carbon consumption process in respiration, while long-term exposure to high temperature will lead to carbon consumption or starvation of plants, thus limiting the growth of cool-season turfgrasses[20]. Studies have shown that under high temperature stress conditions, carbohydrates including glucose, sucrose, and starch in perennial ryegrass[40], tall fescue[25], creeping bentgrass[41], kentucky bluegrass[26], Festuca rubra[26] and Trifolium repens[26] accumulated, and heat-tolerant varieties exhibited higher carbohydrate contents than that in heat-sensitive varieties. These results indicated that carbon metabolism was closely related to heat tolerance of cool-season turfgrasses. However, the molecular mechanism of carbon metabolism during heat stress response of cool-season turfgrasses is still unclear.
Transpiration and osmotic regulation
-
In the early stage of heat stress, transpiration of plant leaves increases, and water vapor is released to the environment through stomata to reduce leaf temperature[42]. With the prolonged duration of high temperature, the water evaporation of plant leaves is excessive, and the stomata is gradually reduced or closed, followed by declined transpiration, and photosynthesis[42,43]. Similar results were observed in perennial ryegrass and tall fescue, where transpiration rates increased during the first 9 d of heat stress[44]. Under the condition of sufficient soil moisture, the increase of plant transpiration can effectively reduce leaf temperature, so as to maintain plant physiological function at high temperature stress. Under high temperature stress, plants adjust cellular osmotic pressure through accumulation of compatible solutes, which are a group of highly soluble organic molecules acting as osmoprotectants to stabilize cellular proteins[24]. The compatible solutes not only serve as a stress signal, but also play crucial roles in mitigating the stress-induced plant injury possibly by maintaining photosynthesis, antioxidant enzyme activity, and nonenzymatic antioxidant compound levels, thereby reducing ROS content and enhancing plant cell osmotic potential[45,46]. Under high temperature stress, the increase of osmoprotectants in plants can effectively buffer the water loss caused by transpiration, thus maintaining the physiological function of cells and reducing the damage of high temperature to plants[46]. The content of soluble sugar and proline significantly increased in Kentucky bluegrass, perennial ryegrass, and tall fescue under high temperature stress, and heat-tolerant varieties demonstrated superior growth rates, tiller numbers and antioxidant activity, and also exhibited higher soluble sugar and proline contents than those in heat-sensitive varieties[17,46−48]. Exogenous application of proline may enhance heat tolerance of creeping bentgrass by increasing endogenous proline content[49]. Therefore, understanding the regulatory mechanism of compatible solutes (such as soluble sugar and proline) will help improve the heat tolerance of cool-season turfgrasses.
Phytohormone
-
Phytohormones, such as auxin, abscisic acid (ABA), salicylic acid (SA), jasmonate acids, cytokinin (CTK) and ethylene (ET), serve as crucial endogenous signaling molecules in plants and play pivotal roles in regulating plant growth and development as well as abiotic stress responses[50−55]. Li et al. investigated the alterations in endogenous hormone levels in heat-tolerant and heat-sensitive varieties of perennial ryegrass under high temperature stress, and observed that maintaining appropriate levels of indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), and SA, as well as delaying the increase in ABA content and the decrease in gibberellin content, may contribute to enhancing the thermotolerance of perennial ryegrass[53]. The ABA content exhibited an initial increase followed by a subsequent decrease in creeping bentgrass under high temperature stress, whereas the ET and CTK contents gradually declined[54]. The ABA content in Kentucky bluegrass gradually increased under high temperature stress, while the IAA content exhibited an initial increase followed by a subsequent decrease[55]. Exogenous ABA, SA, CTK, and ET significantly enhanced the heat tolerance of various cool-season turfgrasses (Supplemental Table S1). ABA, ET and SA function as pivotal hormones in plant response to abiotic stress including heat through regulating stress responsive genes and senescence related genes, whereas IAA and CTK are important hormones in maintaining plant growth under heat stress condition[50−53]. Hormone metabolisms are complex and the homeostasis of endogenous hormones is important for proper growth and development of cool-season turfgrasses under heat stress condition.
-
With the rapid development of next-generation sequencing technology, high-throughput RNA sequencing has become extensively employed in investigating the stress response mechanism of turfgrasses. Currently, transcriptomic methods have been employed by researchers to investigate the response of Kentucky bluegrass[56], tall fescue[19,57], creeping bentgrass[58], and perennial ryegrass[17,57,59] as well as other cool-season turfgrass species to high temperature stress. These studies aim to elucidate key genes involved in the response and explore the molecular mechanisms underlying turfgrass adaptation to high temperature.
Qiong et al. conducted transcriptome analysis on two Kentucky bluegrass varieties subjected to 40 °C stress and observed that heat-sensitive Kentucky bluegrass exhibited a higher number of up-regulated differentially expressed genes than that of heat-tolerant variety, including HEAT STRESS TRANSCRIPTION FACTORS (HSFs, such as HSFA2, HSFA3, HSFB1 and HSFC1) and HEAT SHOCK PROTEINS (HSPs, such as HSP70 and HSP81)[56]. Liu et al. conducted transcriptome analysis of tall fescue and bermudagrass (Cynodon dactylon) under 42 °C stress, and found that high temperature stress induced the specific expression of F-box genes and ABA pathway genes in tall fescue[19]. The transcriptomic analysis of creeping bentgrass in response to high temperature stress revealed a close association between the heat tolerance and expressions changes of four miRNA, including cca-miR156b, miR398s, aly-miR159c-3p and ata-miR408-3p[58]. The transcriptome data of perennial ryegrass after heat stress treatment showed that genes involving in antioxidant response, plant hormones, signal transduction, and cellular metabolic pathways were enriched under high temperature stress[59]. Although a large number of genes related to heat tolerance of cool-season turfgrasses have been identified and cloned based on RNA sequencing, the detailed functions and regulatory mechanisms of these genes during turfgrass heat stress response remain to be characterized.
Transcriptional regulatory networks in response to high temperature stress
-
Plant HSFs are the most important components of transcriptional regulatory networks in response to high temperature stress[60]. Under high temperature conditions, plant HSFs activate heat stress-responsive genes (HSRs) by binding to heat shock elements (HSEs, 5'-nGAAnnTTCn-3') in HSRs promoters, thereby enhancing plant heat tolerance[60,61]. The heat stress-responsive genes encompass HSPs and other molecular chaperones, ROS scavenging enzymes, metabolic balance-protecting enzymes, plant hormone synthesis-related enzymes, and other transcription factors[60,61]. Based on the characteristics of DNA-binding domain and oligomerization domain in its amino acid sequence, HSFs can be divided into three classes: A, B and C. HSFA and HSFC have similar functions, often acting as positive regulators of high temperature stress, while HSFB mainly acts as inhibitors[60,62]. In total, 25 HSFs have been systematically identified in perennial ryegrass, and nine HSF genes were significantly induced by high temperature stress[63]. Seventy-four HSF genes were identified in tall fescue based on transcriptome data, and 34 HSF genes were significantly induced at high temperature stress[57]. The overexpression of tall fescue FaHSFA2c has been shown to enhance the heat tolerance of both tall fescue and Arabidopsis, as well as restore the heat-sensitive phenotype observed in Arabidopsis hsfa2 mutants[64]. Italian ryegrass (L. multiflorum) LmHSFA5 enhanced plant heat and drought tolerance by activating the expression of LmHSP18.2 and LmAPX2[65]. The heat tolerance function of class C HSF gene in cool-season turfgrasses has been recently investigated. Overexpression of perennial ryegrass LpHSFC1b and LpHSFC2b and tall fescue FaHSFC1b increased the heat tolerance of transgenic Arabidopsis and activated the expression of downstream heat stress response genes[17,63,66]. The above results indicate that HSFs are closely related to heat tolerance of cool-season turfgrasses, and functional characterization of HSFs target genes provides further clues to understand heat stress response to high temperature.
Heat shock proteins play a crucial role as molecular chaperones in plant thermoregulatory networks, facilitating the refolding or degradation of denatured proteins[67]. HSPs can be classified into five classes based on their molecular weight: HSP100, HSP90, HSP70, HSP60, and small heat shock proteins[68,69]. Heterologous expression of rice (Oryza sativa) OsHSP26 significantly enhanced the antioxidant and heat tolerance of tall fescue[70]. Tall fescue FaHSP17.8-CII enhances plant heat tolerance by modulating transcriptional memory by remodeling photosystem II and ROS signaling[71]. Creeping bentgrass AsHSP26.8 negatively regulates plant heat tolerance by regulating auxin related genes, HSPs, HSFs and other stress-related genes[72].
In addition to HSF genes, other family genes also play an important role in the plant transcriptional regulation network at high temperature, such as F-box, MULTIPROTEIN-BRIDGING FACTOR 1c (MBF1C), NON-YELLOW COLORING 1 (NYC1), STAYGREEN (SGR) and HOMEOBOX (HOX) genes (Fig. 2)[12,19,37,73−75]. Heterologous expression of tall fescue and bermudagrass F-box genes significantly increased the heat tolerance of transgenic Arabidopsis[19]. Heterologous expression of wheat (Triticum aestivum) TaMBF1C significantly increased the heat tolerance of perennial ryegrass[74]. Zhang et al. conducted heat tolerance identification and molecular marker experiments on 98 varieties of perennial ryegrass, revealing a close association between heat tolerance and four chlorophyll catabolism genes (NYC1, NYC1-like (NOL), SGR, and PHEOPHYTINASE (PPH))[76]. Subsequent investigations demonstrated that the interference of NOL and SGR in perennial ryegrass could effectively delay heat-induced leaf senescence and enhance leaf greenness retention[12,37,75]. The expression levels of HOX6, HOX8, and HOX24 in perennial ryegrass exhibited a negative correlation with heat stress, whereas the expression levels of HOX21 showed a positive correlation with heat stress[73]. These studies characterized the detailed functions of heat stress responsive genes and provide genetic resources for molecular breeding of heat tolerant cool-season turfgrasses in the future.
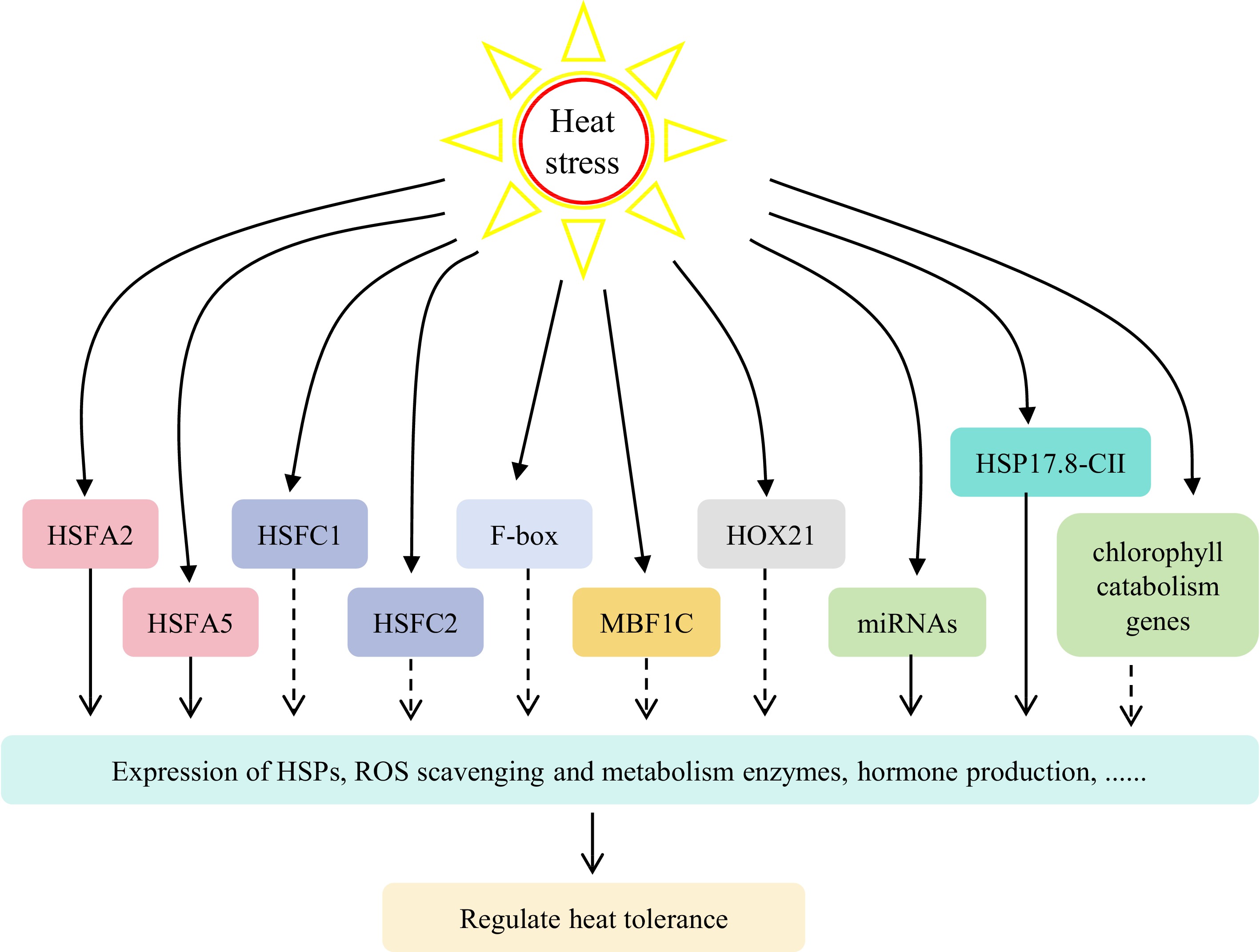
Figure 2.
The regulatory network involved in heat stress response in cool-season turfgrasses. Heat stress induces expression of heat stress-related genes (HSFs, F-box, MBF1C, HOXs, etc) or miRNA, which controls the expression of downstream genes to regulate plant heat tolerance. HSFs, heat stress transcription factor family genes; MBF1C, multiprotein-bridging factor 1c; HOX21, HOMEOBOX gene 21; miRNA, microRNAs.
The function of microRNAs (miRNAs) in response to high temperature stress
-
The microRNAs, as small noncoding regulatory RNAs, perform posttranscriptional regulation by facilitating mRNA degradation (Fig. 2)[77]. miRNAs have been demonstrated to play a pivotal role in the response of turfgrass to high temperature stress[58,78−82]. Li et al. found that the expression profiles of miRNA in the two genotypes of tall fescue were significantly different through small RNA sequencing, and identified four miRNAs (including miR7758, miR5568c-5p, miR5813, and miR9774) significantly induced by high temperature[82]. Analysis of creeping bentgrass small RNA data revealed that nine miRNAs (including miR398s, cca-miR156b, aly-miR159c-3p, ata-miR408-3p, vvi-miR845c, ama-miR156, novel-24223, novel-2964, and novel-10098) were closely related to heat tolerance of creeping bentgrass[58]. Based on the comprehensive analysis of transcriptome and small RNA data, the miRNA-RNA regulated heat tolerance network in perennial ryegrass was constructed, and 20 miRNAs (such as miR5658-z, miR5185-y, miR1144-z, novel-m0258-5p, novel-m0163-3p and novel-m0008-5p) and their corresponding 51 target genes (such as LpCOMT, LpLOX, LpPPH, LpNAC, LpDDP and LpLAC) were identified to be involved in the regulation of heat tolerance in perennial ryegrass[79]. Ectopic expression of rice OsmiR408 significantly enhanced the thermotolerance of perennial ryegrass[80]. Rice OsmiR393 may increase the heat tolerance of creeping bentgrass by regulating the expression of AsHSP17 and AsHSP26.7[81]. Small RNA sequencing identified a large number of miRNAs and target genes involving in heat stress response in cool-season turfgrasses[82]. The data showed that small RNAs and their targets were extensively changed under heat stress condition. Although a large number of miRNAs related to heat tolerance of cool-season turfgrasses have been identified based on small RNA sequencing technology, functions and mechanisms of miRNA regulated pathways need to be characterized in cool-season turfgrasses under heat stress condition.
-
Several cultivation and maintenance management strategies enhanced the heat tolerance of cool-season turfgrasses, including application of exogenous small molecule substances or microorganisms, optimization of nutrient and water management, and stress acclimation[50,2]. Exogenous application of phytohormones and growth regulators proved to effectively enhance the heat tolerance of cool-season turfgrasses, including abscisic acid, melatonin, polyamines, cytokinin, salicylic acid, brassinosteroids, ethylene, gibberellin, strigolactones, jasmonate acids and paclobutrazole. Additionally, osmoregulatory substances (proline), inorganic substances (calcium and silicon), gas molecules (nitric oxide and carbon dioxide), other small molecules (γ-aminobutyric acid, inorganic nitrogen, ascorbic acid, citric acid, butanediol and hydrogen peroxide) and microorganisms (Arbuscular mycorrhizal and Aspergillus aculeatus) also showed protective effects on heat stress tolerance in cool-season turfgrasses (for details, see Supplemental Table S1 and associated references). Previous studies have shown that heat acclimation enhanced plants tolerance to high temperature, that is, plants can acquire stress memory through short-term heat stress treatment to survive under long-term and more severe heat stress condition[70,83,84]. High temperature acclimation diminished chloroplast damage and decreased the contents of antioxidant ascorbic acid and glutathione in tall fescue and perennial ryegrass, thereby improving the heat tolerance of plants[84]. Attention should be paid to the fact that these research results were obtained under laboratory conditions. However, the heat tolerances of cool-season turfgrasses were affected by many factors such as environment and management. Therefore, further field experiments are needed to test these research results.
Breeding of heat tolerant turfgrass varieties
-
The conventional breeding method serves as the fundamental approach for cultivating new turfgrass varieties that possess superior heat tolerance and agronomic characteristics[85]. Conventional breeding approaches typically relies on phenotypic selection associated with heat tolerance, enabling the successful transfer of heat-tolerant traits to specific varieties exhibiting favorable agronomic characteristics through accurate evaluation and selection of exceptional tolerant varieties or breeding lines[21,86]. The effects of high temperature on seed germination rate, tiller number, growth rate, root activity, chlorophyll, soluble sugar and proline content in leaves of cool-season turfgrass can be used as an important index to evaluate the heat resistance of cool-season turfgrass. At present, numerous turfgrass varieties with exceptional quality were obtained through conventional breeding. For example, Oregro Seeds Inc (Albany, NY, USA) used tall fescue 'K31' and other tall fescue varieties ('Stargrazer', 'Orygun' and 'Fawn') as parents to breed 'K32' varieties which can adapt to extensive maintenance conditions and have excellent durability. In the US, germiplasms of cool season turfgrass with improved heat stress tolerance were screened and selected at two different research farms, including tall fescue, perennial ryegrass and bentgrass species[87]. Therefore, many widely used cool season turfgrass come from conventional breeding approaches.
The utilization of genetic engineering in breeding heat-tolerant turfgrass is a more efficient and time-saving approach compared to conventional breeding methods. However, genetic engineering breeding may cause serious ecological crisis due to genetic drift, and the safety of transgenic plants must be considered. The presence of an efficient genetic transformation system is the prerequisite and basis for molecular breeding in turfgrass. Agrobacterium-mediated genetic transformation method has been successfully applied in perennial ryegrass[88], tall fescue[89], creeping bentgrass[90], and Kentucky bluegrass[91]. In addition, gene gun transformation was also successfully applied in perennial ryegrass[92], tall fescue[93]. The CRISPR/Cas9 system can realize accurate improvement of plant stress tolerance, yield and quality through precise editing of a genome, which can greatly promote the creation of heat-tolerant germplasm of turfgrass[94]. The establishment and application of CRISPR/Cas9 system for perennial ryegrass and tall fescue have also been successfully accomplished[92,95,96]. The application of CRISPR/Cas9 system and genetic transformation technology in cool-season turfgrasses could not only shorten the breeding process and improve the breeding efficiency, but accurately improve the ecological adaptability and ornamental quality of turfgrass. The efficient application of CRISPR/Cas9 systems requires high-quality genomes as a support. With the continuous updates of high throughput sequencing technology, more genome data of cool-season turfgrasses will be released to promote the genetic engineering breeding of turfgrasses.
Challenges and perspectives of research on the heat tolerance of cool-season turfgrasses
-
Numerous studies have been conducted on the heat tolerance of cool-season turfgrasses to primarily focus on the evaluation of heat tolerance, growth and development, physiological and biochemical aspects. However, the transcriptional regulatory network of turfgrass in response to heat stress still needs to be further characterized. Considering the fact that plant response to high temperature is a highly intricate process encompassing complex transcriptional regulation, posttranscriptional regulation, epigenetic regulation, as well as protein and metabolite balance, it becomes feasible to employ a combination of genomics, transcriptomics, epigenomics, proteomics, and metabolomics for systematic characterization of regulatory networks in response to high temperature and delving deeper into pivotal genes associated with heat tolerance. The results of these studies will provide genetic resources and serve as a reference for breeding new turfgrass varieties exhibiting high heat tolerance and exceptional agronomic traits.
Currently, despite the existence of numerous studies on exogenous substances mitigating high temperature stress in cool-season turfgrasses, the majority studies predominantly concentrated on individual plant hormones or small molecules, the evaluation of germplasm thermotolerance, and physiological level changes in response to heat stress. Less attention was paid regarding genes associated with plant hormone metabolic pathways and their molecular mechanisms within high temperature regulation networks. In the future, a comprehensive molecular analysis on how exogenous substances regulate the heat tolerance of turfgrass is worthy to be conducted. These efforts will contribute to establishing a solid theoretical foundation for both turfgrass maintenance and tolerance breeding.
The findings from studies on other plant species should also be extrapolated to turfgrasses. For instance, the overexpression of wheat TaMBF1C and rice OsmiR408 in perennial ryegrass has been shown to significantly enhance the thermotolerance of transgenic perennial ryegrass[74,80]. Turfgrass can be categorized into cool-season turfgrasses and warm-season turfgrasses based on their climate and regional origins. To explore the key regulatory genes associated with heat tolerance traits in these two types of turfgrass, a systematic comparison utilizing genomic, transcriptomic, and metabolomics techniques may serve as an effective approach to uncovering their natural differences in heat tolerance.
Research on turfgrass heat tolerance necessitates accurate phenotypic identification. The evaluation system for heat tolerance (for example, measurements of electrolyte leakage, malondialdehyde, chlorophyll, proline and antioxidant enzyme activities) has drawbacks due to low measurement accuracy, destructiveness, time and effort requirements, and limited applicability. These limitations fail to meet the demands of rapidly advancing turfgrass tolerance research and significantly impede the exploration of heat-tolerant resources in turfgrass. A systematic high-throughput phenotyping platform developed for maize[97] and rice[98] has the potential to be established in turfgrass. Subsequently, the high-flux turfgrass phenotyping platform for cool-season turfgrasses could be established using advanced technologies such as visible light imaging, spectral imaging, thermal imaging, fluorescence imaging, and other cutting-edge techniques. This system aims to achieve accurate identification of plant phenotypes in a dynamic and nondestructive manner, thereby facilitating rapid development in the improvement and breeding of heat-tolerant turfgrasses.
-
The authors confirm contribution to the paper as follows: study conception and design: Sun T, Chan Z; figure preparation: Sun T, Wang W; draft manuscript preparation and revision: Sun T, Chan Z. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by the National Key Research and Development Program of China 2023YFE0123200 to Zhulong Chan, the National Natural Science Foundation of China 32201451 to Tianxiao Sun, and 32071884 and 32371761 to Zhulong Chan.
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Exogenous substances enhanced heat tolerance of cool season turfgrass species.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Sun T, Wang W, Chan Z. 2024. How do cool-season turfgrasses respond to high temperature: progress and challenges. Grass Research 4: e010 doi: 10.48130/grares-0024-0008
How do cool-season turfgrasses respond to high temperature: progress and challenges
- Received: 26 November 2023
- Revised: 16 March 2024
- Accepted: 21 March 2024
- Published online: 10 April 2024
Abstract: The utilization of cool-season turfgrasses is widespread in urban greening, ecological restoration, and sports fields. The primary limiting factor affecting its growth and application is considered to be high temperature stress. Under heat stress condition, a range of physiological and morphological traits will be modulated in cool-season turfgrasses, resulting in a deterioration of lawn quality and subsequently impacting the ornamental and functional value of lawns. In this review, we summarize physiological and morphological changes in cool-season turfgrasses caused by high temperature stress. The research progress in molecular characterization of high temperature regulatory networks was further summarized. Approaches for improving cool-season turfgrasses thermotolerance were proposed. We further put forward challenges and perspectives of research on heat tolerance of cool-season turfgrasses, aiming to provide references for the research on characterization of heat tolerance mechanism and breeding heat tolerant cold-season turfgrass.













